Wed, Apr 24, 2024
Volume 5, Issue 1 (Winter 2019)
Iran J Neurosurg 2019, 5(1): 1-14 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Karki M, Roka Y B. Pterional Surgery vs. Endoscopic Endonasal Transsphenoidal Surgery for the Resection of Tuberculum Sellae Meningioma: A Systematic Review of Ophthalmological and Surgical Outcomes. Iran J Neurosurg 2019; 5 (1) :1-14
URL: http://irjns.org/article-1-116-en.html
URL: http://irjns.org/article-1-116-en.html
1- Department of Neurosurgery, Skull Base Tumor Research Center, Neuro-Cardio and Multispeciality Hospital Biratnagar, Morang, Nepal , jigyasu86@gmail.com
2- Department of Neurosurgery, Skull Base Tumor Research Center, Neuro-Cardio and Multispeciality Hospital Biratnagar, Morang, Nepal
2- Department of Neurosurgery, Skull Base Tumor Research Center, Neuro-Cardio and Multispeciality Hospital Biratnagar, Morang, Nepal
Full Text [PDF 945 kb]
(1617 Downloads)
| Abstract (HTML) (4051 Views)
Full Text: (1795 Views)
1. Introduction
Tuberculum Sellae Meningioma (TSM) is a distinct group of meningiomas originating from a number of sites, including the tuberculum sellae, diaphragma sellae, planum sphenoidale and chiasmatic sulcus. TSM represents 4%-10% of all intracranial meningiomas [1-3]. The first case of TSM was reported by Steward in 1899 as an incidental finding [4]. The first complete removal of a TSM was performed by Harvey Cushing in 1916 using a unilateral, sub-frontal approach [5]. As the tumor grows, it displaces, stretches or even encases vital structures. The optic nerve may be displaced superiorly and the internal carotid arteries may shift to the lateral, and if the tumor extends backward, it may push the pituitary stalk posteriorly [6].
These tumors are typically situated in a suprasellar midline position, displacing the optic chiasma posteriorly and slightly superiorly and the optic nerve laterally [3]. They can thus occupy either a prechiasmatic and/or infrachiasmatic location [2, 7]. The narrow anatomical relationship between this area and the optic apparatus explains the early visual disturbances that are the most common presentations upon patients’ admission, secondary to the displacement of the optic chiasma and nerves [8-11]. The resection of TSMs is surgically challenging due to their proximity to the neurovascular structures and is further complicated by the firm, dense and rubbery consistency of tumors [12, 13].
Various transcranial surgical approaches have been traditionally used to remove TSMs [14-18]. After the introduction of the operative microscope, almost 50 years after Cushing’s initial resection, Yasargil laid the foundation for the pterional approach in the 1970s [19, 20]. The disadvantages of the pterional approach include the narrow space and angle and the risk of profuse bleeding when removing the tumor [1, 9, 12]. The risk of retraction injury to the brain and requiring an additional basal exposure before early tumor vascularization are also plausible [21, 22]. Additionally, the contralateral bony optic canal is difficult to open in the unilateral pterional approach.
In an effort to overcome these obstacles and improve visualization and minimize injury to the optic nerve and chiasma and ensure blood supply to these structures, EETS has been used to resect TSMs [23-26]. Since the first description of EETS by Weiss M., there has been a steady interest in using the endonasal approach to remove tumors of the sellar, parasellar and anterior skull bases [22-24, 27-29]. Recently, EETS has been used to remove TSMs, and studies have shown that tumors can be removed effectively and safely by this method [22, 30, 31]. Both EETS and TPS have advantages and disadvantages for patients and neurosurgeons, and the surgical approach chosen for TSM must always be supported by evidence from scientific studies.
However, studies on this subject have been scarce. To assess the outcomes and benefits of TPS and EETS for the removal of TSM and inform proper and careful clinical decisions on the best surgical approach for TSMs, this meta-analysis was conducted on studies about TSMs removed by TPS and EETS.
2. Methods & Materials/ Patients
Search strategy and study design
A PUBMED and MEDLINE database search was performed to retrieve articles published on TSM in 2002-2014. The following keywords were queried singly and in combination: Meningioma, tuberculum sellae, diaphragma sellae and planum sphenoidale, pterional, resection and outcomes. For the purpose of comparison, a similar search was carried out using the following terms: Meningioma, tuberculum sellae, diaphragma sellae, planum sphenoidale, endoscopy, transsphenoidal, resection and outcomes.
This search was limited to traditional transcranial surgery, i.e. TPS and EETS. All the publications describing the outcomes of TSM surgery were selected; however, the review articles, commentaries and editorials were not selected because they did not include original data. For a more accurate comparison of TPS and EETS, reports describing TSM resection before 2002 were not selected, because reports on EETS have only come to exist in the last decade. Figure 1 presents a PRISMA flow diagram describing the search strategy used in the study.
Data extraction and inclusion criteria
All the included series were reviewed for study design, methodology and patient characteristics, as reported in Table 1. The total number of patients was extracted for each study and divided by treatment strategy. The reports describing transcranial approaches, except for pterional and microscopic/or endoscope-assisted and microscopic transsphenoidal surgery for TSMs, were excluded from the analysis. Only reports describing pure microscopic pterional surgery and pure EETS for TSMs were included in this analysis. Only TSM-distinct groups of meningioma originating from tuberculum sellae, diaphragma sellae and planum sphenoidale were included in this research.
Data reporting the patients’ age and gender, the number of cases, pre-operative visual deficits (either deficits in visual acuity or visual field or both), arterial encasement, optic canal involvement, tumor size (maximum diameter) and tumor volume were extracted from the published studies. Pre-operative visual deficits, headache and endocrine abnormalities were taken as major pre-operative clinical features. The objective outcomes extracted from the published series included visual outcomes (improved, stable and worsened), extent of resection (GTR and STR), peri-operative complications and operative mortality.
Disparity was observed in the post-operative visual function assessment among the series between the formal visual field testing and the subjective assessment method; however, both were taken together as the means of assessment of visual function. GTR was based on the intraoperative surgeon’s assessment, and on the post-operative computed tomography or magnetic imaging for the series that had this information available.
The “related articles” function was used to obtain any relevant articles. Likewise, the references of the included articles were reviewed to locate additional relevant articles. An assessment of bias was made only at the outcome level rather than at the individual study level, because only case series and case reports were included in the analysis. To avoid duplicate patients, as in the case of multiple papers published by the same authors/institutions, only the reports with the largest relevant articles were included. An article by Wang et al. [23] describing a case series with the EETS removal of TSM was excluded because the same author had reviewed the same case series in a recent series [30].
Similarly, De Divitis et al. had reviewed one manuscript and a patient series that had been included in a previous series [22, 24]. In such instances, the studied patients were included only once. Case reports describing less than five patients were also excluded. The studies associated with endoscopic-assisted microscopic transsphenoidal surgery were also excluded.
Statistical analysis
Data were analyzed in SPSS V. 17 The analysis of the categorical variables was performed by the Chi-square test and the t-test was used for the continuous variables. Fisher’s exact test was applied if there were equal or less than five values per cell, as appropriate. Two-tailed tests were performed for each analysis and were considered statistically significant if P value was <0.05.
3. Results
Study and patient characteristics
Out of the 90 studies retrieved from PUBMED and MEDLINE using the intended list of keywords (tuberculum sellae, meningioma, pterional and endoscopic transsphenoidal), only 21 met the inclusion criteria. Therefore, 69 studies were excluded from the search because they did not have original data, did not report the outcomes or did not differentiate endoscopic and pterional outcomes from studies that included a mixture of approaches. The search criteria (Figure 1) provided eight studies, reporting on 334 patients who had undergone pterional surgery for TSM (eight articles). During the same period, 13 studies were published that reported on the outcomes in 178 patients who had undergone EETS resection for TSMs (13 articles). Table 1 presents the characteristics and primary findings of the 21 studies.
Table 2 outlines the pre-operative characteristics of the patients and their tumors in studies conducted on TPS and EETS. In the TPS series, there were 334 patients with a Mean±SD age of 54.2±3.1 (range: 49-58.3) years with a mean follow-up of 56.6 (range: 3-198) months. The major presenting symptoms included visual deficits in 97% of the cases (306.334), headache in 23.4% (64.273) and endocrine abnormalities in 7% (7.100). In the EETS series, there were 178 patients with a Mean±SD age of 55.5±5.9 (range: 39.5-63.8) years with a mean follow-up of 15.5 (range: 1-98) months.
The major presenting symptoms included in the present search were visual deficits in 79% of the cases (127.160), headache in 20% (30.151) and endocrine abnormalities in 3% (3.97). A total of 34% of the male and 66% of the female subjects had undergone TPS and 33% of the male and 67% of the female subjects had undergone EETS for their TSM resection. Statistically significant differences were noticed in terms of the patients’ age between the TPS and EETS groups (P<0.0001). Similarly, pre-operative visual deficits also differed significantly between the TPS and EETS groups (97% vs. 79%; P=0.0022).
The volume of tumor, however, was comparatively larger in the TPS groups compared to the endoscopic groups (42.9 cm3 vs. 8.5 cm3), comprising a statistically significant difference (P=0.0006), although there were no statistically significant differences in tumor size between the two groups (P=0.9080; 2.68 vs. 2.63 cm). Although the percentage of patients followed-up with was 78% in the TPS group and 90% in the EETS group, the mean follow-up duration (in months) was longer in the TPS groups compared to the EETS groups (56.6 months vs. 15.5 months; P=0.0005). These two studies did not differ significantly in any other patient characteristics or presenting symptoms.
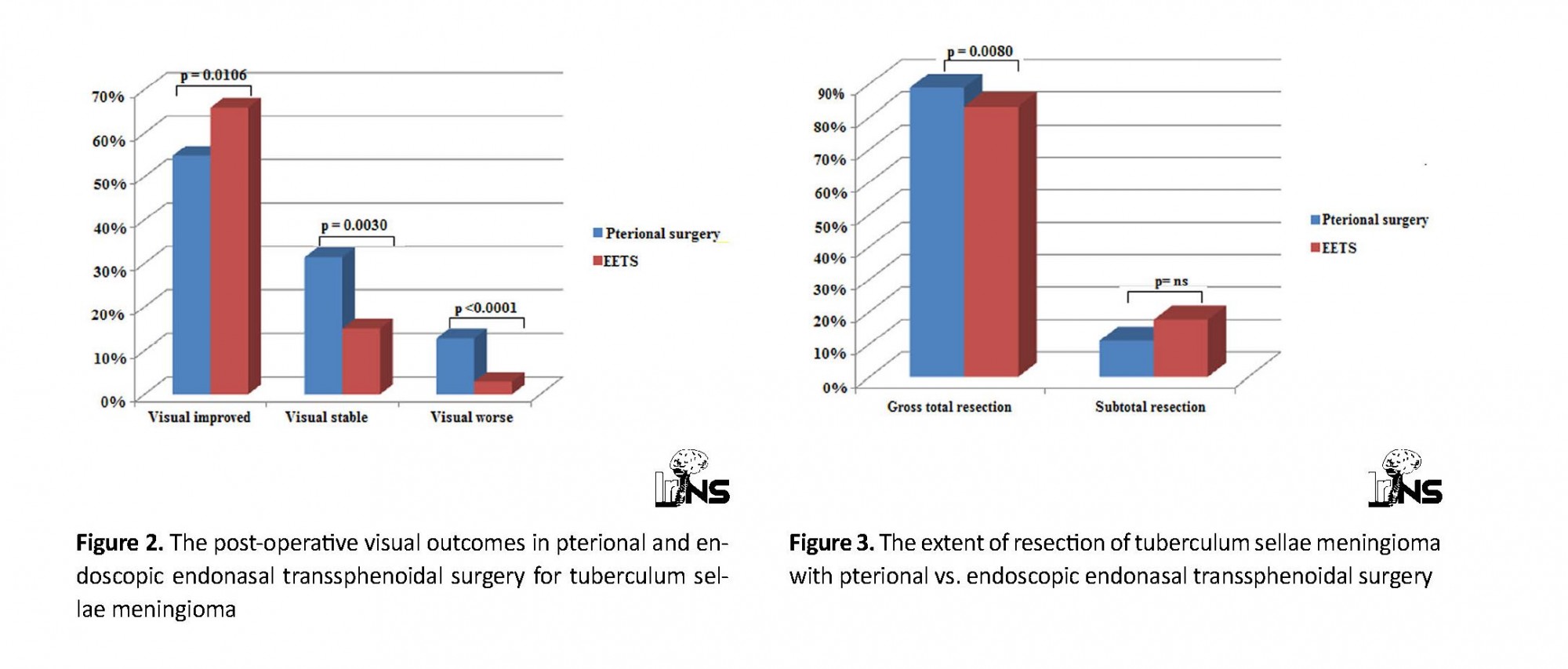
Table 3 presents a comparison of the outcomes and peri-operative complications between EETS and TPS for the removal of TSM. Visual improvement was reported in 67% of the cases (114.173) in the EETS groups and 55% (171.312) in the TPS groups, which comprise a significant difference (P=0.0106). Similarly, visual stability was observed in 15% of the cases (26.173) in the endoscopic surgery groups and 13.4% of the cases (98.312) in the TPS groups, which comprise a significant difference (P=0.0030) (Figure 2).
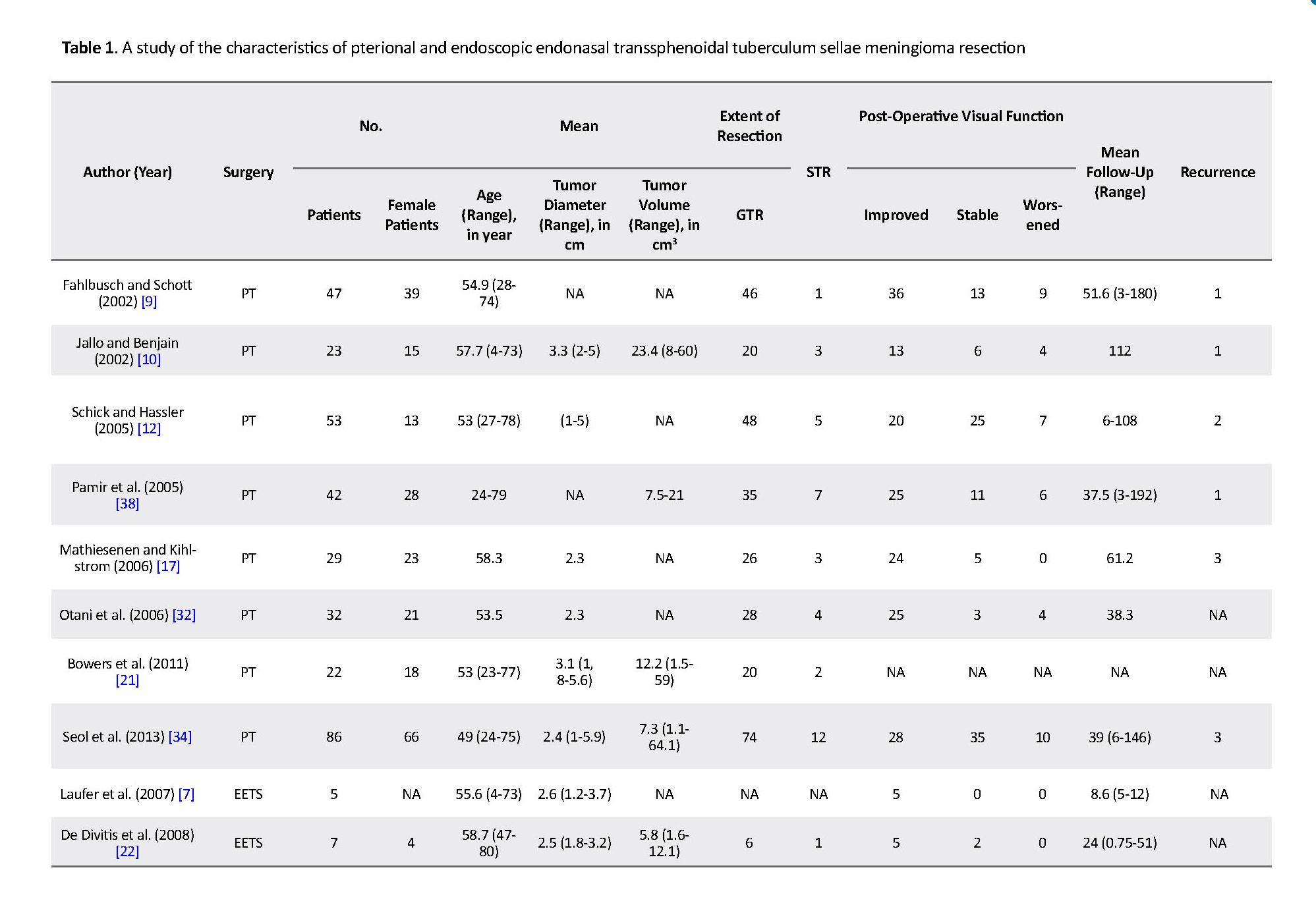
Visual worsening associated with endoscopic groups was significantly lower compared to the TPS groups (3% vs. 13%; P<0.0001). The Gross Total Resection (GTR) and Subtotal Resection (STR) were 89% (297.334) and 11% (37.334) in the TPS groups. In the endoscopic surgery groups, GTR and STR were 83.2% (144.173) and 17% (29.173), respectively. GTR was significantly higher in the TPS groups compared to the endoscopic surgery groups (89% vs. 83.2%; P=0.0080) (Figure 3).
In the TPS groups, the most common peri-operative complications were infections (pneumonia, meningitis and bone flap/wound infection) in 5%, CSF leak in 6%, permanent Diabetes Insipidus (DI) in 3.4%, hemorrhage in 5% and anosmia in 1%. In the endoscopic surgery groups, the most common peri-operative complications were CSF leak in 25%, infection in 5.4% and permanent DI in 11.1%. There was recurrence in 4% of the cases (11.280) in the TPS groups and 6.4% (6.93) in the endoscopic groups, which does not comprise a significant difference (P=0.8867). Peri-operative mortality was reported as 6% (7.123) in the TPS groups and 1.2% (1.82) in the endoscopic groups, which comprise a significant difference (P=0.0486). There were no statistically significant differences in terms of the other peri-operative complications (Table 3).
4. Discussion
This study analyzed case series reporting TSMs surgically removed by TPS and EETS plus their visual and resection outcomes. Essentially, the study aimed to compare the effectiveness and safety of TPS and EETS for visual improvement and total resection. Visual improvement was generally associated with EETS and Gross Total Resection (GTR) with TPS. Visual outcomes comprise one of the most important factors to consider in the surgical treatment of TSMs [30, 32, 33]. Post-operative visual improvement was significantly higher with EETS compared to TPS for the resection of TSMs (66% vs. 55%; P=0.0106). Fahlbusch and Scott reported an 80% improvement and 20% worsening in visual functioning after pterional craniotomy for the removal of TSMs.
In a recent study, Soel et al. [34] also reported improved visual function in only 40%, no changes in 50% and worsened visual function in 14.2% of the cases after TPS for the resection of TSMs. In a meta-analysis, Clark et al. [35] found that pure EETS led to a higher post-operative visual improvement than transcranial surgery for the resection of TSMs (87% vs. 59%; P<0.05). Divitis et al. [22] also reported improved post-operative visual function in 71% of their patients and no changes in 29% after EETS for the removal of TSMs.
Similarly, Komotar et al. [36] reported that 69.1% of the patients showed an improved visual function, while this function was stable in 18.2% and had worsened in 12.7% after endoscopic transsphenoidal surgery; with transcranial surgery, however, 58.7% of the patients showed visual improvement, 27.1% had stable visual function and 14.2% showed worsened visual function. Several studies did not report any significant differences in terms of visual outcome. Li et al. [37] found that if the greater wings of the sphenoid are removed, the TPS approach provides a shorter length of exposure to TSMs.
Nonetheless, since the area below the ipsilateral optic nerve and chiasma are not well visualized with the pterional approach, studies have reported that 0-20% of the patients experience visual worsening post-operatively [9, 17, 38]. The present review found a higher rate of post-operative visual worsening in the TPS group compared to the EETS group (13% vs. 3%; P<0.0001). The higher rate of post-operative visual improvement and lower rate of visual worsening after EETS compared to pterional craniotomy may be a contributing factor [7, 9, 30, 31, 39].
GTR remains the goal of meningioma surgery in most cases regardless of the adopted approach; however, the approach taken shall help reduce the risk of morbidity, such as visual worsening in TSM [8, 9]. Complete resection should not be achieved at the cost of increased surgical morbidity, such as the risk of visual worsening and hypothalamic dysfunction [40]. Surgical resection should be complete as much as possible so as to avoid the risk of recurrence. Overall, the GTR rates reported vary from 50% to 80% in endoscopic transsphenoidal surgery and from 70% to 100% in transcranial surgery [23-25, 29, 31, 41, 42].
Similarly, Komotar et al. [36] reported a higher rate of GTR in transcranial surgery compared to endoscopic transsphenoidal surgery (84.1% vs. 74.7%; P=0.041). Nonetheless, a study conducted by De Divitis et al. [22] showed GTR rates of 83.3% for transcranial surgery and 86.4% for EETS. The present analysis showed a significantly higher rate of GTR in TSMs removed by TPS compared to those removed by EETS (90% vs. 83.2%; P=0.0080), and this observation is not different from that reported by Fahlbusch and Scott, who showed a GTR of 97.9% with the TPS approach without post-operative MRI in all the cases and without any association between the degree of resection and the Simpson grade [9].
Chokyu et al. and Nakamura et al. reported 91.7% of complete resection in TSMs associated with the degree of resection with Simpson grading system [1, 43]. It is plausible for pterional craniotomy to allow an increase in the range of surgical instrument maneuverability, which helps resect large tumors with more lateral extension, vascular encasement and/or significant optic canal involvement, as in some of the reports on transcranial series [21, 44-46]. CSF drainage from the cisterns and the opening of the carotid and sylvian cistern and the lamina terminalis (in some cases) make the frontal lobe fall behind and need less retraction, which facilitates a wide exposure, clear visibility of the tumor, Internal Carotid Artery (ICA) and optic apparatus over the suprasellar space and may be the main reason for the higher rate of complete respectability of TSMs accomplished [47].
Bower et al. reported that resecting TSMs or its dural attachment from over the optic nerve in the optic canal or above or lateral to the anterior clinoid process is difficult in EETS. In the endonasal transsphenoidal route, however, the inferomedial and superomedial aspects of the optic canal are readily exposed. Nonetheless, the superior, superolateral and lateral aspects of the optic canal cannot be easily accessed from below, because of obstruction by the optic nerve and chiasma. The TPS approach is therefore better for addressing optic canal involvement and resecting tumors if the lateral extension of the dural attachement is superior to the optic canal and anterior to the clinoid process. One study reported a 90% GTR of TSMs with optic canal invasion after EETS, with a recurrence rate of 0%-12% [48].
As reported by some series, the extent of resection, histological grading of the tumor, length of post-operative follow-up period and mode and quality of assessment of the tumor are factors affecting the risk of recurrence [9, 36, 49, 50]. The present study also showed that the recurrence rate was higher in EETS than pterional surgery, but the difference was not statistically significant (6.4%, 6.93; 4%, 11.280; P=0.8867). The mean duration of follow-up was also shorter in EETS compared to TPS by 56.6 months (range: 3-192) and 15.5 months (range: 1-98), comprising a statistically significant difference (P=0.0005).
The present review found that a shorter duration of follow-up and lower extent of resection of tumors result in a higher rate of tumor recurrence according to some of the reviewed studies [9, 17]. The recurrence rate for TSMs ranged from 0% to 40% in the purely-endoscopic studies [21, 48, 51] and from 2.1% to 3.5% in the pterional studies [9, 12, 34]. Comparative studies of EETS and transcranial surgery for the removal of TSMs conducted by Komotar and Clark also reported shorter durations of follow-up in the endoscopic group compared to the craniotomy group [35, 36]. Even though meningioma is a benign and slow-growing tumor, there is a chance of recurrence [52, 53].
A longer follow-up is thus necessary in endoscopic transsphenoidal surgery in order to evaluate the long-term surgical outcomes of this approach and compare its recurrence rates with the rate associated with the craniotomy approach. Radiotherapy, fractionated stereotactic radiotherapy and observational are adjuvant treatments of choice, as they help arrest the growth of small tumors and the residual tumor after subtotal resection [54, 55]. Stereotactic radio-surgery is associated with an improved accuracy and less complications compared to conventional radiotherapy [56, 57].
Although EETS has many advantages, its disadvantages are also numerous and significant. The most well-known drawback of EETS is its high risk of CSF leakage, which can occur with the removal of the dura and bone underlying meningioma [22, 23, 29, 31]. Bone removal anterior to the tuberculum sellae is associated with a significantly high risk of CSF fistula compared to the standard transsphenoidal approach to sellae [24, 58]. In the present study, the CSF leak rate was 6% in TPS and 25% in EETS after the removal of TSMs, but the difference was not significant statistically (P=0.6038). Some of the endoscopic series reported CSF leak between 0% and 40% after the resection of TSMs [23, 25].
Similarly, Fatemi et al. reported a 14% CSF leakage rate with endoscopic surgery and a 0% rate with transcranial surgery [29]. In contrast, CSF leakage is rare with transcranial surgery. A CSF leakage usually occurs from the frontal sinus with craniotomy for the resection of TSMs [43]. The use of a peri-cranial flap with or without adipose tissue has been suggested and successfully applied as the most popular reconstruction technique [1, 59]. Fahlbusch and Scott showed a 6.4% rate of CSF leakage with the pterional craniotomy of TSMs [9].
Over the years, various reconstruction techniques have been developed and successfully applied for the reconstruction of leakage after EETS [60, 61]; most recently, the evolution of vascularized nasal septal mucosal flap reconstruction has decreased CSF leakage dramatically, i.e. to 16.1% (P<0.0001), which seems to be more effective because it facilitates the healing process [31, 62]. Since 2010, reports on CSF leakage rate have shown a significant reduction close to <5%, owing to vascularized nasal septal flap and multilayer closure (gasket seal technique) techniques [31, 63]. Nonetheless, CFS leakage is still an issue with EETS.
Diabetes Insipidus (DI) is also a common post-operative endocrinological complication [24, 30]. The present study found the rate of DI as 14% in EETS and 4% in TPS. Although there was a higher rate of DI in EETS, the difference was not statistically significant (P>0.05). Gadgil et al. [64] reported permanent DI in 2.7% of the cases after EETS and in 0.3% after transcranial surgery. Permanent DI was noted in 11.1% of the cases with EETS and 3.4% of the cases with TPS. EETS is more likely than TPS to damage the small perforating thalamic arteries or pituitary stalk/gland.
Furthermore, injury to the brain parenchyma with a subsequent seizure and hemorrhage in transcranial surgery have been shown to occur less frequently with EETS [22]. The present analysis showed a high rate of seizure in the TPS group and this observation is in line with some earlier case series, but hemorrhage was slightly higher in EETS, as an endoscopic approach to this space is controversial [13, 65]. Similarly, infection (including pneumonia, wound infection and meningitis) was common with both TPS and EETS. Some studies showed that respiratory infection (aspiration pneumonia) is the leading cause of death after surgery [38].
Schick and Hassler reported that two patients died as a result of cardioembolic embolism leading to brain stem infarction, and Divitis et al. reported that one patient died due to an unexpected massive intraventricular hemorrhage. The mortality rate was slightly higher with TPS than EETS (5.6% vs. 1.2%; P=0.0486); overall, however, peri-operative complications were not significantly different between the two procedures [12, 66].
The limitations of this study include: The visual outcomes were assessed by different methods, the resection of tumors were typically reported as gross total and subtotal in both EETS and TPS and was therefore not gradable by the Simpson grading system, which complicated the direct comparison of the results [7, 9, 10, 12, 21, 29, 38, 48, 64, 67]. Future studies are recommended to further address these limitations.
5. Conclusion
For the resection of TSMs, EETS produced a higher rate of visual improvement, a lower rate of visual worsening, a lower rate of gross total resection and a higher rate of CSF leakage compared to TPS. Patients’ characteristics and surgeons’ degree of comfort with one approach over the other are crucial for the choice of surgical strategy for the resection of TSMs. EETS is highly probable to provide a safe and effective option over TPS for TSM resection.
Ethical Considerations
Compliance with ethical guidelines
All the patients gave informed consent prior to inclusion in this study. no formal consent was required for this type of retrospective study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceiving and designing the study, data collection and manuscript drafting: Mohan Karki; Data collection and statistical analysis and provided technical feedback about the study design and analyses: Yam Bahadur Roka.
Conflict of interest
All the authors certify that they have no affiliations with or involvement in any organizations or entity with any financial or non-financial interests.
Acknowledgements
We would like to express our gratitude to Sabu Pokhrel for collecting the data and also to Dr. Firouz Afjal and Dr. Alok Jha for their valuable revision of our illustrations.
References
Nakamura M, Roser F, Struck M, Vorkapic P, Samii M. Tuberculum sellae meningiomas: Clinical outcome considering different surgical approaches. Neurosurgery. 2006; 59(5):1019-29. [DOI:10.1227/01.NEU.0000245600.92322.06] [PMID]
Goel A, Muzumdar D, Desai KI. Tuberculum sellae meningioma: A report on management on the basis of a surgical experience with 70 patients. Journal of Neurosurgery. 2002; 51(6):1358-64. [DOI:10.1097/00006123-200212000-00005] [PMID]
Chi JH, McDermott MW. Tuberculum sellae meningiomas. Neurosurgical Focus. 2003; 14(6):1-6. [DOI:10.3171/foc.2003.14.6.6]
Stewart J. The symptomatology of tumors involving the hypophysis cerebri. Transactions of the Association of American Physicians. 1899; 14:282-9.
Cushing HW, Eisenhardt L. Meningiomas: Their classification, regional behaviour, life history, and surgical end results. New York: Hafner Publishing Company; 1962.
Kinjo T, al-Mefty O, Ciric I. Diaphragma sellae meningiomas. Journal of Neurosurgery. 1995; 36(6):1082-92. [DOI:10.1227/00006123-199506000-00003] [PMID]
Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. Journal of Neurosurgery. 2007; 106(3):400-6. [DOI:10.3171/jns.2007.106.3.400] [PMID]
Ciric I, Rosenblatt S. Suprasellar meningiomas. Journal of Neurosurgery. 2001; 49(6):1372-7. [DOI:10.1097/00006123-200112000-00014] [PMID]
Fahlbusch R, Schott W. Pterional surgery of suprasellar meningiomas of the tuberculum sellae and planum sphenoidale: Surgical results with special consideration of ophthalmological and endocrinological results. Journal of Neurosurgery. 2002; 96(2):235-43. [DOI:10.3171/jns.2002.96.2.0235] [PMID]
Jallo GI, Benjamin V. Tuberculum sellae meningiomas: microsurgical anatomy and surgical technique. Journal of Neurosurgery. 2002; 51(6):1432-40. [DOI:10.1097/00006123-200212000-00013]
Zevgaridis D, Medele R, Müller A, Hischa A, Steiger HJ. Meningiomas of the sellar region presenting with visual impairment: Impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir. 2001; 143(5):471-6. [DOI:10.1007/s007010170076] [PMID]
Schick U, Hassler W. Surgical management of tuberculum sellae meningiomas: Involvement of the optic canal and visual outcome. Journal of Neurology, Neurosurgery, and Psychiatry. 2005; 76(7):977-83. [DOI:10.1136/jnnp.2004.039974] [PMID] [PMCID]
Symon L, Rosenstein J. Surgical management of suprasellar meningioma: Part 1: The influence of tumor size, duration of symptoms, and microsurgery on surgical outcome in 101 consecutive cases. Journal of Neurosurgery. 1984; 61(4):633-41. [DOI:10.3171/jns.1984.61.4.0633] [PMID]
Benjamin V, Russell SM. The microsurgical nuances of resecting tuberculum sellae meningiomas. Journal of Neurosurgery. 2005; 56(suppl. 2):411-7. [DOI:10.1227/01.NEU.0000144783.07688.BC] [PMID]
Noguchi A, Balasingam V, McMenomey SO, Delashaw Jr JB. Supraorbital craniotomy for parasellar lesions: Technical note. Journal of Neurosurgery. 2005; 102(5):951-5. [DOI:10.3171/jns.2005.102.5.0951] [PMID]
Landeiro JA, Gonçalves MB, Guimarães RD, Klescoski J, Correa JLA, Lapenta MA, et al. Tuberculum sellae meningiomas: Surgical considerations. Arq Neuropsiquiatr. 2010; 68(3):424-9. [DOI:10.1590/S0004-282X2010000300019] [PMID]
Mathiesen T, Kihlström L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Journal of Neurosurgery. 2006; 59(3):570-6. [DOI:10.1227/01.NEU.0000228683.79123.F9] [PMID]
Sade B, Lee JH. High incidence of optic canal involvement in tuberculum sellae meningiomas: Rationale for aggressive skull base approach. Surgical Neurology. 2009; 72(2):118-23. [DOI:10.1016/j.surneu.2008.08.007] [PMID]
Arifin MZ, Mardjono I, Sidabutar R, Wirjomartani BA, Faried A. Pterional approach versus unilateral frontal approach on tuberculum sellae meningioma: Single centre experiences. Asian Journal of Neurosurgery. 2012; 7(1):21-4. [DOI:10.4103/1793-5482.95691] [PMID] [PMCID]
Margalit N, Kesler A, Ezer H, Freedman S, Ram Z. Tuberculum and diaphragma sella meningioma-surgical technique and visual outcome in a series of 20 cases operated over a 2.5-year period. Acta Neurochirurgica. 2007; 149(12):1199-204. [DOI:10.1007/s00701-007-1280-4] [PMID]
Bowers CA, Altay T, Couldwell WT. Surgical decision-making strategies in tuberculum sellae meningioma resection. Neurosurgical Focus. 2011; 30(5):E1 [DOI:10.3171/2011.2.FOCUS1115] [PMID]
de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O. Tuberculum sellae meningiomas: High route or low route? A series of 51 consecutive cases. Journal of Neurosurgery. 2008; 62(3):556-63. [DOI:10.1227/01.neu.0000317303.93460.24] [PMID]
Wang Q, Lu XJ, Li B, Ji WY, Chen KL. Extended endoscopic endonasal transsphenoidal removal of tuberculum sellae meningiomas: A preliminary report. Journal of Clinical Neuroscience. 2009; 16(7):889-93. [DOI:10.1016/j.jocn.2008.10.003] [PMID]
de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O, Esposito I. Endoscopic transnasal resection of anterior cranial fossa meningiomas. Neurosurgery Focus. 2008; 25(6):E8. [DOI:10.3171/FOC.2008.25.12.E8] [PMID]
Cook SW, Smith Z, Kelly DF. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: Technical note. Journal of Neurosurgery. 2004; 55(1):239-46. [DOI:10.1227/01.NEU.0000126952.51782.4D] [PMID]
Prevedello DM, Thomas A, Gardner P, Snyderman CH, Carrau RL, Kassam AB. Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: Technical case report. Journal of Neurosurgery. 2007; 60(Suppl. 4):E401. [DOI:10.1227/01.NEU.0000255359.94571.91] [PMID]
Weiss M. Transnasal transsphenoidal approach. In: Apuzzo MLJ (ed). Surgery of the Third Ventricle Baltimore. Philadelphia: Williams & Wilkins; 1987.
Kitano M, Taneda M, Nakao Y. Postoperative improvement in visual function in patients with tuberculum sellae meningiomas: Results of the extended transsphenoidal and transcranial approaches. Journal of Neurosurgery. 2007; 107(2):337-46. [DOI:10.3171/JNS-07/08/0337] [PMID]
Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Journal of Neurosurgery. 2009; 64(suppl. 5):ons269-87. [DOI:10.1227/01.NEU.0000327857.22221.53] [PMID]
Wang Q, Lu XJ, Ji WY, Yan ZC, Xu J, Ding YS, et al. Visual outcome after extended endoscopic endonasal transsphenoidal surgery For tuberculum sellae meningiomas. World Neurosurgery. 2010; 73(6):694-700. [DOI:10.1016/j.wneu.2010.04.007] [PMID]
Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Journal of Neurosurgery. 2008; 63(1):36-54. [DOI:10.1227/01.NEU.0000335069.30319.1E] [PMID]
Otani N, Muroi C, Yano H, Khan N, Pangalu A, Yonekawa Y. Surgical management of tuberculum sellae meningioma: Role of selective extradural anterior clinoidectomy. Brithish Journal of Neurosurgery. 2006; 20(30):129-38. [DOI:10.1080/02688690600776747] [PMID]
Park CK, Jung HW, Yang SY, Seol HJ, Paek SH, Kim DG. Surgically treated tuberculum sellae and diaphragm sellae meningiomas: The importance of short-term visual outcome. Journal of Neurosurgery. 2006; 59(2):238-43. [DOI:10.1227/01.NEU.0000223341.08402.C5] [PMID]
Seol HJ, Park HY, Nam DH, Kong DS, Lee JI, Kim JH, et al. Clinical outcomes of tuberculum sellae meningiomas focusing on reversibility of postoperative visual function. Acta Neurochirurgica. 2013; 155(1):25-31. [DOI:10.1007/s00701-012-1551-6] [PMID]
Clark AJ, Jahangiri A, Garcia RM, George JR, Sughrue ME, McDermott MW, et al. Endoscopic surgery for tuberculum sellae meningiomas: A systematic review and meta-analysis. Neurosurgical Review. 2013; 36(3):349-59. [DOI:10.1007/s10143-013-0458-x] [PMID]
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurgery. 2012; 77(5-6):713-24. [DOI:10.1016/j.wneu.2011.08.025] [PMID]
Li X, Liu M, Liu Y, Zhu S. Surgical management of tuberculum sellae meningiomas. Journal of Clinical Neuroscience. 2007; 14(12):1150-4. [DOI:10.1016/j.jocn.2006.09.003] [PMID]
Pamir M, Özduman K, Belirgen M, Kilic T, Özek M. Outcome determinants of pterional surgery for tuberculum sellae meningiomas. Acta Neurochirurgica. 2005; 147(11):1121-30. [DOI:10.1007/s00701-005-0625-0] [PMID]
Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. Journal of Neurosurgery. 2005; 102(5):832-41. [DOI:10.3171/jns.2005.102.5.0832] [PMID]
Ehlers N, Malmros R. The suprasellar meningioma. A review of the literature and presentation of a series of 31 cases. Acta Ophthalmologica. Supplement. 1973:1-74. [PMID]
Panigrahi M, Varaprasad G. Transcranial/transnasal approach for nonpituitary sellar lesions. Surgical Neurology. 2009; 72(6):643-4. [DOI:10.1016/j.surneu.2009.05.013] [PMID]
Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: A 10‐year experience. Journal of Neurosurgery. 2008; 63(4):244-56. [DOI:10.1227/01.NEU.0000327025.03975.BA] [PMID]
Chokyu I, Goto T, Ishibashi K, Nagata T, Ohata K. Bilateral subfrontal approach for tuberculum sellae meningiomas in long-term postoperative visual outcome: Clinical article. Journal of Neurosurgery. 2011; 115(4):802-10. [DOI:10.3171/2011.5.JNS101812] [PMID]
Kaptain GJ, Vincent DA, Sheehan JP, Laws Jr ER. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Journal of Neurosurgery. 2001; 49(1):94-101. [DOI:10.1227/00006123-200107000-00014] [PMID]
Ceylan S, Koc K, Anik I. Extended endoscopic approaches for midline skull-base lesions. Neurosurgical Review. 2009; 32(3):309-19. [DOI:10.1007/s10143-009-0201-9] [PMID]
Dehdashti AR, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: Indications and limitations. Journal of Neurosurgery. 2009; 64(4):677-89. [DOI:10.1227/01.NEU.0000339121.20101.85] [PMID]
Bergland R. The arterial supply of the human optic chiasm. Journal of Neurosurgery. 1969; 31(3):327-34. [DOI:10.3171/jns.1969.31.3.0327] [PMID]
Koutourousiou M, Fernandez-Miranda JC, Stefko ST, Wang EW, Snyderman CH, Gardner PA. Endoscopic endonasal surgery for suprasellar meningiomas: experience with 75 patients: Clinical article. Journal of Neurosurgery. 2014; 120(6):1326-39. [DOI:10.3171/2014.2.JNS13767] [PMID]
Rosenstein J, Symon L. Surgical management of suprasellar meningioma: Part 2: Prognosis for visual function following craniotomy. Journal of Neurosurgery. 1984; 61(4):642-8. [DOI:10.3171/jns.1984.61.4.0642] [PMID]
Finn JE, Mount LA. Meningiomas of the tuberculum sellae and planum sphenoidale: A review of 83 cases. Archives of Ophthalmology. 1974; 92(1):23-7. [DOI:10.1001/archopht.1974.01010010027007] [PMID]
Ebner F, Bornemann A, Wilhelm H, Ernemann U, Honegger J. Tuberculum sellae meningioma symptomatic during pregnancy: Pathophysiological considerations. Acta Neurochirurgica. 2008; 150(2):189-93. [DOI:10.1007/s00701-007-1417-5] [PMID]
Brihaye J, Brihaye-van Geertruyden M, editors. Management and surgical outcome of suprasellar meningiomas. Acta Neurochirurgica's Supplement. 1988; 42:124-9. [DOI:10.1007/978-3-7091-8975-7_25] [PMID]
Andrews BT, Wilson CB. Suprasellar meningiomas: The effect of tumor location on postoperative visual outcome. Journal of Neurosurgery. 1988; 69(4):523-8. [DOI:10.3171/jns.1988.69.4.0523] [PMID]
McGregor JM, Sarkar A. Stereotactic radiosurgery and stereotactic radiotherapy in the treatment of skull base meningiomas. Otolaryngologic Clinics of North America. 2009; 42(4):677-88. [DOI:10.1016/j.otc.2009.04.010] [PMID]
Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas: A retrospective analysis of 140 patients treated from 1967 to 1990. Journal of Neurosurgery. 1994; 80(2):195-201. [DOI:10.3171/jns.1994.80.2.0195] [PMID]
Elia A, Shih H, Loeffler J. Stereotactic radiation treatment for benign meningiomas. Neurosurgery Focus. 2007; 23(4):E5. [DOI:10.3171/foc.2007.23.4.6]
Pollock B, Stafford S, Link M. Gamma knife radiosurgery for skull base meningiomas. Neurosurgery Clinics of North America. 2000; 11(4):659-66. [DOI:10.1016/S1042-3680(18)30091-3]
Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: Surgical experience in 105 cases. Journal of Neurosurgery. 2004; 55(3):539-50. [DOI:10.1227/01.NEU.0000134287.19377.A2] [PMID]
Mahmoud M, Nader R, Al-Mefty O. Optic canal involvement in tuberculum sellae meningiomas: Influence on approach, recurrence, and visual recovery. Journal of Neurosurgery. 2010; 67(3 Suppl Operative):ons108-19. [DOI:10.1227/01.NEU.0000383153.75695.24] [PMID]
Cappabianca P, Cavallo LM, Esposito F, Valente V, de Divitiis E. Sellar repair in endoscopic endonasal transsphenoidal surgery: Results of 170 cases. Journal of Neurosurgery. 2002; 51(6):1365-72. [DOI:10.1097/00006123-200212000-00006] [PMID]
Cavallo LM, Messina A, Esposito F, de Divitiis O, Fabbro MD, de Divitiis E, et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. Journal of Neurosurgery. 2007; 107:713-20. [DOI:10.3171/JNS-07/10/0713] [PMID]
Zanation AM, Carrau RL, Snyderman CH, Germanwala AV, Gardner PA, Prevedello DM, et al. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. The American Journal of Rhinology & Allergy. 2009; 23(5):518-21. [DOI:10.2500/ajra.2009.23.3378] [PMID]
Patel MR, Shah RN, Snyderman CH, Carrau RL, Germanwala AV, Kassam AB, et al. Pericranial flap for endoscopic anterior skull‐base reconstruction: Clinical outcomes and radioanatomic analysis of preoperative planning. Journal of Neurosurgery. 2010; 66(3):506-12. [DOI:10.1227/01.NEU.0000365620.59677.FF] [PMID]
Gadgil N, Thomas JG, Takashima M, Yoshor D. Endoscopic resection of tuberculum sellae meningiomas. Journal of Neurological Surgery Part B: Skull Base. 2013; 74(4):201-10. [DOI:10.1055/s-0033-1342922] [PMID] [PMCID]
Kassam A, Snyderman C, Carrau R, Gardner P, Mintz A. Endoneurosurgical hemostasis techniques: Lessons learned from 400 cases. Neurosurgery Focus. 2005; 19(1):E7. [DOI:10.3171/foc.2005.19.1.8]
De Divitiis E, Cavallo LM, Esposito F, Stella L, Messina A. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Journal of Neurosurgery. 2007; 61(5):229-38. [DOI:10.1227/01.neu.0000303221.63016.f2] [PMID]
Khan OH, Krischek B, Holliman D, Klironomos G, Kucharczyk W, Vescan A, et al. Pure endoscopic expanded endonasal approach for olfactory groove and tuberculum sellae meningiomas. Journal of Clinical Neuroscience. 2014; 21(6):927-33 . [DOI:10.1016/j.jocn.2013.10.015] [PMID]
Van Gompel JJ, Frank G, Pasquini E, Zoli M, Hoover J, Lanzino G. Expanded endonasal endoscopic resection of anterior fossa meningiomas: report of 13 cases and meta-analysis of the literature. Neurosurgical focus. 2011; 30(5):E15.
Ceylan S, Koc K, Anık I. Extended endoscopic transphenoidal approach for tuberculum sellae meningiomas. Acta neurochirurgica. 2011; 153(1):1-9.
Chowdhury FH, Haque MR, Goel AH, Kawsar KA. Endoscopic endonasal extended transsphenoidal removal of tuberculum sellae meningioma (TSM): an experience of six cases. British journal of neurosurgery. 2012; 26(5):692-9. [DOI: 10.3109/02688697.2012.673648]
Bohman LE, Stein SC, Newman JG, Palmer JN, Adappa ND, Khan A, et al. Endoscopic versus open resection of tuberculum sellae meningiomas: a decision analysis. Journal Otorhinolaryngol Relat Spec. 2012;74(5):255-63. [DOI: 10.1159/000343794]
Attia M, Kandasamy J, Jakimovski D, Bedrosian J, Alimi M, Lee DL, et al. The importance and timing of optic canal exploration and decompression during endoscopic endonasal resection of tuberculum sella and planum sphenoidale meningiomas. Operative Neurosurgery. 2012; 71(suppl_1):ons58-67. [DOI: 10.1227/NEU.0b013e318258e23d]
Type of Study: Research |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |