Sat, Apr 20, 2024
Volume 5, Issue 2 (Spring 2019)
Iran J Neurosurg 2019, 5(2): 71-78 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezvani M, Tabesh H, Adimi M, Alavi M, Zareian A, Rahiminejad M et al . The Effect of Intravenous Tranexamic Acid Before and During Spinal Surgery in Bleeding Volume During and After Surgical
Operation. Iran J Neurosurg 2019; 5 (2) :71-78
URL: http://irjns.org/article-1-121-en.html
URL: http://irjns.org/article-1-121-en.html
Majid Rezvani1 
 , Homayoun Tabesh1
, Homayoun Tabesh1 
 , Meisam Adimi *
, Meisam Adimi * 
 2, Mohammad Alavi1
2, Mohammad Alavi1 
 , Abolfazl Zareian1
, Abolfazl Zareian1 
 , Mitra Rahiminejad1
, Mitra Rahiminejad1 
 , Mehrdad Lari1
, Mehrdad Lari1 


 , Homayoun Tabesh1
, Homayoun Tabesh1 
 , Meisam Adimi *
, Meisam Adimi * 
 2, Mohammad Alavi1
2, Mohammad Alavi1 
 , Abolfazl Zareian1
, Abolfazl Zareian1 
 , Mitra Rahiminejad1
, Mitra Rahiminejad1 
 , Mehrdad Lari1
, Mehrdad Lari1 

1- Medical School of Isfahan University of Medical Sciences, Isfahan, Iran
2- Medical School of Isfahan University of Medical Sciences, Isfahan, Iran , meisammeisami04@gmail.com
2- Medical School of Isfahan University of Medical Sciences, Isfahan, Iran , meisammeisami04@gmail.com
Full Text [PDF 739 kb]
(1031 Downloads)
| Abstract (HTML) (3488 Views)
Full Text: (1586 Views)
1. Introduction
Laminectomy and laminotomy are the most common treatments for discectomy. Lumbar disc herniation has been associated with lumbar canal stenosis in 15% to 45% of patients with disc herniation. Canal stenosis is considered to be a degenerative disorder with neurological symptoms in lower limbs, which can reduce the quality of life in patients [1, 2]. Decompression with laminectomy is the most common surgical treatment for lumbar canal stenosis, which is performed to alleviate pain and reduce symptoms. [3-5] Bleeding during surgery is one of the most common surgical complications [1-5].
Tranexamic Acid (TA) prevents lysis of blood clots through plasmin deactivation. TA is the chemical analog for lysin and combines with plasminogen and plasmin, which inhibits their ability to bind with lysin residue in fibrin, therefore preventing fibrinolysis. Studies have reported that TA decreases blood loss and that patients require less transfusion of blood products under general anesthesia during urologic, orthopedic, cardiovascular and liver transplant surgeries [6-11]. Besides, prophylactic transfusion of antifibrinolytic products, such as TA has been shown to improve hemostasis. However, the results are still controversial [12-17].
Local TA in epidural area has been shown to significantly reduce blood loss after surgical laminectomy in the first and second days after surgery as well as total blood loss, therefore reducing duration of hospitalization [13]. Local TA has also been shown to reduce blood loss in patients undergoing spinal surgery using screw fixation [15].
To date, the effect of intravenous TA in reducing blood loss as well as assessing its adverse effects in spinal surgeries has not been well studied. In this study, we decided to determine the effect of tranexamic acid in reducing blood loss through its antifibrinolytic features in patients undergoing laminectomy. Surgeon satisfaction with operation field, as well as hemodynamic parameters of the patients, and adverse effects of TA were also evaluated.
2. Methods & Materials/Patients
In this study, we designed a randomized clinical trial to evaluate the effect of intravenous TA on reducing blood loss during and after laminectomy in patients with canal stenosis and spinal fractures undergoing surgery in 2016 at Alzahra Hospital, affiliated with Isfahan University of Medical Sciences. This study was approved by Iranian Registry of Clinical Trial (IRCT20130311012782N34). In this study, 100 patients diagnosed with canal stenosis and spinal fractures in radiological assessment were randomly divided into two groups.
Inclusion criteria included being under 60 years old with thoracic/lumbosacral spinal stenosis, or thoracic/ lumbosacral spinal fractures that needed surgery. Patients with diabetes, neuropathic disorders, and previous spinal surgery were excluded. Exclusion criteria were patients older than 60 years, with positive drug history of anticoagulants, such as aspirin and dipyridamole, abnormal PT, PTT and INR, positive previous thrombotic incidence, history of abnormal bleeding and previous lumbar surgery. All patients were operated in prone position under general anesthesia.
In this study, 140 patients with inclusion criteria were selected and randomly allocated to two groups of 70 patients with Random Allocation Software. Because including cases of narrow lumbar canal and vertebral fractures makes the groups nonhomogeneous, we matched the patients for demographic variables, medical history and type of disease in order to avoid bias.
One group received TA and the other was selected as the control group. The patients were divided randomly in order, one receiving and the next, not receiving the drug. Prior to surgery, we described the possible benefits, adverse effects, and the method of the study for the participants. After selection of participants, demographic data consisting of age, sex, past medical history such as hypertension, diabetes, cardiovascular diseases and smoking history were all gathered.
Physical examination and radiographic features were used to diagnose canal stenosis. To eliminate the interobserver bias, one expert radiologist reported the radiographic findings to diagnose the disease. Criteria used for diagnosis by the radiologist were hypertrophy of ligamentum flavum and facet joints.
One surgical approach and technique was used for all surgeries. Also, the method of anesthesia is same for all patients. Patients in the treatment group received 1 gram of intravenous TA (Transec, Rasht Pharmaceutical Company, Iran) and another intravenous dose of 250 milligram one hour after the beginning of the surgery. Bleeding in the surgical field was suctioned and its volume was measured at the end of the surgery.
Bleeding after the surgery was measured through hemovac drain and the figures were recorded separately for the first 24 and 48 hours. The person who recorded the data was blinded to whether the patient received tranexamic acid or not. The need for transfusion and its volume, as well as the length of hospital stay was compared in the two groups. Chi-square test, student sample t-test and variance analysis were used for intergroup comparisons as indicated. P<0.05 was considered as statistically significant. Statistical package for social sciences software (SPSS for windows, V. 23; Chicago, IL) was used for the analysis.
3. Results
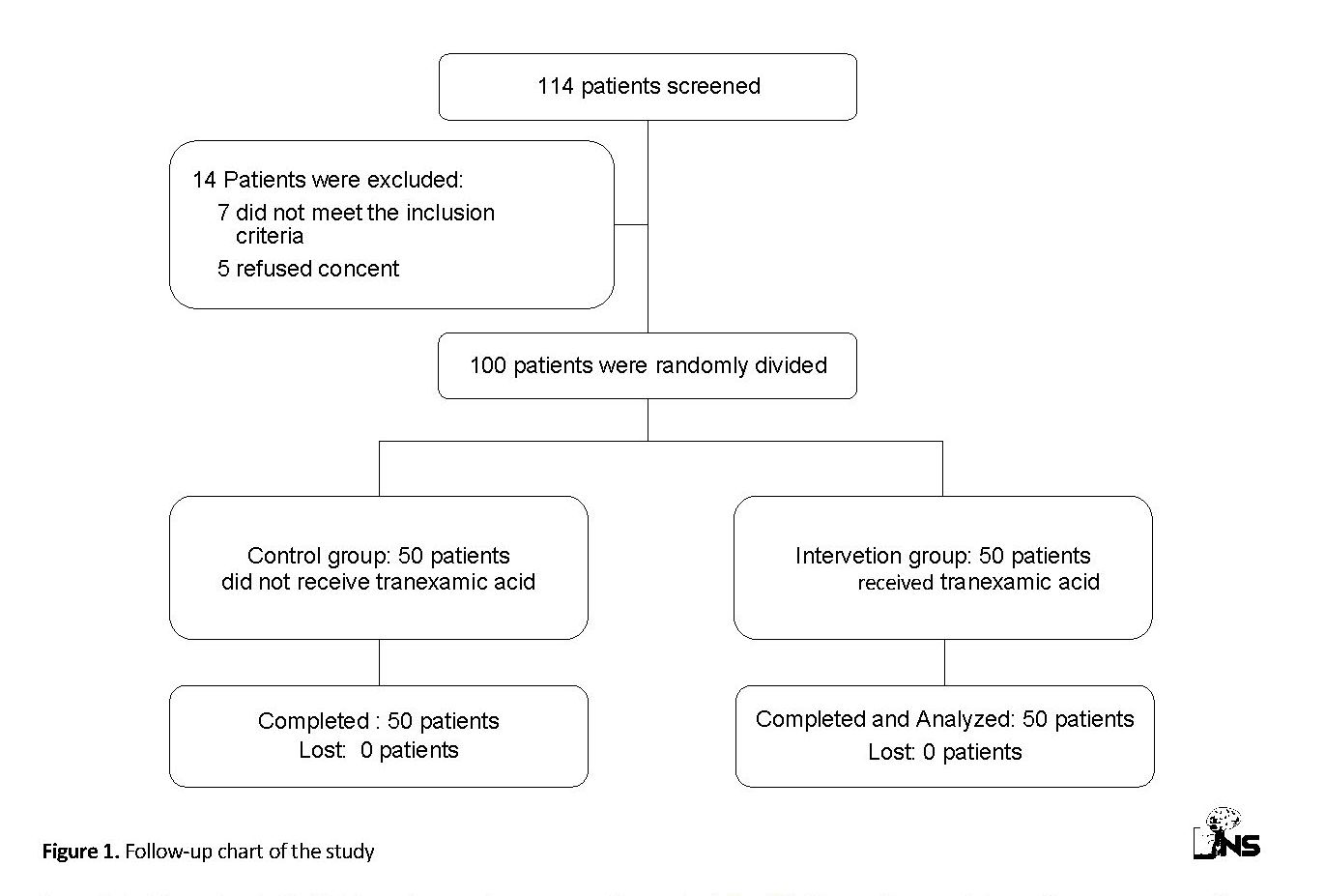
One hundred and fourteen patients were screened for eligibility. Fourteen patients were excluded from the study (five patients refused to accept the informed consent and seven patients did not meet the inclusion criteria). One hundred eligible patients were randomly assigned to two groups. One group received intravenous TA and the other was selected as the control group. All patients in both groups completed the study follow-up period and were included in the data analysis (Figure 1).
Laminectomy and laminotomy are the most common treatments for discectomy. Lumbar disc herniation has been associated with lumbar canal stenosis in 15% to 45% of patients with disc herniation. Canal stenosis is considered to be a degenerative disorder with neurological symptoms in lower limbs, which can reduce the quality of life in patients [1, 2]. Decompression with laminectomy is the most common surgical treatment for lumbar canal stenosis, which is performed to alleviate pain and reduce symptoms. [3-5] Bleeding during surgery is one of the most common surgical complications [1-5].
Tranexamic Acid (TA) prevents lysis of blood clots through plasmin deactivation. TA is the chemical analog for lysin and combines with plasminogen and plasmin, which inhibits their ability to bind with lysin residue in fibrin, therefore preventing fibrinolysis. Studies have reported that TA decreases blood loss and that patients require less transfusion of blood products under general anesthesia during urologic, orthopedic, cardiovascular and liver transplant surgeries [6-11]. Besides, prophylactic transfusion of antifibrinolytic products, such as TA has been shown to improve hemostasis. However, the results are still controversial [12-17].
Local TA in epidural area has been shown to significantly reduce blood loss after surgical laminectomy in the first and second days after surgery as well as total blood loss, therefore reducing duration of hospitalization [13]. Local TA has also been shown to reduce blood loss in patients undergoing spinal surgery using screw fixation [15].
To date, the effect of intravenous TA in reducing blood loss as well as assessing its adverse effects in spinal surgeries has not been well studied. In this study, we decided to determine the effect of tranexamic acid in reducing blood loss through its antifibrinolytic features in patients undergoing laminectomy. Surgeon satisfaction with operation field, as well as hemodynamic parameters of the patients, and adverse effects of TA were also evaluated.
2. Methods & Materials/Patients
In this study, we designed a randomized clinical trial to evaluate the effect of intravenous TA on reducing blood loss during and after laminectomy in patients with canal stenosis and spinal fractures undergoing surgery in 2016 at Alzahra Hospital, affiliated with Isfahan University of Medical Sciences. This study was approved by Iranian Registry of Clinical Trial (IRCT20130311012782N34). In this study, 100 patients diagnosed with canal stenosis and spinal fractures in radiological assessment were randomly divided into two groups.
Inclusion criteria included being under 60 years old with thoracic/lumbosacral spinal stenosis, or thoracic/ lumbosacral spinal fractures that needed surgery. Patients with diabetes, neuropathic disorders, and previous spinal surgery were excluded. Exclusion criteria were patients older than 60 years, with positive drug history of anticoagulants, such as aspirin and dipyridamole, abnormal PT, PTT and INR, positive previous thrombotic incidence, history of abnormal bleeding and previous lumbar surgery. All patients were operated in prone position under general anesthesia.
In this study, 140 patients with inclusion criteria were selected and randomly allocated to two groups of 70 patients with Random Allocation Software. Because including cases of narrow lumbar canal and vertebral fractures makes the groups nonhomogeneous, we matched the patients for demographic variables, medical history and type of disease in order to avoid bias.
One group received TA and the other was selected as the control group. The patients were divided randomly in order, one receiving and the next, not receiving the drug. Prior to surgery, we described the possible benefits, adverse effects, and the method of the study for the participants. After selection of participants, demographic data consisting of age, sex, past medical history such as hypertension, diabetes, cardiovascular diseases and smoking history were all gathered.
Physical examination and radiographic features were used to diagnose canal stenosis. To eliminate the interobserver bias, one expert radiologist reported the radiographic findings to diagnose the disease. Criteria used for diagnosis by the radiologist were hypertrophy of ligamentum flavum and facet joints.
One surgical approach and technique was used for all surgeries. Also, the method of anesthesia is same for all patients. Patients in the treatment group received 1 gram of intravenous TA (Transec, Rasht Pharmaceutical Company, Iran) and another intravenous dose of 250 milligram one hour after the beginning of the surgery. Bleeding in the surgical field was suctioned and its volume was measured at the end of the surgery.
Bleeding after the surgery was measured through hemovac drain and the figures were recorded separately for the first 24 and 48 hours. The person who recorded the data was blinded to whether the patient received tranexamic acid or not. The need for transfusion and its volume, as well as the length of hospital stay was compared in the two groups. Chi-square test, student sample t-test and variance analysis were used for intergroup comparisons as indicated. P<0.05 was considered as statistically significant. Statistical package for social sciences software (SPSS for windows, V. 23; Chicago, IL) was used for the analysis.
3. Results
One hundred and fourteen patients were screened for eligibility. Fourteen patients were excluded from the study (five patients refused to accept the informed consent and seven patients did not meet the inclusion criteria). One hundred eligible patients were randomly assigned to two groups. One group received intravenous TA and the other was selected as the control group. All patients in both groups completed the study follow-up period and were included in the data analysis (Figure 1).
Table 1 shows baseline demographics, clinical data and surgical results in the two study groups. No significant difference was noted between TA group and control group for mean age, sex distribution, type of disease, laminectomy, fixation, duration of operation, transfusion, length of hospital stay and hemoglobin before and after surgery (P≥0.05). TA group experienced significantly less bleeding during surgery than the control group (433 ml versus 522 ml respectively, P=0.009). Also, during the first 24 hours after surgery, bleeding volume was 159.2 ml in TA group and 196.4 ml in the control group (P=0.011). During the second 24 hours after surgery, bleeding volume was similar between the two groups (P=0.112).
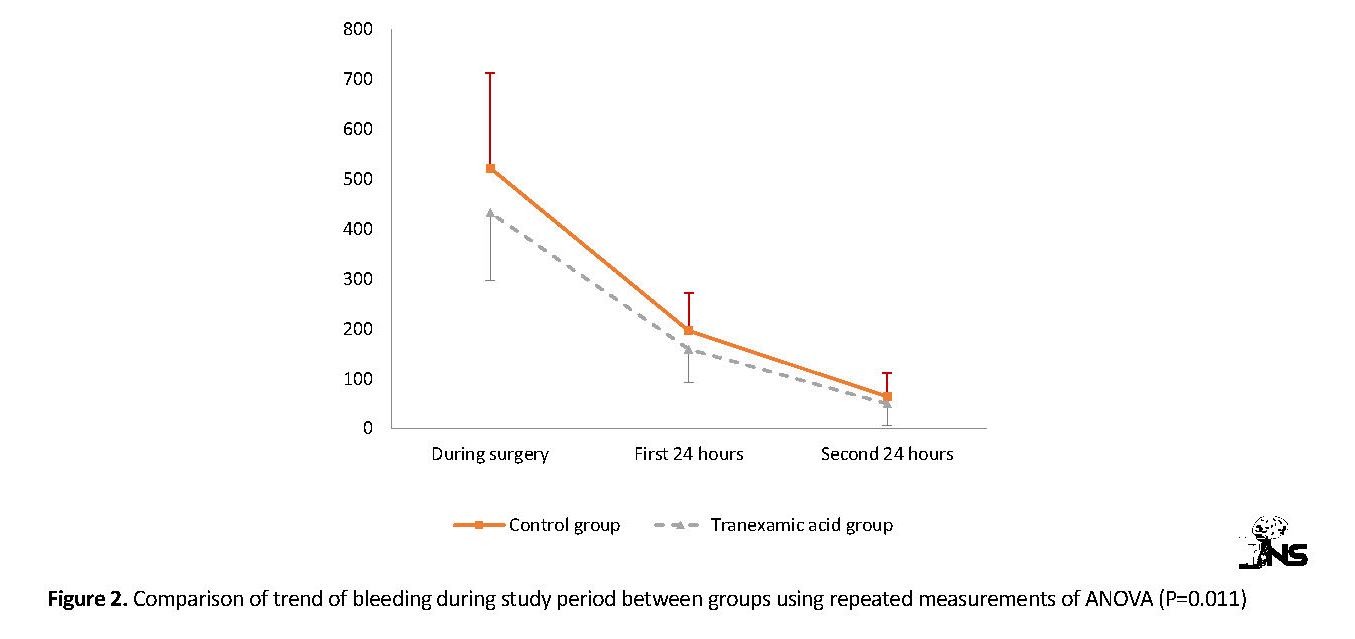
Figure 2 shows the trend of bleeding during study period between the groups using repeated measurements of ANOVA. Both groups experience a decrease in bleeding levels during the study period, but the decrease slope was significantly more in TA group compared with the control group (P=0.011). Also, Figure 3 shows the changes in hemoglobin level during study period between the two groups.
The values of hemoglobin in both groups slowly decreased and the trend of decrease was not significantly different between TA group and the control group (P=0.154). All patients received prophylactic dosage of enoxaparin in the first 24 hours after the surgery. During hospitalization period after the surgery, four patients suffered from Deep Vein Thrombosis (DVT). These four patients were all female. Three of them were in TA group and one patient in the control group.
4. Discussion
In this study, we evaluated the effect of intravenous TA on reducing blood loss during and after laminectomy in patients undergoing spinal surgery. TA administration for reducing blood loss has been suggested in several studies [18, 19]. TA was first used in cardiac surgery to reduce blood loss [6-11]. Recently, there is an increasing interest in using TA for reducing bleeding, especially after the publication of CRASH-2 [20], which is the largest published trauma trial up to now. Currently, the role of fibrinolysis in bleeding is being closely observed [14-17, 21].
TA (trans-4-(aminomethyl) cyclohexanecarboxylic acid) is a synthet=ic derivative of lysine which competitively inhibits the activation of plasminogen to the serine protease, plasmin, via binding to Kringle domains. TA is also a competitive inhibitor of tissue plasminogen activator. It blocks the lysine-binding sites of plasminogen, resulting in inhibition of plasminogen activation and fibrin binding to plasminogen, which impairs fibrinolysis [22]. TA can also directly inhibit plasmin activity, but higher doses are required to reduce plasmin formation [15-17].
TA is about ten times as potent as aminocaproic acid in vitro, and binds more strongly than aminocaproic acid to both the strong and weak receptor sites of the plasminogen in a ratio corresponding to the difference in potency between the compounds. TA is distributed throughout all body tissues and the plasma half-life is 120 minutes [15-17, 23-25].
In our study, intravenous TA significantly reduced bleeding during surgery, as well as the blood loss in the first 24 hours after the surgery. Transfusion rate in TA group was less than that in the control group. However, the difference between the two groups was not statistically significant. Thus, no significant effect was observed in transfusion rate during surgery. Several studies have shown that despite the reduction in bleeding-induced mortality, administration of TA does not reduce transfusion requirements. However, in studies relating to traumatic situations, blood is given empirically and blood losses are not accurately measured. Besides, it is confirmed that blood transfusion practice varies worldwide [18-20, 26].
We administered a loading dose of TA at the beginning of the surgery, as well as a maintenance dose one hour later to evaluate its effect. Previous data show that timing of TA administration is also important. Further analysis of CRASH-2 showed that treatment given in the first 3 hours reduced the risk of death due to bleeding, even with a more significant reduction in the first hour compared with between 1 and 3 hours. However, treatment given after 3 hours seemed to increase death due to bleeding [27]. Thus, TA should be given as early as possible to traumatic patients with bleeding. TA is less effective, and could be harmful in traumatic patients admitted late after injury [28-30].
In the present study, although transfusion rate was lower in TA group, no statistical difference was observed between the two groups in terms of blood transfusion requirement. A systematic review in 2012 [29], reflecting the increased interest in TA over the intervening years, identified 129 trials between 1972 and 2011 that included 10488 patients. In this meta-analysis, TA reduced the probability of receiving a blood transfusion by a third. This effect remained when the analysis was restricted to trials using adequate allocation concealment. Fewer deaths occurred in the TA group, however, there was considerable uncertainty when the analysis was restricted to trials using adequate concealment.
The authors concluded that cumulative meta-analysis showed reliable evidence that TA reduces the need for transfusion. Another systematic review of 104 randomly assigned trials examined whether the effect of TA on blood loss varies with the extent of surgical bleeding. The results suggest that despite variation in the magnitude of blood loss between procedures and the heterogeneity of the studies included, the use of TA was associated with an overall reduction in surgical bleeding by about a third. This reduction in bleeding with TA is almost identical to the reduction in the risk of receiving a blood transfusion with TA suggesting, as expected in the closely monitored environment of an operating theater, that unlike traumatic bleeding in CRASH-2, blood transfusion use was closely titrated to blood loss [20, 22, 27-30].
Strong evidence that TA reduces blood transfusion in surgery has been available for many years. Further trials on the effect of TA on blood transfusion are unlikely to add useful new information. However, the effect of TA on thromboembolic events and mortality remains uncertain. Surgical patients should be made aware of this evidence so that they can make an informed choice [22, 27-30].
Spine surgery is usually associated with a large amount of blood loss, necessitating blood transfusions. [1-4] Blood loss-associated morbidity can be because of direct risks, such as hypotension and organ damage, or as a result of blood transfusions [22, 30]. TA administration significantly reduced intraoperative, postoperative, and total blood loss. TA led to a reduction in proportion of patients who received a blood transfusion as compared with placebo [29, 30].
Four patients suffered from DVT in this study. They were all female, three in the TA group and one in the control group. In other studies, mortality data were reported in only a third of the included trials, and less than half of the reported data on deep vein thrombosis, and pulmonary embolism. Data from studies and meta-analyses for over a decade suggest that TA reduces blood transfusion in surgical patients, yet its effect on thromboembolic events and mortality remains uncertain [29].
5. Conclusion
In spinal surgeries, TA administration in the beginning of the process reduces surgical bleeding during surgery and in the first 24 hours after surgery. Transfusion requirements, reduction in hemoglobin level, duration of operation and length of hospital stay were independent of TA administration in this study. TA administration may have adverse effects on hospitalization period, related to complications such as DVT, especially in women. However, future studies are needed to conclude the advantages and disadvantages of TA administration in spinal surgeries.
Ethical Considerations
Compliance with ethical guidelines
Patients signed a written informed consent to participate in the study. This study complied with the principles of the declaration of Helsinki. The ethics committee of Isfahan University of Medical Sciences approved the study protocol.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed equally in performing the project and preparing the manuscript.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgements
This article is the result of a doctoral dissertation in neurosurgery, which was approved and implemented by the Department of Research and Technology of Isfahan Medical School. Therefore, the authors of this article are grateful for their support.
References
Du Bois M, Szpalski M, Donceel P. Patients at risk for long-term sick leave because of low back pain. The Spine Journal. 2009; 9(5):350-9. [DOI:10.1016/j.spinee.2008.07.003] [PMID]
Crook J, Milner R, Schultz IZ, Stringer B. Determinants of occupational disability following a low back injury: A critical review of the literature. Journal of Occupational Rehabilitation. 2002; 12(4):277-95. [DOI:10.1023/A:1020278708861] [PMID]
Hara N, Oka H, Yamazaki T, Takeshita K, Murakami M, Hoshi K, et al. Predictors of residual symptoms in lower extremities after decompression surgery on lumbar spinal stenosis. European Spine Journal. 2010; 19(11):1849-54. [DOI:10.1007/s00586-010-1374-1] [PMID] [PMCID]
Hansraj KK, Cammisa FP Jr, O’Leary PF, Crockett HC, Fras CI, Cohen MS, et al. Decompressive surgery for typical lumbar spinal stenosis. Clinical Orthopaedics and Related Research. 2001; (384):10-7. [DOI:10.1097/00003086-200103000-00003] [PMID]
Gelalis ID, Stafilas KS, Korompilias AV, Zacharis KC, Beris AE, Xenakis TA. Decompressive surgery for degenerative lumbar spinal stenosis: Long-term results. International Orthopaedics. 2006; 30(1):59-63. [DOI:10.1007/s00264-005-0030-6] [PMID] [PMCID]
Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999; 57(6):1005-32. [DOI:10.2165/00003495-199957060-00017] [PMID]
Vermeulen M, Lindsay KW, Murray GD, Cheah F, Hijdra A, Muizelaar JP, et al. Antifibrinolytic treatment in subarachnoid hemorrhage. The New England Journal of Medicine. 1984; 311(7):432-7. [DOI:10.1056/NEJM198408163110703] [PMID]
Berntorp E, Follrud C, Lethagen S. No increased risk of venous thrombosis in women taking tranexamic acid. Thrombosis and Haemostasis. 2001; 86(2):714-5. [DOI:10.1055/s-0037-1616122] [PMID]
Ido K, Neo M, Asada Y, Kondo K, Morita T, Sakamoto T, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Archives of Orthopaedic and Trauma Surgery. 2000; 120(9):518-20. [DOI:10.1007/s004029900132] [PMID]
Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesthesia & Analgesia. 2001; 93(1):82-7. [DOI:10.1097/00000539-200107000-00018] [PMID]
Grant JA, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery: The efficacy of tranexamic acid. Journal of Pediatric Orthopaedics. 2009; 29(3):300-4. [DOI:10.1097/BPO.0b013e31819a85de] [PMID]
Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004; 112(2):154-7. [DOI:10.1016/s0301-2115(03)00287-2] [PMID]
Fremes SE, Wong BI, Lee E, Mai R, Christakis GT, McLean RF, et al. Metaanalysis of prophylactic drug treatment in the prevention of postoperative bleeding. The Annals of Thoracic Surgery. 1994; 58(6):1580-8. [DOI:10.1016/0003-4975(94)91636-5] [PMID]
Horrow JC, Hlavacek J, Strong MD, Collier W, Brodsky I, Goldman SM, et al. Prophylactic tranexamic acid decreases bleeding after cardiac operations. The Journal of Thoracic and Cardiovascular Surgery. 1990; 99(1):70-4. [DOI:10.1016/S0022-5223(19)35634-X]
Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995; 82(2):383-92. [DOI:10.1097/00000542-199502000-00009] [PMID]
Hamada H, Senami M, Fujii K, Sera K, Kobayashi A, Kuroda M. Prophylactic hemostatic drugs do not reduce hemorrhage: Thromboelastographic study during upper abdominal surgery. Journal of Anesthesia. 1995; 9(1):32-5. [DOI:10.1007/BF02482032] [PMID]
Lozano M, Basora M, Peidro L, Merino I, Segur JM, Pereira A, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sanguinis. 2008; 95(1):39-44. [DOI:10.1111/j.1423-0410.2008.01045.x] [PMID]
Saberi H, Miri SM, Poordel Namdar M. [The effects of topically applied tranexamic acid on reduction of post-laminectomy hemorrhage (Persian)]. Tehran University Medical Journal. 2010; 68(9):527-33.
Krohn CD, Sørensen R, Lange JE, Riise R, Bjørnsen S, Brosstad F. Tranexamic acid given into the wound reduces postoperative blood loss by half in major orthopaedic surgery. The European Journal of Surgery. Supplement. 2003; (588):57-61. [PMID]
CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. The Lancet. 2010; 376(9734):23-32. [DOI:10.1016/S0140-6736(10)60835-5] [PMID]
Mannucci PM. Hemostatic drugs. The New England Journal of Medicine. 1998; 339(4):245-53. [DOI:10.1056/NEJM199807233390407] [PMID]
Åstedt B. Clinical pharmacology of tranexamic acid. Scandinavian Journal of Gastroenterology. 1987; 22(Suupl. 137):22-5. [DOI:10.3109/00365528709089756]
Miller RA, May MW, Hendry WF, Whitfield HN, Wickham JE. The prevention of secondary haemorrhage after prostatectomy: The value of antifibrinolytic therapy. British Journal of Urology. 1980; 52(1):26-8. [DOI:10.1111/j.1464-410X.1980.tb02914.x] [PMID]
Forbes C, Barr R, Reid G, Thomson C, Prentice C, Mc Nicol G, et al. Tranexamic acid in control of haemorrhage after dental extraction in haemophilia and Christmas disease. British Medical Journal. 1972; 2(5809):311-3. [DOI:10.1136/bmj.2.5809.311] [PMID] [PMCID]
Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: A case-control study using the General Practice Research Database. BJOG: An International Journal of Obstetrics & Gynaecology. 2009; 116(1):91-7. [DOI:10.1111/j.1471-0528.2008.01926.x] [PMID]
Lindoff C, Rybo G, Åstedt B. Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thrombosis and Haemostasis. 1993; 70(2):238-40. [DOI:10.1055/s-0038-1649475] [PMID]
CRASH-2 collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. The Lancet. 2011; 377(9771):1096-101. [DOI:10.1016/S0140-6736(11)60278-X] [PMID]
Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2011; (1):CD001886. [DOI:10.1002/14651858.CD001886.pub4] [PMID]
Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012; 344:e3054. [DOI:10.1136/bmj.e3054] [PMID] [PMCID]
Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. The British Journal of Surgery. 2013; 100(10):1271-9. [DOI:10.1002/bjs.9193] [PMID]
4. Discussion
In this study, we evaluated the effect of intravenous TA on reducing blood loss during and after laminectomy in patients undergoing spinal surgery. TA administration for reducing blood loss has been suggested in several studies [18, 19]. TA was first used in cardiac surgery to reduce blood loss [6-11]. Recently, there is an increasing interest in using TA for reducing bleeding, especially after the publication of CRASH-2 [20], which is the largest published trauma trial up to now. Currently, the role of fibrinolysis in bleeding is being closely observed [14-17, 21].
TA (trans-4-(aminomethyl) cyclohexanecarboxylic acid) is a synthet=ic derivative of lysine which competitively inhibits the activation of plasminogen to the serine protease, plasmin, via binding to Kringle domains. TA is also a competitive inhibitor of tissue plasminogen activator. It blocks the lysine-binding sites of plasminogen, resulting in inhibition of plasminogen activation and fibrin binding to plasminogen, which impairs fibrinolysis [22]. TA can also directly inhibit plasmin activity, but higher doses are required to reduce plasmin formation [15-17].
TA is about ten times as potent as aminocaproic acid in vitro, and binds more strongly than aminocaproic acid to both the strong and weak receptor sites of the plasminogen in a ratio corresponding to the difference in potency between the compounds. TA is distributed throughout all body tissues and the plasma half-life is 120 minutes [15-17, 23-25].
In our study, intravenous TA significantly reduced bleeding during surgery, as well as the blood loss in the first 24 hours after the surgery. Transfusion rate in TA group was less than that in the control group. However, the difference between the two groups was not statistically significant. Thus, no significant effect was observed in transfusion rate during surgery. Several studies have shown that despite the reduction in bleeding-induced mortality, administration of TA does not reduce transfusion requirements. However, in studies relating to traumatic situations, blood is given empirically and blood losses are not accurately measured. Besides, it is confirmed that blood transfusion practice varies worldwide [18-20, 26].
We administered a loading dose of TA at the beginning of the surgery, as well as a maintenance dose one hour later to evaluate its effect. Previous data show that timing of TA administration is also important. Further analysis of CRASH-2 showed that treatment given in the first 3 hours reduced the risk of death due to bleeding, even with a more significant reduction in the first hour compared with between 1 and 3 hours. However, treatment given after 3 hours seemed to increase death due to bleeding [27]. Thus, TA should be given as early as possible to traumatic patients with bleeding. TA is less effective, and could be harmful in traumatic patients admitted late after injury [28-30].
In the present study, although transfusion rate was lower in TA group, no statistical difference was observed between the two groups in terms of blood transfusion requirement. A systematic review in 2012 [29], reflecting the increased interest in TA over the intervening years, identified 129 trials between 1972 and 2011 that included 10488 patients. In this meta-analysis, TA reduced the probability of receiving a blood transfusion by a third. This effect remained when the analysis was restricted to trials using adequate allocation concealment. Fewer deaths occurred in the TA group, however, there was considerable uncertainty when the analysis was restricted to trials using adequate concealment.
The authors concluded that cumulative meta-analysis showed reliable evidence that TA reduces the need for transfusion. Another systematic review of 104 randomly assigned trials examined whether the effect of TA on blood loss varies with the extent of surgical bleeding. The results suggest that despite variation in the magnitude of blood loss between procedures and the heterogeneity of the studies included, the use of TA was associated with an overall reduction in surgical bleeding by about a third. This reduction in bleeding with TA is almost identical to the reduction in the risk of receiving a blood transfusion with TA suggesting, as expected in the closely monitored environment of an operating theater, that unlike traumatic bleeding in CRASH-2, blood transfusion use was closely titrated to blood loss [20, 22, 27-30].
Strong evidence that TA reduces blood transfusion in surgery has been available for many years. Further trials on the effect of TA on blood transfusion are unlikely to add useful new information. However, the effect of TA on thromboembolic events and mortality remains uncertain. Surgical patients should be made aware of this evidence so that they can make an informed choice [22, 27-30].
Spine surgery is usually associated with a large amount of blood loss, necessitating blood transfusions. [1-4] Blood loss-associated morbidity can be because of direct risks, such as hypotension and organ damage, or as a result of blood transfusions [22, 30]. TA administration significantly reduced intraoperative, postoperative, and total blood loss. TA led to a reduction in proportion of patients who received a blood transfusion as compared with placebo [29, 30].
Four patients suffered from DVT in this study. They were all female, three in the TA group and one in the control group. In other studies, mortality data were reported in only a third of the included trials, and less than half of the reported data on deep vein thrombosis, and pulmonary embolism. Data from studies and meta-analyses for over a decade suggest that TA reduces blood transfusion in surgical patients, yet its effect on thromboembolic events and mortality remains uncertain [29].
5. Conclusion
In spinal surgeries, TA administration in the beginning of the process reduces surgical bleeding during surgery and in the first 24 hours after surgery. Transfusion requirements, reduction in hemoglobin level, duration of operation and length of hospital stay were independent of TA administration in this study. TA administration may have adverse effects on hospitalization period, related to complications such as DVT, especially in women. However, future studies are needed to conclude the advantages and disadvantages of TA administration in spinal surgeries.
Ethical Considerations
Compliance with ethical guidelines
Patients signed a written informed consent to participate in the study. This study complied with the principles of the declaration of Helsinki. The ethics committee of Isfahan University of Medical Sciences approved the study protocol.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed equally in performing the project and preparing the manuscript.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgements
This article is the result of a doctoral dissertation in neurosurgery, which was approved and implemented by the Department of Research and Technology of Isfahan Medical School. Therefore, the authors of this article are grateful for their support.
References
Du Bois M, Szpalski M, Donceel P. Patients at risk for long-term sick leave because of low back pain. The Spine Journal. 2009; 9(5):350-9. [DOI:10.1016/j.spinee.2008.07.003] [PMID]
Crook J, Milner R, Schultz IZ, Stringer B. Determinants of occupational disability following a low back injury: A critical review of the literature. Journal of Occupational Rehabilitation. 2002; 12(4):277-95. [DOI:10.1023/A:1020278708861] [PMID]
Hara N, Oka H, Yamazaki T, Takeshita K, Murakami M, Hoshi K, et al. Predictors of residual symptoms in lower extremities after decompression surgery on lumbar spinal stenosis. European Spine Journal. 2010; 19(11):1849-54. [DOI:10.1007/s00586-010-1374-1] [PMID] [PMCID]
Hansraj KK, Cammisa FP Jr, O’Leary PF, Crockett HC, Fras CI, Cohen MS, et al. Decompressive surgery for typical lumbar spinal stenosis. Clinical Orthopaedics and Related Research. 2001; (384):10-7. [DOI:10.1097/00003086-200103000-00003] [PMID]
Gelalis ID, Stafilas KS, Korompilias AV, Zacharis KC, Beris AE, Xenakis TA. Decompressive surgery for degenerative lumbar spinal stenosis: Long-term results. International Orthopaedics. 2006; 30(1):59-63. [DOI:10.1007/s00264-005-0030-6] [PMID] [PMCID]
Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999; 57(6):1005-32. [DOI:10.2165/00003495-199957060-00017] [PMID]
Vermeulen M, Lindsay KW, Murray GD, Cheah F, Hijdra A, Muizelaar JP, et al. Antifibrinolytic treatment in subarachnoid hemorrhage. The New England Journal of Medicine. 1984; 311(7):432-7. [DOI:10.1056/NEJM198408163110703] [PMID]
Berntorp E, Follrud C, Lethagen S. No increased risk of venous thrombosis in women taking tranexamic acid. Thrombosis and Haemostasis. 2001; 86(2):714-5. [DOI:10.1055/s-0037-1616122] [PMID]
Ido K, Neo M, Asada Y, Kondo K, Morita T, Sakamoto T, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Archives of Orthopaedic and Trauma Surgery. 2000; 120(9):518-20. [DOI:10.1007/s004029900132] [PMID]
Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesthesia & Analgesia. 2001; 93(1):82-7. [DOI:10.1097/00000539-200107000-00018] [PMID]
Grant JA, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery: The efficacy of tranexamic acid. Journal of Pediatric Orthopaedics. 2009; 29(3):300-4. [DOI:10.1097/BPO.0b013e31819a85de] [PMID]
Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004; 112(2):154-7. [DOI:10.1016/s0301-2115(03)00287-2] [PMID]
Fremes SE, Wong BI, Lee E, Mai R, Christakis GT, McLean RF, et al. Metaanalysis of prophylactic drug treatment in the prevention of postoperative bleeding. The Annals of Thoracic Surgery. 1994; 58(6):1580-8. [DOI:10.1016/0003-4975(94)91636-5] [PMID]
Horrow JC, Hlavacek J, Strong MD, Collier W, Brodsky I, Goldman SM, et al. Prophylactic tranexamic acid decreases bleeding after cardiac operations. The Journal of Thoracic and Cardiovascular Surgery. 1990; 99(1):70-4. [DOI:10.1016/S0022-5223(19)35634-X]
Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995; 82(2):383-92. [DOI:10.1097/00000542-199502000-00009] [PMID]
Hamada H, Senami M, Fujii K, Sera K, Kobayashi A, Kuroda M. Prophylactic hemostatic drugs do not reduce hemorrhage: Thromboelastographic study during upper abdominal surgery. Journal of Anesthesia. 1995; 9(1):32-5. [DOI:10.1007/BF02482032] [PMID]
Lozano M, Basora M, Peidro L, Merino I, Segur JM, Pereira A, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sanguinis. 2008; 95(1):39-44. [DOI:10.1111/j.1423-0410.2008.01045.x] [PMID]
Saberi H, Miri SM, Poordel Namdar M. [The effects of topically applied tranexamic acid on reduction of post-laminectomy hemorrhage (Persian)]. Tehran University Medical Journal. 2010; 68(9):527-33.
Krohn CD, Sørensen R, Lange JE, Riise R, Bjørnsen S, Brosstad F. Tranexamic acid given into the wound reduces postoperative blood loss by half in major orthopaedic surgery. The European Journal of Surgery. Supplement. 2003; (588):57-61. [PMID]
CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. The Lancet. 2010; 376(9734):23-32. [DOI:10.1016/S0140-6736(10)60835-5] [PMID]
Mannucci PM. Hemostatic drugs. The New England Journal of Medicine. 1998; 339(4):245-53. [DOI:10.1056/NEJM199807233390407] [PMID]
Åstedt B. Clinical pharmacology of tranexamic acid. Scandinavian Journal of Gastroenterology. 1987; 22(Suupl. 137):22-5. [DOI:10.3109/00365528709089756]
Miller RA, May MW, Hendry WF, Whitfield HN, Wickham JE. The prevention of secondary haemorrhage after prostatectomy: The value of antifibrinolytic therapy. British Journal of Urology. 1980; 52(1):26-8. [DOI:10.1111/j.1464-410X.1980.tb02914.x] [PMID]
Forbes C, Barr R, Reid G, Thomson C, Prentice C, Mc Nicol G, et al. Tranexamic acid in control of haemorrhage after dental extraction in haemophilia and Christmas disease. British Medical Journal. 1972; 2(5809):311-3. [DOI:10.1136/bmj.2.5809.311] [PMID] [PMCID]
Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: A case-control study using the General Practice Research Database. BJOG: An International Journal of Obstetrics & Gynaecology. 2009; 116(1):91-7. [DOI:10.1111/j.1471-0528.2008.01926.x] [PMID]
Lindoff C, Rybo G, Åstedt B. Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thrombosis and Haemostasis. 1993; 70(2):238-40. [DOI:10.1055/s-0038-1649475] [PMID]
CRASH-2 collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. The Lancet. 2011; 377(9771):1096-101. [DOI:10.1016/S0140-6736(11)60278-X] [PMID]
Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2011; (1):CD001886. [DOI:10.1002/14651858.CD001886.pub4] [PMID]
Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012; 344:e3054. [DOI:10.1136/bmj.e3054] [PMID] [PMCID]
Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. The British Journal of Surgery. 2013; 100(10):1271-9. [DOI:10.1002/bjs.9193] [PMID]
Type of Study: Clinical Trial |
Subject:
Spine
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |