Thu, Apr 25, 2024
Volume 5, Issue 2 (Spring 2019)
Iran J Neurosurg 2019, 5(2): 54-62 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghodsi S R, Esmaili Dezaki H, Alizadeh M, Namazi Z. Post-Market Surveillance Study of a Skull Flap Fixation Device: Cranfixer. Iran J Neurosurg 2019; 5 (2) :54-62
URL: http://irjns.org/article-1-156-en.html
URL: http://irjns.org/article-1-156-en.html
1- DanaWell Company, Tehran University of Medical Sciences, Tehran, Iran , roholahghodsi@gmail.com (Corresponding author)
2- DanaWell Company, Tehran University of Medical Sciences, Tehran, Iran
3- . Department of Tissue Engineering, School of Advanced Technologies, Shahrekord University of Medical Sciences, Shahrekord, Iran
4- Department of Dental Biomaterials, School of Dentistry, Shahid Beheshti Univeristy of Medical Sciences, Tehran, Iran
2- DanaWell Company, Tehran University of Medical Sciences, Tehran, Iran
3- . Department of Tissue Engineering, School of Advanced Technologies, Shahrekord University of Medical Sciences, Shahrekord, Iran
4- Department of Dental Biomaterials, School of Dentistry, Shahid Beheshti Univeristy of Medical Sciences, Tehran, Iran
Full Text [PDF 880 kb]
(1207 Downloads)
| Abstract (HTML) (3479 Views)
3. Results
As mentioned before, patients with at least two followups are reported in this study. Figure 4 shows the implanted Cranfixers in some postoperative images.
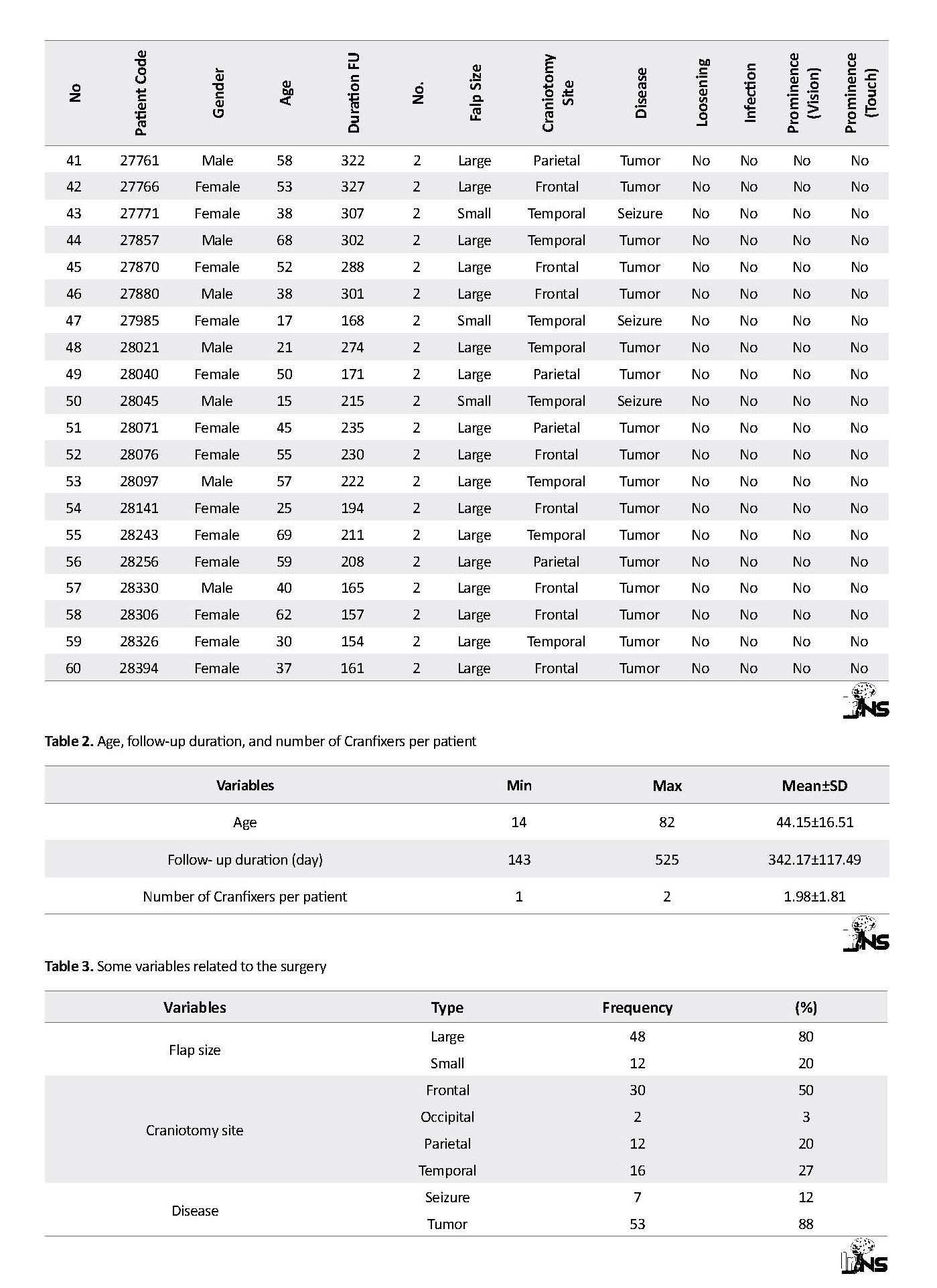
Table 1 presents patient demographics: gender, age, follow-up duration, number of Cranfixers used, size of bone flap, location of bone flap in the skull, patient’s disease, flap loosening, infection and prominence (visual/tactile). In the following, the variables and the data are presented in tables and charts. The variables are: 1) gender, 2) age, 3) duration of the follow-up, 4) number of Cranfixers used for each patient, 5) flap size, 6) location of defect on the skull, 7) disease, 8) flap loosening, 9) infection, and 10) prominence (visual/tactile) (Table 1). The quantitative variables are described in Table 2. The mean age of patients was 44 years old (ranged: 14-82).
Another important quantitative variable is the follow-up duration. Among 95 patients, 60 individuals were selected with at least two follow-ups. The mean follow-up duration was 342 days while the longest duration of 525 days belonged to a 14-year-old girl. In most surgeries, two Cranfixers were used for each patient. Totally, 118 Cranfixers were implanted in 60 patients.
Table 3 presents some variables about the surgery. Most of the dissection flaps were large (80%) and located on frontal (50%) region. After the frontal region, the temporal and parietal regions were ranked second and third, respectively, in frequent surgical sites. Tumor was the most prevalent cause of surgery in this study (88%). The relationships of craniotomy location with flap size and diseases are shown in Table 4. In most cases, large flaps were in frontal region. We considered flap size of >16 mm2 as large. Chi-square was used to assess statistical significance of different variables. The calculated P-value proves the significance of these relationships (Table 5).
Finally, the most important variables, the objectives of this study, are presented in Table 6. Totally, there was no evidence of loosening. Infection was reported only in one case (1.7%). The cause of infection was investigated thorough product tracking form and the LOT number. Two Cranfixers were used for the infected case with same LOT number and sterilization date. The documentation and standard indicators show that both of these Cranfixers were sterilized in a standard manner, but only one of them was infected. As a result, based on these pieces of evidence, the probability that the infection had been caused by the product is very slight (P<0.001). The responsible surgeon believed the silk stitch may have caused the infection.
Another important characteristic of Cranfixer is prominence after surgery. In five cases, the Cranfixer was touchable through scalp (8.3%) and just three of them were visible if carefully inspected (5%). This characteristic is not usually of clinical importance, but it may compromise patient’s appearance and self-confidence. Therefore, it must be considered in future versions of Cranfixer.
4. Discussion
This study supports previous studies regarding biocompatibility of PMMA. Cranfixer revealed good performance regarding flap fixation and was easy to use according to the surgeon. Thickness of the device is a point for further revision and development.
Our clinical investigation lasted up to 18 months, and we observed the following characteristics of Cranfixer: 1. Good performance in the flap fixation; 2. Excellent biocompatibility; 3. user-friendliness and ease to use from the surgeons’ point of view. Furthermore, the thickness of Cranfixer should be revised without loss of strength and product performance.
Ethical Considerations
Compliance with ethical guidelines
The Case Report Form (CRF) and informed consent of this study were designed and used according to the approved procedure of Food and Drug Administration of Iran. The questionnaire and method of this study were evaluated and approved by the Research Deputy of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.657).
Funding
The financial support of the project was provided by Darman Afarin Noandish Afagh (DanaWell) company (IRCT20190528043740N1).
Authors contributions
Conceiving and designing the study: Seyed Roholah Ghodsi, Zahra Namazi; Data collection and manuscript drafting: Hosein Esmaili Dezaki, Zahra Faraji; Data collection and statistical analysis: Morteza Alizadeh, Hosein Esmaili Dezaki; Study design and analyses; Seyed Roholah Ghodsi, Zahra Namazi, Morteza Alizadeh
Conflict of interest
The authors with DanaWell affiliation have indirect financial interest in the subject matter discussed in this paper.
References
Julian OC, Lopez-Belio M, Dye WS, Javid H, Grove WJ. The median sternal incision in intracardiac surgery with extracorporeal circulation; A general evaluation of its use in heart surgery. Surgery. 1957; 42(4):753-61. [PMID]
Blair GA, Fannin TF, Gordon DS. Titanium-strip cranioplasty. British Medical Journal. 1976; 2(6041):907-8. [DOI:10.1136/bmj.2.6041.907] [PMID] [PMCID]
Estin D, Troffkin N, Heilman CB. Bone flap fixation with titanium clamps: A new technique. Surgical Neurology. 2000; 53(4):391-4. [DOI:10.1016/S0090-3019(00)00186-5] [PMID]
Van Loock K, Menovsky T, Kamerling N, De Ridder D. Cranial bone flap fixation using a new device (Cranial LoopTM). Minimally Invasive Neurosurgery: MIN. 2011; 54(3):119-24. [DOI:10.1055/s-0031-1283171] [PMID]
Thomson LA, Law FC, James KH, Matthew CA, Rushton N. Biocompatibility of particulate polymethylmethacrylate bone cements: A comparative study in vitro and in vivo. Biomaterials. 1992; 13(12):811-8. [DOI:10.1016/0142-9612(92)90173-L]
Fiaschi P, Pavanello M, Imperato A, Dallolio V, Accogli A, Capra V, et al. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: A single-center experience. Journal of Neurosurgery: Pediatrics. 2016; 17(6):705-10. [DOI:10.3171/2015.10.PEDS15489] [PMID]
Full Text: (1096 Views)
1. Introduction
Craniotomy is a common surgical procedure in neurosurgery. After craniotomy, the removed piece of bone must be returned. Various fixation techniques are used for securing the bone in place. These techniques vary from making holes in the bone and simple suturing to fixation with the biocompatible implantable fixators.
Suturing technique is a basic method in which a stainless steel wire is used to secure the fixation [1]. Holes are drilled in the flap and the adjacent bone which are twisted with a wire that passes through the holes. In addition, the strips are used to fix skull flaps in place with screws [2].
Craniotomy is a common surgical procedure in neurosurgery. After craniotomy, the removed piece of bone must be returned. Various fixation techniques are used for securing the bone in place. These techniques vary from making holes in the bone and simple suturing to fixation with the biocompatible implantable fixators.
Suturing technique is a basic method in which a stainless steel wire is used to secure the fixation [1]. Holes are drilled in the flap and the adjacent bone which are twisted with a wire that passes through the holes. In addition, the strips are used to fix skull flaps in place with screws [2].
The most novel technique is clamping the flap. The clamping method is simple to use and reduces the surgery time. Clamps are made of different materials such as titanium and PEEK [3, 4]. Cranfixer is made of Poly-Methyl Methacrylate (PMMA), a well-known long-term biocompatible material that has long been used [5, 6]. Cranfixer was approved by Iran’s Ministry of Health and Medical Education after qualification review and related standard audition for biocompatibility, mechanical strength, and easy handling. We examined and reported surgeon experience and patient satisfaction in the present study. Cranfixer is fixed with silk sutures as shown in Figure 1. This is an easy and applicable mechanism. In Figure 2, a flap fixation is shown with two Cranfixers.
2. Methods and Materials/Patients
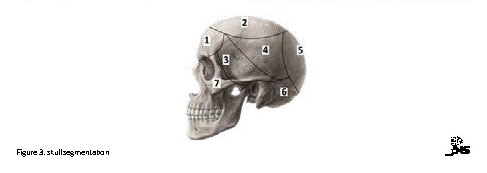
Sixty patients who had at least two follow-ups were included in this study. The main cause of surgery was brain tumors. Patients’ age ranged from 14 to 82 years (mean age=44). There were equal numbers of male and female. Cranfixer was used in different parts of the skull as shown in Figure 3.
Sixty patients who had at least two follow-ups were included in this study. The main cause of surgery was brain tumors. Patients’ age ranged from 14 to 82 years (mean age=44). There were equal numbers of male and female. Cranfixer was used in different parts of the skull as shown in Figure 3.
Different measures were chosen to evaluate infection, device loosening and prominence through scalp (both visual and touch) from surgeons’ and patients’ perspective. Follow-ups ranged from 143 to 523 days with a mean follow-up of 342 days. Pearson’s chi-square method was used for statistical analysis.
Characteristics and demographics of the participants were summarized with descriptive statistics. Patients were referred for therapy and selected from the age range of 14 to 82 years old, consisting of males and females. It was not possible to blind the surgeons because they were familiar with Cranfixer. Clinical Research Form/Case Report Form (CRF) was developed according to the guidelines of FDA of Iran and CONSORT statement.
The first part of this study obtained data from the surgeon’s experience at the time of surgery. The second part of data was collected from patient follow-ups in two rounds. Comorbidities like immunodeficiency were not observed in these follow-ups.
The first part of this study obtained data from the surgeon’s experience at the time of surgery. The second part of data was collected from patient follow-ups in two rounds. Comorbidities like immunodeficiency were not observed in these follow-ups.
Cranfixers were used in frontal, temporal, parietal and occipital regions of the skull. Complications such as infection, loosening, visual prominence and tactile prominence were included in statistical analysis. According to the surgical history, patients were followed up 143 to 523 days to assess the instances listed. Pearson’s chi-square was used for statistical analysis.
In accordance with the ethical principles and the national norms and standards for conducting medical research in Iran, the questionnaire and method of this study were evaluated and approved by the Research Deputy of Tehran University of Medical Sciences (IR. TUMS.VCR.REC.1397.657)
3. Results
As mentioned before, patients with at least two followups are reported in this study. Figure 4 shows the implanted Cranfixers in some postoperative images.
Table 1 presents patient demographics: gender, age, follow-up duration, number of Cranfixers used, size of bone flap, location of bone flap in the skull, patient’s disease, flap loosening, infection and prominence (visual/tactile). In the following, the variables and the data are presented in tables and charts. The variables are: 1) gender, 2) age, 3) duration of the follow-up, 4) number of Cranfixers used for each patient, 5) flap size, 6) location of defect on the skull, 7) disease, 8) flap loosening, 9) infection, and 10) prominence (visual/tactile) (Table 1). The quantitative variables are described in Table 2. The mean age of patients was 44 years old (ranged: 14-82).
Another important quantitative variable is the follow-up duration. Among 95 patients, 60 individuals were selected with at least two follow-ups. The mean follow-up duration was 342 days while the longest duration of 525 days belonged to a 14-year-old girl. In most surgeries, two Cranfixers were used for each patient. Totally, 118 Cranfixers were implanted in 60 patients.
Table 3 presents some variables about the surgery. Most of the dissection flaps were large (80%) and located on frontal (50%) region. After the frontal region, the temporal and parietal regions were ranked second and third, respectively, in frequent surgical sites. Tumor was the most prevalent cause of surgery in this study (88%). The relationships of craniotomy location with flap size and diseases are shown in Table 4. In most cases, large flaps were in frontal region. We considered flap size of >16 mm2 as large. Chi-square was used to assess statistical significance of different variables. The calculated P-value proves the significance of these relationships (Table 5).
Finally, the most important variables, the objectives of this study, are presented in Table 6. Totally, there was no evidence of loosening. Infection was reported only in one case (1.7%). The cause of infection was investigated thorough product tracking form and the LOT number. Two Cranfixers were used for the infected case with same LOT number and sterilization date. The documentation and standard indicators show that both of these Cranfixers were sterilized in a standard manner, but only one of them was infected. As a result, based on these pieces of evidence, the probability that the infection had been caused by the product is very slight (P<0.001). The responsible surgeon believed the silk stitch may have caused the infection.
Another important characteristic of Cranfixer is prominence after surgery. In five cases, the Cranfixer was touchable through scalp (8.3%) and just three of them were visible if carefully inspected (5%). This characteristic is not usually of clinical importance, but it may compromise patient’s appearance and self-confidence. Therefore, it must be considered in future versions of Cranfixer.
4. Discussion
This study supports previous studies regarding biocompatibility of PMMA. Cranfixer revealed good performance regarding flap fixation and was easy to use according to the surgeon. Thickness of the device is a point for further revision and development.
Our clinical investigation lasted up to 18 months, and we observed the following characteristics of Cranfixer: 1. Good performance in the flap fixation; 2. Excellent biocompatibility; 3. user-friendliness and ease to use from the surgeons’ point of view. Furthermore, the thickness of Cranfixer should be revised without loss of strength and product performance.
Ethical Considerations
Compliance with ethical guidelines
The Case Report Form (CRF) and informed consent of this study were designed and used according to the approved procedure of Food and Drug Administration of Iran. The questionnaire and method of this study were evaluated and approved by the Research Deputy of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.657).
Funding
The financial support of the project was provided by Darman Afarin Noandish Afagh (DanaWell) company (IRCT20190528043740N1).
Authors contributions
Conceiving and designing the study: Seyed Roholah Ghodsi, Zahra Namazi; Data collection and manuscript drafting: Hosein Esmaili Dezaki, Zahra Faraji; Data collection and statistical analysis: Morteza Alizadeh, Hosein Esmaili Dezaki; Study design and analyses; Seyed Roholah Ghodsi, Zahra Namazi, Morteza Alizadeh
Conflict of interest
The authors with DanaWell affiliation have indirect financial interest in the subject matter discussed in this paper.
References
Julian OC, Lopez-Belio M, Dye WS, Javid H, Grove WJ. The median sternal incision in intracardiac surgery with extracorporeal circulation; A general evaluation of its use in heart surgery. Surgery. 1957; 42(4):753-61. [PMID]
Blair GA, Fannin TF, Gordon DS. Titanium-strip cranioplasty. British Medical Journal. 1976; 2(6041):907-8. [DOI:10.1136/bmj.2.6041.907] [PMID] [PMCID]
Estin D, Troffkin N, Heilman CB. Bone flap fixation with titanium clamps: A new technique. Surgical Neurology. 2000; 53(4):391-4. [DOI:10.1016/S0090-3019(00)00186-5] [PMID]
Van Loock K, Menovsky T, Kamerling N, De Ridder D. Cranial bone flap fixation using a new device (Cranial LoopTM). Minimally Invasive Neurosurgery: MIN. 2011; 54(3):119-24. [DOI:10.1055/s-0031-1283171] [PMID]
Thomson LA, Law FC, James KH, Matthew CA, Rushton N. Biocompatibility of particulate polymethylmethacrylate bone cements: A comparative study in vitro and in vivo. Biomaterials. 1992; 13(12):811-8. [DOI:10.1016/0142-9612(92)90173-L]
Fiaschi P, Pavanello M, Imperato A, Dallolio V, Accogli A, Capra V, et al. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: A single-center experience. Journal of Neurosurgery: Pediatrics. 2016; 17(6):705-10. [DOI:10.3171/2015.10.PEDS15489] [PMID]
Type of Study: Clinical Trial |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |