Fri, Apr 19, 2024
Volume 8, Issue 1 (Continuous publishing 2022)
Iran J Neurosurg 2022, 8(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Vadiee G, Eghlidos Z, Hosseini S A, Rezakhah A. Hemichorea-Hemiballismus in a Patient With a Large Right Cerebellopontine Angle Meningioma: A Case Report. Iran J Neurosurg 2022; 8 (1) : 6
URL: http://irjns.org/article-1-295-en.html
URL: http://irjns.org/article-1-295-en.html
1- Department of Neurosurgery, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
2- Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
3- Department of Neurosurgery, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran , amirrezakhah@yahoo.com
2- Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
3- Department of Neurosurgery, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran , amirrezakhah@yahoo.com
Full Text [PDF 1695 kb]
(467 Downloads)
| Abstract (HTML) (1364 Views)
Full Text: (357 Views)
1. Background and Importance
Meningiomas (previously known as meningeal neoplasms) are the most common primary intracranial tumors in adults [1]. Meningiomas account for about 33% of all intracranial neoplasms incidentally diagnosed via radiological imaging; when considering symptomatic cases, this percentage drops to 15% of such neoplasms. Meningiomas are two to three times more common in middle-aged women than in their male counterparts [2]. Although brain tumors are rarely associated with movement abnormalities, they may rarely be associated with movement disorders, such as parkinsonism, chorea, hemiballismus, and dystonia [3, 4]. Our focus in this report was on movement disorders of the hemichorea-hemiballismus type.
Chorea, derived from the Greek word that means dance, refers to irregular, fast, involuntary movements that can occur anywhere in the body [5]. As the pathophysiology of chorea is not fully understood, the classification of sporadic chorea is based on its etiology [6]. The etiologies include brain lesions secondary to inherited disease, structural brain lesions, electrolyte imbalances, hypoglycemia, vascular disorders, infections, drugs, and autoimmune diseases [5, 7-9]. If these movements are limited to one side of the body, they are referred to as hemichorea [5].
Hemiballismus, which is a type of chorea, involves the extensive, violent, involuntary movements of one limb or multiple limbs on one side of the body. This condition has various causes, the most common of which are vascular conditions, including strokes involving the middle cerebral or posterior cerebral arteries and non-ketotic hyperglycemia [10, 11]. In this report, we investigated a case of large right cerebellopontine meningioma with compressive effects on the medulla and pons accompanied by hemichorea-hemiballismus.
2. Case Presentation
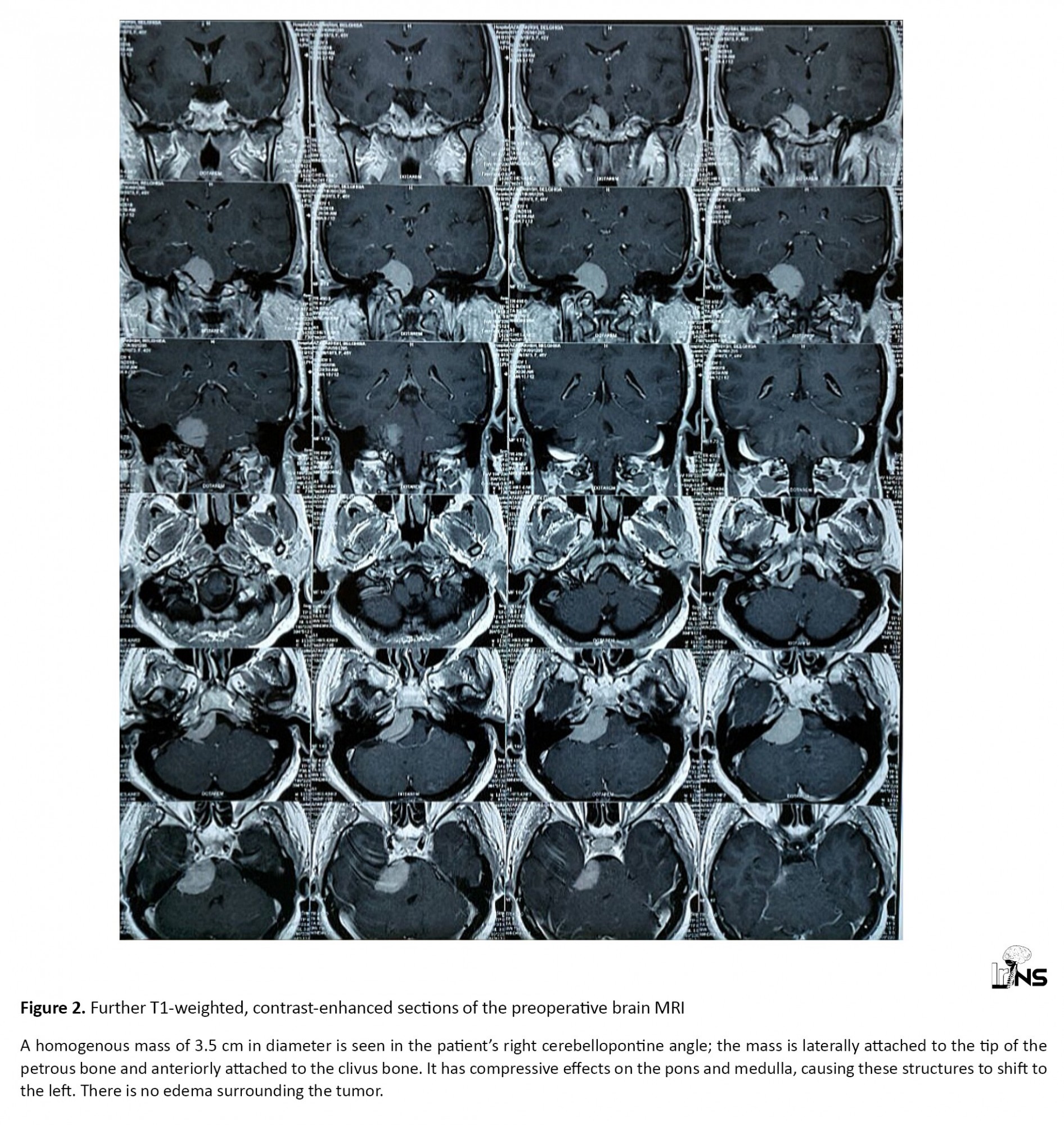
A 45-year-old woman presented with abnormal movements of the right upper and lower limbs commencing several months beforehand. However, the main complaints of the patient were severe headaches (non-responsive to analgesics), convulsions, blurred vision, and intermittent altered consciousness; she was referred to our center for further examination. Upon physical examination, abnormal movements of the right face and right upper and lower limbs were apparent. The patient’s right hand was kept in a dystonic posture during the examination and while walking. The patient would smile suddenly during the examination for no apparent reason. According to the nurses’ reports and the patient’s companions, there were no irregular movements while sleeping. On examination, however, there were no other findings related to cognitive-functional pathologies. There was no family history of similar illness and no other congenital diseases were found in the patient’s history. The movement symptoms had developed slowly. The patient and her companions were unable to recall the precise onset of the involuntary movements, the site, from which they commenced, and the manner, in which they appeared. Upon admission, the vital signs of the patient were as follows: BP (blood pressure): 130/80; HR (heart rate): 75; RR (respiratory rate): 15; O2 SAT (oxygen saturation): 98%. On brain magnetic resonance imaging (MRI), a homogenous mass of 3.5 cm in diameter was found in the patient’s right CPA; the mass was laterally attached to the tip of the petrous bone and anteriorly attached to the clivus bone. It had compressive effects on the pons and medulla, causing these structures to shift to the left. There was no edema surrounding the tumor (Figures 1 and 2).
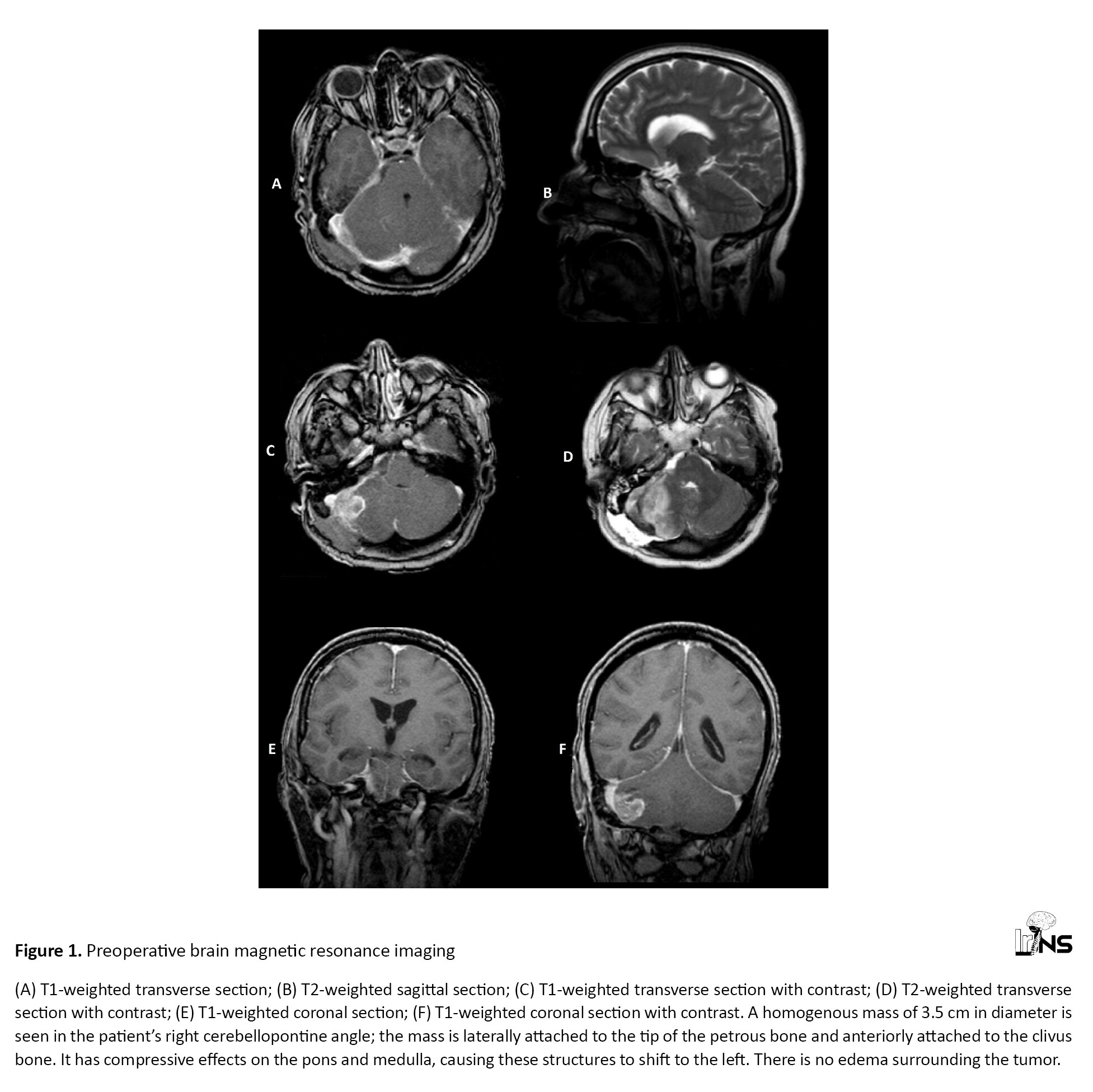
A diagnosis of meningioma was made for the patient. The patient’s EEG (electroencephalogram) was normal. All tests and investigations, including complete blood count (CBC), electrolytes, ESR, ANA, RF, TSH (erythrocyte sedimentation rate, anti-nuclear antibody, rheumatoid factor, thyroid-stimulating hormone) and antiphospholipid AB were reported as normal. After considering the risks and benefits of surgery and discussing the options and possible outcomes with the patient, mass resection surgery was selected and performed. The patient’s movement disorders completely resolved immediately after the operation during admission to the neurosurgery ward. For follow-up, the patient was examined in the clinic one and two months after surgery. No movement disorders were observed in either of these visits.
3. Discussion
Movement disorder is one of the most common signs of brain tumors, and its early recognition is of great importance for undertaking effective measures to treat the underlying cause. The presence of movement disorders during brain tumors may be explained by lacunar infarcts of the basal ganglia and their associated lesions, thalamic lesions and swelling of the surrounding tissue, external compression of adjacent structures, and blockage of the vascular supply to the basal ganglia. According to other reports, the compression of the midbrain caused by the tumor may be the reason behind the associated movement disorders [4].
In most cases of movement disorders, the lesion or mass exists externally. These lesions can be in the temporal, parietal, or frontal lobes. Furthermore, meningiomas are mostly responsible for such presentation, though movement disorders, including Parkinson’s tremors, have also been reported in other tumors, such as glioma or metastases [4]. Subdural hemorrhage has also been reported as a cause of Parkinsonism [12, 13]. In the study by Wakai et al., meningioma of the anterior third ventricle was reported as a cause of parkinsonism [14]. Benincasa et al. reported a case of hemi-Parkinsonism in a patient with frontal meningioma in 2008 [15].
In our patient, based on the MRI, the hemichorea-hemiballismus movement abnormalities could be due to compression or disruption of the extrapyramidal system, as damage to the extrapyramidal system leads to various movement disorders [16]. The extrapyramidal system is composed of several tracts (reticulospinal, vestibulospinal, rubrospinal, and tectospinal) and nuclei. It plays an essential role in maintaining posture and regulating involuntary motor functions. A part of its fibers is separated and descended through the contralateral side, while the remainder continues descending ipsilaterally. The rubrospinal and tectospinal tracts decussate at the midbrain region. In our case, the meningioma was located below the decussation of the fibers, which can be the reason why the patient’s symptoms were ipsilateral. The mechanism behind the symptoms may be extrinsic compression of the extrapyramidal fibers by the meningioma, though direct mechanical pressure and/or torsion of the basal ganglia by the mass might also have caused the dysfunction of these nuclei [4]. Fortunately, our patient had an excellent response to surgery, with no movement disorders being observed during follow-up.
4. Conclusion
The present study reports the first case of movement disorder caused by a meningioma in the CPA. Although meningiomas are usually considered benign tumors, they can, as noted in the case report, exert mechanical mass effects on adjacent structures following gradual enlargement. This can lead to various movement disorders, even if the tumor is in an unusual location. Hence, all patients with movement disorders must be examined thoroughly, with the neurological examination being of utmost importance. The present case indicates the variety of causes and manifestations of movement disorders, particularly dystonia and hemiballismus secondary to benign meningioma, even in less common sites.
Ethical Considerations
Compliance with ethical guidelines
Written informed consent was obtained from the patient for publication of this case report (including images) following the de-identification of data. The study followed the ethical guidelines of the 1975 Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Gholamreza Vadiee, Amir Rezakhah; Data collection: Zahra Eghlidos, Seyed Ali Hosseini; Data analysis and interpretation: Gholamreza Vadiee, Amir Rezakhah; Drafting the article: Zahra Eghlidos, Seyed Ali Hosseini; Critically revising the article: Zahra Eghlidos, Seyed Ali Hosseini, Gholamreza Vadiee, Amir Rezakhah; Reviewing the submitted version of the manuscript: Zahra Eghlidos, Seyed Ali Hosseini, Gholamreza Vadiee, Amir Rezakhah; Approving the final version of the manuscript: Zahra Eghlidos, Seyed Ali Hosseini, Gholamreza Vadiee, Amir Rezakhah.
Conflict of interest
The authors declared no conflict of interest
References
Meningiomas (previously known as meningeal neoplasms) are the most common primary intracranial tumors in adults [1]. Meningiomas account for about 33% of all intracranial neoplasms incidentally diagnosed via radiological imaging; when considering symptomatic cases, this percentage drops to 15% of such neoplasms. Meningiomas are two to three times more common in middle-aged women than in their male counterparts [2]. Although brain tumors are rarely associated with movement abnormalities, they may rarely be associated with movement disorders, such as parkinsonism, chorea, hemiballismus, and dystonia [3, 4]. Our focus in this report was on movement disorders of the hemichorea-hemiballismus type.
Chorea, derived from the Greek word that means dance, refers to irregular, fast, involuntary movements that can occur anywhere in the body [5]. As the pathophysiology of chorea is not fully understood, the classification of sporadic chorea is based on its etiology [6]. The etiologies include brain lesions secondary to inherited disease, structural brain lesions, electrolyte imbalances, hypoglycemia, vascular disorders, infections, drugs, and autoimmune diseases [5, 7-9]. If these movements are limited to one side of the body, they are referred to as hemichorea [5].
Hemiballismus, which is a type of chorea, involves the extensive, violent, involuntary movements of one limb or multiple limbs on one side of the body. This condition has various causes, the most common of which are vascular conditions, including strokes involving the middle cerebral or posterior cerebral arteries and non-ketotic hyperglycemia [10, 11]. In this report, we investigated a case of large right cerebellopontine meningioma with compressive effects on the medulla and pons accompanied by hemichorea-hemiballismus.
2. Case Presentation
A 45-year-old woman presented with abnormal movements of the right upper and lower limbs commencing several months beforehand. However, the main complaints of the patient were severe headaches (non-responsive to analgesics), convulsions, blurred vision, and intermittent altered consciousness; she was referred to our center for further examination. Upon physical examination, abnormal movements of the right face and right upper and lower limbs were apparent. The patient’s right hand was kept in a dystonic posture during the examination and while walking. The patient would smile suddenly during the examination for no apparent reason. According to the nurses’ reports and the patient’s companions, there were no irregular movements while sleeping. On examination, however, there were no other findings related to cognitive-functional pathologies. There was no family history of similar illness and no other congenital diseases were found in the patient’s history. The movement symptoms had developed slowly. The patient and her companions were unable to recall the precise onset of the involuntary movements, the site, from which they commenced, and the manner, in which they appeared. Upon admission, the vital signs of the patient were as follows: BP (blood pressure): 130/80; HR (heart rate): 75; RR (respiratory rate): 15; O2 SAT (oxygen saturation): 98%. On brain magnetic resonance imaging (MRI), a homogenous mass of 3.5 cm in diameter was found in the patient’s right CPA; the mass was laterally attached to the tip of the petrous bone and anteriorly attached to the clivus bone. It had compressive effects on the pons and medulla, causing these structures to shift to the left. There was no edema surrounding the tumor (Figures 1 and 2).

A diagnosis of meningioma was made for the patient. The patient’s EEG (electroencephalogram) was normal. All tests and investigations, including complete blood count (CBC), electrolytes, ESR, ANA, RF, TSH (erythrocyte sedimentation rate, anti-nuclear antibody, rheumatoid factor, thyroid-stimulating hormone) and antiphospholipid AB were reported as normal. After considering the risks and benefits of surgery and discussing the options and possible outcomes with the patient, mass resection surgery was selected and performed. The patient’s movement disorders completely resolved immediately after the operation during admission to the neurosurgery ward. For follow-up, the patient was examined in the clinic one and two months after surgery. No movement disorders were observed in either of these visits.

3. Discussion
Movement disorder is one of the most common signs of brain tumors, and its early recognition is of great importance for undertaking effective measures to treat the underlying cause. The presence of movement disorders during brain tumors may be explained by lacunar infarcts of the basal ganglia and their associated lesions, thalamic lesions and swelling of the surrounding tissue, external compression of adjacent structures, and blockage of the vascular supply to the basal ganglia. According to other reports, the compression of the midbrain caused by the tumor may be the reason behind the associated movement disorders [4].
In most cases of movement disorders, the lesion or mass exists externally. These lesions can be in the temporal, parietal, or frontal lobes. Furthermore, meningiomas are mostly responsible for such presentation, though movement disorders, including Parkinson’s tremors, have also been reported in other tumors, such as glioma or metastases [4]. Subdural hemorrhage has also been reported as a cause of Parkinsonism [12, 13]. In the study by Wakai et al., meningioma of the anterior third ventricle was reported as a cause of parkinsonism [14]. Benincasa et al. reported a case of hemi-Parkinsonism in a patient with frontal meningioma in 2008 [15].
In our patient, based on the MRI, the hemichorea-hemiballismus movement abnormalities could be due to compression or disruption of the extrapyramidal system, as damage to the extrapyramidal system leads to various movement disorders [16]. The extrapyramidal system is composed of several tracts (reticulospinal, vestibulospinal, rubrospinal, and tectospinal) and nuclei. It plays an essential role in maintaining posture and regulating involuntary motor functions. A part of its fibers is separated and descended through the contralateral side, while the remainder continues descending ipsilaterally. The rubrospinal and tectospinal tracts decussate at the midbrain region. In our case, the meningioma was located below the decussation of the fibers, which can be the reason why the patient’s symptoms were ipsilateral. The mechanism behind the symptoms may be extrinsic compression of the extrapyramidal fibers by the meningioma, though direct mechanical pressure and/or torsion of the basal ganglia by the mass might also have caused the dysfunction of these nuclei [4]. Fortunately, our patient had an excellent response to surgery, with no movement disorders being observed during follow-up.
4. Conclusion
The present study reports the first case of movement disorder caused by a meningioma in the CPA. Although meningiomas are usually considered benign tumors, they can, as noted in the case report, exert mechanical mass effects on adjacent structures following gradual enlargement. This can lead to various movement disorders, even if the tumor is in an unusual location. Hence, all patients with movement disorders must be examined thoroughly, with the neurological examination being of utmost importance. The present case indicates the variety of causes and manifestations of movement disorders, particularly dystonia and hemiballismus secondary to benign meningioma, even in less common sites.
Ethical Considerations
Compliance with ethical guidelines
Written informed consent was obtained from the patient for publication of this case report (including images) following the de-identification of data. The study followed the ethical guidelines of the 1975 Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Gholamreza Vadiee, Amir Rezakhah; Data collection: Zahra Eghlidos, Seyed Ali Hosseini; Data analysis and interpretation: Gholamreza Vadiee, Amir Rezakhah; Drafting the article: Zahra Eghlidos, Seyed Ali Hosseini; Critically revising the article: Zahra Eghlidos, Seyed Ali Hosseini, Gholamreza Vadiee, Amir Rezakhah; Reviewing the submitted version of the manuscript: Zahra Eghlidos, Seyed Ali Hosseini, Gholamreza Vadiee, Amir Rezakhah; Approving the final version of the manuscript: Zahra Eghlidos, Seyed Ali Hosseini, Gholamreza Vadiee, Amir Rezakhah.
Conflict of interest
The authors declared no conflict of interest
References
- Shaikh N, Dixit K, Raizer J. Recent advances in managing/understanding meningioma. F1000Research. 2018; 7:F1000 Faculty Rev-490. [DOI:10.12688/f1000research.13674.1] [PMID] [PMCID]
- Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991; 11(6):1087-106. [DOI:10.1148/radiographics.11.6.1749851] [PMID]
- Krauss JK, Nobbe F, Wakhloo AK, Mohadjer M, Vach W, Mundinger F. Movement disorders in astrocytomas of the basal ganglia and the thalamus. Journal of Neurology, Neurosurgery & Psychiatry. 1992; 55(12):1162-7. [DOI:10.1136/jnnp.55.12.1162] [PMID] [PMCID]
- Bhatoe H. Movement disorders caused by brain tumours. Neurology India. 1999; 47(1):40. https://neurologyindia.com/article.asp?issn=0028-3886;year=1999;volume=47;issue=1;spage=40;epage=2;aulast=Bhatoe
- Ropper AH, Adams R, Victor M, Samuels MA. Adams and Victor’s principles of neurology: McGraw Hill Medical; 2005. https://www.google.com/books/edition/Adams_and_Victor_s_Principles_of_Neurolo/fsCfe040fOsC?hl=en
- Piccolo I, Defanti CA, Soliveri P, Volontè MA, Cislaghi G, Girotti F. Cause and course in a series of patients with sporadic chorea. Journal of Neurology. 2003; 250(4):429-35. [DOI:10.1007/s00415-003-1010-7] [PMID]
- Bhidayasiri R, Truong DD. Chorea and related disorders. Postgraduate Medical Journal. 2004; 80(947):527-34. [DOI:10.1136/pgmj.2004.019356] [PMID] [PMCID]
- Mencacci NE, Carecchio M. Recent advances in genetics of chorea. Current Opinion in Neurology. 2016; 29(4):486-95. [DOI:10.1097/WCO.0000000000000352] [PMID] [PMCID]
- Paraskevas GP, Vlachos GS, Vassilopoulou S, Anagnostou E, Spengos K, Zis V. Hypoglycemia-induced hemichorea in a patient with Fahr’s syndrome. Neurological Sciences. 2012; 33(6):1397-9. [DOI:10.1007/s10072-012-1096-8] [PMID]
- Ghika-Schmid F, Ghika J, Regli F, Bogousslavsky J. Hyperkinetic movement disorders during and after acute stroke: The Lausanne Stroke Registry. Journal of The Neurological Sciences. 1997; 146(2):109-16. [DOI:10.1016/S0022-510X(96)00290-0]
- Hawley JS, Weiner WJ. Hemiballismus: Current concepts and review. Parkinsonism & Related Disorders. 2012; 18(2):125-9. [DOI:10.1016/j.parkreldis.2011.08.015] [PMID]
- Syndyk R, Khan I. Parkinsonism due to subdural hematoma. Journal of Neurosurgery. 1983; 58(2):298-9. [DOI:10.3171/jns.1983.58.2.0298] [PMID]
- Turjanski N, Pentland B, Lees AJ, Brooks DJ. Parkinsonism associated with acute intracranial hematomas: An [18F] dopa positron-emission tomography study. Movement Disorders. 1997; 12(6):1035-8. [DOI:10.1002/mds.870120630] [PMID]
- Wakai S, Nakamura K, Niizaki K, Nagai M, Nishizawa T, Yokoyama S, et al. Meningioma of the anterior third ventricle presenting with parkinsonism. Surgical Neurology. 1984; 21(1):88-92. [DOI:10.1016/0090-3019(84)90408-7]
- Benincasa D, Romano A, Mastronardi L, Pellicano C, Bozzao A, Pontieri FE. Hemiparkinsonism due to frontal meningioma. Acta Neurologica Belgica. 2008; 108(1):29-32. https://www.actaneurologica.be/pdfs/2008-1/07-Benincasa%20et%20al.pdf
- Lee J, Muzio MR. Neuroanatomy, extrapyramidal system. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PMID]
Type of Study: Case report |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






