Wed, Apr 24, 2024
Volume 3, Issue 3 (12-2017)
Iran J Neurosurg 2017, 3(3): 89-94 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tabesh H, Kamangar M, Tabesh A, Rastgoo A, Mohammadhosseini E, Raeissi-dehkordi M. The Effect of Applying Topical Vancomycin Powder on Prevention of Surgical Site Infection in Patients Undergoing Spinal Surgery Using Implants . Iran J Neurosurg 2017; 3 (3) :89-94
URL: http://irjns.org/article-1-90-en.html
URL: http://irjns.org/article-1-90-en.html
Homayoun Tabesh1 
 , Mohammad Kamangar *
, Mohammad Kamangar * 
 2, Aryan Tabesh3
2, Aryan Tabesh3 
 , Amin Rastgoo1
, Amin Rastgoo1 
 , Ehsan Mohammadhosseini1
, Ehsan Mohammadhosseini1 
 , Mehrnaz Raeissi-dehkordi4
, Mehrnaz Raeissi-dehkordi4 


 , Mohammad Kamangar *
, Mohammad Kamangar * 
 2, Aryan Tabesh3
2, Aryan Tabesh3 
 , Amin Rastgoo1
, Amin Rastgoo1 
 , Ehsan Mohammadhosseini1
, Ehsan Mohammadhosseini1 
 , Mehrnaz Raeissi-dehkordi4
, Mehrnaz Raeissi-dehkordi4 

1- Department of Neurosurgery, School of Medicine, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
2- Department of Neurosurgery, School of Medicine, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran , dr_mkamangar@yahoo.com
3- Department of Biochemistry, University of Washington, Seattle, Washington, United States
4- Department of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2- Department of Neurosurgery, School of Medicine, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran , dr_mkamangar@yahoo.com
3- Department of Biochemistry, University of Washington, Seattle, Washington, United States
4- Department of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Full Text [PDF 610 kb]
(1658 Downloads)
| Abstract (HTML) (5589 Views)
According to Table 2, the average surgery time in case and control groups were 3.32±0.68(h) and 3.38±0.75(h), respectively which had no significant difference between the two groups (P=0.69). Also, the mean of implant length in case and control group are 5.49±1.47 and 5.1±1.34 mm respectively and no statistically difference between the two groups was seen (P=0.36)
The mean time of hospitalization in the case group was 5.06±1.38 days while 5.28±1.01 days was obtained for the control group. The observed difference was not statistically significant between the two groups (P=0.21).
During the study, only one case showed SSI who belonged to the case group but in the opposite group no surgical infection happened, despite that, no significant difference was observed between the groups (P=0.32).
The patient with SSI was 83 years old and the duration of surgery for him was 5 hours, but in the non-infected group, the mean age was 51.9±14.5 years old and the average surgery time was 3.41±0.73 hours, both had a significant difference with that of the patient with SSI (P=0.035 and P=0.033, respectively). Yet, other variables such as length of implant, patient’s BMI, hospitalization time, sex and underlying disease had no effect on causing infection after surgery.
4. Discussion
This study aimed to determine the effect of local vancomycin powder in surgical site for preventing post-surgical infection in a patient undergoing spinal surgery using implants. Infection after surgery is one of the important challenges after spinal surgery which would extend hospitalization time and increase the costs. Furthermore, infection after surgery can influence the expected results of the surgical procedure, in addition, it demands a long-term antibiotic therapy and can cause wound dehiscence and displacement of the implant [1].
Spinal procedures with implantation of an instrument are usually lengthy; therefore, they can increase the risk of infection [7]. There are different methods of decreasing the risk of infection such as using systemic antibiotics. Despites these methods, SSI is still a major challenge which triggered our motive to initiate this research where in addition to systemic antibiotics, we used local vancomycin in surgical site and studied its effect [7].
Our study which contained 100 patients (50 control, 50 cases) encountered only one case (1%) of SSI belonging to case group so applying this intervention had no significant effect on decreasing the chance of infection. Although do not have many studies that evaluated the role of topical vancomycin in preventing SSI in Iran, because of thorough consideration and care during surgical procedure, the rate of SSI after this type of surgery is low and there is no need for additional prophylactic measures.One of the reasons of lack of a significant difference between the two groups of case and control may be the small size of samples compared to the incidence of infection, the other reason may be attributed to the use of systemic vancomycin as a prophylactic antibiotic in our center which is in contrast to the routine of many other centers which use cefazolin.
Many studies have shown that vancomycin is an effective and efficient antibiotic in preventing surgical site infection in spinal surgery. A systematic review was conducted in 2015 by Bakhsheshian and colleagues. Through reviewing 671 articles on effects of various antibiotics to prevent surgical site infection in spinal surgery, they discovered 18 articles examining the effect of topical application of vancomycin. According to 13 clinical trials, topical vancomycin reduced the risk of surgical site infection as much as 27% while no serious side effect has been reported from topical use of vancomycin [8].
In another systematic review by Kang and colleagues in 2015, the effect of topical vancomycin in preventing SSI in spinal surgery was confirmed [9].
In a study in 2016 by Cheung and colleagues, the effect of topical vancomycin at surgery site had a positive effect on reducing infections after surgery [10].
Our study found that SSI had a direct relation with age and length of operation so that in older patients and lengthy operations, the chance of infection was more. Different studies and experiences have shown that most of the patients who need spinal instrumentation are middle or old age and naturally the probability of underlying disease and as a result, the chance of infection in this group of patients, is high. Therefore, in addition to prophylactic antibiotics other precautions should be considered for them.
5. Conclusion
Keeping routine sterility consideration during operation is effective enough for preventing surgical site infection and additional tasks such as using topical vancomycin are not necessary. Meanwhile, it is suggested that another study with a larger sample size be performed to evaluate the effect of topical vancomycin more reliably.
The low number of sample size, the short period of follow-up, the number of observers for each variant, the variation of surgeons working as chief residents in each case (experience) were the limitations of this study.
Acknowledgments
This article is extracted from a thesis approved in Isfahan University of Medical Sciences.
Conflict of Interest
The authors' contribution is as follows: Conception and Design: Homayoun Tabesh; Data Collection: Homayoun Tabesh, Mohammad Kamangar, Aryan Tabesh, Amin Rastgoo, Ehsan Mohammadhosseini, Mehrnaz Raeissi-dehkordi; Drafting the Article: Mohammad Kamangar, Homayoun Tabesh, Amin Rastgoo, Ehsan Mohammadhosseini; Critically Revising the Article: Mohammad Kamangar, Homayoun Tabesh, Aryan Tabesh; Reviewing Submitted Version of the Manuscript: All authors; and Approving the Final Version of the Manuscript: All authors.
References
Full Text: (1599 Views)
1. Introduction
Spinal surgery complications may include post-operative infection, Deep Vein Thrombosis (DVT), Pulmonary thromboembolism, vision loss, instrumentation failure after spinal surgery, pseudarthrosis and hematoma which may cause serious systemic infection or necessitate surgical revision [1]. Prevalence of surgical site infection is estimated to be approximately 1.9% in the United States [2]. According to some statistics, these figures may vary from 4% to 11%, thus, with a minimum incidence, 700,000 patients are annually involved with post-operative infections only in US which will face a significant increase with reducing preventive measures [3]. Early mobilization of the patient and if possible avoiding long-term hospitalization, improving the nutritional status, proper bathing, correct placement of the surgical site dressing and even improving the mental health of patients help to reduce surgical site infection [4].
Owing to the high resistance of nosocomial infections against antibiotics and lack of sufficient concentration of antibiotics at surgical site due to immobilization of the patients and reduced peripheral blood flow, we still are facing some sort of surgical site infection [5].Vancomycin is an antibiotic that is effective on battling with aerobic and anaerobic gram-positive bacteria [6]. Since most of SSIs are caused by gram-positive cocci and due to decreased blood flow of hospitalized patients in the beds after spinal surgeries, this study aimed to determine the effect of local vancomycin powder in the surgical site on preventing post-surgical infection in a patient that had undergone spinal surgery using implants.
2. Methods & Materials/Patients
This is a clinical trial study which was carried out from February 2015 to June 2016 in Al-Zahra Hospital, Isfahan University of Medical Sciences, Iran. The study was approved by Ethics Committee of Isfahan University of Medical Sciences (Confirmation Code: IR.MUI.REC.1395.3.113).
Inclusion criteria
The study population included the patients with degenerative spinal instability who had undergone spinal surgery with instrument implantation. The candidate patients for spinal surgery with instrument implantation who had signed the written informed consent for participation in the study were enrolled.
Exclusion criteria
The exclusion criteria were sensitivity to vancomycin, failure in follow-up and surgeries for infectious factors like osteomyelitis and discitis.
Required sample size
The sample size required for this study was assigned by sample size determination equation to compare two proportions considering 95% confidence level, 80% strength of the test, estimated 15% infection rate in surgical site [5] and the minimum significant difference between two groups which was 0.2, for 50 people in each group.
Methodology
By randomized block method, 100 patients undergoing spinal surgery were divided into two groups of 50 as case and control groups The demographic information, pre-existing health condition, weight and height of patients hospitalized the day before surgery were all recorded in a specific check list. Preparations before surgery including a visit by anesthesiologist, consultation with a specialist if needed, and other necessary tests were performed.
Method of surgery
In the morning of surgery, Foley catheter was inserted and prophylactic intravenous antibiotic including 1 gram of ceftazidime and 1 gram of vancomycin were infused 30 to 60 minutes before skin incision. Povidone-iodine was used two times in surgical site before operation and the patient was covered with a sterile drape.
Before starting surgery, surgeon and assistant surgeon and scrub nurse washed their hands with povidone-iodine at least for 5 minutes. At the end of surgery for patients in case group, 1 gram of vancomycin in 20 milliliters of normal saline was poured into the wound but not for the control group. Vacuum drain was placed in surgical site then the wound was repaired with nylon. Surgery time was recorded, wound care was the same for both groups and wound dress was changed every other day. Patients were hospitalized for 4 to 7 days and the antibiotics were administered to all patients including 1 gram of Ceftazidime every 8 hours and 1 gram vancomycin each 12 hours for 3 days after surgery. The drain was removed after 2 or 3 days.
The patients were informed about signs and symptoms of wound infection including fever, erythema, swelling of the surgical site or wound after discharge which may necessitate them to refer for an urgent visit. The patients were examined at the 10th, 30th and 90th day of surgery for detecting any probable infection. In case of infection, sample from the wound was obtained for culture and antibiogram.
Statistical analysis
The data were analyzed by chi-square (for comparison of nominal data between the two groups) and t-test (for comparison of quantitative data between the two groups) using SPSS software (Version 24). P<0.05 was considered as the significance level.
3. Results
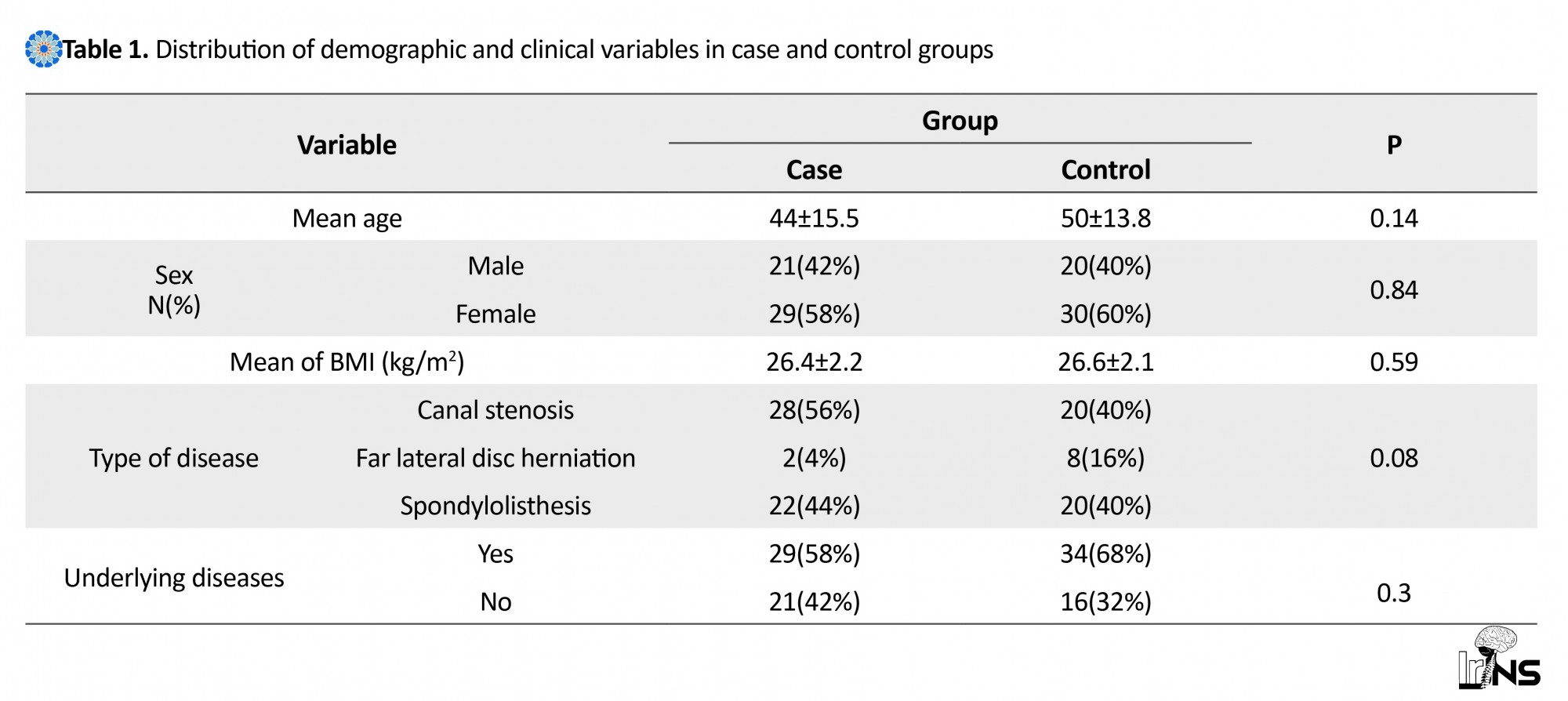
In this study, 100 patients underwent surgery in two groups of case and control. In Table 1, the distribution of demographic and clinical variables of the two groups are shown. According to the findings, there was no significant difference between these groups considering factors such as age, sex, BMI, type of disease and other clinical variables.
Spinal surgery complications may include post-operative infection, Deep Vein Thrombosis (DVT), Pulmonary thromboembolism, vision loss, instrumentation failure after spinal surgery, pseudarthrosis and hematoma which may cause serious systemic infection or necessitate surgical revision [1]. Prevalence of surgical site infection is estimated to be approximately 1.9% in the United States [2]. According to some statistics, these figures may vary from 4% to 11%, thus, with a minimum incidence, 700,000 patients are annually involved with post-operative infections only in US which will face a significant increase with reducing preventive measures [3]. Early mobilization of the patient and if possible avoiding long-term hospitalization, improving the nutritional status, proper bathing, correct placement of the surgical site dressing and even improving the mental health of patients help to reduce surgical site infection [4].
Owing to the high resistance of nosocomial infections against antibiotics and lack of sufficient concentration of antibiotics at surgical site due to immobilization of the patients and reduced peripheral blood flow, we still are facing some sort of surgical site infection [5].Vancomycin is an antibiotic that is effective on battling with aerobic and anaerobic gram-positive bacteria [6]. Since most of SSIs are caused by gram-positive cocci and due to decreased blood flow of hospitalized patients in the beds after spinal surgeries, this study aimed to determine the effect of local vancomycin powder in the surgical site on preventing post-surgical infection in a patient that had undergone spinal surgery using implants.
2. Methods & Materials/Patients
This is a clinical trial study which was carried out from February 2015 to June 2016 in Al-Zahra Hospital, Isfahan University of Medical Sciences, Iran. The study was approved by Ethics Committee of Isfahan University of Medical Sciences (Confirmation Code: IR.MUI.REC.1395.3.113).
Inclusion criteria
The study population included the patients with degenerative spinal instability who had undergone spinal surgery with instrument implantation. The candidate patients for spinal surgery with instrument implantation who had signed the written informed consent for participation in the study were enrolled.
Exclusion criteria
The exclusion criteria were sensitivity to vancomycin, failure in follow-up and surgeries for infectious factors like osteomyelitis and discitis.
Required sample size
The sample size required for this study was assigned by sample size determination equation to compare two proportions considering 95% confidence level, 80% strength of the test, estimated 15% infection rate in surgical site [5] and the minimum significant difference between two groups which was 0.2, for 50 people in each group.
Methodology
By randomized block method, 100 patients undergoing spinal surgery were divided into two groups of 50 as case and control groups The demographic information, pre-existing health condition, weight and height of patients hospitalized the day before surgery were all recorded in a specific check list. Preparations before surgery including a visit by anesthesiologist, consultation with a specialist if needed, and other necessary tests were performed.
Method of surgery
In the morning of surgery, Foley catheter was inserted and prophylactic intravenous antibiotic including 1 gram of ceftazidime and 1 gram of vancomycin were infused 30 to 60 minutes before skin incision. Povidone-iodine was used two times in surgical site before operation and the patient was covered with a sterile drape.
Before starting surgery, surgeon and assistant surgeon and scrub nurse washed their hands with povidone-iodine at least for 5 minutes. At the end of surgery for patients in case group, 1 gram of vancomycin in 20 milliliters of normal saline was poured into the wound but not for the control group. Vacuum drain was placed in surgical site then the wound was repaired with nylon. Surgery time was recorded, wound care was the same for both groups and wound dress was changed every other day. Patients were hospitalized for 4 to 7 days and the antibiotics were administered to all patients including 1 gram of Ceftazidime every 8 hours and 1 gram vancomycin each 12 hours for 3 days after surgery. The drain was removed after 2 or 3 days.
The patients were informed about signs and symptoms of wound infection including fever, erythema, swelling of the surgical site or wound after discharge which may necessitate them to refer for an urgent visit. The patients were examined at the 10th, 30th and 90th day of surgery for detecting any probable infection. In case of infection, sample from the wound was obtained for culture and antibiogram.
Statistical analysis
The data were analyzed by chi-square (for comparison of nominal data between the two groups) and t-test (for comparison of quantitative data between the two groups) using SPSS software (Version 24). P<0.05 was considered as the significance level.
3. Results
In this study, 100 patients underwent surgery in two groups of case and control. In Table 1, the distribution of demographic and clinical variables of the two groups are shown. According to the findings, there was no significant difference between these groups considering factors such as age, sex, BMI, type of disease and other clinical variables.
According to Table 2, the average surgery time in case and control groups were 3.32±0.68(h) and 3.38±0.75(h), respectively which had no significant difference between the two groups (P=0.69). Also, the mean of implant length in case and control group are 5.49±1.47 and 5.1±1.34 mm respectively and no statistically difference between the two groups was seen (P=0.36)
The mean time of hospitalization in the case group was 5.06±1.38 days while 5.28±1.01 days was obtained for the control group. The observed difference was not statistically significant between the two groups (P=0.21).
During the study, only one case showed SSI who belonged to the case group but in the opposite group no surgical infection happened, despite that, no significant difference was observed between the groups (P=0.32).
The patient with SSI was 83 years old and the duration of surgery for him was 5 hours, but in the non-infected group, the mean age was 51.9±14.5 years old and the average surgery time was 3.41±0.73 hours, both had a significant difference with that of the patient with SSI (P=0.035 and P=0.033, respectively). Yet, other variables such as length of implant, patient’s BMI, hospitalization time, sex and underlying disease had no effect on causing infection after surgery.
4. Discussion
This study aimed to determine the effect of local vancomycin powder in surgical site for preventing post-surgical infection in a patient undergoing spinal surgery using implants. Infection after surgery is one of the important challenges after spinal surgery which would extend hospitalization time and increase the costs. Furthermore, infection after surgery can influence the expected results of the surgical procedure, in addition, it demands a long-term antibiotic therapy and can cause wound dehiscence and displacement of the implant [1].
Spinal procedures with implantation of an instrument are usually lengthy; therefore, they can increase the risk of infection [7]. There are different methods of decreasing the risk of infection such as using systemic antibiotics. Despites these methods, SSI is still a major challenge which triggered our motive to initiate this research where in addition to systemic antibiotics, we used local vancomycin in surgical site and studied its effect [7].
Our study which contained 100 patients (50 control, 50 cases) encountered only one case (1%) of SSI belonging to case group so applying this intervention had no significant effect on decreasing the chance of infection. Although do not have many studies that evaluated the role of topical vancomycin in preventing SSI in Iran, because of thorough consideration and care during surgical procedure, the rate of SSI after this type of surgery is low and there is no need for additional prophylactic measures.One of the reasons of lack of a significant difference between the two groups of case and control may be the small size of samples compared to the incidence of infection, the other reason may be attributed to the use of systemic vancomycin as a prophylactic antibiotic in our center which is in contrast to the routine of many other centers which use cefazolin.
Many studies have shown that vancomycin is an effective and efficient antibiotic in preventing surgical site infection in spinal surgery. A systematic review was conducted in 2015 by Bakhsheshian and colleagues. Through reviewing 671 articles on effects of various antibiotics to prevent surgical site infection in spinal surgery, they discovered 18 articles examining the effect of topical application of vancomycin. According to 13 clinical trials, topical vancomycin reduced the risk of surgical site infection as much as 27% while no serious side effect has been reported from topical use of vancomycin [8].
In another systematic review by Kang and colleagues in 2015, the effect of topical vancomycin in preventing SSI in spinal surgery was confirmed [9].
In a study in 2016 by Cheung and colleagues, the effect of topical vancomycin at surgery site had a positive effect on reducing infections after surgery [10].
Our study found that SSI had a direct relation with age and length of operation so that in older patients and lengthy operations, the chance of infection was more. Different studies and experiences have shown that most of the patients who need spinal instrumentation are middle or old age and naturally the probability of underlying disease and as a result, the chance of infection in this group of patients, is high. Therefore, in addition to prophylactic antibiotics other precautions should be considered for them.
5. Conclusion
Keeping routine sterility consideration during operation is effective enough for preventing surgical site infection and additional tasks such as using topical vancomycin are not necessary. Meanwhile, it is suggested that another study with a larger sample size be performed to evaluate the effect of topical vancomycin more reliably.
The low number of sample size, the short period of follow-up, the number of observers for each variant, the variation of surgeons working as chief residents in each case (experience) were the limitations of this study.
Acknowledgments
This article is extracted from a thesis approved in Isfahan University of Medical Sciences.
Conflict of Interest
The authors' contribution is as follows: Conception and Design: Homayoun Tabesh; Data Collection: Homayoun Tabesh, Mohammad Kamangar, Aryan Tabesh, Amin Rastgoo, Ehsan Mohammadhosseini, Mehrnaz Raeissi-dehkordi; Drafting the Article: Mohammad Kamangar, Homayoun Tabesh, Amin Rastgoo, Ehsan Mohammadhosseini; Critically Revising the Article: Mohammad Kamangar, Homayoun Tabesh, Aryan Tabesh; Reviewing Submitted Version of the Manuscript: All authors; and Approving the Final Version of the Manuscript: All authors.
References
- Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: A systematic review. Spine. 2010; 35(9S):S125-37. doi: 10.1097/brs.0b013e3181d8342c
- Fan Y, Wei Z, Wang W, Tan L, Jiang H, Tian L, et al. The incidence and distribution of surgical site infection in mainland China: A meta-analysis of 84 prospective observational studies. Scientific Reports. 2014; 4(1). doi: 10.1038/srep06783
- ETHICON. Comprehensive Approach to Prevention surgical-Site infections. Journal of Neurosurgery. 2004; 54(4):54.
- Cirak B, Alptekin M, Palaoglu S, Ozcan OE, Ozgen T. Surgical therapy for lumbar spinal stenosis: evaluation of 300 cases. Neurosurgical Review. 2001; 24(2-3):80–2. doi: 10.1007/pl00014585
- Berger E. Late postoperative results in 1000 work related lumbar spine conditions. Surgical Neurology. 2000; 54(2):101–8. doi: 10.1016/s0090-3019(00)00283-4
- Heshmati R. [Iranian generic pharmacopeia (Persian)]. Tehran: Hejrat; 2006.
- Traynelis V, Kasliwal M, Tan L. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surgical Neurology International. 2013; 4(6):392. doi: 10.4103/2152-7806.120783
- Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurgery. 2015; 83(5):816–23. doi: 10.1016/j.wneu.2014.12.033
- Kang DG, Holekamp TF, Wagner SC, Lehman RA. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: A systematic literature review. The Spine Journal. 2015; 15(4):762–70. doi: 10.1016/j.spinee.2015.01.030
- Cheung JPY, Luk KDK. Complications of anterior and posterior cervical spine surgery. Asian Spine Journal. 2016; 10(2):385-400. doi: 10.4184/asj.2016.10.2.385
Type of Study: Research |
Subject:
Gamma Knife Radiosurgery
References
1. Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: A systematic review. Spine. 2010; 35(9S):S125-37. doi: 10.1097/brs.0b013e3181d8342c [DOI:10.1097/BRS.0b013e3181d8342c]
2. Fan Y, Wei Z, Wang W, Tan L, Jiang H, Tian L, et al. The incidence and distribution of surgical site infection in mainland China: A meta-analysis of 84 prospective observational studies. Scientific Reports. 2014; 4(1). doi: 10.1038/srep06783 [DOI:10.1038/srep06783]
3. ETHICON. Comprehensive Approach to Prevention surgical-Site infections. Journal of Neurosurgery. 2004; 54(4):54.
4. Cirak B, Alptekin M, Palaoglu S, Ozcan OE, Ozgen T. Surgical therapy for lumbar spinal stenosis: evaluation of 300 cases. Neurosurgical Review. 2001; 24(2-3):80–2. doi: 10.1007/pl00014585 [DOI:10.1007/PL00014585]
5. Berger E. Late postoperative results in 1000 work related lumbar spine conditions. Surgical Neurology. 2000; 54(2):101–8. doi: 10.1016/s0090-3019(00)00283-4 [DOI:10.1016/S0090-3019(00)00283-4]
6. Heshmati R. [Iranian generic pharmacopeia (Persian)]. Tehran: Hejrat; 2006.
7. Traynelis V, Kasliwal M, Tan L. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surgical Neurology International. 2013; 4(6):392. doi: 10.4103/2152-7806.120783 [DOI:10.4103/2152-7806.120783]
8. Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurgery. 2015; 83(5):816–23. doi: 10.1016/j.wneu.2014.12.033 [DOI:10.1016/j.wneu.2014.12.033]
9. Kang DG, Holekamp TF, Wagner SC, Lehman RA. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: A systematic literature review. The Spine Journal. 2015; 15(4):762–70. doi: 10.1016/j.spinee.2015.01.030 [DOI:10.1016/j.spinee.2015.01.030]
10. Cheung JPY, Luk KDK. Complications of anterior and posterior cervical spine surgery. Asian Spine Journal. 2016; 10(2):385-400. doi: 10.4184/asj.2016.10.2.385 [DOI:10.4184/asj.2016.10.2.385]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |