Sat, Apr 20, 2024
Volume 4, Issue 4 (Autumn 2018)
Iran J Neurosurg 2018, 4(4): 205-212 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azar M, Kazemi F, Jahanbakhshi A, Jalessi M, Amini E, Kazemi F, et al . Outcomes of Gamma Knife Radiosurgery in Patients With Parasagittal Meningioma. Iran J Neurosurg 2018; 4 (4) :205-212
URL: http://irjns.org/article-1-133-en.html
URL: http://irjns.org/article-1-133-en.html
Maziar Azar1 

 , Farid Kazemi2
, Farid Kazemi2 

 , Amin Jahanbakhshi3
, Amin Jahanbakhshi3 
 , Maryam Jalessi4
, Maryam Jalessi4 

 , Elahe Amini5
, Elahe Amini5 

 , Foad Kazemi6
, Foad Kazemi6 

 , Feyzollah Ebrahimnia6
, Feyzollah Ebrahimnia6 
 , Hessam Rahatlou *
, Hessam Rahatlou * 

 7
7


 , Farid Kazemi2
, Farid Kazemi2 

 , Amin Jahanbakhshi3
, Amin Jahanbakhshi3 
 , Maryam Jalessi4
, Maryam Jalessi4 

 , Elahe Amini5
, Elahe Amini5 

 , Foad Kazemi6
, Foad Kazemi6 

 , Feyzollah Ebrahimnia6
, Feyzollah Ebrahimnia6 
 , Hessam Rahatlou *
, Hessam Rahatlou * 

 7
7
1- MD. Professor of Neurosurgery, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
2- MD. Associate Professor of Neurosurgery, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
3- Assistant Professor of Neurosurgery, Skull Base Research Center, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran
4- MD. Associate Professor of Otolaryngology- Head and Neck Surgery, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
5- MD. Research assistant, ENT and Head & Neck Research Center and Department, Iran University of Medical Sciences , Tehran, Iran
6- MD. Neurosurgeon, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
7- MD. Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran , dr.h.rahatlou@gmail.com
2- MD. Associate Professor of Neurosurgery, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
3- Assistant Professor of Neurosurgery, Skull Base Research Center, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran
4- MD. Associate Professor of Otolaryngology- Head and Neck Surgery, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
5- MD. Research assistant, ENT and Head & Neck Research Center and Department, Iran University of Medical Sciences , Tehran, Iran
6- MD. Neurosurgeon, Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran
7- MD. Skull Base Research Center, Iran University of Medical Sciences, Tehran, Iran , dr.h.rahatlou@gmail.com
Keywords: Radiosurgery, Parasagittal, meningioma, Superior sagittal sinus, Treatment outcome, Cerebral edema
Full Text [PDF 544 kb]
(1281 Downloads)
| Abstract (HTML) (4373 Views)
Full Text: (1785 Views)
1. Introduction
arasagittal Meningioma (PSM) is the second most common region for intracranial meningioma accounting for (21-31%) of all meningioma cases [1]. The management of PSM may be complicated because of its partial or complete invasion to adjacent critical structures, particularly the superior sagittal sinus, which can cause severe brain edema and venous infarction [1]. Although the surgical resection of the tumor is the first line of treatment for meningioma, Gamma Knife Radiosurgery (GKRS) has been proved as an appropriate alternative in the last decades. Because, PSM operation bears a high risk of morbidity and mortality, using the gamma knife is another choice to prevent the various risks of operation such as bleeding, infection, and permanent neurovascular complications [2, 3].
However, GKRS has some morbidities because of its radiation effects on susceptible adjacent structures, most notably the superior sagittal sinus, wall, and lumen, as well as the veins affected in the radiation field [2]. PSMs are at particular risk for venous occlusive complications, which leads to brain edema, especially in comparison with skull base lesions [2, 4, 5].
Considering the value of stereotactic radiosurgery in PSMs, which has been under debate recently, we performed this study to evaluate the outcomes of GKRS in patients with PSM in Iran Gamma Knife Center.
2. Methods and Materials/Patients
Study design
In this descriptive, retrospective cross-sectional study, we reviewed the records of 61 patients with PSM diagnosed and confirmed by either CT scan or MRI, who underwent GKRS from 2003 to 2013. The study was conducted in Iran Gamma Knife Center, Tehran.
Data collection
The hospital records of the survived patients were examined to collect their demographic characteristics and medical records, including age, sex, primary clinical signs and symptoms, underlying diseases, radiosurgical parameters such as the maximum dose, marginal dose, isodose, and the mean follow-up time. Tumor size and volume were recorded according to MRI reported by two expert radiologists. The exclusion criteria included missing follow-up visits, having multiple meningiomas, having a history of other brain tumors, being younger than 18 years, and using fractionated GKRS. After GKRS, the patients were assessed by MRI every six months in the first year and, then, every year for three years routinely.
The gamma knife device was the Elekta Model C, Version 5.34, Type C. To perform GKRS, after preparing the patient, the stereotactic frame was attached to head, using four pins at a depth of about 2mm. Then, MRI, CT scan, or brain angiography were performed to obtain appropriate stereotactic images. All tumor characteristics, including volume and distance from sensitive points, were determined accurately and used in designing the treatment plan. After designing, we used the gamma knife device, which was a hemisphere comprising 201 cobalt sources and 60 radiation directions.
In this study, we assessed the radiological outcome of treatment, including tumor volume in the last follow-up after treatment. Moreover, we evaluated the determinants and short-term complications, including headache, vertigo, nausea, and muscle weakness following GKRS, as well as the patients’ Progression-free Survival (PFS).
Statistical analysis
Descriptive statistics were used to analyze demographic and clinical data. Quantitative variables were reported as Mean±SD and qualitative variables as percentage and ratio. A Kaplan-Meier plot was constructed to assess PFS. All statistical analyses were performed, using SPSS V. 22 and P values less than 0.05 were considered the statistical significance.
3. Results
Sixty-one patients with PSM were evaluated, of whom 32(52%) were men (Mean±SD age: 49.70±16.95 y) and 29(47.5%) were women (Mean±SD age: 52.19±13.05 y). There was no significant difference among the participants in age and sex. Moreover, 45(73.8%) patients had a history of operation, and 16(26.2%) had no previous operation or radiotherapy. Of 45 patients with a history of operation, 30 patients experienced one, nine experienced two, and five experienced three operations. Four patients with a history of operation also received postoperative radiotherapy. Primary symptoms were headache (37%), seizure (22%), paraparesis (10%), vertigo (8%), nausea and vomiting (8%), and cognitive disorder (5%).
Radiologic outcomes following gamma knife radiosurgery
The Mean±SD follow-up time was 30.28±27.48 months (range: 2-120mon). Six patients were followed-up for less than six months, and one patient died in the fifth month of follow-up. Table 1 presents the radiosurgery parameters of the patients.
arasagittal Meningioma (PSM) is the second most common region for intracranial meningioma accounting for (21-31%) of all meningioma cases [1]. The management of PSM may be complicated because of its partial or complete invasion to adjacent critical structures, particularly the superior sagittal sinus, which can cause severe brain edema and venous infarction [1]. Although the surgical resection of the tumor is the first line of treatment for meningioma, Gamma Knife Radiosurgery (GKRS) has been proved as an appropriate alternative in the last decades. Because, PSM operation bears a high risk of morbidity and mortality, using the gamma knife is another choice to prevent the various risks of operation such as bleeding, infection, and permanent neurovascular complications [2, 3].
However, GKRS has some morbidities because of its radiation effects on susceptible adjacent structures, most notably the superior sagittal sinus, wall, and lumen, as well as the veins affected in the radiation field [2]. PSMs are at particular risk for venous occlusive complications, which leads to brain edema, especially in comparison with skull base lesions [2, 4, 5].
Considering the value of stereotactic radiosurgery in PSMs, which has been under debate recently, we performed this study to evaluate the outcomes of GKRS in patients with PSM in Iran Gamma Knife Center.
2. Methods and Materials/Patients
Study design
In this descriptive, retrospective cross-sectional study, we reviewed the records of 61 patients with PSM diagnosed and confirmed by either CT scan or MRI, who underwent GKRS from 2003 to 2013. The study was conducted in Iran Gamma Knife Center, Tehran.
Data collection
The hospital records of the survived patients were examined to collect their demographic characteristics and medical records, including age, sex, primary clinical signs and symptoms, underlying diseases, radiosurgical parameters such as the maximum dose, marginal dose, isodose, and the mean follow-up time. Tumor size and volume were recorded according to MRI reported by two expert radiologists. The exclusion criteria included missing follow-up visits, having multiple meningiomas, having a history of other brain tumors, being younger than 18 years, and using fractionated GKRS. After GKRS, the patients were assessed by MRI every six months in the first year and, then, every year for three years routinely.
The gamma knife device was the Elekta Model C, Version 5.34, Type C. To perform GKRS, after preparing the patient, the stereotactic frame was attached to head, using four pins at a depth of about 2mm. Then, MRI, CT scan, or brain angiography were performed to obtain appropriate stereotactic images. All tumor characteristics, including volume and distance from sensitive points, were determined accurately and used in designing the treatment plan. After designing, we used the gamma knife device, which was a hemisphere comprising 201 cobalt sources and 60 radiation directions.
In this study, we assessed the radiological outcome of treatment, including tumor volume in the last follow-up after treatment. Moreover, we evaluated the determinants and short-term complications, including headache, vertigo, nausea, and muscle weakness following GKRS, as well as the patients’ Progression-free Survival (PFS).
Statistical analysis
Descriptive statistics were used to analyze demographic and clinical data. Quantitative variables were reported as Mean±SD and qualitative variables as percentage and ratio. A Kaplan-Meier plot was constructed to assess PFS. All statistical analyses were performed, using SPSS V. 22 and P values less than 0.05 were considered the statistical significance.
3. Results
Sixty-one patients with PSM were evaluated, of whom 32(52%) were men (Mean±SD age: 49.70±16.95 y) and 29(47.5%) were women (Mean±SD age: 52.19±13.05 y). There was no significant difference among the participants in age and sex. Moreover, 45(73.8%) patients had a history of operation, and 16(26.2%) had no previous operation or radiotherapy. Of 45 patients with a history of operation, 30 patients experienced one, nine experienced two, and five experienced three operations. Four patients with a history of operation also received postoperative radiotherapy. Primary symptoms were headache (37%), seizure (22%), paraparesis (10%), vertigo (8%), nausea and vomiting (8%), and cognitive disorder (5%).
Radiologic outcomes following gamma knife radiosurgery
The Mean±SD follow-up time was 30.28±27.48 months (range: 2-120mon). Six patients were followed-up for less than six months, and one patient died in the fifth month of follow-up. Table 1 presents the radiosurgery parameters of the patients.
According to our investigations, 5(8.2%) patients died. Of these patients, one was a woman, and four were men. Two of them had a history of operation; one patient experienced three operations, and another one underwent one operation. Radiologically, increased tumor volume was seen in three patients, who died because of tumor growth and its complications. There was no change in the tumor volume in two patients, of whom one suffered from an extensive stroke within five months and died, but the cause of death was not recorded for the other patient. Radiologic tumor control was achieved in (91.8%) of the patients, in whom the tumor volume decreased in 30 (49.2%) patients and remained unchanged in 26 (42.6%) patients. An increase in tumor volume was noticed in five (8.2%) patients, of whom three died and two underwent operations.
Clinical outcomes following gamma knife radiosurgery
There was no significant difference in age at the onset of symptoms between those who died and patients who survived. The Mean±SD age of diagnosis was 61±18.76 years in patients who died and 49.9±14.61 years in patients who survived.
Eleven patients, who responded to corticosteroid therapy, developed a refractory headache after GKRS because of the evidence of brain edema. There was a marked improvement in headache, nausea, vomiting, and vertigo in (56.9%) of the patients (during six months follow-up), while no change was seen in (35.3%) of the patients. Five patients, who had a history of operation, reported the worsening of muscle weakness, headache, and vertigo. Two of these patients had three operations (one of these two had a history of radiotherapy following operation), two had one operation, and one had a history of radiotherapy followed by an operation. Table 2 presents different radiologic tumor outcomes in the last follow-up.
Clinical outcomes following gamma knife radiosurgery
There was no significant difference in age at the onset of symptoms between those who died and patients who survived. The Mean±SD age of diagnosis was 61±18.76 years in patients who died and 49.9±14.61 years in patients who survived.
Eleven patients, who responded to corticosteroid therapy, developed a refractory headache after GKRS because of the evidence of brain edema. There was a marked improvement in headache, nausea, vomiting, and vertigo in (56.9%) of the patients (during six months follow-up), while no change was seen in (35.3%) of the patients. Five patients, who had a history of operation, reported the worsening of muscle weakness, headache, and vertigo. Two of these patients had three operations (one of these two had a history of radiotherapy following operation), two had one operation, and one had a history of radiotherapy followed by an operation. Table 2 presents different radiologic tumor outcomes in the last follow-up.
Tumor outcomes and progression-free survival
There was no significant difference between radiosurgical parameters and tumor volume in radiological tumor-related outcomes or clinical outcomes. Gender did not correlate with various tumor outcomes, as well.
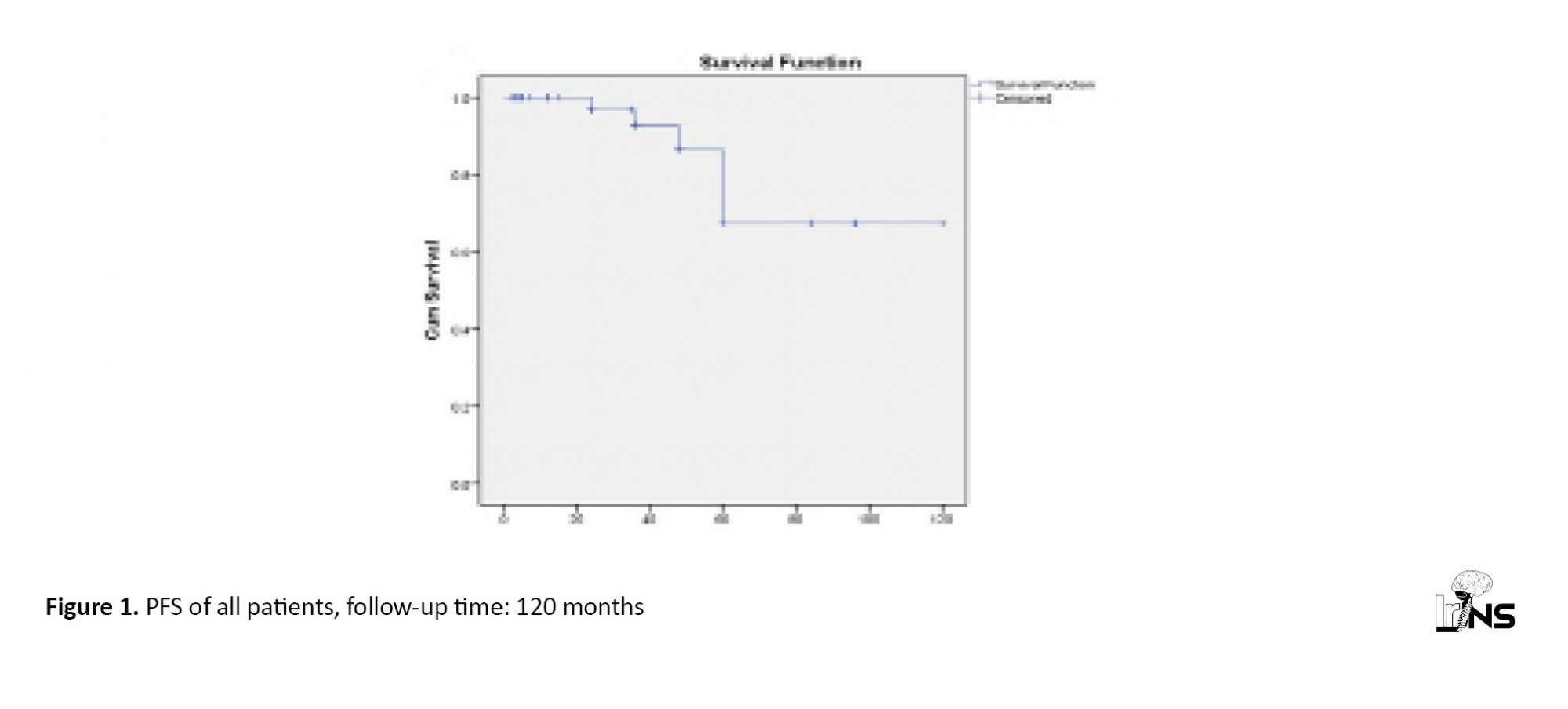
According to Kaplan-Meier analysis, the overall PFS was (98.6%) in 12 months, 96.9±0.03% in 24 months, 92±0.04% in 36 months, 86.6±0.07% in 48 months, and 67.04±13.4% in 60 months (Figure 1). There was no significant difference in PFS between the operated and non-operated groups.
There was no significant difference between radiosurgical parameters and tumor volume in radiological tumor-related outcomes or clinical outcomes. Gender did not correlate with various tumor outcomes, as well.
According to Kaplan-Meier analysis, the overall PFS was (98.6%) in 12 months, 96.9±0.03% in 24 months, 92±0.04% in 36 months, 86.6±0.07% in 48 months, and 67.04±13.4% in 60 months (Figure 1). There was no significant difference in PFS between the operated and non-operated groups.
4. Discussion
Because PSMs can invade critical adjacent structures such as the superior sagittal sinus and large cerebral veins, surgeons may encounter difficulties in the management of these tumors. Therefore, the aim of the treatment is not only removing the tumor mass but also its control after removal [1, 6, 7]. A bulk of studies in the literature have demonstrated that the tumor resection surgery of PSM is associated with a significant risk of recurrence [1, 6, 8-14]. Recent investigations detected tumor recurrence in (27.2%) during the mean follow-up time of 84.4 months [8]. Raza et al. reported an 11% recurrence rate after microsurgical resection in 61 patients with PSM with a mean follow-up of 41 months [6]. Similarly, Pettersson-Segerlind J et al. reported a long-term recurrence rate of 47% in a 25-year follow-up that increased with increasing Simpson Grades [10]. Despite these facts, radiosurgery showed more effectiveness in controlling PSM [11, 15]. Such studies were performed to evaluate the safety and efficacy of radiosurgery in PSMs’ tumor control. They stated that radiosurgery had no mortality and no risk of sinus thrombosis, as well [16]. Hadelsberg et al. reported 4% risk of headaches after radiosurgery [16]; Escribano Mesa et al. however, reported 32.8% of motor deficit after the operation, which was most followed by headache, seizures, dysphasia, loss of vision, and head tumescence [8]. It has been proved that radiosurgery had more advantages over open surgery with less harm to critical structures like arteries, veins, and venous sinuses, at least at clinically-performed radiation doses. Therefore, it can be an appropriate therapy when critical vessels are invaded by tumor mass and its cure by the conventional operation is accompanied by a high risk of sinus occlusion and thrombosis [16].
According to the investigation of 61 patients diagnosed with PSM during 30.28 months of follow-up time (range: 2-120 months) after GKRS, the actuarial radiologic tumor control was 91.8%. This result agrees with the available literature. Seo et al. reported the tumor control rate of 91.7% in 424 patients over five years [17]. Hadelsberg et al. after studying on 74 patients reported that parasagittal tumors were controlled in 90.6% of the patients during the mean follow-up time of 49 months [16]. Hesagawa et al. achieved the tumor control rate of 87% in 119 lesions, including convexity, parasagittal, and falcine meningiomas, of which 49 (41%) lesions regressed, 52(44%) cases remained stable, and 18(15%) cases had in-field tumor progression [18]. We achieved nearly better results of tumor volume decreasing in 30(49.2%) patients. This variable remained unchanged in 26(42.6%) patients and increased in five (8.2%) patients, of whom three died, and two underwent operation.
Furthermore, using the Kaplan-Meier analysis, we found PFS as (98.6%), 92±0.04%, and 67.04±13.4% in one, three, and five years, respectively. PFS in a study performed by Sheehan et al. at two, three, and fivw years was (98%), (90%), and (90%), respectively [19]. Hesagawa et al. reported the actuarial five and ten years PFS rate of (78%) and (57%), respectively [18]. Many investigations have reported the predictors of PFS. Ding et al. declared that the independent predictors of tumor PFS were tumor location (parasagittal), prior resection, and younger age [15]. It was considered that tumor volume and margin dose were not associated with PFS, as we concluded in our study [20]. Seo et al. mentioned female sex and the history of craniotomy as associated factors with tumor progression [17]. However, we found no association between the history of operation and PFS rate and no significant difference between genders, as well.
Although GKRS has more advantages because of lower morbidities compared with the operation, we cannot fully protect neurovascular structures, particularly dural venous sinuses invaded by some of the tumors from radiation exposure [21, 22]. Moreover, studies have shown that the rate of stereotactic radiosurgery side effects is higher in non-basal regions, especially in the parasagittal area [4, 5]. PSMs are at particular risk for venous occlusive complications, which leads to brain edema [2]. The etiology is still unclear, but the secretion of vasoactive substances in the parasagittal region after radiosurgery may be involved [23].
In our study, 56.9% of the cases represent notably with recurrence in their symptoms, including headache, nausea, vomiting, and vertigo during 6 months of follow-up after administrating GKRS. Nevertheless, 11 (18.03%) patients developed a refractory headache due to brain edema, of whom all responded to corticosteroid therapy. It was justified in the literature that gamma knife therapy could significantly improve symptoms like headache in the new or recurrent cases of meningioma [3]. However, many studies declared that edema developed mostly in tumors around the critical structures such as midline and sagittal sinus in comparison with skull-based meningioma [5, 20, 24-26]. It seems that PSMs are more probably radio-resistant than other meningiomas and trend to present edema following radiosurgery, as well [18, 27, 28].
In a study by Sheehan et al. on 61 patients with a median follow-up time of 28 months, edema progression after GKRS in 77 parasagittal and parafalcine tumors were reported in (40%) of tumors, of which (26%) regressed in time. Also, brain edema regressed in (25%) and remained stable (23%) [19]. Evidence has indicated the anatomical location of tumor and its invasion to venous sinus (parasagittal location), tumor volume, margin, and maximal dose in radiosurgery administration, a well as the history of pretreatment edema, and sagittal sinus occlusion are potential factors associated with post-radiosurgical edema [26, 28].
One study has demonstrated that tumors accompanied by preexisting edema were more likely to present progressive edema after administrating radiosurgery, and prior resection was not related to the occurrence of new or worsening edema, as well [19]. Furthermore, Kollova et al. found that a margin dose greater than 16 Gy was a risk factor associated with edema [3]. However, we found no significant association between radiologic findings (most notably edema) and radiosurgical parameters such as maximum dose delivery, marginal dose, and isodose, as well as tumor volume.
Moreover, in our investigation, five patients, who had a history of operation, reported the worsening of muscle weakness, headache, and vertigo. Two of these patients had three operations (one of them had a history of radiotherapy following operation), two had one operation, and one had a history of radiotherapy followed by an operation. There was no change in (35.3%) of the patients.
Hesagawa et al. found that initial treatment by gamma knife could expose patients to peritumoral edema, particularly in the first three months after radiosurgery. They reported an edema rate of 50% (21 of 42 patients) in a group with the initial treatment of gamma knife compared with 13% (8 of 61) of peritumoral edema occurrence, with a previous operation [18]. Our data showed that of those 11 patients, who developed edema, only four patients had a history of at least one resection operation, and seven patients underwent the initial treatment of GKRS. Consequently, stereotactic radiosurgery is truly a potential to control tumor growth by itself, though it seems to be effective as an adjuvant therapy because of the risk of progressive peritumoral edema, particularly in PSMs.
Among the limitations of this study were overlooking the evaluation of the patients’ endocrine function and the short follow-up time. The investigation of these parameters in other studies with larger sample sizes may provide an accurate assessment of GKRS’s effects in PSMs.
5. Conclusions
In conclusion, GKRS is a safe and effective choice as the first or second line of treatment of PSM. We did not find any association among the patients’ age, tumor size, and radiosurgery parameters as potential factors associated with edema following radiosurgery.
Considering the critical location of parasagittal tumors, the combined treatment approach of radiosurgery with microsurgery may reduce the complications, particularly conditions, in which the tumor has completely invaded within the superior sagittal sinus and total resection accompanied by the high risk of mortality. Because radiosurgery management of PSMs encounters such limitations, we suggest long-term follow-up to diagnose life-threatening complications, including brain edema.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We would like to appreciate our colleagues for their efforts in the scientific implementation of the project.
References
Because PSMs can invade critical adjacent structures such as the superior sagittal sinus and large cerebral veins, surgeons may encounter difficulties in the management of these tumors. Therefore, the aim of the treatment is not only removing the tumor mass but also its control after removal [1, 6, 7]. A bulk of studies in the literature have demonstrated that the tumor resection surgery of PSM is associated with a significant risk of recurrence [1, 6, 8-14]. Recent investigations detected tumor recurrence in (27.2%) during the mean follow-up time of 84.4 months [8]. Raza et al. reported an 11% recurrence rate after microsurgical resection in 61 patients with PSM with a mean follow-up of 41 months [6]. Similarly, Pettersson-Segerlind J et al. reported a long-term recurrence rate of 47% in a 25-year follow-up that increased with increasing Simpson Grades [10]. Despite these facts, radiosurgery showed more effectiveness in controlling PSM [11, 15]. Such studies were performed to evaluate the safety and efficacy of radiosurgery in PSMs’ tumor control. They stated that radiosurgery had no mortality and no risk of sinus thrombosis, as well [16]. Hadelsberg et al. reported 4% risk of headaches after radiosurgery [16]; Escribano Mesa et al. however, reported 32.8% of motor deficit after the operation, which was most followed by headache, seizures, dysphasia, loss of vision, and head tumescence [8]. It has been proved that radiosurgery had more advantages over open surgery with less harm to critical structures like arteries, veins, and venous sinuses, at least at clinically-performed radiation doses. Therefore, it can be an appropriate therapy when critical vessels are invaded by tumor mass and its cure by the conventional operation is accompanied by a high risk of sinus occlusion and thrombosis [16].
According to the investigation of 61 patients diagnosed with PSM during 30.28 months of follow-up time (range: 2-120 months) after GKRS, the actuarial radiologic tumor control was 91.8%. This result agrees with the available literature. Seo et al. reported the tumor control rate of 91.7% in 424 patients over five years [17]. Hadelsberg et al. after studying on 74 patients reported that parasagittal tumors were controlled in 90.6% of the patients during the mean follow-up time of 49 months [16]. Hesagawa et al. achieved the tumor control rate of 87% in 119 lesions, including convexity, parasagittal, and falcine meningiomas, of which 49 (41%) lesions regressed, 52(44%) cases remained stable, and 18(15%) cases had in-field tumor progression [18]. We achieved nearly better results of tumor volume decreasing in 30(49.2%) patients. This variable remained unchanged in 26(42.6%) patients and increased in five (8.2%) patients, of whom three died, and two underwent operation.
Furthermore, using the Kaplan-Meier analysis, we found PFS as (98.6%), 92±0.04%, and 67.04±13.4% in one, three, and five years, respectively. PFS in a study performed by Sheehan et al. at two, three, and fivw years was (98%), (90%), and (90%), respectively [19]. Hesagawa et al. reported the actuarial five and ten years PFS rate of (78%) and (57%), respectively [18]. Many investigations have reported the predictors of PFS. Ding et al. declared that the independent predictors of tumor PFS were tumor location (parasagittal), prior resection, and younger age [15]. It was considered that tumor volume and margin dose were not associated with PFS, as we concluded in our study [20]. Seo et al. mentioned female sex and the history of craniotomy as associated factors with tumor progression [17]. However, we found no association between the history of operation and PFS rate and no significant difference between genders, as well.
Although GKRS has more advantages because of lower morbidities compared with the operation, we cannot fully protect neurovascular structures, particularly dural venous sinuses invaded by some of the tumors from radiation exposure [21, 22]. Moreover, studies have shown that the rate of stereotactic radiosurgery side effects is higher in non-basal regions, especially in the parasagittal area [4, 5]. PSMs are at particular risk for venous occlusive complications, which leads to brain edema [2]. The etiology is still unclear, but the secretion of vasoactive substances in the parasagittal region after radiosurgery may be involved [23].
In our study, 56.9% of the cases represent notably with recurrence in their symptoms, including headache, nausea, vomiting, and vertigo during 6 months of follow-up after administrating GKRS. Nevertheless, 11 (18.03%) patients developed a refractory headache due to brain edema, of whom all responded to corticosteroid therapy. It was justified in the literature that gamma knife therapy could significantly improve symptoms like headache in the new or recurrent cases of meningioma [3]. However, many studies declared that edema developed mostly in tumors around the critical structures such as midline and sagittal sinus in comparison with skull-based meningioma [5, 20, 24-26]. It seems that PSMs are more probably radio-resistant than other meningiomas and trend to present edema following radiosurgery, as well [18, 27, 28].
In a study by Sheehan et al. on 61 patients with a median follow-up time of 28 months, edema progression after GKRS in 77 parasagittal and parafalcine tumors were reported in (40%) of tumors, of which (26%) regressed in time. Also, brain edema regressed in (25%) and remained stable (23%) [19]. Evidence has indicated the anatomical location of tumor and its invasion to venous sinus (parasagittal location), tumor volume, margin, and maximal dose in radiosurgery administration, a well as the history of pretreatment edema, and sagittal sinus occlusion are potential factors associated with post-radiosurgical edema [26, 28].
One study has demonstrated that tumors accompanied by preexisting edema were more likely to present progressive edema after administrating radiosurgery, and prior resection was not related to the occurrence of new or worsening edema, as well [19]. Furthermore, Kollova et al. found that a margin dose greater than 16 Gy was a risk factor associated with edema [3]. However, we found no significant association between radiologic findings (most notably edema) and radiosurgical parameters such as maximum dose delivery, marginal dose, and isodose, as well as tumor volume.
Moreover, in our investigation, five patients, who had a history of operation, reported the worsening of muscle weakness, headache, and vertigo. Two of these patients had three operations (one of them had a history of radiotherapy following operation), two had one operation, and one had a history of radiotherapy followed by an operation. There was no change in (35.3%) of the patients.
Hesagawa et al. found that initial treatment by gamma knife could expose patients to peritumoral edema, particularly in the first three months after radiosurgery. They reported an edema rate of 50% (21 of 42 patients) in a group with the initial treatment of gamma knife compared with 13% (8 of 61) of peritumoral edema occurrence, with a previous operation [18]. Our data showed that of those 11 patients, who developed edema, only four patients had a history of at least one resection operation, and seven patients underwent the initial treatment of GKRS. Consequently, stereotactic radiosurgery is truly a potential to control tumor growth by itself, though it seems to be effective as an adjuvant therapy because of the risk of progressive peritumoral edema, particularly in PSMs.
Among the limitations of this study were overlooking the evaluation of the patients’ endocrine function and the short follow-up time. The investigation of these parameters in other studies with larger sample sizes may provide an accurate assessment of GKRS’s effects in PSMs.
5. Conclusions
In conclusion, GKRS is a safe and effective choice as the first or second line of treatment of PSM. We did not find any association among the patients’ age, tumor size, and radiosurgery parameters as potential factors associated with edema following radiosurgery.
Considering the critical location of parasagittal tumors, the combined treatment approach of radiosurgery with microsurgery may reduce the complications, particularly conditions, in which the tumor has completely invaded within the superior sagittal sinus and total resection accompanied by the high risk of mortality. Because radiosurgery management of PSMs encounters such limitations, we suggest long-term follow-up to diagnose life-threatening complications, including brain edema.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We would like to appreciate our colleagues for their efforts in the scientific implementation of the project.
References
- DiMeco F, Li KW, Casali C, Ciceri E, Giombini S, Filippini G, et al. Meningiomas invading the superior sagittal sinus: Surgical experience in 108 cases. Neurosurgery. 2004; 55(6):1263-74. [DOI:10.1227/01.NEU.0000333779.73940.C4] [PMID]
- Conti A, Pontoriero A, Salamone I, Siragusa C, Midili F, La Torre D, et al. Protecting venous structures during radiosurgery for parasagittal meningiomas. Neurosurgical Focus. 2009; 27(5):E11. [DOI:10.3171/2009.8.FOCUS09-157] [PMID]
- Kollova A, Liscak R, Novotny J, Vladyka V, Simonova G, Janouskova L. Gamma Knife surgery for benign meningioma. Journal of Neurosurgery. 2007; 107(2):325-36. [DOI:10.3171/JNS-07/08/0325] [PMID]
- Tanaka M, Imhof HG, Schucknecht B, Kollias S, Yonekawa Y, Valavanis A. Correlation between the efferent venous drainage of the tumor and peritumoral edema in intracranial meningiomas: superselective angiographic analysis of 25 cases. Journal of Neurosurgery. 2006; 104(3):382-8. [DOI:10.3171/jns.2006.104.3.382] [PMID]
- Vermeulen S, Young R, Li F, Meier R, Raisis J, Klein S, et al. A comparison of single fraction radiosurgery tumor control and toxicity in the treatment of basal and nonbasal meningiomas. Stereotactic and Functional Neurosurgery. 1999; 72:60-6. [DOI:10.1159/000056440] [PMID]
- Raza SM, Gallia GL, Brem H, Weingart JD, Long DM, Olivi A. Perioperative and long-term outcomes from the management of parasagittal meningiomas invading the superior sagittal sinus. Neurosurgery. 2010; 67(4):885-93. [DOI:10.1227/NEU.0b013e3181ef2a18] [PMID]
- Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008; 62(1):53-8. [DOI:10.1227/01.NEU.0000311061.72626.0D] [PMID]
- Escribano Mesa JA, Alonso Morillejo E, Parron Carreno T, Huete Allut A, Narro Donate JM, Mendez Roman P, et al. Risk of recurrence in operated parasagittal meningiomas: A logistic binary regression model. World Neurosurgery. 2018; 110:e112-e8. [DOI:10.1016/j.wneu.2017.10.087] [PMID]
- Gatterbauer B, Gevsek S, Hoftberger R, Lutgendorf-Caucig C, Ertl A, Mallouhi A, et al. Multimodal treatment of parasagittal meningiomas: A single-center experience. Journal of Neurosurgery. 2017; 127(6):1249-56. [DOI:10.3171/2016.9.JNS161859] [PMID]
- Pettersson-Segerlind J, Orrego A, Lonn S, Mathiesen T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurgery. 2011; 76(6):564-71. [DOI:10.1016/j.wneu.2011.05.015] [PMID]
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. Journal of Neurology, Neurosurgery, and Psychiatry. 1957; 20(1):22-39. [DOI:10.1136/jnnp.20.1.22] [PMID] [PMCID]
- Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB, J, Rhoton AL. Benign meningiomas: Primary treatment selection affects survival. International Journal of Radiation Oncology, Biology, Physics. 1997; 39(2):427-36. [DOI:10.1016/S0360-3016(97)00317-9]
- Omay SB, Barnett GH. Surgical navigation for meningioma surgery. Journal of Neuro-Oncology. 2010; 99(3):357-64. [DOI:10.1007/s11060-010-0359-6] [PMID]
- Ryu HS, Moon KS, Lee KH, Jang WY, Jung TY, Kim IY, et al. Recurred intracranial meningioma: A retrospective analysis for treatment outcome and prognostic factor. Brain Tumor Research and Treatment. 2017; 5(2):54-63. [DOI:10.14791/btrt.2017.5.2.54] [PMID] [PMCID]
- Ding D, Xu Z, McNeill IT, Yen CP, Sheehan JP. Radiosurgery for parasagittal and parafalcine meningiomas. Journal of Neurosurgery. 2013; 119(4):871-7. [DOI:10.3171/2013.6.JNS13110] [PMID]
- Hadelsberg U, Nissim U, Cohen ZR, Spiegelmann R. LINAC radiosurgery in the management of parasagittal meningiomas. Stereotactic and Functional Neurosurgery. 2015; 93(1):10-6. [DOI:10.1159/000368440] [PMID]
- Seo Y, Kim DG, Kim JW, Han JH, Chung HT, Paek SH. Long-term outcomes after gamma knife radiosurgery for benign meningioma: A single institution’s experience with 424 patients. Neurosurgery. 2018; 83(5):1040-9. [DOI:10.1093/neuros/nyx585] [PMID]
- Hasegawa T, Kida Y, Yoshimoto M, Iizuka H, Ishii D, Yoshida K. Gamma Knife surgery for convexity, parasagittal, and falcine meningiomas. Journal of Neurosurgery. 2011; 114(5):1392-8. [DOI:10.3171/2010.11.JNS10112] [PMID]
- Sheehan JP, Lee CC, Xu Z, Przybylowski CJ, Melmer PD, Schlesinger D. Edema following Gamma Knife radiosurgery for parasagittal and parafalcine meningiomas. Journal of Neurosurgery. 2015; 123(5):1287-93. [DOI:10.3171/2014.12.JNS142159] [PMID]
- Kondziolka D, Patel AD, Kano H, Flickinger JC, Lunsford LD. Long-term outcomes after gamma knife radiosurgery for meningiomas. American Journal of Clinical Oncology. 2016; 39(5):453-7. [DOI:10.1097/COC.0000000000000080] [PMID]
- Azar M, Kazemi F, Jahanbakhshi A, Chanideh I, Jalessi M, Amini E, et al. Gamma knife radiosurgery for cavernous sinus meningiomas: analysis of outcome in 166 patients. Stereotactic and Functional Neurosurgery. 2017; 95(4):259-67. [DOI:10.1159/000478024] [PMID]
- Lee CC, Sheehan JP, Kano H, Akpinar B, Martinez-Alvarez R, Martinez-Moreno N, et al. Gamma knife radiosurgery for hemangioma of the cavernous sinus. Journal of Neurosurgery. 2017; 126(5):1498-505. [DOI:10.3171/2016.4.JNS152097] [PMID]
- Bitzer M, Wockel L, Luft AR, Wakhloo AK, Petersen D, Opitz H, et al. The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. Journal of Neurosurgery. 1997; 87(3):368-73. [DOI:10.3171/jns.1997.87.3.0368] [PMID]
- Ganz JC, Schrottner O, Pendl G. Radiation-induced edema after Gamma Knife treatment for meningiomas. Stereotactic and Functional Neurosurgery. 1996; 66(Suppl. 1):129-33. [DOI:10.1159/000099778] [PMID]
- Cai R, Barnett GH, Novak E, Chao ST, Suh JH. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010; 66(3):513-22. [DOI:10.1227/01.NEU.0000365366.53337.88] [PMID]
- Sheehan JP, Cohen-Inbar O, Ruangkanchanasetr R, Bulent Omay S, Hess J, Chiang V, et al. Post-radiosurgical edema associated with parasagittal and parafalcine meningiomas: A multicenter study. Journal of Neuro-Oncology. 2015; 125(2):317-24. [DOI:10.1007/s11060-015-1911-1] [PMID]
- Kim KH, Kang SJ, Choi JW, Kong DS, Seol HJ, Nam DH, et al. Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. Journal of Neurosurgery. 2018 130(5):1740-9. [DOI:10.3171/2017.12.JNS171943]
- Kalapurakal JA, Silverman CL, Akhtar N, Laske DW, Braitman LE, Boyko OB, et al. Intracranial meningiomas: Factors that influence the development of cerebral edema after stereotactic radiosurgery and radiation therapy. Radiology. 1997; 204(2):461-5. [DOI:10.1148/radiology.204.2.9240536] [PMID]
Type of Study: Research |
Subject:
Gamma Knife Radiosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




.PNG)