Sat, Apr 20, 2024
Volume 3, Issue 2 (9-2017)
Iran J Neurosurg 2017, 3(2): 51-57 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zareian L, Azarhomayoun A, Alimohamadi M, Khajavi M, Razeghi-Jahromi S. Impact of Acute Phase Epigallocatechin-3-gallate Supplementation on Consciousness and S100B Serum Levels in TBI Patients: A Double Blind Randomized Clinical Trial
. Iran J Neurosurg 2017; 3 (2) :51-57
URL: http://irjns.org/article-1-87-en.html
URL: http://irjns.org/article-1-87-en.html
Leila Zareian1 
 , Amir Azarhomayoun2
, Amir Azarhomayoun2 
 , Maysam Alimohamadi3
, Maysam Alimohamadi3 
 , Mohammadreza Khajavi4
, Mohammadreza Khajavi4 
 , Soodeh Razeghi-Jahromi *
, Soodeh Razeghi-Jahromi * 

 5
5

 , Amir Azarhomayoun2
, Amir Azarhomayoun2 
 , Maysam Alimohamadi3
, Maysam Alimohamadi3 
 , Mohammadreza Khajavi4
, Mohammadreza Khajavi4 
 , Soodeh Razeghi-Jahromi *
, Soodeh Razeghi-Jahromi * 

 5
5
1- MSc, Animal Physiology Department, Basic Sciences Faculty, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
2- MD, Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences (TUMS), Tehran, Iran
3- MD, Brain and Spinal Cord Injury Research Center (BASIR), Neuroscience Institute, Tehran University of Medical Sciences (TUMS), Tehran, Iran
4- MD, Department of Anesthesiology, Sina hospital, Tehran University of Medical Sciences, Tehran, Iran
5- PhD, Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran , razeghi@sina.tums.ac.i
2- MD, Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences (TUMS), Tehran, Iran
3- MD, Brain and Spinal Cord Injury Research Center (BASIR), Neuroscience Institute, Tehran University of Medical Sciences (TUMS), Tehran, Iran
4- MD, Department of Anesthesiology, Sina hospital, Tehran University of Medical Sciences, Tehran, Iran
5- PhD, Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran , razeghi@sina.tums.ac.i
Keywords: Epigallocatechin-3-gallate, Head Trauma, Neuroprotection, S100B Protein, Traumatic Brain Injury
Full Text [PDF 464 kb]
(2402 Downloads)
| Abstract (HTML) (8720 Views)
Statistical Analysis
The distribution of continuous variables was assessed using Kolmogorov–Smirnov test. To compare the continuous variables between the two groups, the independent -samples t-test and Mann–Whitney U-test were used for data with normal and non-normal distributions, respectively. For a before-after comparison of the variable levels, paired sample t-test and Wilcoxon were used for the data with normal and non-normal distribution, respectively. P-value less than 0.05 was considered as statistically significant for all tests. The data were analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA).
Results
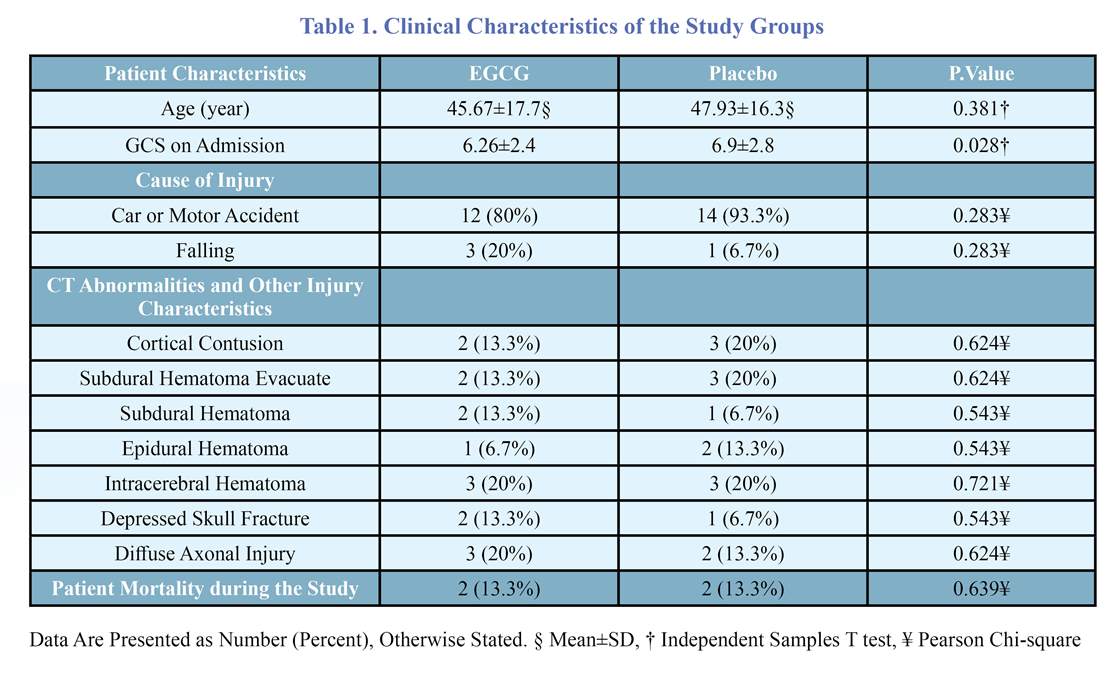
The baseline characteristics of the patients are presented in table 1. The two groups had no significant differences in baseline characteristics. As shown in table 2, seven days following TBI, the last GCS was significantly higher in the EGCG group. Alteration of GCS was measured as last GCS on ICU admission. The EGCG group had significantly higher GCS improvement than the control group after seven days: the mean GCS level increased by 2.93 units in the EGCG group while having an average of 0.14-unit deterioration in the control group at the same time.
Alteration of S100B level was calculated as T2-T1. The average S100 levels decreased in both groups after seven days of admission. The amount of reduction in S100B levels was greater in EGCG than that in the control group (23.96 pg/ml versus 18.6 pg/ml) but the difference was not statistically significant (P= 0.209).
Although the overall duration of ICU stay was not significantly different among the two groups, the duration of mechanical ventilation was significantly shorter in the EGCG group (5.1 versus 9.8 days) (Table 3).
The patients were divided into those who did not have any clinically significant abnormality of the serum gluocose, Hb/Hct and platelet levels (not requiring therapeutic intervention) and those who required therapeutic intervention for abnormalities of these parameters. The prevalence of these potentially confounding factors was not statistically significant between the two groups of the study (Table3).
Discussion
In this study, we evaluated the effect of EGCG of green tea on the clinical outcome (consciousness, duration of ICU stay, mechanical ventilation, and serum S100B levels) of the patients with moderate to severe TBI. Our results show a beneficial effect of EGCG supplementation on improvement of GCS and shortening of the mechanical ventilation period in these patients. These results are in consistence with the previous experimental studies: Itoh et al. reported that seven days of oral supplementation with 0.1% solution of EGCG in water for brain injury-induced rats could reduce neuronal damages [18]. Hau et al. reported that intraperitoneal injection of epigallocatechin-3-gallate solution has neuroprotective effects in brain damage-induced rats by protecting them against oxidative damages to the brain. They concluded that EGCG showed neuroprotective effects by reducing free radical damages and preventing apoptosis in damaged neurons [19,20].
A number of reports proposed S100B serum level as a valid biomarker for brain damage [21]. Previous studies showed that the injection of five mg S100B protein to rat cerebellar vermis had anti-apoptotic effects [22]. Some studies show that S100B probably binds to the neuronal membrane RAGE receptors in low concentrations and increases the survival of neurons by producing against reactive oxygen species (ROS) and increasing the expression of anti-apoptotic factors [22-25]. The findings of current study show that EGCG supplementation results in reducing S100Β protein compared with the control group, although the difference between the two groups was not statistically significant.
Pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) have an important role in the pathophysiology of cerebral edema and brain damage after traumatic insult [26,27]. Factors with anti-inflammatory properties can increase the blood flow in damaged tissues, and subsequently improve the process of wound healing and recovery [27]. Experimental evidence suggests that EGCG could inhibit TNF-alpha gene expression [28,29]. In a study performed on an animal model of multiple sclerosis, the combination of glatiramer acetate (GA) and EGCG had favorable effects on neuronal survival and axonal growth by reduced reactive oxygen species generation and reduction in the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) [28]. To the best of our knowledge, until the date no human study has assessed the effect of EGCG supplementation on the secondary neural damages caused by trauma [30].
To control the confounding effect of underlying mental illness and/or internal organ injury on the clinical outcome of TBI, we excluded these patients from our study. The other possible source of confounding was hematologic and metabolic derangements which may affect the clinical outcome in TBI patients. Statistical analysis revealed that prevalence of these parameters was not statistically different among the two groups, and thus may not serve as a source of confounding. The major limitation of this study was the small sample size of the study. Due to the overwhelming predominance of TBI among men in our country, we only included male patients in this study. Furthermore, impact of the findings of this study on the long-term clinical outcome of TBI patients is a very important issue that needs to be evaluated by larger studies with longer follow-up periods.
Conclusion
The results of this study show that acute phase supplementing with EGCG might have favorable effects on consciousness levels and duration of mechanical ventilation in early phase after moderate to severe TBI. According to the availability and low cost of this product, we recommend early treatment with EGCG as a part of the integral management of patients with TBI admitted to ICU. Acknowledgement
We would like to appreciate the staff of Sina hospital ICU for their kind cooperation. Also, we would like to thank Ms. Hamideh Abbasi for her assistance in assessing S100B level.
Funding
This study was supported by a grant from Iranian Center of Neurological Research, Tehran University of Medical Sciences.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contribution
Conception and Design: Leila Zareian, Soodeh Razeghi Jahromi
Data Collection: Leila Zareian, Amir Azarhomayoun
Drafting the Article: Leila Zareian, Amir Azarhomayoun, Mohammadreza Khajavi
Critically Revising the Article: Maysam Alimohamadi
Reviewed Submitted Version of the Manuscript: All Authors
Approved the Final Version of the Manuscript: All Authors
References
1. Bruns JR, Hauser WA. The epidemiology of traumatic brain injury. a review. Epilepsia. 2003; 44: 2-10.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99: 4-9.
3. Leon-Carion j, Barroso Y marrtin JM. Epidemiology of traumatic brain injury and subarachnoid hemorrhage. J Pituitary. 2005; 8: 197-202.
4. Faria A, Meireles M, Fernandes I, Santos-Buelga C, Gonzalez-Manzano S, Dueñas M, de Freitas V, Mateus N, Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014 Apr 15;149:190-6.
5. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma. 2007;24 Suppl 1:S7-13.
6. Alexander M. Mild traumatic brain injury. pathophysiology, natural history, and clinical management. Neurology. 1995; 45(7): 50 - 60.
7. Kuptniratsaikul V, Kovindha A, Suethanapornkal S.Complications during the rehabilitation period in thai patients with stroke: A Multi center prospective study. AMJ Phys Med Rehabil. 2009; 88(2): 92-99.
8.Castellano C. Psychopharmacology of memory modulation: evidence hormones.J Behave Brain Res. 1996; 34(8): 1-21.
9. Rosenfeldt F, Wilson M. population, pathophysiology and therapy. J Exp Gerontol. 2013;48:45-54.
10. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1 and mitochondrial biogenesis in cerebral ischemia. J Mol Sci . 2011; 12: 7199–215.
11- Higdon J. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions.J Food Sci Nutr. 2003;43: 89-143.
12. Anna K. Flavonoids and the CNS, Molecules. 2011; 16: 1471-1485.
13. Croft KD. (1998). The chemistry and biological effects of flavonoids and phenolic acids. Ann NY Acad Sci. 854:435.
14. Middleton E J. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 2000; 439:175-82.
15. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging, and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845-50
16. BloomfieldSM, Mcknney J, Smith L, Brisman. Reliability of S100B in predicting severity of central nervous system injury. Neurocritic Care. 2007; 6:121-138.
17. Woertgen C, Holzschuh M, Metz C, Brawanski A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J Trauma. 1999; 47: 1126-1130.
18. Itoh T, Tabuchi M, Mizuguchi N, Imano M, Tsubaki M, Nishida S. Neuroprotective effect of epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J Neural Transm. 2013 ;120(5):767–783.
19. Hua W, Hui-Chin Tu, and Jeng-Yung.Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. J BMC Neuroscience. 2011; 12:52.
20. Zink BJ, SymzdYnjer-Chodobska J, Chodobski A. Emerging concepts in the pathophysiology of traumatic brain injury. Psychiat Clin North Am. 2010; 33: 741-756.
21. Karpiak SE, Serokosz M, Rapport MM. Effects of antisera to S100 protein and to synaptic membrane fraction on maze performance and EEG. Brain Res. 1976; 313- 21.
22. Kleindienst A, McGinn M, Harvey H, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22(2):645– 655.
23. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging, and antioxidative effects of flavonoids. Free Radic Biol Med . 1994; 16:845-5.
24. Mirrahimi B, Mortazavi A, Nouri M, Ketabchi E, Amirjamshidi A, Ashouri A, Khajavi M, Mojtahedzadeh M. Effect of magnesium on functional outcome and paraclinical parameters of patients undergoing supratentorial craniotomy for brain tumors: a randomized controlled trial. Acta Neurochir. 2015; 157(6):985.
25. Etezadi F, Aklamli M, Najafi A, Khajavi M, Shariat Moharari R, Mirrahimi B, Mortazavi SA, Mojtahedzadeh M. Evaluation of the anti-inflammatory effects of peri-operative infusion of magnesium sulfate on the microsurgical procedures for intracranial tumors. Anesth Pain Med. 2014;30;4(5):e22379.
26. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1 and mitochondrial biogenesis in cerebral ischemia. J Mol Sci. 2011; 12: 7199–215.
27. Chan PH.Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001; 21(1):2-14.
28. Yun HJ, Yoo WH, Han MK, Lee YR, KiLee SI. Epigallocatechin-3-gallate suppresses T NF a lpha -induced p roduction of M MP-1 a nd -3 i n r heumatoid arthritis synovial fibroblasts. Rheumatol Int. 2008;29(1): 23–9.
29. Renno WM, Saleh F, Klepacek I, Al-Khaledi G, Ismael H, Asfar S. Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr Neurosci. 2006; 9(1-2): 41-7.
30. Herges K. Neuroprotective Effect of Combination Therapy of Glatiramer Acetate and Epigallocatechin-3-Gallate in Neuroinflammation. PLoS ONE. 2011; 6(10): e25456. https://doi.org/10.1371/journal.pone.0025456
Full Text: (3912 Views)
Introduction
Traumatic brain injury (TBI) is the leading cause of disability and death in all age groups worldwide [1]. TBI occurs as a result of direct mechanical insult to the brain followed by secondary events resulting in cell degeneration and death. Secondary insults include excitotoxic damage, blood brain barrier disruption, free radical production, calcium-mediated damage, neuro-inflammation, and hypoxia due to circulatory disturbance [2-4]. Since the primary insult is not preventable, investigations are mainly focused on reversing the secondary insults [5,6]. Recent studies have shown that oxidative stress has a central role in the pathogenesis of traumatic brain damage [5,6]. Oxidative stress enhances free radical generation that subsequently activates apoptotic pathways which results in cell death. Neurodegeneration might continue for months after TBI.
Green tea polyphenol, epigallocatechin-3-gallate (EGCG), has a variety of biological functions and health benefits including anti-obesity [7], anti-inflammatory [8], anti-atherogenic [9] and neuro-protective [10] properties.
EGCG is also known as a very potent antioxidant in biological systems. Several mechanisms have been proposed for antioxidant effects of EGCG including free radical scavenging, attenuation of lipid peroxidation by inhibiting xanthine oxidase activation and blockade of inducible nitric oxide synthase [11-13].
Recent in vivo and in vitro studies suggest the protective role of EGCG against free radical-induced neuronal damage [14,15]. Studies have indicated that S100B, a member of the S100 protein family, is abundant in the nervous system where it is predominantly expressed in astrocytes, oligodendrocytes, and Schwann cells. When secreted by astrocytes, S100B has neurotrophic effects during development and nerve regeneration at physiologic concentrations. However, high concentrations of S100B have shown to be neurotoxic. It seems that S100B serum levels correlates with clinical outcome after TBI [16]. Some studies suggested S100B as a biomarker for determining the severity of brain injury and also monitoring therapeutic intervention [17]. An increment in S100B level was reported immediately after TBI. The level was reported to drop after treatment. There is no evidence about the impact of administration of EGCG on the outcome of the patients with TBI in the acute phase. This study is a randomized clinical trial to evaluate the potential benefit of EGCG on the early clinical outcome and serum levels of S100B biomarker of the patients suffering from moderate to severe TBI.
Methods and Materials/Patients
Male patients (16-65 years old) admitted to a referral trauma center, intensive care unit of Sina hosipital in Tehran, Iran, with moderate to severe TBI from October 2014 to October 2015 were enrolled in this study. The inclusion criteria of this study were GCS of 4-12, enteral nutrition starting within 24 hours after admission and mechanical ventilation. The procedure and protocol of the study was approved by the Ethic Committee of Tehran University of Medical Sciences (clinical trials.gov ID: NCT02731495). Written informed consent was obtained from patients’ relatives.
Data Collection and Supplementation
After recording anamnesis, the patients were examined physically. Emergency care started in the emergency room. Patients with GCS higher than 12 or lower than 4, internal organ bleeding, obvious fractures in their limbs, history of underlying neurologic, metabolic, or psychiatric disorder, alcohol or drug abuse, and vegetarian diet were excluded from the study. Basic information including age, sex, vital signs, GCS, and clinical symptoms at the time of admission were recorded.
All patients received enteral nutrition with the same formula and protocol. The patients were divided into two groups using random balanced blocks: (1) receiving EGCG supplement (n=15) and (2) receiving placebo (control group, n=15). CONSORT flow diagram of the study is shown in figure 1. EGCG supplement was in form of 400 mg oral capsules with the purity of 80% catechin (Vitamilife, U.S.). EGCG powder of each capsule was dissolved in 10 ml deionized water and given to patients via gavage (one capsule per day) for seven days by a nurse who was blinded to the treatment. The control group received only 10 ml of deionized water via gavage for a week by the same nurse. During that week, GCS of the patients was recorded by a neurosurgery resident blinded to the intervention. Vital signs and serum level of glucose, hematocrit/hemoglobin, and platelets were recorded every day at 9:00 AM for one week. Moreover, the durations of mechanical ventilation and ICU stay were recorded.
In order to analyze S100B protein, a 5 ml peripheral venous blood sample was taken from patients on admission (T1) and 24 hours after the last gavage with EGCG (T2) and stored at -80°C. Human S100B ELISA kit (BioVendor - Laboratorni medicina a.s. Cat# RD 192090100) was used to quantify the level of S100B protein according to the manufacturer protocol. The primary outcome in this study was serum level of the S100B biomarker on admission and after one week of supplementation with either placebo or EGCG.
Traumatic brain injury (TBI) is the leading cause of disability and death in all age groups worldwide [1]. TBI occurs as a result of direct mechanical insult to the brain followed by secondary events resulting in cell degeneration and death. Secondary insults include excitotoxic damage, blood brain barrier disruption, free radical production, calcium-mediated damage, neuro-inflammation, and hypoxia due to circulatory disturbance [2-4]. Since the primary insult is not preventable, investigations are mainly focused on reversing the secondary insults [5,6]. Recent studies have shown that oxidative stress has a central role in the pathogenesis of traumatic brain damage [5,6]. Oxidative stress enhances free radical generation that subsequently activates apoptotic pathways which results in cell death. Neurodegeneration might continue for months after TBI.
Green tea polyphenol, epigallocatechin-3-gallate (EGCG), has a variety of biological functions and health benefits including anti-obesity [7], anti-inflammatory [8], anti-atherogenic [9] and neuro-protective [10] properties.
EGCG is also known as a very potent antioxidant in biological systems. Several mechanisms have been proposed for antioxidant effects of EGCG including free radical scavenging, attenuation of lipid peroxidation by inhibiting xanthine oxidase activation and blockade of inducible nitric oxide synthase [11-13].
Recent in vivo and in vitro studies suggest the protective role of EGCG against free radical-induced neuronal damage [14,15]. Studies have indicated that S100B, a member of the S100 protein family, is abundant in the nervous system where it is predominantly expressed in astrocytes, oligodendrocytes, and Schwann cells. When secreted by astrocytes, S100B has neurotrophic effects during development and nerve regeneration at physiologic concentrations. However, high concentrations of S100B have shown to be neurotoxic. It seems that S100B serum levels correlates with clinical outcome after TBI [16]. Some studies suggested S100B as a biomarker for determining the severity of brain injury and also monitoring therapeutic intervention [17]. An increment in S100B level was reported immediately after TBI. The level was reported to drop after treatment. There is no evidence about the impact of administration of EGCG on the outcome of the patients with TBI in the acute phase. This study is a randomized clinical trial to evaluate the potential benefit of EGCG on the early clinical outcome and serum levels of S100B biomarker of the patients suffering from moderate to severe TBI.
Methods and Materials/Patients
Male patients (16-65 years old) admitted to a referral trauma center, intensive care unit of Sina hosipital in Tehran, Iran, with moderate to severe TBI from October 2014 to October 2015 were enrolled in this study. The inclusion criteria of this study were GCS of 4-12, enteral nutrition starting within 24 hours after admission and mechanical ventilation. The procedure and protocol of the study was approved by the Ethic Committee of Tehran University of Medical Sciences (clinical trials.gov ID: NCT02731495). Written informed consent was obtained from patients’ relatives.
Data Collection and Supplementation
After recording anamnesis, the patients were examined physically. Emergency care started in the emergency room. Patients with GCS higher than 12 or lower than 4, internal organ bleeding, obvious fractures in their limbs, history of underlying neurologic, metabolic, or psychiatric disorder, alcohol or drug abuse, and vegetarian diet were excluded from the study. Basic information including age, sex, vital signs, GCS, and clinical symptoms at the time of admission were recorded.
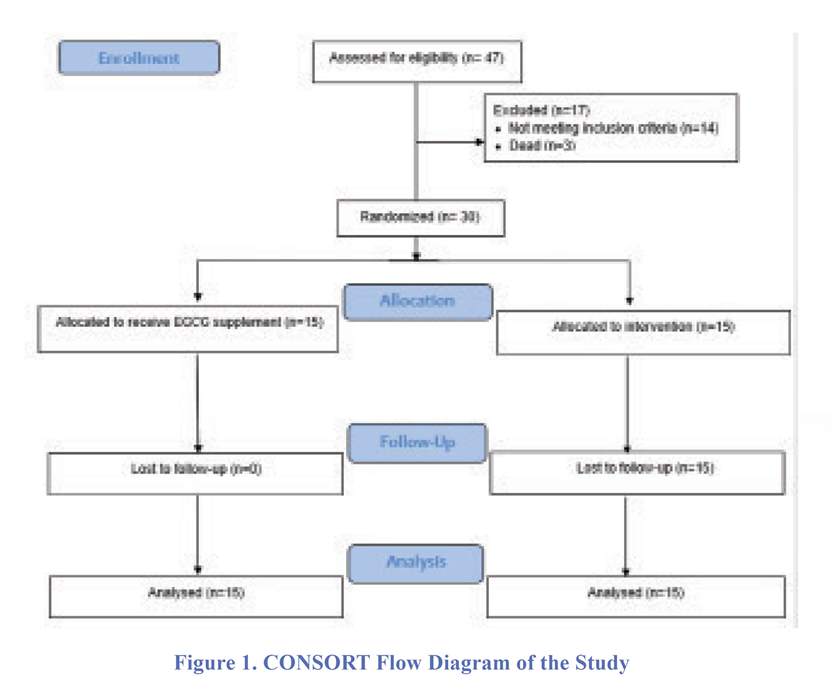
All patients received enteral nutrition with the same formula and protocol. The patients were divided into two groups using random balanced blocks: (1) receiving EGCG supplement (n=15) and (2) receiving placebo (control group, n=15). CONSORT flow diagram of the study is shown in figure 1. EGCG supplement was in form of 400 mg oral capsules with the purity of 80% catechin (Vitamilife, U.S.). EGCG powder of each capsule was dissolved in 10 ml deionized water and given to patients via gavage (one capsule per day) for seven days by a nurse who was blinded to the treatment. The control group received only 10 ml of deionized water via gavage for a week by the same nurse. During that week, GCS of the patients was recorded by a neurosurgery resident blinded to the intervention. Vital signs and serum level of glucose, hematocrit/hemoglobin, and platelets were recorded every day at 9:00 AM for one week. Moreover, the durations of mechanical ventilation and ICU stay were recorded.
In order to analyze S100B protein, a 5 ml peripheral venous blood sample was taken from patients on admission (T1) and 24 hours after the last gavage with EGCG (T2) and stored at -80°C. Human S100B ELISA kit (BioVendor - Laboratorni medicina a.s. Cat# RD 192090100) was used to quantify the level of S100B protein according to the manufacturer protocol. The primary outcome in this study was serum level of the S100B biomarker on admission and after one week of supplementation with either placebo or EGCG.
Statistical Analysis
The distribution of continuous variables was assessed using Kolmogorov–Smirnov test. To compare the continuous variables between the two groups, the independent -samples t-test and Mann–Whitney U-test were used for data with normal and non-normal distributions, respectively. For a before-after comparison of the variable levels, paired sample t-test and Wilcoxon were used for the data with normal and non-normal distribution, respectively. P-value less than 0.05 was considered as statistically significant for all tests. The data were analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA).
Results
The baseline characteristics of the patients are presented in table 1. The two groups had no significant differences in baseline characteristics. As shown in table 2, seven days following TBI, the last GCS was significantly higher in the EGCG group. Alteration of GCS was measured as last GCS on ICU admission. The EGCG group had significantly higher GCS improvement than the control group after seven days: the mean GCS level increased by 2.93 units in the EGCG group while having an average of 0.14-unit deterioration in the control group at the same time.
Alteration of S100B level was calculated as T2-T1. The average S100 levels decreased in both groups after seven days of admission. The amount of reduction in S100B levels was greater in EGCG than that in the control group (23.96 pg/ml versus 18.6 pg/ml) but the difference was not statistically significant (P= 0.209).
Although the overall duration of ICU stay was not significantly different among the two groups, the duration of mechanical ventilation was significantly shorter in the EGCG group (5.1 versus 9.8 days) (Table 3).
The patients were divided into those who did not have any clinically significant abnormality of the serum gluocose, Hb/Hct and platelet levels (not requiring therapeutic intervention) and those who required therapeutic intervention for abnormalities of these parameters. The prevalence of these potentially confounding factors was not statistically significant between the two groups of the study (Table3).
Discussion
In this study, we evaluated the effect of EGCG of green tea on the clinical outcome (consciousness, duration of ICU stay, mechanical ventilation, and serum S100B levels) of the patients with moderate to severe TBI. Our results show a beneficial effect of EGCG supplementation on improvement of GCS and shortening of the mechanical ventilation period in these patients. These results are in consistence with the previous experimental studies: Itoh et al. reported that seven days of oral supplementation with 0.1% solution of EGCG in water for brain injury-induced rats could reduce neuronal damages [18]. Hau et al. reported that intraperitoneal injection of epigallocatechin-3-gallate solution has neuroprotective effects in brain damage-induced rats by protecting them against oxidative damages to the brain. They concluded that EGCG showed neuroprotective effects by reducing free radical damages and preventing apoptosis in damaged neurons [19,20].
A number of reports proposed S100B serum level as a valid biomarker for brain damage [21]. Previous studies showed that the injection of five mg S100B protein to rat cerebellar vermis had anti-apoptotic effects [22]. Some studies show that S100B probably binds to the neuronal membrane RAGE receptors in low concentrations and increases the survival of neurons by producing against reactive oxygen species (ROS) and increasing the expression of anti-apoptotic factors [22-25]. The findings of current study show that EGCG supplementation results in reducing S100Β protein compared with the control group, although the difference between the two groups was not statistically significant.
Pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) have an important role in the pathophysiology of cerebral edema and brain damage after traumatic insult [26,27]. Factors with anti-inflammatory properties can increase the blood flow in damaged tissues, and subsequently improve the process of wound healing and recovery [27]. Experimental evidence suggests that EGCG could inhibit TNF-alpha gene expression [28,29]. In a study performed on an animal model of multiple sclerosis, the combination of glatiramer acetate (GA) and EGCG had favorable effects on neuronal survival and axonal growth by reduced reactive oxygen species generation and reduction in the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) [28]. To the best of our knowledge, until the date no human study has assessed the effect of EGCG supplementation on the secondary neural damages caused by trauma [30].
To control the confounding effect of underlying mental illness and/or internal organ injury on the clinical outcome of TBI, we excluded these patients from our study. The other possible source of confounding was hematologic and metabolic derangements which may affect the clinical outcome in TBI patients. Statistical analysis revealed that prevalence of these parameters was not statistically different among the two groups, and thus may not serve as a source of confounding. The major limitation of this study was the small sample size of the study. Due to the overwhelming predominance of TBI among men in our country, we only included male patients in this study. Furthermore, impact of the findings of this study on the long-term clinical outcome of TBI patients is a very important issue that needs to be evaluated by larger studies with longer follow-up periods.
Conclusion
The results of this study show that acute phase supplementing with EGCG might have favorable effects on consciousness levels and duration of mechanical ventilation in early phase after moderate to severe TBI. According to the availability and low cost of this product, we recommend early treatment with EGCG as a part of the integral management of patients with TBI admitted to ICU. Acknowledgement
We would like to appreciate the staff of Sina hospital ICU for their kind cooperation. Also, we would like to thank Ms. Hamideh Abbasi for her assistance in assessing S100B level.
Funding
This study was supported by a grant from Iranian Center of Neurological Research, Tehran University of Medical Sciences.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contribution
Conception and Design: Leila Zareian, Soodeh Razeghi Jahromi
Data Collection: Leila Zareian, Amir Azarhomayoun
Drafting the Article: Leila Zareian, Amir Azarhomayoun, Mohammadreza Khajavi
Critically Revising the Article: Maysam Alimohamadi
Reviewed Submitted Version of the Manuscript: All Authors
Approved the Final Version of the Manuscript: All Authors
References
1. Bruns JR, Hauser WA. The epidemiology of traumatic brain injury. a review. Epilepsia. 2003; 44: 2-10.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99: 4-9.
3. Leon-Carion j, Barroso Y marrtin JM. Epidemiology of traumatic brain injury and subarachnoid hemorrhage. J Pituitary. 2005; 8: 197-202.
4. Faria A, Meireles M, Fernandes I, Santos-Buelga C, Gonzalez-Manzano S, Dueñas M, de Freitas V, Mateus N, Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014 Apr 15;149:190-6.
5. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma. 2007;24 Suppl 1:S7-13.
6. Alexander M. Mild traumatic brain injury. pathophysiology, natural history, and clinical management. Neurology. 1995; 45(7): 50 - 60.
7. Kuptniratsaikul V, Kovindha A, Suethanapornkal S.Complications during the rehabilitation period in thai patients with stroke: A Multi center prospective study. AMJ Phys Med Rehabil. 2009; 88(2): 92-99.
8.Castellano C. Psychopharmacology of memory modulation: evidence hormones.J Behave Brain Res. 1996; 34(8): 1-21.
9. Rosenfeldt F, Wilson M. population, pathophysiology and therapy. J Exp Gerontol. 2013;48:45-54.
10. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1 and mitochondrial biogenesis in cerebral ischemia. J Mol Sci . 2011; 12: 7199–215.
11- Higdon J. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions.J Food Sci Nutr. 2003;43: 89-143.
12. Anna K. Flavonoids and the CNS, Molecules. 2011; 16: 1471-1485.
13. Croft KD. (1998). The chemistry and biological effects of flavonoids and phenolic acids. Ann NY Acad Sci. 854:435.
14. Middleton E J. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 2000; 439:175-82.
15. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging, and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845-50
16. BloomfieldSM, Mcknney J, Smith L, Brisman. Reliability of S100B in predicting severity of central nervous system injury. Neurocritic Care. 2007; 6:121-138.
17. Woertgen C, Holzschuh M, Metz C, Brawanski A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J Trauma. 1999; 47: 1126-1130.
18. Itoh T, Tabuchi M, Mizuguchi N, Imano M, Tsubaki M, Nishida S. Neuroprotective effect of epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J Neural Transm. 2013 ;120(5):767–783.
19. Hua W, Hui-Chin Tu, and Jeng-Yung.Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. J BMC Neuroscience. 2011; 12:52.
20. Zink BJ, SymzdYnjer-Chodobska J, Chodobski A. Emerging concepts in the pathophysiology of traumatic brain injury. Psychiat Clin North Am. 2010; 33: 741-756.
21. Karpiak SE, Serokosz M, Rapport MM. Effects of antisera to S100 protein and to synaptic membrane fraction on maze performance and EEG. Brain Res. 1976; 313- 21.
22. Kleindienst A, McGinn M, Harvey H, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22(2):645– 655.
23. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging, and antioxidative effects of flavonoids. Free Radic Biol Med . 1994; 16:845-5.
24. Mirrahimi B, Mortazavi A, Nouri M, Ketabchi E, Amirjamshidi A, Ashouri A, Khajavi M, Mojtahedzadeh M. Effect of magnesium on functional outcome and paraclinical parameters of patients undergoing supratentorial craniotomy for brain tumors: a randomized controlled trial. Acta Neurochir. 2015; 157(6):985.
25. Etezadi F, Aklamli M, Najafi A, Khajavi M, Shariat Moharari R, Mirrahimi B, Mortazavi SA, Mojtahedzadeh M. Evaluation of the anti-inflammatory effects of peri-operative infusion of magnesium sulfate on the microsurgical procedures for intracranial tumors. Anesth Pain Med. 2014;30;4(5):e22379.
26. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1 and mitochondrial biogenesis in cerebral ischemia. J Mol Sci. 2011; 12: 7199–215.
27. Chan PH.Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001; 21(1):2-14.
28. Yun HJ, Yoo WH, Han MK, Lee YR, KiLee SI. Epigallocatechin-3-gallate suppresses T NF a lpha -induced p roduction of M MP-1 a nd -3 i n r heumatoid arthritis synovial fibroblasts. Rheumatol Int. 2008;29(1): 23–9.
29. Renno WM, Saleh F, Klepacek I, Al-Khaledi G, Ismael H, Asfar S. Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr Neurosci. 2006; 9(1-2): 41-7.
30. Herges K. Neuroprotective Effect of Combination Therapy of Glatiramer Acetate and Epigallocatechin-3-Gallate in Neuroinflammation. PLoS ONE. 2011; 6(10): e25456. https://doi.org/10.1371/journal.pone.0025456
Type of Study: Clinical Trial |
Subject:
Neurotrauma
References
1. Bruns JR, Hauser WA. The epidemiology of traumatic brain injury. a review. Epilepsia. 2003; 44: 2-10. [DOI:10.1046/j.1528-1157.44.s10.3.x] [PMID]
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99: 4-9. [DOI:10.1093/bja/aem131] [PMID]
3. Leon-Carion j, Barroso Y marrtin JM. Epidemiology of traumatic brain injury and subarachnoid hemorrhage. J Pituitary. 2005; 8: 197-202. [DOI:10.1007/s11102-006-6041-5] [PMID]
4. Faria A, Meireles M, Fernandes I, Santos-Buelga C, Gonzalez-Manzano S, Due-as M, de Freitas V, Mateus N, Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014 Apr 15;149:190-6. [DOI:10.1016/j.foodchem.2013.10.095] [PMID]
5. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma. 2007;24 Suppl 1:S7-13. [PMID]
6. Alexander M. Mild traumatic brain injury. pathophysiology, natural history, and clinical management. Neurology. 1995; 45(7): 50 - 60. [DOI:10.1212/WNL.45.7.1253]
7. Kuptniratsaikul V, Kovindha A, Suethanapornkal S.Complications during the rehabilitation period in thai patients with stroke: A Multi center prospective study. AMJ Phys Med Rehabil. 2009; 88(2): 92-99. [DOI:10.1097/PHM.0b013e3181909d5f] [PMID]
8. Castellano C. Psychopharmacology of memory modulation: evidence hormones.J Behave Brain Res. 1996; 34(8): 1-21. [DOI:10.1016/0166-4328(96)00200-8]
9. Rosenfeldt F, Wilson M. population, pathophysiology and therapy. J Exp Gerontol. 2013;48:45-54. [DOI:10.1016/j.exger.2012.03.010] [PMID]
10. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1 and mitochondrial biogenesis in cerebral ischemia. J Mol Sci . 2011; 12: 7199–215. [DOI:10.3390/ijms12107199] [PMID]
11. Higdon J. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions.J Food Sci Nutr. 2003;43: 89-143. [DOI:10.1080/10408690390826464]
12. Anna K. Flavonoids and the CNS, Molecules. 2011; 16: 1471-1485. [DOI:10.3390/molecules16021471] [PMID]
13. Croft KD. (1998). The chemistry and biological effects of flavonoids and phenolic acids. Ann NY Acad Sci. 854:435. [DOI:10.1111/j.1749-6632.1998.tb09922.x] [PMID]
14. Middleton E J. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 2000; 439:175-82. [DOI:10.1007/978-1-4615-5335-9_13]
15. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging, and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845-50 [DOI:10.1016/0891-5849(94)90202-X]
16. BloomfieldSM, Mcknney J, Smith L, Brisman. Reliability of S100B in predicting severity of central nervous system injury. Neurocritic Care. 2007; 6:121-138. [DOI:10.1007/s12028-007-0008-x] [PMID]
17. Woertgen C, Holzschuh M, Metz C, Brawanski A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J Trauma. 1999; 47: 1126-1130. [DOI:10.1097/00005373-199912000-00026] [PMID]
18. Itoh T, Tabuchi M, Mizuguchi N, Imano M, Tsubaki M, Nishida S. Neuroprotective effect of epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J Neural Transm. 2013 ;120(5):767–783. [DOI:10.1007/s00702-012-0918-4] [PMID]
19. Hua W, Hui-Chin Tu, and Jeng-Yung.Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. J BMC Neuroscience. 2011; 12:52. [DOI:10.1186/1471-2202-12-52] [PMID]
20. Zink BJ, SymzdYnjer-Chodobska J, Chodobski A. Emerging concepts in the pathophysiology of traumatic brain injury. Psychiat Clin North Am. 2010; 33: 741-756. [DOI:10.1016/j.psc.2010.08.005] [PMID]
21. Karpiak SE, Serokosz M, Rapport MM. Effects of antisera to S100 protein and to synaptic membrane fraction on maze performance and EEG. Brain Res. 1976; 313- 21. [DOI:10.1016/0006-8993(76)90885-4]
22. Kleindienst A, McGinn M, Harvey H, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22(2):645– 655. [DOI:10.1089/neu.2005.22.645] [PMID]
23. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging, and antioxidative effects of flavonoids. Free Radic Biol Med . 1994; 16:845-5. [DOI:10.1016/0891-5849(94)90202-X]
24. Mirrahimi B, Mortazavi A, Nouri M, Ketabchi E, Amirjamshidi A, Ashouri A, Khajavi M, Mojtahedzadeh M. Effect of magnesium on functional outcome and paraclinical parameters of patients undergoing supratentorial craniotomy for brain tumors: a randomized controlled trial. Acta Neurochir. 2015; 157(6):985. [DOI:10.1007/s00701-015-2376-x] [PMID]
25. Etezadi F, Aklamli M, Najafi A, Khajavi M, Shariat Moharari R, Mirrahimi B, Mortazavi SA, Mojtahedzadeh M. Evaluation of the anti-inflammatory effects of peri-operative infusion of magnesium sulfate on the microsurgical procedures for intracranial tumors. Anesth Pain Med. 2014;30;4(5):e22379.
26. Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1 and mitochondrial biogenesis in cerebral ischemia. J Mol Sci. 2011; 12: 7199–215. [DOI:10.3390/ijms12107199] [PMID] [PMCID]
27. Chan PH.Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001; 21(1):2-14. [DOI:10.1097/00004647-200101000-00002] [PMID]
28. Yun HJ, Yoo WH, Han MK, Lee YR, KiLee SI. Epigallocatechin-3-gallate suppresses T NF a lpha -induced p roduction of M MP-1 a nd -3 i n r heumatoid arthritis synovial fibroblasts. Rheumatol Int. 2008;29(1): 23–9. [DOI:10.1007/s00296-008-0597-5] [PMID]
29. Renno WM, Saleh F, Klepacek I, Al-Khaledi G, Ismael H, Asfar S. Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr Neurosci. 2006; 9(1-2): 41-7. [DOI:10.1080/10284150600576705] [PMID]
30. Herges K. Neuroprotective Effect of Combination Therapy of Glatiramer Acetate and Epigallocatechin-3-Gallate in Neuroinflammation. PLoS ONE. 2011; 6(10): e25456. [DOI:10.1371/journal.pone.0025456]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |