Wed, Dec 17, 2025
Volume 4, Issue 2 (Spring 2018)
Iran J Neurosurg 2018, 4(2): 93-100 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rahmanian A, Derakhshan N, Alibai E A. Outcome of In-Hospital Rebleeding and Early Aneurysm Rupture at the Referral Center. Iran J Neurosurg 2018; 4 (2) :93-100
URL: http://irjns.org/article-1-117-en.html
URL: http://irjns.org/article-1-117-en.html
1- Department of Neurosurgery, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
2- Department of Neurosurgery, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran ,nima_med83@yahoo.com
2- Department of Neurosurgery, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran ,
Full Text [PDF 835 kb]
(1753 Downloads)
| Abstract (HTML) (6548 Views)
In-hospital rebleeding and early intraoperative rupture occurred at different situations and time frames between initiation of brain CT angiography and intraoperative visualization of aneurysm neck. Two patients suffered rebleeding during CTA, two in the ICU before the planned operation (on the next operative day as the standard of our center), three during induction of neuro-anesthesia, two during incision and craniotomy, three during opening of dura matter and before frontal lobe retraction, and 10 during frontal lobe retraction.
According to mRS and GOS after 6 months, 15(68.2%) patients had favorable outcomes (MRS of 0, 1, and 2 plus GOS of 4 and 5) and 7(31.2%) died (Table 2).
Full Text: (2453 Views)
1. Introduction
Cerebral aneurysms are the commonest cause of spontaneous Subarachnoid Hemorrhage (SAH) worldwide. Despite recent advances in aggressive endovascular therapy, surgical techniques, and neurocritical care, aneurysmal Subarachnoid Hemorrhage (aSAH) is still associated with high rate of mortality and morbidity. Many deaths after aSAH are due to the initial hemorrhage and occur very rapidly [1]. However, in hospitalized patients, even in neurovascular centers with pioneering early definitive treatment [2], rebleeding is still the most important preventable cause of poor outcome, followed by vasospasm, and hydrocephalus [3].
Rebleeding occurs at a rate of about 3.4% to 17.3 % according to different studies[3, 4]. Rebleeding rates have significantly declined since the establishment of “modern management” [2] principles which emphasize on the early surgical or endovascular intervention. However, despite the inclusion of early management in the acute phase of aSAH within less than three days of the initial hemorrhage, rebleeding still occurs and is associated with high mortality and morbidity [4, 5].
Risk factors for rebleeding are low Glasgow Coma Scale (GCS) score [3, 4, 6], poor Hunt and Hess (H&H) clinical grades 3 or 4, high systolic blood pressure [4, 7], systolic blood pressure variability [8], intraventricular or intracerebral hematoma [4, 9], number of aneurysms [10], and undergoing angiographic evaluation within 6 hours of SAH [4]. Genotyping assays performed on blood samples of patients with aSAH have detected common endothelin Single Nucleotide Polymorphism (SNP) on blood samples of patients with aSAH and identified TT genotype of EDN1 G/T SNP as an independent risk factor [11].
Many authors agree that rebleedings are more prevalent within the first 24 hours, whereas others believe that they occur at a later period after initial hemorrhage [6, 12]. The data regarding the effect of aneurysm size on rebleeding risk are variable and inconsistent [13-15]; however, most neurovascular authorities agree that certain features (e.g. blood-blister like carotid aneurysms) [16, 17] and projections [18] leave the aneurysm at a higher risk for re-rupturing.
Previous studies indicate that mortality of aggressive treatment of patients, who experience rebleeding, is as high as 50% to 80 % and the survivors had a poor functional outcome [8, 19, 20]. Data regarding the efficacy of antifibrinolytics in prevention of rebleeding are equivocal [21] and early surgery still remains the only hope they have for survival. Here, following the establishment of “modern management” in our neurovascular unit, we reviewed the demographic features, risk factors, operative data and functional outcome of all 22 salvageable patients with aSAH, who experienced rebleeding or intraoperative rupture at the referral hospital prior to successful clipping of their aneurysms.
This study aims at describing the surgical technique and functional outcome of patients (and its determinants), who experienced early in-hospital rebleeding at this tertiary neurovascular center.
2. Methods and Materials/Patients
Study population
In this retrospective study, a total of 648 patients with anterior circulation aneurysms, who were operated between September 2010 and September 2017 at Neurovascular Unit of Namazi Hospital (main referral neurovascular center in southern Iran) [22, 23], were investigated. After exclusion of those who were lost to follow up and the ones whose hospital records were incomplete and inaccessible, 617 were remained eligible as the reference population. Of them, 22 patients suffered from in-hospital rebleeding and early rupture between the diagnostic neuroimaging modality (CT angiography) and visualization of aneurysm neck for dissection. They were included in this survey.
Surgical technique
Regardless of routine neuro-anesthetic protocols for every patient with SAH due to cerebral aneurysm, rebleeding was diagnosed differently in each situation and surgery was performed. The current policy in our neurovascular center is diagnostic confirmation of aneurysm by CT Angiography (CTA) and saving 4-vessel catheter angiography for those who require confirmatory evaluations as well as collateral circulation survey. All salvageable 22 cases of in-hospital rebleedings had been diagnosed by CTA. Four patients who suffered from early rupture and in-hospital rebleeding prior to surgery were operated urgently after their sudden loss of consciousness and confirmation of rebleeding by brain CT scan.
Before anesthesia, rebleeding was noticed via sudden changes in GCS as well as patients’ pupils and a feel of severe sudden-onset headache in conscious patients. During and after induction of anesthesia, a sudden increase in blood pressure accompanied by decrease in heart rate (i.e. the Cushing phenomenon) in a previously stable patient was identified as rebleeding. After opening the dura matter through frontal lobe retraction, a sudden aggressive brain swelling was the case. We used a Frazier suction tube 12 French, and bipolar cautery to perform a wedge shaped frontal lobectomy to access the internal carotid artery through the fastest route (Figure 1B). Then temporary clip is applied on main feeder artery and using a cotton-ball and suction, a pilot clip is applied on the bleeder point which is further re-adjusted to occlude the aneurysm sac (or reconstruct the arterial lumen in cases of blister-like carotid aneurysms via double-clip technique).
Clipping time was considered the time between opening the dura matter and confirmation of successful clipping by video-angiography. Successful occlusion of aneurysm and patency of parent artery and branches are confirmed via Indocyanine Green (ICG) video-angiography as well as intraoperative Doppler ultrasonography. We carried out lateral supra-orbital craniotomy for all these patients.
Data collection
An information form was filled out for each patient by reviewing hospital records, critical care documents, as well as surgery notes, and finally the contact details. Functional outcome after 6 months was obtained through phone calls as well as out-patient records that stated in terms of the Glasgow Outcome Scale (GOS) and the modified Rankin Scale (mRS).
Statistical analysis
All the statistical analyses were performed in SPSS (SPSS Inc., Chicago, Illinois, USA) version 22.0. The obtained data are reported as mean±SD. The Independent t test, 1-way ANOVA, Kruskal-Wallis and Mann-Whitney tests were utilized to examine the effect of each variable on the outcome of patients after 6 months, as appropriate.
3. Results
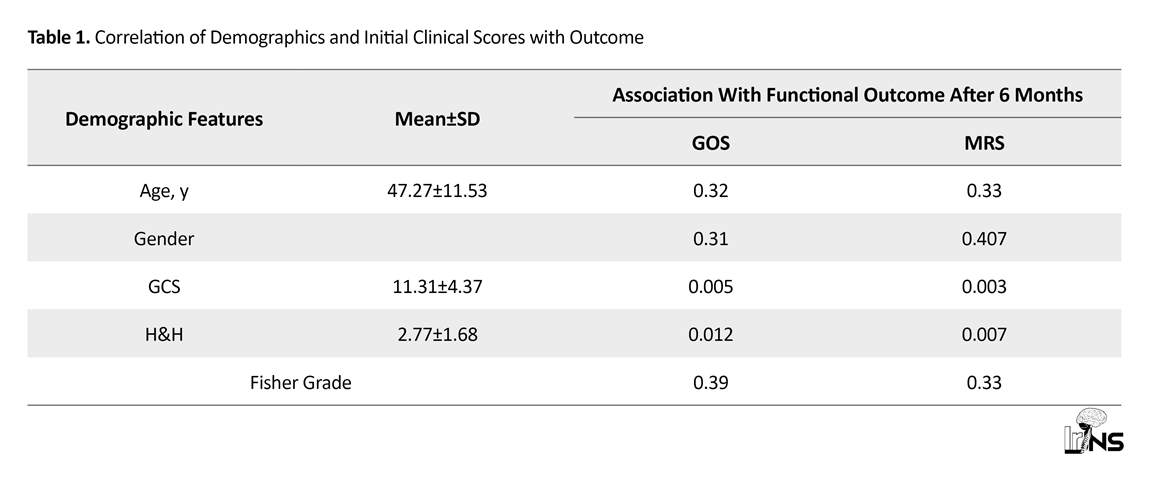
Between September 2010 and September 2017, 617 patients underwent operation due to anterior circulation aneurysms. Of them, 22(3.56%) suffered from aneurysm in-hospital rebleeding and early intra-operative rupture at the referral hospital. The patients had a Mean±SD age of 47.27±11.53 years. They comprised 13 men and 9 women (M: F ratio 1.44) compared to the reference population with the Mean±SD age of 48.12±4.42 and M: F ratio of 0.95. Neither age nor gender were associated with functional outcome at 6-month follow-up. GCS and H&H clinical grade at admission were correlated with the outcome. Most patients had modified Fischer grade 3 or 4 on CT scans (86.36%), but no correlation was found between Fisher grade and presence of IVH and ICH with functional outcome.
Cerebral aneurysms are the commonest cause of spontaneous Subarachnoid Hemorrhage (SAH) worldwide. Despite recent advances in aggressive endovascular therapy, surgical techniques, and neurocritical care, aneurysmal Subarachnoid Hemorrhage (aSAH) is still associated with high rate of mortality and morbidity. Many deaths after aSAH are due to the initial hemorrhage and occur very rapidly [1]. However, in hospitalized patients, even in neurovascular centers with pioneering early definitive treatment [2], rebleeding is still the most important preventable cause of poor outcome, followed by vasospasm, and hydrocephalus [3].
Rebleeding occurs at a rate of about 3.4% to 17.3 % according to different studies[3, 4]. Rebleeding rates have significantly declined since the establishment of “modern management” [2] principles which emphasize on the early surgical or endovascular intervention. However, despite the inclusion of early management in the acute phase of aSAH within less than three days of the initial hemorrhage, rebleeding still occurs and is associated with high mortality and morbidity [4, 5].
Risk factors for rebleeding are low Glasgow Coma Scale (GCS) score [3, 4, 6], poor Hunt and Hess (H&H) clinical grades 3 or 4, high systolic blood pressure [4, 7], systolic blood pressure variability [8], intraventricular or intracerebral hematoma [4, 9], number of aneurysms [10], and undergoing angiographic evaluation within 6 hours of SAH [4]. Genotyping assays performed on blood samples of patients with aSAH have detected common endothelin Single Nucleotide Polymorphism (SNP) on blood samples of patients with aSAH and identified TT genotype of EDN1 G/T SNP as an independent risk factor [11].
Many authors agree that rebleedings are more prevalent within the first 24 hours, whereas others believe that they occur at a later period after initial hemorrhage [6, 12]. The data regarding the effect of aneurysm size on rebleeding risk are variable and inconsistent [13-15]; however, most neurovascular authorities agree that certain features (e.g. blood-blister like carotid aneurysms) [16, 17] and projections [18] leave the aneurysm at a higher risk for re-rupturing.
Previous studies indicate that mortality of aggressive treatment of patients, who experience rebleeding, is as high as 50% to 80 % and the survivors had a poor functional outcome [8, 19, 20]. Data regarding the efficacy of antifibrinolytics in prevention of rebleeding are equivocal [21] and early surgery still remains the only hope they have for survival. Here, following the establishment of “modern management” in our neurovascular unit, we reviewed the demographic features, risk factors, operative data and functional outcome of all 22 salvageable patients with aSAH, who experienced rebleeding or intraoperative rupture at the referral hospital prior to successful clipping of their aneurysms.
This study aims at describing the surgical technique and functional outcome of patients (and its determinants), who experienced early in-hospital rebleeding at this tertiary neurovascular center.
2. Methods and Materials/Patients
Study population
In this retrospective study, a total of 648 patients with anterior circulation aneurysms, who were operated between September 2010 and September 2017 at Neurovascular Unit of Namazi Hospital (main referral neurovascular center in southern Iran) [22, 23], were investigated. After exclusion of those who were lost to follow up and the ones whose hospital records were incomplete and inaccessible, 617 were remained eligible as the reference population. Of them, 22 patients suffered from in-hospital rebleeding and early rupture between the diagnostic neuroimaging modality (CT angiography) and visualization of aneurysm neck for dissection. They were included in this survey.
Surgical technique
Regardless of routine neuro-anesthetic protocols for every patient with SAH due to cerebral aneurysm, rebleeding was diagnosed differently in each situation and surgery was performed. The current policy in our neurovascular center is diagnostic confirmation of aneurysm by CT Angiography (CTA) and saving 4-vessel catheter angiography for those who require confirmatory evaluations as well as collateral circulation survey. All salvageable 22 cases of in-hospital rebleedings had been diagnosed by CTA. Four patients who suffered from early rupture and in-hospital rebleeding prior to surgery were operated urgently after their sudden loss of consciousness and confirmation of rebleeding by brain CT scan.
Before anesthesia, rebleeding was noticed via sudden changes in GCS as well as patients’ pupils and a feel of severe sudden-onset headache in conscious patients. During and after induction of anesthesia, a sudden increase in blood pressure accompanied by decrease in heart rate (i.e. the Cushing phenomenon) in a previously stable patient was identified as rebleeding. After opening the dura matter through frontal lobe retraction, a sudden aggressive brain swelling was the case. We used a Frazier suction tube 12 French, and bipolar cautery to perform a wedge shaped frontal lobectomy to access the internal carotid artery through the fastest route (Figure 1B). Then temporary clip is applied on main feeder artery and using a cotton-ball and suction, a pilot clip is applied on the bleeder point which is further re-adjusted to occlude the aneurysm sac (or reconstruct the arterial lumen in cases of blister-like carotid aneurysms via double-clip technique).
Clipping time was considered the time between opening the dura matter and confirmation of successful clipping by video-angiography. Successful occlusion of aneurysm and patency of parent artery and branches are confirmed via Indocyanine Green (ICG) video-angiography as well as intraoperative Doppler ultrasonography. We carried out lateral supra-orbital craniotomy for all these patients.
Data collection
An information form was filled out for each patient by reviewing hospital records, critical care documents, as well as surgery notes, and finally the contact details. Functional outcome after 6 months was obtained through phone calls as well as out-patient records that stated in terms of the Glasgow Outcome Scale (GOS) and the modified Rankin Scale (mRS).
Statistical analysis
All the statistical analyses were performed in SPSS (SPSS Inc., Chicago, Illinois, USA) version 22.0. The obtained data are reported as mean±SD. The Independent t test, 1-way ANOVA, Kruskal-Wallis and Mann-Whitney tests were utilized to examine the effect of each variable on the outcome of patients after 6 months, as appropriate.
3. Results
Between September 2010 and September 2017, 617 patients underwent operation due to anterior circulation aneurysms. Of them, 22(3.56%) suffered from aneurysm in-hospital rebleeding and early intra-operative rupture at the referral hospital. The patients had a Mean±SD age of 47.27±11.53 years. They comprised 13 men and 9 women (M: F ratio 1.44) compared to the reference population with the Mean±SD age of 48.12±4.42 and M: F ratio of 0.95. Neither age nor gender were associated with functional outcome at 6-month follow-up. GCS and H&H clinical grade at admission were correlated with the outcome. Most patients had modified Fischer grade 3 or 4 on CT scans (86.36%), but no correlation was found between Fisher grade and presence of IVH and ICH with functional outcome.
In-hospital rebleeding and early intraoperative rupture occurred at different situations and time frames between initiation of brain CT angiography and intraoperative visualization of aneurysm neck. Two patients suffered rebleeding during CTA, two in the ICU before the planned operation (on the next operative day as the standard of our center), three during induction of neuro-anesthesia, two during incision and craniotomy, three during opening of dura matter and before frontal lobe retraction, and 10 during frontal lobe retraction.
According to mRS and GOS after 6 months, 15(68.2%) patients had favorable outcomes (MRS of 0, 1, and 2 plus GOS of 4 and 5) and 7(31.2%) died (Table 2).
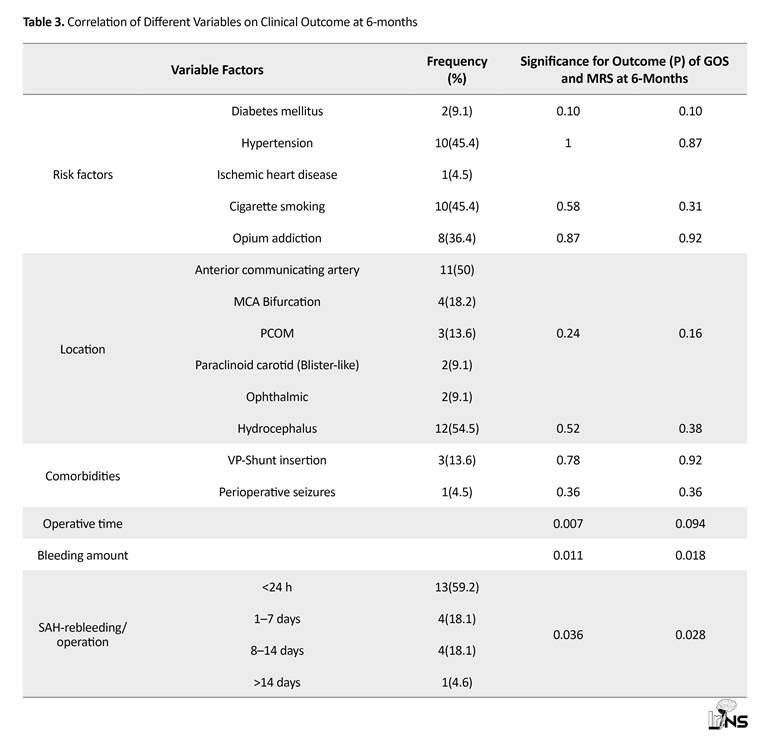
Patient’s risk factors such as Hypertension (HTN), Diabetes Mellitus (DM), Ischemic Heart Disease (IHD), smoking and opium addiction were not correlated with the worse outcome. All patients had one aneurysm and distribution of the location of aneurysm in anterior circulation was demonstrated in Table 3, which did not show any correlation with the outcome. However, the frequency of Anterior Communicating Artery (ACOM) and carotid blister-like location was higher in this subset of patients compared to our reference population.
Perioperative seizures, hydrocephalus and need for ventriculoperitoneal shunt was not accompanied with worse outcome in this group of patients. However, operative time and bleeding amount were directly correlated with worse outcome (P values of 0.07 and 0.018, respectively). The Mean±SD values for clipping time and bleeding amount were 57.95±30.38 min and 893.18(955.22) mL, respectively which are significantly higher than the values for our standard procedure (P<0.001).
The interval between SAH and in-hospital rebleeding or early intra-operative rupture (followed by emergency aneurysm clipping) was less than 24 hours for 13 patients, 2–7 days for 4 patients, 8–14 days for 4 patients and more than 14 days for 1 patient. Earlier rebleeding was found to be associated with worse prognosis in terms of lower GOS (P=0.036) and higher mRS (P=0.028). Of 11 ACOM aneurysms with in-hospital rebleeding and early intraoperative rupture prior to aneurysm neck identification, 10 had superior and 1 had inferior projection.
4. Discussion
Although early surgical and endovascular management of cerebral aneurysms in the past two decades has declined the catastrophic results seen with in-hospital rebleeding and early intraoperative rupture beforehand, it is still the most important preventable comorbidity of aSAH along with vasospasm and hydrocephalus. Genetic studies have now confirmed that endothelin pleomorphism [11, 24-26] plays a crucial role in this scenario and we believe that the road to redemption goes through genomic and proteomic analysis of cerebral aneurysms. Considering demographic features in our series, we found a higher M:F ratio in those who sustained rebleeding; however, no correlation was found between gender and functional outcome. Our data were consistent with previous literature showing that better GCS and H&H grades were associated with better prognosis.
Zhao et al. also believed that patients, who sustain rebleeding will benefit from aggressive treatment [19]. They found that worse clinical grades (in terms of WFNS grade) and lower GCS after rebleeding resulted in poorer functional outcome. However, considering that we included many patients that are believed to sustain re-rupture during anesthesia or surgery, this comparison could not be made.
Notwithstanding high systolic blood pressure risk factor for aneurysm rebleeding [4, 7], we believe that premorbid hypertension does not worsen functional outcome. Diabetes mellitus, ischemic heart disease, smoking and opium addiction were not associated with a poor outcome either.
ACOM (with superior projection) and blood-blister like carotid aneurysm were found as the two locations at risk for rebleeding; however, neither of locations and projections was found to impose a greater risk for poor outcome in this series. Most patients of the presented series suffered from in-hospital rebleeding and early intraoperative rupture within the first 24 hours (59.2%). The interval between initial hemorrhage and rebleeding (followed by emergency clipping) was found to be inversely correlated with outcome which might be due to better feasibility of the aneurysm dissection and stabilization of the clot formed following initial hemorrhage.
Patients, whose operations were longer and suffered more bleeding amount, were also found to have poorer clinical outcomes. Two points should be considered as limitations to the current study. First is the sole inclusion of surgically-managed individuals in the investigation, as the equipment for endovascular management of such cases were not available due to sanctions imposed on Iran. Second is that we only operated anterior circulation aneurysms in the mentioned setting and posterior circulation aneurysms were not subject to this technique and tend to have a further worse prognosis in cases of rebleeding and none happened in the operation room.
Despite several reports on the dismal prognosis of in-hospital rebleeding [8, 19, 20], we found 68.2% favorable outcome (mRS=0, 1 and 2) in our series. We believe that the lobectomy with a large Frazier suction tip (Fr 12) in this technique provides the ideal surgical corridor for access to the aneurysm and its main feeders without causing significant neurologic damages (Figure 1; A, B and C). Even though, this series covers only patients with a single anterior circulation aneurysm, we believe that aggressive treatment should be the rule for similar situations and provide the remarkable chance for a possible favorable outcome.
A limitation of this study is that all 22 patients underwent surgical management and no historical “control” group could be considered for comparison. That is probably due to further experience of our surgical team and the unavailability of endovascular equipment in an emergent setting at this neurovascular center.
5. Conclusion
Almost 3.5% of patients with cerebral aneurysm in anterior circulation suffered from in-hospital rebleeding and early intraoperative rupture at the referral hospital. Despite previous reports of the dismal prognosis of these patients, 15 out of 22 patients in our series survived with favorable outcomes (mRS of 0, 1, and 2 or GOS of 4 or 5). We believe that our surgical technique opens a window of hope for survival of such patients with a considerable chance of favorable outcomes.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
The Authors contributions are as follows: Abdolkarim Rahmanian contributed to describing the surgical technic and conceptualization. Nima Derakhshan helped in writing the original draft and revised in its final format. Ehsan Ali Alibai revised the manuscript critically and contributed to conceptualization and methodology.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Here we want to thank the patients participated in this investigation and also their families who helped with evaluation of outcome at follow-ups. We would also like to thank neurosurgery residents, neurosurgery operating room and Neuro-intensive care unit personnel who helped to make this center the largest tertiary neurovascular center of Southern Iran.
References
Perioperative seizures, hydrocephalus and need for ventriculoperitoneal shunt was not accompanied with worse outcome in this group of patients. However, operative time and bleeding amount were directly correlated with worse outcome (P values of 0.07 and 0.018, respectively). The Mean±SD values for clipping time and bleeding amount were 57.95±30.38 min and 893.18(955.22) mL, respectively which are significantly higher than the values for our standard procedure (P<0.001).
The interval between SAH and in-hospital rebleeding or early intra-operative rupture (followed by emergency aneurysm clipping) was less than 24 hours for 13 patients, 2–7 days for 4 patients, 8–14 days for 4 patients and more than 14 days for 1 patient. Earlier rebleeding was found to be associated with worse prognosis in terms of lower GOS (P=0.036) and higher mRS (P=0.028). Of 11 ACOM aneurysms with in-hospital rebleeding and early intraoperative rupture prior to aneurysm neck identification, 10 had superior and 1 had inferior projection.
4. Discussion
Although early surgical and endovascular management of cerebral aneurysms in the past two decades has declined the catastrophic results seen with in-hospital rebleeding and early intraoperative rupture beforehand, it is still the most important preventable comorbidity of aSAH along with vasospasm and hydrocephalus. Genetic studies have now confirmed that endothelin pleomorphism [11, 24-26] plays a crucial role in this scenario and we believe that the road to redemption goes through genomic and proteomic analysis of cerebral aneurysms. Considering demographic features in our series, we found a higher M:F ratio in those who sustained rebleeding; however, no correlation was found between gender and functional outcome. Our data were consistent with previous literature showing that better GCS and H&H grades were associated with better prognosis.
Zhao et al. also believed that patients, who sustain rebleeding will benefit from aggressive treatment [19]. They found that worse clinical grades (in terms of WFNS grade) and lower GCS after rebleeding resulted in poorer functional outcome. However, considering that we included many patients that are believed to sustain re-rupture during anesthesia or surgery, this comparison could not be made.
Notwithstanding high systolic blood pressure risk factor for aneurysm rebleeding [4, 7], we believe that premorbid hypertension does not worsen functional outcome. Diabetes mellitus, ischemic heart disease, smoking and opium addiction were not associated with a poor outcome either.
ACOM (with superior projection) and blood-blister like carotid aneurysm were found as the two locations at risk for rebleeding; however, neither of locations and projections was found to impose a greater risk for poor outcome in this series. Most patients of the presented series suffered from in-hospital rebleeding and early intraoperative rupture within the first 24 hours (59.2%). The interval between initial hemorrhage and rebleeding (followed by emergency clipping) was found to be inversely correlated with outcome which might be due to better feasibility of the aneurysm dissection and stabilization of the clot formed following initial hemorrhage.
Patients, whose operations were longer and suffered more bleeding amount, were also found to have poorer clinical outcomes. Two points should be considered as limitations to the current study. First is the sole inclusion of surgically-managed individuals in the investigation, as the equipment for endovascular management of such cases were not available due to sanctions imposed on Iran. Second is that we only operated anterior circulation aneurysms in the mentioned setting and posterior circulation aneurysms were not subject to this technique and tend to have a further worse prognosis in cases of rebleeding and none happened in the operation room.
Despite several reports on the dismal prognosis of in-hospital rebleeding [8, 19, 20], we found 68.2% favorable outcome (mRS=0, 1 and 2) in our series. We believe that the lobectomy with a large Frazier suction tip (Fr 12) in this technique provides the ideal surgical corridor for access to the aneurysm and its main feeders without causing significant neurologic damages (Figure 1; A, B and C). Even though, this series covers only patients with a single anterior circulation aneurysm, we believe that aggressive treatment should be the rule for similar situations and provide the remarkable chance for a possible favorable outcome.
A limitation of this study is that all 22 patients underwent surgical management and no historical “control” group could be considered for comparison. That is probably due to further experience of our surgical team and the unavailability of endovascular equipment in an emergent setting at this neurovascular center.
5. Conclusion
Almost 3.5% of patients with cerebral aneurysm in anterior circulation suffered from in-hospital rebleeding and early intraoperative rupture at the referral hospital. Despite previous reports of the dismal prognosis of these patients, 15 out of 22 patients in our series survived with favorable outcomes (mRS of 0, 1, and 2 or GOS of 4 or 5). We believe that our surgical technique opens a window of hope for survival of such patients with a considerable chance of favorable outcomes.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
The Authors contributions are as follows: Abdolkarim Rahmanian contributed to describing the surgical technic and conceptualization. Nima Derakhshan helped in writing the original draft and revised in its final format. Ehsan Ali Alibai revised the manuscript critically and contributed to conceptualization and methodology.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Here we want to thank the patients participated in this investigation and also their families who helped with evaluation of outcome at follow-ups. We would also like to thank neurosurgery residents, neurosurgery operating room and Neuro-intensive care unit personnel who helped to make this center the largest tertiary neurovascular center of Southern Iran.
References
- Hillman J, von Essen C, Leszniewski W, Johansson I. Significance of “ultra-early” rebleeding in subarachnoid hemorrhage. Journal of Neurosurgery. 1988; 68(6):901-907. [DOI:10.3171/jns.1988.68.6.0901] [PMID]
- Roos YBWEM, Beenen LFM, Groen RJM, Albrecht KW, Vermeulen M. Timing of surgery in patients with aneurysmal subarachnoid haemorrhage: rebleeding is still the major cause of poor outcome in neurosurgical units that aim at early surgery. Journal of Neurology, Neurosurgery & Psychiatry. 1997; 63(4):490-93. [DOI:10.1136/jnnp.63.4.490]
- Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Archives of Neurology. 2005; 62(3):410-16. [DOI:10.1001/archneur.62.3.410] [PMID]
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. Journal of Neurosurgery. 1996; 84(1):35-42. [DOI:10.3171/jns.1996.84.1.0035] [PMID]
- Tang C, Zhang TS, Zhou LF. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage: A meta-analysis. PLoS ONE. 2014; 9(6):e99536. [DOI:10.1371/journal.pone.0099536] [PMID] [PMCID]
- Brilstra EH, Rinkel GJ, Algra A, van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology. 2000; 55(11):1656-60. [DOI:10.1212/WNL.55.11.1656] [PMID]
- De Marchis GM, Lantigua H, Schmidt JM, et al. Impact of premorbid hypertension on haemorrhage severity and aneurysm rebleeding risk after subarachnoid haemorrhage. Journal of Neurology, Neurosurgery & Psychiatry. 2014; 85(1):56-9. [DOI:10.1136/jnnp-2013-305051] [PMID]
- Lin QS, Ping-Chen YXL, Lin ZY, Yu LH, Dai LS, Kang DZ. Systolic blood pressure variability is a novel risk factor for rebleeding in acute subarachnoid hemorrhage: A case-control study. Medicine. 2016; 95(11):e3028. [DOI:10.1097/MD.0000000000003028]
- Anevrizmal KZBAT. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage based on the analysis of on-admission information. Turkish Neurosurgery. 2012; 22(6):675-81.
- Beck J, Raabe A, Szelenyi A, Berkefeld J, Gerlach R, Setzer M, et al. Sentinel headache and the risk of rebleeding after aneurysmal subarachnoid hemorrhage. Stroke. 2006; 37(11):2733-37. [DOI:10.1161/01.STR.0000244762.51326.e7] [PMID]
- Beck J, Raabe A, Szelenyi A, Berkefeld J, Gerlach R, Setzer M, et al. Endothelin polymorphisms as a risk factor for cerebral aneurysm rebleeding following aneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2017; 157:65-9. [DOI:10.1016/j.clineuro.2017.04.007] [PMID]
- Rosenørn J, Eskesen V, Schmidt K, Rønde F. The risk of rebleeding from ruptured intracranial aneurysms. Journal of Neurosurgery. 1987; 67(3):329-32. [DOI:10.3171/jns.1987.67.3.0329] [PMID]
- Rahmanian A, Ghaffarpasand F, Alibai E, Choque-Velasquez J, Jahromi BR, Hernesniemi J. Surgical outcome of very small intracranial aneurysms utilizing the double clip technique. World Neurosurgery. 2018; 110:e605-e611. [DOI: 10.1016/j.wneu.2017.11.060]
- Boogaarts HD, van Lieshout JH, van Amerongen MJ, de Vries J, Verbeek ALM, Grotenhuis JA, et al. Aneurysm diameter as a risk factor for pretreatment rebleeding: A meta-analysis. Journal of Neurosurgery. 2015; 122(4):921-28. [DOI:10.3171/2014.12.JNS14931] [PMID]
- Rahmanian A, Ghaffarpasand F, Derakhshan N. Surgical outcome of patients with very small intracranial aneurysms: A single-center experience from southern Iran. World Neurosurgery. 2017; 98:470-8. [DOI:10.1016/j.wneu.2016.11.086] [PMID]
- Ren Y, Liu L, Sun H, Liu Y, Li H, Ma L, et al. Microsurgical versus endovascular treatments for blood-blister aneurysms of the internal carotid artery: A retrospective study of 83 patients in a single center. World Neurosurgery. 2018; 109:e615-e624. [DOI:10.1016/j.wneu.2017.10.048]
- Konczalla J, Gessler F, Bruder M, Berkefeld J, Marquardt G, Seifert V. Outcome after subarachnoid hemorrhage from blood blister-like aneurysm rupture depends on age and aneurysm morphology. World Neurosurgery. 2017; 105:944-51. [DOI:10.1016/j.wneu.2017.06.129]
- Fukuda H, Hayashi K, Yoshino K, Koyama T, Lo B, Kurosaki Y, et al. Impact of aneurysm projection on intraoperative complications during surgical clipping of ruptured posterior communicating artery aneurysms. Neurosurgery. 2015; 78(3):381-90. [DOI:10.1227/NEU.0000000000001131] [PMID]
- Zhao B, Yang H, Zheng K, Li Z, Xiong Y, Tan X, et al. Predictors of good functional outcomes and mortality in patients with severe rebleeding after aneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2016; 144:28-32. [DOI:10.1016/j.clineuro.2016.02.042] [PMID]
- Kienzler J, Marbacher S, Remonda L, Soleman J, Ai Schlaeppi J, Leupold U et al. Outcome after in-hospital rebleeding of rupture of intracranial aneurysms. Journal of Neurological Surgery Part A: Central European Neurosurgery. 2016; 77(03):207-21. [DOI:10.1055/s-0035-1570007] [PMID]
- Jan Hillman, Steen Fridriksson, Ola Nilsson, Zhengquan Yu, Hans Säveland, Karl-Erik Jakobsson. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: A prospective randomized study. Journal of Neurosurgery. 2002; 97(4):771-8. [DOI:10.3171/jns.2002.97.4.0771] [PMID]
- Rahmanian A, Derakhshan N, Sisakht AM, Ziarati NK, Shahraki HR, Motamed S. Risk factors for unfavorable outcome in aneurysmal subarachnoid hemorrhage revisited; odds and ends. Bulletin of Emergency & Trauma. 2018; 6(2):13340. [DOI:10.29252/beat-060215] [PMID] [PMCID]
- Rahmanian A, Sisakht A, Derakhshan N, Ziarati N, Shahraki H, Najafi B. Fresh frozen plasma versus albumin in treatment of cerebral vasospasm in subarachnoid hemorrhage: A historical cohort study. World Journal of Surgery. 2018; 1:1002.
- Savastano LE, Bhambri A, Wilkinson DA, Pandey AS. Biology of cerebral aneurysm formation, growth, and rupture. In: Ringer A, editor. Intracranial Aneurysms. Amsterdam: Elsevier; 2018.
- Hendrix P, Foreman PM, Starke RM, Harrigan MR, Fisher WS, Vyas NA, et al. Associations of endothelin polymorphisms and aneurysm size at time of rupture. World Neurosurgery. 2017; 102:253-257. [DOI:10.1016/j.wneu.2017.03.041] [PMID]
- Griessenauer CJ, Starke RM, Foreman PM, Hendrix P, Harrigan MR, Fisher WS, et al. Associations between endothelin polymorphisms and aneurysmal subarachnoid hemorrhage, clinical vasospasm, delayed cerebral ischemia, and functional outcome. Journal of Neurosurgery. 2018; 128(5):1311-7. [PMID]
Type of Study: Research |
Subject:
Vascular Neurosurgery
References
1. Hillman J, von Essen C, Leszniewski W, Johansson I. Significance of "ultra-early" rebleeding in subarachnoid hemorrhage. Journal of Neurosurgery. 1988; 68(6):901-907. [DOI:10.3171/jns.1988.68.6.0901] [PMID] [DOI:10.3171/jns.1988.68.6.0901]
2. Roos YBWEM, Beenen LFM, Groen RJM, Albrecht KW, Vermeulen M. Timing of surgery in patients with aneurysmal subarachnoid haemorrhage: rebleeding is still the major cause of poor outcome in neurosurgical units that aim at early surgery. Journal of Neurology, Neurosurgery & Psychiatry. 1997; 63(4):490-93. [DOI:10.1136/jnnp.63.4.490] [DOI:10.1136/jnnp.63.4.490]
3. Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Archives of Neurology. 2005; 62(3):410-16. [DOI:10.1001/archneur.62.3.410] [PMID] [DOI:10.1001/archneur.62.3.410]
4. Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. Journal of Neurosurgery. 1996; 84(1):35-42. [DOI:10.3171/jns.1996.84.1.0035] [PMID] [DOI:10.3171/jns.1996.84.1.0035]
5. Tang C, Zhang TS, Zhou LF. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage: A meta-analysis. PLoS ONE. 2014; 9(6):e99536. [DOI:10.1371/journal.pone.0099536] [PMID] [PMCID] [DOI:10.1371/journal.pone.0099536]
6. Brilstra EH, Rinkel GJ, Algra A, van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology. 2000; 55(11):1656-60. [DOI:10.1212/WNL.55.11.1656] [PMID] [DOI:10.1212/WNL.55.11.1656]
7. De Marchis GM, Lantigua H, Schmidt JM, et al. Impact of premorbid hypertension on haemorrhage severity and aneurysm rebleeding risk after subarachnoid haemorrhage. Journal of Neurology, Neurosurgery & Psychiatry. 2014; 85(1):56-9. [DOI:10.1136/jnnp-2013-305051] [PMID] [DOI:10.1136/jnnp-2013-305051]
8. Lin QS, Ping-Chen YXL, Lin ZY, Yu LH, Dai LS, Kang DZ. Systolic blood pressure variability is a novel risk factor for rebleeding in acute subarachnoid hemorrhage: A case-control study. Medicine. 2016; 95(11):e3028. [DOI:10.1097/MD.0000000000003028] [DOI:10.1097/MD.0000000000003028]
9. Anevrizmal KZBAT. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage based on the analysis of on-admission information. Turkish Neurosurgery. 2012; 22(6):675-81.
10. Beck J, Raabe A, Szelenyi A, Berkefeld J, Gerlach R, Setzer M, et al. Sentinel headache and the risk of rebleeding after aneurysmal subarachnoid hemorrhage. Stroke. 2006; 37(11):2733-37. [DOI:10.1161/01.STR.0000244762.51326.e7] [PMID] [DOI:10.1161/01.STR.0000244762.51326.e7]
11. Beck J, Raabe A, Szelenyi A, Berkefeld J, Gerlach R, Setzer M, et al. Endothelin polymorphisms as a risk factor for cerebral aneurysm rebleeding following aneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2017; 157:65-9. [DOI:10.1016/j.clineuro.2017.04.007] [PMID] [DOI:10.1016/j.clineuro.2017.04.007]
12. Rosenørn J, Eskesen V, Schmidt K, Rønde F. The risk of rebleeding from ruptured intracranial aneurysms. Journal of Neurosurgery. 1987; 67(3):329-32. [DOI:10.3171/jns.1987.67.3.0329] [PMID] [DOI:10.3171/jns.1987.67.3.0329]
13. Rahmanian A, Ghaffarpasand F, Alibai E, Choque-Velasquez J, Jahromi BR, Hernesniemi J. Surgical outcome of very small intracranial aneurysms utilizing the double clip technique. World Neurosurgery. 2018; 110:e605-e611. [DOI: 10.1016/j.wneu.2017.11.060] [DOI:10.1016/j.wneu.2017.11.060]
14. Boogaarts HD, van Lieshout JH, van Amerongen MJ, de Vries J, Verbeek ALM, Grotenhuis JA, et al. Aneurysm diameter as a risk factor for pretreatment rebleeding: A meta-analysis. Journal of Neurosurgery. 2015; 122(4):921-28. [DOI:10.3171/2014.12.JNS14931] [PMID] [DOI:10.3171/2014.12.JNS14931]
15. Rahmanian A, Ghaffarpasand F, Derakhshan N. Surgical outcome of patients with very small intracranial aneurysms: A single-center experience from southern Iran. World Neurosurgery. 2017; 98:470-8. [DOI:10.1016/j.wneu.2016.11.086] [PMID] [DOI:10.1016/j.wneu.2016.11.086]
16. Ren Y, Liu L, Sun H, Liu Y, Li H, Ma L, et al. Microsurgical versus endovascular treatments for blood-blister aneurysms of the internal carotid artery: A retrospective study of 83 patients in a single center. World Neurosurgery. 2018; 109:e615-e624. [DOI:10.1016/j.wneu.2017.10.048] [DOI:10.1016/j.wneu.2017.10.048]
17. Konczalla J, Gessler F, Bruder M, Berkefeld J, Marquardt G, Seifert V. Outcome after subarachnoid hemorrhage from blood blister-like aneurysm rupture depends on age and aneurysm morphology. World Neurosurgery. 2017; 105:944-51. [DOI:10.1016/j.wneu.2017.06.129] [DOI:10.1016/j.wneu.2017.06.129]
18. Fukuda H, Hayashi K, Yoshino K, Koyama T, Lo B, Kurosaki Y, et al. Impact of aneurysm projection on intraoperative complications during surgical clipping of ruptured posterior communicating artery aneurysms. Neurosurgery. 2015; 78(3):381-90. [DOI:10.1227/NEU.0000000000001131] [PMID] [DOI:10.1227/NEU.0000000000001131]
19. Zhao B, Yang H, Zheng K, Li Z, Xiong Y, Tan X, et al. Predictors of good functional outcomes and mortality in patients with severe rebleeding after aneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2016; 144:28-32. [DOI:10.1016/j.clineuro.2016.02.042] [PMID] [DOI:10.1016/j.clineuro.2016.02.042]
20. Kienzler J, Marbacher S, Remonda L, Soleman J, Ai Schlaeppi J, Leupold U et al. Outcome after in-hospital rebleeding of rupture of intracranial aneurysms. Journal of Neurological Surgery Part A: Central European Neurosurgery. 2016; 77(03):207-21. [DOI:10.1055/s-0035-1570007] [PMID] [DOI:10.1055/s-0035-1570007]
21. Jan Hillman, Steen Fridriksson, Ola Nilsson, Zhengquan Yu, Hans Säveland, Karl-Erik Jakobsson. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: A prospective randomized study. Journal of Neurosurgery. 2002; 97(4):771-8. [DOI:10.3171/jns.2002.97.4.0771] [PMID] [DOI:10.3171/jns.2002.97.4.0771]
22. Rahmanian A, Derakhshan N, Sisakht AM, Ziarati NK, Shahraki HR, Motamed S. Risk factors for unfavorable outcome in aneurysmal subarachnoid hemorrhage revisited; odds and ends. Bulletin of Emergency & Trauma. 2018; 6(2):13340. [DOI:10.29252/beat-060215] [PMID] [PMCID] [DOI:10.29252/beat-060215]
23. Rahmanian A, Sisakht A, Derakhshan N, Ziarati N, Shahraki H, Najafi B. Fresh frozen plasma versus albumin in treatment of cerebral vasospasm in subarachnoid hemorrhage: A historical cohort study. World Journal of Surgery. 2018; 1:1002.
24. Savastano LE, Bhambri A, Wilkinson DA, Pandey AS. Biology of cerebral aneurysm formation, growth, and rupture. In: Ringer A, editor. Intracranial Aneurysms. Amsterdam: Elsevier; 2018. [DOI:10.1016/B978-0-12-811740-8.00002-2]
25. Hendrix P, Foreman PM, Starke RM, Harrigan MR, Fisher WS, Vyas NA, et al. Associations of endothelin polymorphisms and aneurysm size at time of rupture. World Neurosurgery. 2017; 102:253-257. [DOI:10.1016/j.wneu.2017.03.041] [PMID] [DOI:10.1016/j.wneu.2017.03.041]
26. Griessenauer CJ, Starke RM, Foreman PM, Hendrix P, Harrigan MR, Fisher WS, et al. Associations between endothelin polymorphisms and aneurysmal subarachnoid hemorrhage, clinical vasospasm, delayed cerebral ischemia, and functional outcome. Journal of Neurosurgery. 2018; 128(5):1311-7. [PMID] [DOI:10.3171/2016.12.JNS162594] [PMID]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |