Fri, Feb 13, 2026
Volume 4, Issue 1 (Winter 2018)
Iran J Neurosurg 2018, 4(1): 13-18 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azarhomayoun A, Nouri M. In-House Handmade Camera for Indocyanine Green Video Angiography. Iran J Neurosurg 2018; 4 (1) :13-18
URL: http://irjns.org/article-1-125-en.html
URL: http://irjns.org/article-1-125-en.html
1- Gundishapour Academy of Neuroscience, Ahvaz, Iran.
2- Icahn School of Medicine, Mount Sinai, New York, USA ,nouri@gan-ac.ir
2- Icahn School of Medicine, Mount Sinai, New York, USA ,
Full Text [PDF 985 kb]
(4265 Downloads)
| Abstract (HTML) (7711 Views)
Full Text: (3475 Views)
1. Introduction
In operations with vascular preservation or reconstruction as the main goals, such as surgical treatment of brain vascular malformations, certainty of blood flow in vessels is of highest importance. Several methods are available for vascular patency assessment during the surgery, including intraoperative angiography, Doppler ultrasound, and Indocyanine Green (ICG) Video Angiography (VA) [1].
Intraoperative angiography is costly and its setup is not available in many operation theaters. Fluorescence techniques like ICG angiography are easily applicable, as they do not need moving the microscope out of the field and bringing the fluoroscopy in and have no radiation exposure. It helps surgeons with evaluating vascular anatomy, integrity of blood vessels, and physiologic parameters of blood flow [2].
Nowadays microscopes are equipped with filters that allow ICG-VA during surgery. Unfortunately, these microscopes are very expensive and therefore, not available in many centers; especially the third-world countries. Considering these limitations, we constructed a handmade ICG-VA camera for angiography applicable in the operation room or the laboratory for research purposes. In this article, we reviewed the fluorescent properties of ICG briefly and then, described the structure of our device and its preliminary usage in an animal model.
2. Methods and Materials/Patients
ICG properties and camera design
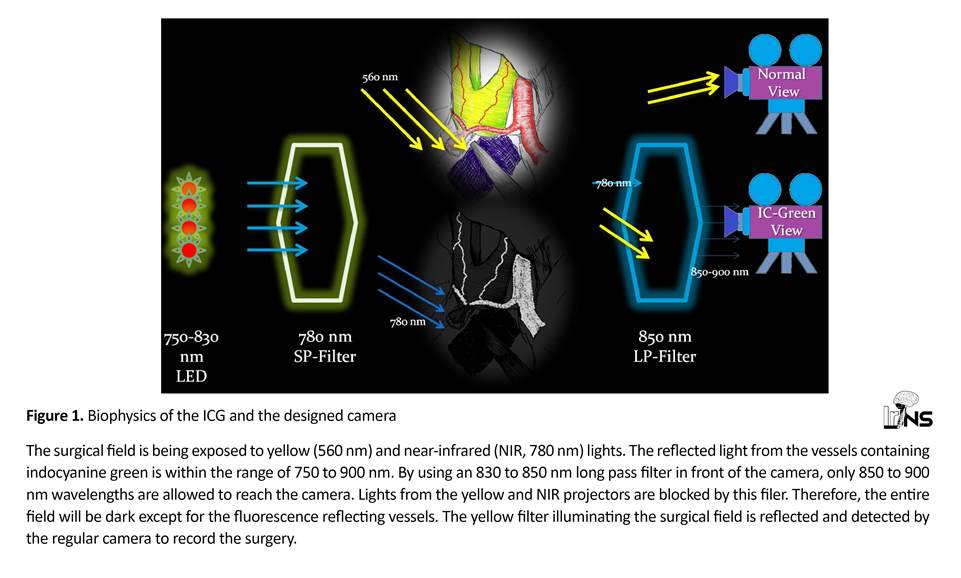
ICG is a fluorescent substance with an excitation wavelength between 600 to 900 nm (peak of absorption 800 nm) in plasma and fluorescence wavelength between 750 to 900 nm (peak 830 nm). Fluorescence happens only if the ICG is bound to proteins (e.g. in blood). Our camera was designed and constructed, based on these properties (Figure 1).
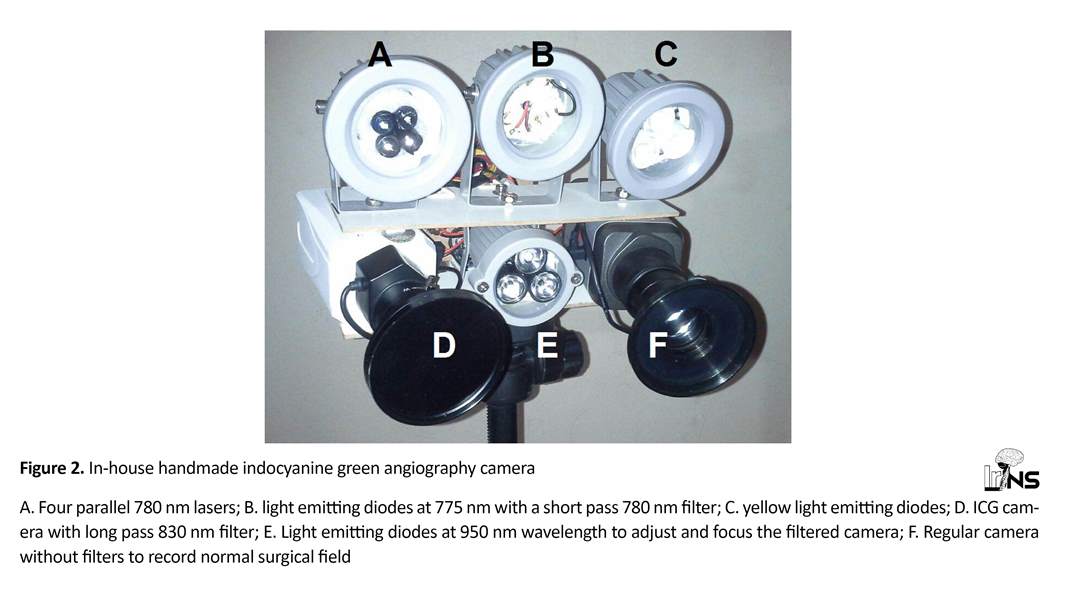
This device is composed of four light sources and 2 cameras (Figure 2). One light source provides pure yellow light (560 nm) to the field for recording the surgical field by a regular camera. Ceiling light cannot be used for this purpose, as most light sources do not provide pure rays and also emit some light near the infrared range (wavelength above 800 nm) which interferes with fluorescence angiography recorded by the other camera. But, as explained below, pure yellow light gets filtered by the ICG camera thus preventing any image artifacts.
The other light source is a projector of 775 nm wavelength (it has a range of 750 to 830 with a 775 peak) with a short-pass filter of 780 nm in front of it to prevent any ray with wavelength above 780 nm from reaching the field. Charged Couple Device (CCD) surveillance cameras (Sony Super HAD II CCD image sensor with 650 TVL resolution, assembled in Iran) were used for recording. One camera is for recording of the surgical field under the yellow light.
The other camera is to record ICG-VA and has a long-pass 830 nm filter (Marumi, Japan) to filter out lights below 850 nm wavelength. Figure 2 shows the device components. Both cameras were connected to a 4- channel Digital Video Recorder (DVR) for simultaneous recording of the videos. The third light source constitutes of four parallel 780 nm lasers to illuminate the center of surgical field. In our experience, this setting results in a sharper image contrast.
Normally, the ICG camera shows a dark image as all visible light (below 830 nm) are filtered; so even the near-infrared light (780 nm) by the second and third projectors is not detected. Therefore, it is impossible to understand if the camera lens is focused on the object.
In operations with vascular preservation or reconstruction as the main goals, such as surgical treatment of brain vascular malformations, certainty of blood flow in vessels is of highest importance. Several methods are available for vascular patency assessment during the surgery, including intraoperative angiography, Doppler ultrasound, and Indocyanine Green (ICG) Video Angiography (VA) [1].
Intraoperative angiography is costly and its setup is not available in many operation theaters. Fluorescence techniques like ICG angiography are easily applicable, as they do not need moving the microscope out of the field and bringing the fluoroscopy in and have no radiation exposure. It helps surgeons with evaluating vascular anatomy, integrity of blood vessels, and physiologic parameters of blood flow [2].
Nowadays microscopes are equipped with filters that allow ICG-VA during surgery. Unfortunately, these microscopes are very expensive and therefore, not available in many centers; especially the third-world countries. Considering these limitations, we constructed a handmade ICG-VA camera for angiography applicable in the operation room or the laboratory for research purposes. In this article, we reviewed the fluorescent properties of ICG briefly and then, described the structure of our device and its preliminary usage in an animal model.
2. Methods and Materials/Patients
ICG properties and camera design
ICG is a fluorescent substance with an excitation wavelength between 600 to 900 nm (peak of absorption 800 nm) in plasma and fluorescence wavelength between 750 to 900 nm (peak 830 nm). Fluorescence happens only if the ICG is bound to proteins (e.g. in blood). Our camera was designed and constructed, based on these properties (Figure 1).
This device is composed of four light sources and 2 cameras (Figure 2). One light source provides pure yellow light (560 nm) to the field for recording the surgical field by a regular camera. Ceiling light cannot be used for this purpose, as most light sources do not provide pure rays and also emit some light near the infrared range (wavelength above 800 nm) which interferes with fluorescence angiography recorded by the other camera. But, as explained below, pure yellow light gets filtered by the ICG camera thus preventing any image artifacts.
The other light source is a projector of 775 nm wavelength (it has a range of 750 to 830 with a 775 peak) with a short-pass filter of 780 nm in front of it to prevent any ray with wavelength above 780 nm from reaching the field. Charged Couple Device (CCD) surveillance cameras (Sony Super HAD II CCD image sensor with 650 TVL resolution, assembled in Iran) were used for recording. One camera is for recording of the surgical field under the yellow light.
The other camera is to record ICG-VA and has a long-pass 830 nm filter (Marumi, Japan) to filter out lights below 850 nm wavelength. Figure 2 shows the device components. Both cameras were connected to a 4- channel Digital Video Recorder (DVR) for simultaneous recording of the videos. The third light source constitutes of four parallel 780 nm lasers to illuminate the center of surgical field. In our experience, this setting results in a sharper image contrast.
Normally, the ICG camera shows a dark image as all visible light (below 830 nm) are filtered; so even the near-infrared light (780 nm) by the second and third projectors is not detected. Therefore, it is impossible to understand if the camera lens is focused on the object.
To solve this problem, the fourth light source was added to emit infrared light (950 nm) which is not filtered by the ICG camera and illuminates the field while we can focus the ICG camera lens. After adjusting the lens, this projector is turned off.
Animal models
Helsinki Declaration for animal care was followed throughout the study in all experiments performed. Balb/c female mice (20-25 g) were purchased form the Faculty of Veterinary Medicine, University of Tehran, Iran. Ketamine 50 mg/kg (Alfasan, Woerden, The Netherlands) and acepromazine 5 mg/kg (Castran, Interchemie, The Netherlands) were used to induce anesthesia for laparotomy.
To have a good source of vessels, mesenteric arteries of the small intestine were exposed. Animal’s tail was used to inject ICG intravenously with an insulin syringe (1 mg/kg). Intraperitoneal injection of sodium pento
Animal models
Helsinki Declaration for animal care was followed throughout the study in all experiments performed. Balb/c female mice (20-25 g) were purchased form the Faculty of Veterinary Medicine, University of Tehran, Iran. Ketamine 50 mg/kg (Alfasan, Woerden, The Netherlands) and acepromazine 5 mg/kg (Castran, Interchemie, The Netherlands) were used to induce anesthesia for laparotomy.
To have a good source of vessels, mesenteric arteries of the small intestine were exposed. Animal’s tail was used to inject ICG intravenously with an insulin syringe (1 mg/kg). Intraperitoneal injection of sodium pento
barbital (120 mg/kg) (Sigma, MO, USA) was given to euthanize the animal after the procedure.
3. Results
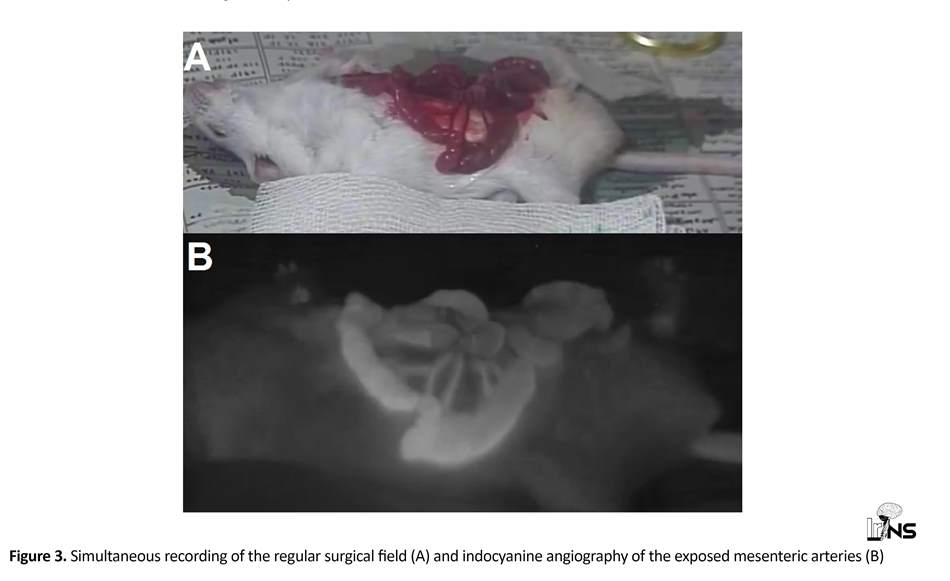
After inducing anesthesia and exposing mesenteric vessels (as mentioned earlier), projector and laser were turned on to illuminate the surgical field. Indocyanine green (SERB, Paris, France) was reconstituted with sterile water for intravenous injections (1 mg/kg). We introduced ICG through central vein of mouse tail after darkening the room. The ICG in the vessels was exposed to the 780 nm light and its fluorescent light was detected by the filtered Camera Connected to DVR and TV (CCTV setup). Simultaneous recording of the surgical field was done by the regular camera under the yellow light (Figure 3).
4. Discussion
There are some reports available of handmade devices for fluorescence applications [3, 4]. Fujisawa et al. constructed an ICG imaging system for sentinel node detection by ICG and successfully used it in 16 patients [3]. Pallota et al. manufactured a custom-made system for ICG detection of lymphatic vessels in flap reconstructed lower extremities to predict lymphedema in these patients [4]. Szyc et al. designed a handhold ICG fluorescence imaging system for sentinel node biopsy [5].
None of the above-mentioned devices were applied for ICG arterial angiography and only used for lymphangiography which does not require high quality imaging and contrast. In our experiment, we tried a stepwise manner to improve the quality of images by using more near-infrared sensitive cameras and also, strengthening the power of illuminating projectors.
ICG angiography is applicable in the operation rooms or laboratories [2]. Intraoperative ICG angiography has been reported to correlate well with the findings of intra- or post-operative conventional angiography [6]. However, this costly technology may not be affordable in many developing countries and the use of low-cost hand-made devices may help in these situations to reduce the cost while maintaining the clinical outcome [7].
Our camera was designed with some limitations. Due to the international sanctions against the country, we lacked accessing high quality products in many instances. For example, using medical grade cameras specifically designed for near-infrared wavelength, instead of CCDs improves the quality of the images significantly. High power emitting diodes and lasers can also improve the contrast and sharpness of the images which were inaccessible for us.
Also, due to the regulations by the Ministry of Health and Medical Educations, we had extremely limited access to the ICG drug which prevented repetition of our experiment. Therefore, we believe that researchers in other developing countries with a wider access to electronic and optic resources may achieve higher quality images with few modifications in our proposed camera. Currently, we are using the camera in our laboratory to develop a blood flow analysis software comparable to those available in the market [2]. We are also working to create the first generation of real-time stereoscopic ICG angiographies (unpublished data).
5. Conclusion
Considering the very low cost of the device and its acceptable image quality, it can be utilized in operation theatres and or laboratories for research purposes.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical principles to be considered in this research.
Funding
The project was financially supported by Jundishapur Academy of Neuroscience and no external fund was received for any part of the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest.
Acknowledgements
The authors extend their gratitude to Professor Yoko Kato from Japan for her dedication to educate young neurosurgeons from developing countries which resulted in our familiarity with intraoperative angiography and development of this project.
References
3. Results
After inducing anesthesia and exposing mesenteric vessels (as mentioned earlier), projector and laser were turned on to illuminate the surgical field. Indocyanine green (SERB, Paris, France) was reconstituted with sterile water for intravenous injections (1 mg/kg). We introduced ICG through central vein of mouse tail after darkening the room. The ICG in the vessels was exposed to the 780 nm light and its fluorescent light was detected by the filtered Camera Connected to DVR and TV (CCTV setup). Simultaneous recording of the surgical field was done by the regular camera under the yellow light (Figure 3).
4. Discussion
There are some reports available of handmade devices for fluorescence applications [3, 4]. Fujisawa et al. constructed an ICG imaging system for sentinel node detection by ICG and successfully used it in 16 patients [3]. Pallota et al. manufactured a custom-made system for ICG detection of lymphatic vessels in flap reconstructed lower extremities to predict lymphedema in these patients [4]. Szyc et al. designed a handhold ICG fluorescence imaging system for sentinel node biopsy [5].
None of the above-mentioned devices were applied for ICG arterial angiography and only used for lymphangiography which does not require high quality imaging and contrast. In our experiment, we tried a stepwise manner to improve the quality of images by using more near-infrared sensitive cameras and also, strengthening the power of illuminating projectors.
ICG angiography is applicable in the operation rooms or laboratories [2]. Intraoperative ICG angiography has been reported to correlate well with the findings of intra- or post-operative conventional angiography [6]. However, this costly technology may not be affordable in many developing countries and the use of low-cost hand-made devices may help in these situations to reduce the cost while maintaining the clinical outcome [7].
Our camera was designed with some limitations. Due to the international sanctions against the country, we lacked accessing high quality products in many instances. For example, using medical grade cameras specifically designed for near-infrared wavelength, instead of CCDs improves the quality of the images significantly. High power emitting diodes and lasers can also improve the contrast and sharpness of the images which were inaccessible for us.
Also, due to the regulations by the Ministry of Health and Medical Educations, we had extremely limited access to the ICG drug which prevented repetition of our experiment. Therefore, we believe that researchers in other developing countries with a wider access to electronic and optic resources may achieve higher quality images with few modifications in our proposed camera. Currently, we are using the camera in our laboratory to develop a blood flow analysis software comparable to those available in the market [2]. We are also working to create the first generation of real-time stereoscopic ICG angiographies (unpublished data).
5. Conclusion
Considering the very low cost of the device and its acceptable image quality, it can be utilized in operation theatres and or laboratories for research purposes.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical principles to be considered in this research.
Funding
The project was financially supported by Jundishapur Academy of Neuroscience and no external fund was received for any part of the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest.
Acknowledgements
The authors extend their gratitude to Professor Yoko Kato from Japan for her dedication to educate young neurosurgeons from developing countries which resulted in our familiarity with intraoperative angiography and development of this project.
References
- Yamada Y, Kato Y, Nouri M. No overtaking! Take a safe trip to aneurysm. Austin Journal of Cerebrovascular Disease & Stroke. 2014; 1(2):1.
- Kato Y, Yamada Y, Sadato A, Nouri M, Cherian I, Tanaka T, Inamasu J. Intraoperative anatomical and hemodynamic analysis of intracerebral arteriovenous malformations by semi-quantitative color-coded indocyanine green videoangiography. Asian Journal of Neurosurgery. 2017; 12(4):638-43. [DOI:10.4103/ajns.AJNS_62_14] [PMID] [PMCID]
- Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. A custom-made, low-cost intraoperative fluorescence navigation system with indocyanine green for sentinel lymph node biopsy in skin cancer. Dermatology. 2011; 222(3):261-8. [DOI: 10.1159/000327080] [PMID]
- Pallotta OJ, van Zanten M, McEwen M, Burrow L, Beesley J, Piller N. Development and validation of a custom made indocyanine green fluorescence lymphatic vessel imager. Journal of Biomedical Optics. 2015; 20(6):066003. [DOI:10.1117/1.JBO.20.6.066003] [PMID]
- Szyc L, Bonifer S, Walter A, Jagemann U, Grosenick D, Macdonald R. Development of a handheld fluorescence imaging camera for intraoperative sentinel lymph node mapping. Journal of Biomedical Optics. 2015; 20(5):051025 [DOI:10.1117/1.JBO.20.5.051025] [PMID]
- Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green video angiography during aneurysm surgery. Journal of Neurosurgery. 2005; 103(6):982-9. [DOI:10.3171/jns.2005.103.6.0982] [PMID]
- Kato Y, Nouri M. Training the next generation of neurosurgeons in developing countries: Mission possible. Iranian Journal of Neurosurgery. 2017; 3(1):6-7. [DOI:10.29252/irjns.3.1.6]
Type of Study: Research |
Subject:
Vascular Neurosurgery
References
1. Yamada Y, Kato Y, Nouri M. No overtaking! Take a safe trip to aneurysm. Austin Journal of Cerebrovascular Disease & Stroke. 2014; 1(2):1.
2. Kato Y, Yamada Y, Sadato A, Nouri M, Cherian I, Tanaka T, Inamasu J. Intraoperative anatomical and hemodynamic analysis of intracerebral arteriovenous malformations by semi-quantitative color-coded indocyanine green videoangiography. Asian Journal of Neurosurgery. 2017; 12(4):638-43. [DOI:10.4103/ajns.AJNS_62_14] [PMID] [PMCID] [DOI:10.4103/ajns.AJNS_62_14]
3. Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. A custom-made, low-cost intraoperative fluorescence navigation system with indocyanine green for sentinel lymph node biopsy in skin cancer. Dermatology. 2011; 222(3):261-8. [DOI: 10.1159/000327080] [PMID] [DOI:10.1159/000327080]
4. Pallotta OJ, van Zanten M, McEwen M, Burrow L, Beesley J, Piller N. Development and validation of a custom made indocyanine green fluorescence lymphatic vessel imager. Journal of Biomedical Optics. 2015; 20(6):066003. [DOI:10.1117/1.JBO.20.6.066003] [PMID] [DOI:10.1117/1.JBO.20.6.066003]
5. Szyc L, Bonifer S, Walter A, Jagemann U, Grosenick D, Macdonald R. Development of a handheld fluorescence imaging camera for intraoperative sentinel lymph node mapping. Journal of Biomedical Optics. 2015; 20(5):051025 [DOI:10.1117/1.JBO.20.5.051025] [PMID] [DOI:10.1117/1.JBO.20.5.051025]
6. Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green video angiography during aneurysm surgery. Journal of Neurosurgery. 2005; 103(6):982-9. [DOI:10.3171/jns.2005.103.6.0982] [PMID] [DOI:10.3171/jns.2005.103.6.0982]
7. Kato Y, Nouri M. Training the next generation of neurosurgeons in developing countries: Mission possible. Iranian Journal of Neurosurgery. 2017; 3(1):6-7. [DOI:10.29252/irjns.3.1.6] [DOI:10.29252/irjns.3.1.6]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |