Fri, Jul 11, 2025
Volume 1, Issue 3 (12-2015)
Iran J Neurosurg 2015, 1(3): 11-15 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shirvani M, Hajimirzabeigi A, Jafari R, Khatami M, Razzaghi A, Yousefzadeh-Chabok S. Microscopic Transsphenoidal Surgery for Pituitary Adenomas in Children and Adolescents. Iran J Neurosurg 2015; 1 (3) :11-15
URL: http://irjns.org/article-1-18-en.html
URL: http://irjns.org/article-1-18-en.html
Masoud Shirvani1 
 , Alireza Hajimirzabeigi2
, Alireza Hajimirzabeigi2 
 , Rozita Jafari3
, Rozita Jafari3 
 , Morteza Khatami4
, Morteza Khatami4 
 , Alireza Razzaghi5
, Alireza Razzaghi5 
 , Shahrokh Yousefzadeh-Chabok *6
, Shahrokh Yousefzadeh-Chabok *6 



 , Alireza Hajimirzabeigi2
, Alireza Hajimirzabeigi2 
 , Rozita Jafari3
, Rozita Jafari3 
 , Morteza Khatami4
, Morteza Khatami4 
 , Alireza Razzaghi5
, Alireza Razzaghi5 
 , Shahrokh Yousefzadeh-Chabok *6
, Shahrokh Yousefzadeh-Chabok *6 


1- MD, Neurosurgeon, Department of Neurosurgery, Milad Hospital, Tehran, Iran
2- MD, Department of Neurosurgery, Milad Hospital, Tehran, Iran
3- Surgeon, Department of Otolaryngology, Head and Neck Surgery, Tracheal Research Center, Shahid Beheshti University of Medical Sciences, Masih Daneshvari, Tehran, Iran
4- MD, Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Guilan, Iran
5- MSc in Epidemiology, Guilan Road Trauma Research Center, Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran
6- MD, Professor of Neurosurgery, Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht, Guilan, Iran
2- MD, Department of Neurosurgery, Milad Hospital, Tehran, Iran
3- Surgeon, Department of Otolaryngology, Head and Neck Surgery, Tracheal Research Center, Shahid Beheshti University of Medical Sciences, Masih Daneshvari, Tehran, Iran
4- MD, Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Guilan, Iran
5- MSc in Epidemiology, Guilan Road Trauma Research Center, Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran
6- MD, Professor of Neurosurgery, Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht, Guilan, Iran
Keywords: Pediatric Pituitary Adenoma, Apoplexy, Transsphenoidal Approach, Functional Pituitary Adenomas, Nonfunctional Pituitary Adenomas
Full Text [PDF 313 kb]
(3349 Downloads)
| Abstract (HTML) (7824 Views)
Full Text: (1800 Views)
Introduction
Changing the diagnosis and registration of pituitary adenomas, improved diagnostics, improved data collection, enhanced awareness of pituitary diseases among physicians and the public, longer life expectancy and/or an actual increase in the incidence of these tumors in the US population reflected an increase in the incidence of pituitary tumors reported between 2004 and 2009 (1). Pituitary adenomas account for roughly 3% of supratentorial lesions in the pediatric population and 3–6% of adenomas that can be surgically treated (2,3). Pituitary adenomas in childhood are usually of secretory type (4). Although rare, these tumors can have a dramatic impact on normal growth (5) and maturation by causing changes in hormonal functions during a critical period of development. Growth hormone is usually the first pituitary hormone to be suppressed in the event of a pituitary tumor. What follows are gonadotropins and thyroid-stimulating hormone and ACTH secretion is usually affected last. In children without GH-secreting adenomas, the common presentations are growth retardation and short stature.
In the case of ACTH-secreting adenomas, growth retardation or arrest is viewed as the direct impact of hypercortisolemia on the developing skeletal system. Most adenoma subtypes cause menstrual irregularity, delayed menarche or amenorrhea in adolescent girls. Non-secreting macro-adenomas in children are infrequent and thus, one can rarely find visual impairment and cranial nerve palsies as a result of their mass effect or hypopituitarism due to compression of the optic chiasm (6). Early detection and surgical treatment may produce a better outcome in pediatric pituitary adenomas. However, there is still some dispute on this topic and some researchers consider 16 to 20 years as the pediatric cut-off age and, accordingly, the incidence of pituitary adenoma ascribed to pediatric populations is thought to be affected by how ‘pediatric’ is defined. For example, a pituitary adenoma is rather rarely diagnosed in childhood and the incidence increases during adolescence which extends till 19 years of age. On the other hand, limitation in the anatomical structure of pediatric patients and surgical instruments make this topic remain a challenging problem (2). To treat pituitary adenomas, surgical techniques should be evaluated with respect to some aspects such as efficacy inachieving the primary objective of the surgery, safety and convenience considering both the patient’s comfort and the costs of treatment (6). As a general rule, radical surgery in the majority of patients with pituitary adenomas is a challenging matter that made approximately 15-20% of patients be re-operated because of tumor recurrence. So, there is a relatively low cure rate for hormone-secreting adenomas, ranging from 15-50% for GH-producing macro-adenomas to 34% for ACTH-producing macro-adenomas (7-9). On the other hand, tumor remnants or repeated surgeries do not change the quality of life for many patients. Also, deciding on a radical surgery must be carefully balanced against the risk and ramifications of surgical morbidity (10). Despite the promising articles about endoscopic endonasal (2) approach, the limitations of endoscopic endonasal (2) approach include post-operative nasal complaints as well as risk for post-operative CSF leak. The remaining diseases of the pituitary gland in the EE approach include subtotal resection, the recurrence of tumors postoperatively and the risk for serious intra-operative complications (11). Microscopic transsphenoidal surgery helps to reduce the incidence of postoperative complications and deficits, decrease the duration of hospital admission, avoid trauma to healthy parenchyma and at the same time, achieves the surgical goal (12). This study aims at describing the presentation, management and subsequent treatment outcomes of children and adolescents diagnosed with a pituitary adenoma in a joint neuroendocrine setting followed up by a single service as well as assessing long-term outcomes in terms of endocrine status and neurology symptoms.
Methods and Materials/Patients
This study is a retrospective assessment of patients under 20 years of age with pituitary adenoma, who underwent a transsphenoidal tumor resection surgery between January 2011 and June 30, 2014. Lab data included serum prolactin (PRL), growth hormone (2), insulin-like growth factor-1 (IGF-1), cortisol (and 24-h urinary free cortisol excretion for patients with suspected Cushing’s syndrome), free thyroxin, thyroid-stimulating hormone(TSH), adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle stimulating hormone (13), testosterone, and estradiol. We have reviewed operative notes retrospectively and analyzed them to assess the pre-operative and post-operative MRI and CT scan images.
Surgical Technique
Microscopic approach
Surgeries were performed on the patients in a supine position. After a medial nasal incision, the septal mucosa was unilaterally detached from the osseous and cartilaginous nasal septae. A nasal speculum was inserted to keep the mucosal tunnel open and an operating microscope was introduced.
A gel foam patch was fixed to the opening in the sella and held in place with fibrin glue. There are specific technical considerations in children and adolescents who undergo an endonasal transsphenoidal procedure. Using surgical technical approach in this study was the same as that of adult patients. Due to small space available in children, small sized blade and surgical instruments suitable for microsurgical approach were used for preventing damages to anatomy. Removal of a minimum section of skull bone was the main goal of surgical team which enables us to properly reconstruct the skull base.
Because of the small size of the nares in children, a narrower and shorter endonasal speculum may be required. As for the pneumatization of the sphenoid sinus in all of our patients, we did not use a driller to reaching the sella along the midline.
Post-surgical follow-up
The success of tumor removal was based on both the surgeon’s intraoperative visual assessment and the findings of MRI scans that were routinely undertaken three months after surgery. For patients with prolactinoma, PRL levels <20 ng/ml in women and <15 ng/ml in men on the first post-operative day were considered to be indicators of remission. For patients with acromegaly, remission was defined as a normal IGF-1 level combined with a basal GH level of <1 μg/l or combined with a GH level of <0.4 μg/l after oral glucose tolerance test suppression. The levels of IGF-1 were age-adjusted. For patients with Cushing’s syndrome, remission was defined as normal levels of 24-h urinary free-cortisol, morning serum cortisol and serum ACTH. In FSH/LH secreting tumors, normalized serum FSH and LH levels after surgery were indicators of remission. All the tests were repeated at least once a year or as required, depending on the patient’s clinical status.Residual disease was reported by the radiologist evaluating the post-operative and 3-month follow-up MRI. Recurrence was defined as the interval tumor growth on MRI between the tumor resection and the last follow-up imaging study.
Results
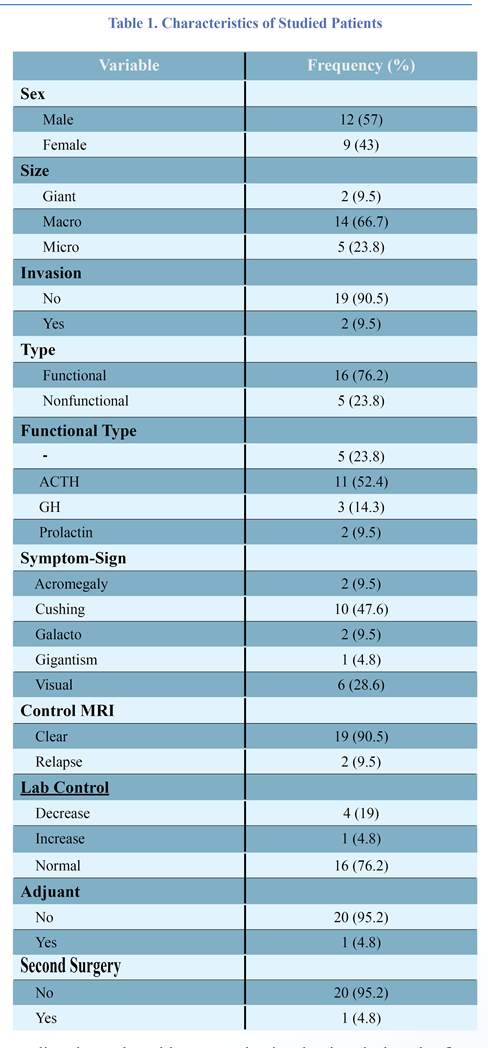
A total of 21 participants were included in the study. About 57.1% (n=12) were male. Their mean age at the diagnosis was 16.28 years (6– 20 years). Almost all had macro size (66.7%, n=14). Most of them had no invasion (90.5%, n=19).According to the results of the patients’ conditions, the functional type (76.2%, n=16) was more than the non-functional one. Most of them were ACTH (52.4%, n=11). The most common symptom in them was Cushing (47.6%, n=10). The post-operative control MRI of most of patients was clear (90.5%, n=19). The lab control in most of them was normal (76.2%, n=16). Apoplexy was seen in 5 patients (33.8%). The characteristics of studied patients are presented in table 1. Gross-total resection (GTR; 100% tumor removal as judged by early post-operative imaging) was intended in most cases and achieved in 19 cases. Residual tumor, classified as intracavernous or extracavernous based on MRI findings, was intracavernous in two of the cases. However, only one of these patients showed evidence of radiologic recurrence during the follow-up period. In two patients, we observed residual tumor growth after partial resection, whereas, after GTR, no recurrence was seen. This difference was not statistically significant. Only a small subgroup of pituitary adenomas showed a higher GTR rate (6.8, 75%). Following the surgery, two children underwent radiotherapy. In our study, disease control was achieved in all of the 5 microadenomas (23.8%), 14 macroadenomas (66.7%), and two other giant adenomas (9.5%). Other than endocrine-related symptoms, the most common complaints were visual field deficits. Six patients had pre-operative visual field deficits. Following the surgery, all of them had complete normalization of their visual disturbance. There were no surgery-related or post-operative deaths or cerebrovascular accidents. Intraoperative CSF leaks occurred in one child who was treated successfully intra-operatively. Four patients were presented with diabetes insipidus, but none of our patients developed permanent diabetes insipidus requiring medical therapy. Also none of the patients experienced post-operative epistaxis or a nasal septum perforation. No patient exhibited panhypopituitarism either. The mean follow-up of all the patients after surgery was 3 years and 8 months (i.e. a range of 25 years old).
Discussion
From a clinicopathological point of view, pituitary adenoma is a benign tumor that can be classified as functional (hormone-secreting) or non-functional. It causes pituitary dysfunctions such as adenoma-related hypersecretion or mass effect-related hypopituitarism. In addition, patients often suffer from visual scope defect, loss of visual acuity, or diplopia induced by oculomotor dysfunction. This last defect is observed especially in the case of cavernous sinus infiltration. Although pituitary tumors are common intracranial neoplasms in the adult population and constitute 8% to 12% fall intracranial neoplasms, they are not commonly found in children (3). In the present study, the maximum age for inclusion was similar to that proposed by the American Academy of Pediatrics (AAP) in the Journal of Pediatric Health Care 2008 and in the study by Jankowski PP et al. (2015). The age limit of pediatrics is set to be from fetal life until 21 years of age) (3). The rather high number of pediatric
patients be set by non-functional pituitary adenoma in our series did not match such patients in other studies (3,14). In this study, for certain reasons, a microscopic transsphenoidal surgery was chosen for pituitary adenomas. Apart from other current techniques, although endonasal transsphenoidal surgery helps to remove these tumors in adults and children with few complications, little morbidity and near zero mortality. The major concern in the use of this surgical technique in children is its likelihood not to provide sufficient exposure for complete tumor resection. Regarding the pediatric population, with whom access through the nares is a challenging technique, is not without limitations. For example, small nares can be an obstacle to introducing of the endoscope unless turbinectomy or ethmoidectomy is performed concurrently. These procedures raise the probability of post-operative rhinological complications including empty nose syndrome (2). Other limitations include clouding of the scope’s lens with blood and moisture, difficult controlling of bleeding complications during surgery and two-dimensional vision, which is the most serious setback of the technique (12,14). On the other hand, the pneumatization of the sphenoid sinus which starts from the 3rd to the 4th year of age and becomes completed in the second decade of life is an important anatomical factor to consider in a transsphenoidal surgery. In contrast, it has been shown in some anatomical and radiological
Changing the diagnosis and registration of pituitary adenomas, improved diagnostics, improved data collection, enhanced awareness of pituitary diseases among physicians and the public, longer life expectancy and/or an actual increase in the incidence of these tumors in the US population reflected an increase in the incidence of pituitary tumors reported between 2004 and 2009 (1). Pituitary adenomas account for roughly 3% of supratentorial lesions in the pediatric population and 3–6% of adenomas that can be surgically treated (2,3). Pituitary adenomas in childhood are usually of secretory type (4). Although rare, these tumors can have a dramatic impact on normal growth (5) and maturation by causing changes in hormonal functions during a critical period of development. Growth hormone is usually the first pituitary hormone to be suppressed in the event of a pituitary tumor. What follows are gonadotropins and thyroid-stimulating hormone and ACTH secretion is usually affected last. In children without GH-secreting adenomas, the common presentations are growth retardation and short stature.
In the case of ACTH-secreting adenomas, growth retardation or arrest is viewed as the direct impact of hypercortisolemia on the developing skeletal system. Most adenoma subtypes cause menstrual irregularity, delayed menarche or amenorrhea in adolescent girls. Non-secreting macro-adenomas in children are infrequent and thus, one can rarely find visual impairment and cranial nerve palsies as a result of their mass effect or hypopituitarism due to compression of the optic chiasm (6). Early detection and surgical treatment may produce a better outcome in pediatric pituitary adenomas. However, there is still some dispute on this topic and some researchers consider 16 to 20 years as the pediatric cut-off age and, accordingly, the incidence of pituitary adenoma ascribed to pediatric populations is thought to be affected by how ‘pediatric’ is defined. For example, a pituitary adenoma is rather rarely diagnosed in childhood and the incidence increases during adolescence which extends till 19 years of age. On the other hand, limitation in the anatomical structure of pediatric patients and surgical instruments make this topic remain a challenging problem (2). To treat pituitary adenomas, surgical techniques should be evaluated with respect to some aspects such as efficacy inachieving the primary objective of the surgery, safety and convenience considering both the patient’s comfort and the costs of treatment (6). As a general rule, radical surgery in the majority of patients with pituitary adenomas is a challenging matter that made approximately 15-20% of patients be re-operated because of tumor recurrence. So, there is a relatively low cure rate for hormone-secreting adenomas, ranging from 15-50% for GH-producing macro-adenomas to 34% for ACTH-producing macro-adenomas (7-9). On the other hand, tumor remnants or repeated surgeries do not change the quality of life for many patients. Also, deciding on a radical surgery must be carefully balanced against the risk and ramifications of surgical morbidity (10). Despite the promising articles about endoscopic endonasal (2) approach, the limitations of endoscopic endonasal (2) approach include post-operative nasal complaints as well as risk for post-operative CSF leak. The remaining diseases of the pituitary gland in the EE approach include subtotal resection, the recurrence of tumors postoperatively and the risk for serious intra-operative complications (11). Microscopic transsphenoidal surgery helps to reduce the incidence of postoperative complications and deficits, decrease the duration of hospital admission, avoid trauma to healthy parenchyma and at the same time, achieves the surgical goal (12). This study aims at describing the presentation, management and subsequent treatment outcomes of children and adolescents diagnosed with a pituitary adenoma in a joint neuroendocrine setting followed up by a single service as well as assessing long-term outcomes in terms of endocrine status and neurology symptoms.
Methods and Materials/Patients
This study is a retrospective assessment of patients under 20 years of age with pituitary adenoma, who underwent a transsphenoidal tumor resection surgery between January 2011 and June 30, 2014. Lab data included serum prolactin (PRL), growth hormone (2), insulin-like growth factor-1 (IGF-1), cortisol (and 24-h urinary free cortisol excretion for patients with suspected Cushing’s syndrome), free thyroxin, thyroid-stimulating hormone(TSH), adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle stimulating hormone (13), testosterone, and estradiol. We have reviewed operative notes retrospectively and analyzed them to assess the pre-operative and post-operative MRI and CT scan images.
Surgical Technique
Microscopic approach
Surgeries were performed on the patients in a supine position. After a medial nasal incision, the septal mucosa was unilaterally detached from the osseous and cartilaginous nasal septae. A nasal speculum was inserted to keep the mucosal tunnel open and an operating microscope was introduced.
A gel foam patch was fixed to the opening in the sella and held in place with fibrin glue. There are specific technical considerations in children and adolescents who undergo an endonasal transsphenoidal procedure. Using surgical technical approach in this study was the same as that of adult patients. Due to small space available in children, small sized blade and surgical instruments suitable for microsurgical approach were used for preventing damages to anatomy. Removal of a minimum section of skull bone was the main goal of surgical team which enables us to properly reconstruct the skull base.
Because of the small size of the nares in children, a narrower and shorter endonasal speculum may be required. As for the pneumatization of the sphenoid sinus in all of our patients, we did not use a driller to reaching the sella along the midline.
Post-surgical follow-up
The success of tumor removal was based on both the surgeon’s intraoperative visual assessment and the findings of MRI scans that were routinely undertaken three months after surgery. For patients with prolactinoma, PRL levels <20 ng/ml in women and <15 ng/ml in men on the first post-operative day were considered to be indicators of remission. For patients with acromegaly, remission was defined as a normal IGF-1 level combined with a basal GH level of <1 μg/l or combined with a GH level of <0.4 μg/l after oral glucose tolerance test suppression. The levels of IGF-1 were age-adjusted. For patients with Cushing’s syndrome, remission was defined as normal levels of 24-h urinary free-cortisol, morning serum cortisol and serum ACTH. In FSH/LH secreting tumors, normalized serum FSH and LH levels after surgery were indicators of remission. All the tests were repeated at least once a year or as required, depending on the patient’s clinical status.Residual disease was reported by the radiologist evaluating the post-operative and 3-month follow-up MRI. Recurrence was defined as the interval tumor growth on MRI between the tumor resection and the last follow-up imaging study.
Results
A total of 21 participants were included in the study. About 57.1% (n=12) were male. Their mean age at the diagnosis was 16.28 years (6– 20 years). Almost all had macro size (66.7%, n=14). Most of them had no invasion (90.5%, n=19).According to the results of the patients’ conditions, the functional type (76.2%, n=16) was more than the non-functional one. Most of them were ACTH (52.4%, n=11). The most common symptom in them was Cushing (47.6%, n=10). The post-operative control MRI of most of patients was clear (90.5%, n=19). The lab control in most of them was normal (76.2%, n=16). Apoplexy was seen in 5 patients (33.8%). The characteristics of studied patients are presented in table 1. Gross-total resection (GTR; 100% tumor removal as judged by early post-operative imaging) was intended in most cases and achieved in 19 cases. Residual tumor, classified as intracavernous or extracavernous based on MRI findings, was intracavernous in two of the cases. However, only one of these patients showed evidence of radiologic recurrence during the follow-up period. In two patients, we observed residual tumor growth after partial resection, whereas, after GTR, no recurrence was seen. This difference was not statistically significant. Only a small subgroup of pituitary adenomas showed a higher GTR rate (6.8, 75%). Following the surgery, two children underwent radiotherapy. In our study, disease control was achieved in all of the 5 microadenomas (23.8%), 14 macroadenomas (66.7%), and two other giant adenomas (9.5%). Other than endocrine-related symptoms, the most common complaints were visual field deficits. Six patients had pre-operative visual field deficits. Following the surgery, all of them had complete normalization of their visual disturbance. There were no surgery-related or post-operative deaths or cerebrovascular accidents. Intraoperative CSF leaks occurred in one child who was treated successfully intra-operatively. Four patients were presented with diabetes insipidus, but none of our patients developed permanent diabetes insipidus requiring medical therapy. Also none of the patients experienced post-operative epistaxis or a nasal septum perforation. No patient exhibited panhypopituitarism either. The mean follow-up of all the patients after surgery was 3 years and 8 months (i.e. a range of 25 years old).
Discussion
From a clinicopathological point of view, pituitary adenoma is a benign tumor that can be classified as functional (hormone-secreting) or non-functional. It causes pituitary dysfunctions such as adenoma-related hypersecretion or mass effect-related hypopituitarism. In addition, patients often suffer from visual scope defect, loss of visual acuity, or diplopia induced by oculomotor dysfunction. This last defect is observed especially in the case of cavernous sinus infiltration. Although pituitary tumors are common intracranial neoplasms in the adult population and constitute 8% to 12% fall intracranial neoplasms, they are not commonly found in children (3). In the present study, the maximum age for inclusion was similar to that proposed by the American Academy of Pediatrics (AAP) in the Journal of Pediatric Health Care 2008 and in the study by Jankowski PP et al. (2015). The age limit of pediatrics is set to be from fetal life until 21 years of age) (3). The rather high number of pediatric
patients be set by non-functional pituitary adenoma in our series did not match such patients in other studies (3,14). In this study, for certain reasons, a microscopic transsphenoidal surgery was chosen for pituitary adenomas. Apart from other current techniques, although endonasal transsphenoidal surgery helps to remove these tumors in adults and children with few complications, little morbidity and near zero mortality. The major concern in the use of this surgical technique in children is its likelihood not to provide sufficient exposure for complete tumor resection. Regarding the pediatric population, with whom access through the nares is a challenging technique, is not without limitations. For example, small nares can be an obstacle to introducing of the endoscope unless turbinectomy or ethmoidectomy is performed concurrently. These procedures raise the probability of post-operative rhinological complications including empty nose syndrome (2). Other limitations include clouding of the scope’s lens with blood and moisture, difficult controlling of bleeding complications during surgery and two-dimensional vision, which is the most serious setback of the technique (12,14). On the other hand, the pneumatization of the sphenoid sinus which starts from the 3rd to the 4th year of age and becomes completed in the second decade of life is an important anatomical factor to consider in a transsphenoidal surgery. In contrast, it has been shown in some anatomical and radiological
studies that sphenoid pneumatization begins during the first months and is usually completed within the first decade of life (13). Thorough radiological evaluation by means of computerized tomography and MRI is needed for investigation of the anatomical variations in the paranasal sinuses. It was a surprise to find none of our patients in need of drilling bones for incompletely pneumatized sphenoid sinuses. The key points for a good surgery are indeed mastering the anatomy of the corresponding locations and being experienced in transsphenoidal surgery especially in pediatric cases. Regarding the pneumatization of the sphenoid sinus, no increased risk was observed of partially pneumatized or non-pneumatized sinus, late postoperative surgical complications in terms of growth abnormality in the face or a nasal problem. Although no volumetric analysis was performed in our study, the majority of tumors had a maximum diameter of greater than 10 mm. Relapse was observed in just two cases of giant adenoma. Our results showed a significant risk of recurrence in tumors that have a maximum diameter of greater than 10 mm. This is, indeed, consistent with the general finding that the risk of recurrence increases with increased tumor size. Tabaee et al. reported a GTR in 89% of patients suffering from pituitary adenomas. It was found that tumor size is the only significant predictor of the extent of tumor removal; that is to say, smaller tumors are more likely to be completely removed. Hofstetter et al. (2012) elaborated upon this finding and found that volumetric analysis is a powerful predictor of resectability because it can reveal a tumor volume greater than 10 cm3 for pituitary adenoma (15,16). Sometimes, a more complete resection is correlated with a decreased rate of tumor recurrence and improved outcomes but this is not the case universally (17). At our institution, however, we found similar or somehow better results in the rate of intra-operatively complications and post-operative residue in our pediatric microscopic endonasal patients than the results reported for endoscopic endonasal surgeries in other series (10,12-14,18,19). In our opinion, fewer post-operative complications of microscopic approach in pediatrics patients is due to lesser exposure which is imposed during surgery. In addition, 3-dimentional view and better resolution which is provided by microscope and possibility usage of wide spectrum of instrument in this technique for exposure and resection of tumor leads to promising results. Although reoperations usually emerged as technically challenging surgeries, no specific technique modification was routinely made for such cases. However, in cases with significant anatomical changes and/or considerable extrasellar residual tumors after the initial surgery, a neuro-navigation system and an ultrasonic probe were used for carotid monitoring. Generally, there was no difference between the complication profiles of these cases (20). To have experienced the transsphenoidal approach is also very important, particularly in the case of ACTH-secreting adenomas. The importance is due to the fact that resection, if done successfully, can often be life-saving for the patient. Considering the low surgical morbidity and the treatment rates discussed previously, it is reasonable to have early surgical management as an alternative to lifelong medical treatment (2). It seems that the initial lower rate of complications such as transitory diabetes insipidus, which are usually not considered as surgery complications and higher rate of successful resection of adenoma in our series are correlated to the extent of operational mastery and the presence of important intraoperative instruments in the beginning. A few instruments at hand were a surgical navigator for the resection of tumors with suprasellar/retrosellar extensions and an intraoperative Doppler for carotid analyzes in cases with important parasellar components. In order to present a complete report of our series, it was decided to include patients who had transitory diabetes insipidus, which is usually not considered as a surgery complication; therefore, it may justify our slightly higher complication rate if compared with the results of other groups. Although this study outlines our results with a purely transsphenoidal microsurgical approach to the resection of pituitary adenomas and raises important issues about recurrence in patients, the design of the study is a limiting factor; clinical presentations, surgical outcomes and post-operative complications were all based on chart reviews, which restricts the analysis of the information provided in charts. In our series of patients, a rate of apoplexy of about 33.8% was observed, which was slightly higher than those presented by other groups of patients. Ours would have sudden deterioration of visual loss as well as headaches, and they had to be operated immediately. Pituitary apoplexy is still a clinical condition that can bring about serious neurologic and endocrine symptoms which may disable sequelae (3). Surprisingly, our patients underwent a surgery and came up with results comparable to other patients. We believe that such results came about due to the incidence of macro-adenomas in our series. Uncommon features of adenoma were observed in our study. A 9-year-old boy with Nelson syndrome and a 13-year-old girl with McCune-Albright syndrome presented gigantism, fibrous dysplasia, and pigmentation (café au lait spots). These patients recovered completely after surgery. No similar cases were reported in other studies.
Conclusion
Surgical approach for treatment of pituitary adenoma is designed for gross total tumor resection and the least harm to the patient, considering anatomical variation and tumor invasion to the adjacent area. It also needs a highly-experienced surgeon and state-of-the-art surgical equipment. In our study, all patients underwent microscopic transsphenoidal surgery due to limitation of endoscopic approach in pediatric and avoided wide anatomical deficit. Doing a comparative study between these two approaches will bring about promising result.
Funding
None.
Conflicts of Interest
The authors have no conflicts of interest.
References
1. Gittleman H, Ostrom QT, Farah PD, Ondracek A, Chen Y, Wolinsky Y, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009: Clinical article. Journal of neurosurgery. 2014;121(3):527-35.
2. Tarapore PE, Sughrue ME, Blevins L, Auguste KI, Gupta N, Kunwar S. Microscopic endonasal transsphenoidal pituitary adenomectomy in the pediatric population: Clinical article. Journal of Neurosurgery: Pediatrics. 2011;7(5):501-9.
3. Steele CA, MacFarlane IA, Blair J, Cuthbertson DJ, Didi M, Mallucci C, et al. Pituitary adenomas in childhood, adolescence and young adulthood: presentation, management, endocrine and metabolic outcomes. European Journal of Endocrinology. 2010;163(4):515-22.
4. Taşkapılıoğlu MÖ, Yilmazlar S, Eren E, Tarım O, Moralı Güler T. Transnasal Transsphenoidal Surgical Method in Pediatric Pituitary Adenomas. Pediatric neurosurgery. 2014;50(3):128-32.
5. Gondim JA, Almeida JPC, Albuquerque LAF, Schops M, Gomes E, Ferraz T, et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(2):174-83.
6. Cho HJ, Kim H, Kwak YJ, Seo JW, Paek SH, Sohn C-H, et al. Clinicopathologic analysis of pituitary adenoma: A single institute experience. Journal of Korean medical science. 2014;29(3):405-10.
7. Flier JS, Underhill LH, Klibanski A, Zervas NT. Diagnosis and management of hormone-secreting pituitary adenomas. New England Journal of Medicine. 1991;324(12):822-31.
8. Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. The Journal of Clinical Endocrinology & Metabolism. 1998;83(10):3411-8.
9. Ross DA, Wilson CB. Results of transsphenoidal microsurgery for growth hormone-secreting pituitary adenoma in a series of 214 patients. Journal of Neurosurgery. 1988;68(6):854-67.
10. Bodhinayake I, Ottenhausen M, Mooney MA, Kesavabhotla K, Christos P, Schwarz JT, et al. Results and risk factors for recurrence following endoscopic endonasal transsphenoidal surgery for pituitary adenoma. Clinical neurology and neurosurgery. 2014;119:75-9.
11. Cheng R, Tian H, Gao W, Li Z. A comparison between endoscopic transsphenoidal surgery and traditional trans-sphenoidal microsurgery for functioning pituitary adenomas. Journal of International Medical Research. 2011;39(5):1985-93.
12. Jankowski PP, Crawford JR, Khanna P, Malicki DM, Ciacci JD, Levy ML. Pituitary tumor apoplexy in adolescents. World neurosurgery. 2015;83(4):644-51.
13. Storr HL, Alexandraki KI, Martin L, Isidori AM, Kaltsas G, Monson JP, et al. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing's disease. European Journal of Endocrinology. 2011;164(5):667-74.
14. Rigante M, Massimi L, Parrilla C, Galli J, Caldarelli M, Di Rocco C, et al. Endoscopic transsphenoidal approach versus microscopic approach in children. International journal of pediatric otorhinolaryngology. 2011;75(9):1132-6.
15. Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012;15(3):450-63.
16. Shin SS, Gardner PA, Stefko ST, Madhok R, Fernandez-Miranda JC, Snyderman CH. Endoscopic endonasal approach for nonvestibular schwannomas. Neurosurgery. 2011;69(5):1046-57.
17. Locatelli D, Massimi L, Rigante M, Custodi V, Paludetti G, Castelnuovo P, et al. Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. International journal of pediatric otorhinolaryngology. 2010;74(11):1298-302.
18. Sughrue ME, Chang EF, Tyrell JB, Kunwar S, Wilson CB, Blevins Jr LS. Pre-operative dopamine agonist therapy improves post-operative tumor control following prolactinoma resection. Pituitary. 2009;12(3):158-64.
19. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2012:jnnp-2012-303194.
20. Chone CT, Sampaio MH, Sakano E, Paschoal JR, Garnes HM, Queiroz L, et al. Endoscopic endonasal transsphenoidal resection of pituitary adenomas: preliminary evaluation of consecutive cases. Brazilian journal of otorhinolaryngology. 2014;80(2):146-51.
Conclusion
Surgical approach for treatment of pituitary adenoma is designed for gross total tumor resection and the least harm to the patient, considering anatomical variation and tumor invasion to the adjacent area. It also needs a highly-experienced surgeon and state-of-the-art surgical equipment. In our study, all patients underwent microscopic transsphenoidal surgery due to limitation of endoscopic approach in pediatric and avoided wide anatomical deficit. Doing a comparative study between these two approaches will bring about promising result.
Funding
None.
Conflicts of Interest
The authors have no conflicts of interest.
References
1. Gittleman H, Ostrom QT, Farah PD, Ondracek A, Chen Y, Wolinsky Y, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009: Clinical article. Journal of neurosurgery. 2014;121(3):527-35.
2. Tarapore PE, Sughrue ME, Blevins L, Auguste KI, Gupta N, Kunwar S. Microscopic endonasal transsphenoidal pituitary adenomectomy in the pediatric population: Clinical article. Journal of Neurosurgery: Pediatrics. 2011;7(5):501-9.
3. Steele CA, MacFarlane IA, Blair J, Cuthbertson DJ, Didi M, Mallucci C, et al. Pituitary adenomas in childhood, adolescence and young adulthood: presentation, management, endocrine and metabolic outcomes. European Journal of Endocrinology. 2010;163(4):515-22.
4. Taşkapılıoğlu MÖ, Yilmazlar S, Eren E, Tarım O, Moralı Güler T. Transnasal Transsphenoidal Surgical Method in Pediatric Pituitary Adenomas. Pediatric neurosurgery. 2014;50(3):128-32.
5. Gondim JA, Almeida JPC, Albuquerque LAF, Schops M, Gomes E, Ferraz T, et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(2):174-83.
6. Cho HJ, Kim H, Kwak YJ, Seo JW, Paek SH, Sohn C-H, et al. Clinicopathologic analysis of pituitary adenoma: A single institute experience. Journal of Korean medical science. 2014;29(3):405-10.
7. Flier JS, Underhill LH, Klibanski A, Zervas NT. Diagnosis and management of hormone-secreting pituitary adenomas. New England Journal of Medicine. 1991;324(12):822-31.
8. Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. The Journal of Clinical Endocrinology & Metabolism. 1998;83(10):3411-8.
9. Ross DA, Wilson CB. Results of transsphenoidal microsurgery for growth hormone-secreting pituitary adenoma in a series of 214 patients. Journal of Neurosurgery. 1988;68(6):854-67.
10. Bodhinayake I, Ottenhausen M, Mooney MA, Kesavabhotla K, Christos P, Schwarz JT, et al. Results and risk factors for recurrence following endoscopic endonasal transsphenoidal surgery for pituitary adenoma. Clinical neurology and neurosurgery. 2014;119:75-9.
11. Cheng R, Tian H, Gao W, Li Z. A comparison between endoscopic transsphenoidal surgery and traditional trans-sphenoidal microsurgery for functioning pituitary adenomas. Journal of International Medical Research. 2011;39(5):1985-93.
12. Jankowski PP, Crawford JR, Khanna P, Malicki DM, Ciacci JD, Levy ML. Pituitary tumor apoplexy in adolescents. World neurosurgery. 2015;83(4):644-51.
13. Storr HL, Alexandraki KI, Martin L, Isidori AM, Kaltsas G, Monson JP, et al. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing's disease. European Journal of Endocrinology. 2011;164(5):667-74.
14. Rigante M, Massimi L, Parrilla C, Galli J, Caldarelli M, Di Rocco C, et al. Endoscopic transsphenoidal approach versus microscopic approach in children. International journal of pediatric otorhinolaryngology. 2011;75(9):1132-6.
15. Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012;15(3):450-63.
16. Shin SS, Gardner PA, Stefko ST, Madhok R, Fernandez-Miranda JC, Snyderman CH. Endoscopic endonasal approach for nonvestibular schwannomas. Neurosurgery. 2011;69(5):1046-57.
17. Locatelli D, Massimi L, Rigante M, Custodi V, Paludetti G, Castelnuovo P, et al. Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. International journal of pediatric otorhinolaryngology. 2010;74(11):1298-302.
18. Sughrue ME, Chang EF, Tyrell JB, Kunwar S, Wilson CB, Blevins Jr LS. Pre-operative dopamine agonist therapy improves post-operative tumor control following prolactinoma resection. Pituitary. 2009;12(3):158-64.
19. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2012:jnnp-2012-303194.
20. Chone CT, Sampaio MH, Sakano E, Paschoal JR, Garnes HM, Queiroz L, et al. Endoscopic endonasal transsphenoidal resection of pituitary adenomas: preliminary evaluation of consecutive cases. Brazilian journal of otorhinolaryngology. 2014;80(2):146-51.
Type of Study: Research |
Subject:
Gamma Knife Radiosurgery
References
1. Gittleman H, Ostrom QT, Farah PD, Ondracek A, Chen Y, Wolinsky Y, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009: Clinical article. Journal of neurosurgery. 2014;121(3):527-35. [DOI:10.3171/2014.5.JNS131819] [PMID]
2. Tarapore PE, Sughrue ME, Blevins L, Auguste KI, Gupta N, Kunwar S. Microscopic endonasal transsphenoidal pituitary adenomectomy in the pediatric population: Clinical article. Journal of Neurosurgery: Pediatrics. 2011;7(5):501-9. [DOI:10.3171/2011.2.PEDS10278] [PMID]
3. Steele CA, MacFarlane IA, Blair J, Cuthbertson DJ, Didi M, Mallucci C, et al. Pituitary adenomas in childhood, adolescence and young adulthood: presentation, management, endocrine and metabolic outcomes. European Journal of Endocrinology. 2010;163(4):515-22. [DOI:10.1530/EJE-10-0519] [PMID]
4. Taşkapılıoğlu MÖ, Yilmazlar S, Eren E, Tarım O, Moralı Güler T. Transnasal Transsphenoidal Surgical Method in Pediatric Pituitary Adenomas. Pediatric neurosurgery. 2014;50(3):128-32. [DOI:10.1159/000381862] [PMID]
5. Gondim JA, Almeida JPC, Albuquerque LAF, Schops M, Gomes E, Ferraz T, et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(2):174-83. [DOI:10.1007/s11102-010-0280-1] [PMID]
6. Cho HJ, Kim H, Kwak YJ, Seo JW, Paek SH, Sohn C-H, et al. Clinicopathologic analysis of pituitary adenoma: A single institute experience. Journal of Korean medical science. 2014;29(3):405-10. [DOI:10.3346/jkms.2014.29.3.405] [PMID] [PMCID]
7. Flier JS, Underhill LH, Klibanski A, Zervas NT. Diagnosis and management of hormone-secreting pituitary adenomas. New England Journal of Medicine. 1991;324(12):822-31. [DOI:10.1056/NEJM199103213241207] [PMID]
8. Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. The Journal of Clinical Endocrinology & Metabolism. 1998;83(10):3411-8. [DOI:10.1210/jcem.83.10.5111] [PMID]
9. Ross DA, Wilson CB. Results of transsphenoidal microsurgery for growth hormone-secreting pituitary adenoma in a series of 214 patients. Journal of Neurosurgery. 1988;68(6):854-67. [DOI:10.3171/jns.1988.68.6.0854] [PMID]
10. Bodhinayake I, Ottenhausen M, Mooney MA, Kesavabhotla K, Christos P, Schwarz JT, et al. Results and risk factors for recurrence following endoscopic endonasal transsphenoidal surgery for pituitary adenoma. Clinical neurology and neurosurgery. 2014;119:75-9. [DOI:10.1016/j.clineuro.2014.01.020] [PMID]
11. Cheng R, Tian H, Gao W, Li Z. A comparison between endoscopic transsphenoidal surgery and traditional trans-sphenoidal microsurgery for functioning pituitary adenomas. Journal of International Medical Research. 2011;39(5):1985-93. [DOI:10.1177/147323001103900545] [PMID]
12. Jankowski PP, Crawford JR, Khanna P, Malicki DM, Ciacci JD, Levy ML. Pituitary tumor apoplexy in adolescents. World neurosurgery. 2015;83(4):644-51. [DOI:10.1016/j.wneu.2014.12.026] [PMID]
13. Storr HL, Alexandraki KI, Martin L, Isidori AM, Kaltsas G, Monson JP, et al. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing's disease. European Journal of Endocrinology. 2011;164(5):667-74. [DOI:10.1530/EJE-10-1120] [PMID]
14. Rigante M, Massimi L, Parrilla C, Galli J, Caldarelli M, Di Rocco C, et al. Endoscopic transsphenoidal approach versus microscopic approach in children. International journal of pediatric otorhinolaryngology. 2011;75(9):1132-6. [DOI:10.1016/j.ijporl.2011.06.004] [PMID]
15. Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012;15(3):450-63. [DOI:10.1007/s11102-011-0350-z] [PMID]
16. Shin SS, Gardner PA, Stefko ST, Madhok R, Fernandez-Miranda JC, Snyderman CH. Endoscopic endonasal approach for nonvestibular schwannomas. Neurosurgery. 2011;69(5):1046-57. [PMID]
17. Locatelli D, Massimi L, Rigante M, Custodi V, Paludetti G, Castelnuovo P, et al. Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. International journal of pediatric otorhinolaryngology. 2010;74(11):1298-302. [DOI:10.1016/j.ijporl.2010.08.009] [PMID]
18. Sughrue ME, Chang EF, Tyrell JB, Kunwar S, Wilson CB, Blevins Jr LS. Pre-operative dopamine agonist therapy improves post-operative tumor control following prolactinoma resection. Pituitary. 2009;12(3):158-64. [DOI:10.1007/s11102-008-0135-1] [PMID]
19. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2012:jnnp-2012-303194.
20. Chone CT, Sampaio MH, Sakano E, Paschoal JR, Garnes HM, Queiroz L, et al. Endoscopic endonasal transsphenoidal resection of pituitary adenomas: preliminary evaluation of consecutive cases. Brazilian journal of otorhinolaryngology. 2014;80(2):146-51. [DOI:10.5935/1808-8694.20140030] [PMID]
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |