Mon, Feb 9, 2026
Volume 6, Issue 4 (Autumn 2020)
Iran J Neurosurg 2020, 6(4): 195-202 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tabibkhooei A, Azar M, Alizadeh S, Aslaninia A. Effects of Temporary Clips on Somatosensory Evoked Potentials in Anterior Circulation of Brain Aneurysm Surgery Among Rasul Akram Hospital Patients During 2017-2018. Iran J Neurosurg 2020; 6 (4) :195-202
URL: http://irjns.org/article-1-239-en.html
URL: http://irjns.org/article-1-239-en.html
1- Neurosurgery Research Center, Rasul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Neurosurgery Research Center, Rasul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran. ,dr.aslaninia@gmail.com
2- Neurosurgery Research Center, Rasul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran. ,
Full Text [PDF 784 kb]
(1667 Downloads)
| Abstract (HTML) (8500 Views)
Full Text: (1640 Views)
1. Introduction
Different strategies can be applied to provide cytoprotection during cerebrovascular surgery. The most rational and direct approach to reduce ischemia is probably limiting the ischemic length. Temporary arterial obstruction for CEA (Carotid Endarterectomy) and aneurysm dissection, as well as permanent clipping, are now known as common and standard methods in surgeries. However, maintaining optimal obstruction time by emphasizing on anatomic level of obstruction and artery blockage area, plays an important role. Duration of focal brain ischemia which can safely be tracked (without any clinical consequence), varies for different situations and mainly depends on the vascular area [1-5]. Cerebrovascular brain incidents especially brain aneurysm rupture are known as a major cause of death and disability [6]. Despite all progressions, in emergency divisions, brain aneurysm is an important cause of demise and disability in Iran as well as other countries, which mostly leads to severe effects [7].
Treatment of intracranial aneurysms is progressing and several technical innovations have taken place especially in the recent 2 decades. Improvements in morbidity and mortality rate have apparently been achieved through better diagnosis, rapid recovery of ruptured aneurysm, and developments in managing clinical methods [8-10]. Using temporary clips is a standard method for brain aneurysm surgery and corresponding rupture or preventing aneurysm rupture during dissection [11]. However, employing temporary clips elevates the risk of hypoperfusion in the territory of arteries, and the resulting additional post-op deficit is still considered a terrible complication.
Neuromonitoring technique can theoretically be utilized during surgery to alarm for brain hypoperfusion and reduce these kinds of complications. Monitoring Somatosensory Evoked Potential (SSEP) and corresponding changes are used to identify cerebral ischemia and help to predict neuronal injuries during using temporary clips in brain aneurysm surgeries [12]. This approach is limited to integrated performance evaluation for somatosensory and cortex paths [13]. Motor Evoked Potential (MEP) monitoring provides more information about subcortical structures, motor cortex, and related paths and shows more sensitivity for recognizing insufficient bloodstream in MCA (Middle Cerebral Artery) and anterior choroidal artery, compared with SSEP monitoring. MEP monitoring may be restricted because of any interruption caused by patients’ head moving during surgery. There is a strong correlation between the decrease of brain bloodstream with the decrease of amplitude and increase of latency in SSEP. In spite of the many existing reports on SSEP conducted during brain aneurysm surgery, the proper time of locating a temporary clip is still a matter of question. The time of releasing vascular occlusions for restarting brain blood circulation varies from person to person and depends on arterial collateral arteries.
There is a common agreement on temporary vascular obstruction in aneurysm surgery for short and repeated clipping periods that leads to a higher level of safety and lower degrees of risks for a neurological defect after surgery, compared to long periods of blockage, but no deterministic data can verify this common agreement e.g., Christopher Wallace, the author of Youmans’ book, uses a technique routinely in microsurgical aneurysm surgery in which each temporary clipping lasts less than 3 minutes and there are at least 5 minutes as interval period between every 2 clippings.
2. Methods and Materials/Patients
This study was conducted as a clinical trial for candidate patients of anterior cerebral circulation aneurysm surgery during 2017-2018 in Rasul Akram Hospital, Tehran, Iran. In order to investigate the anatomical status of aneurysm and collateral arteries, medical imaging including brain angiography or CT scan angiography, and MR angiography was performed for all patients. SSEP monitoring was performed related to the median nerve in the contralateral wrist to examine the Middle Cerebral Artery (MCA) and posterior tibialis nerve in the contralateral ankle to examine the Anterior Cerebral Artery (ACA) during the surgery procedure. Incentive parameters with a power of 5 to 25 milliampere, corresponding duration of 0.2 milliseconds, and waves with a frequency of 3.3 Hz were registered. Before locating temporary clips, SSEP was extracted as a baseline from every patient and then recorded. Temporary clips were performed for each patient during dissection of the aneurysm 3 times on average and each clip was released after at most 3 minutes or in case of any significant change in monitoring. SSEP changes for any clip and at the end of any minute were registered in form of latency percentage increase and amplitude percentage decrease compared to baseline. After locating the permanent clip and at the end of the surgery, the final SSEP was registered to compare with any changes over the baseline. Critical significant SSEP changes were defined as an amplitude reduction of the cerebral evoked potential of greater than 50%, latency delay of greater than 10%.
All patients were admitted to ICU after surgery and were evaluated for any neurological deficit. Brain CT scan was immediately performed after surgery for all patients and control CT scans were done to identify delayed ischemic changes. Besides, delayed CT scan was performed as a 2-month follow-up plan.
3. Results
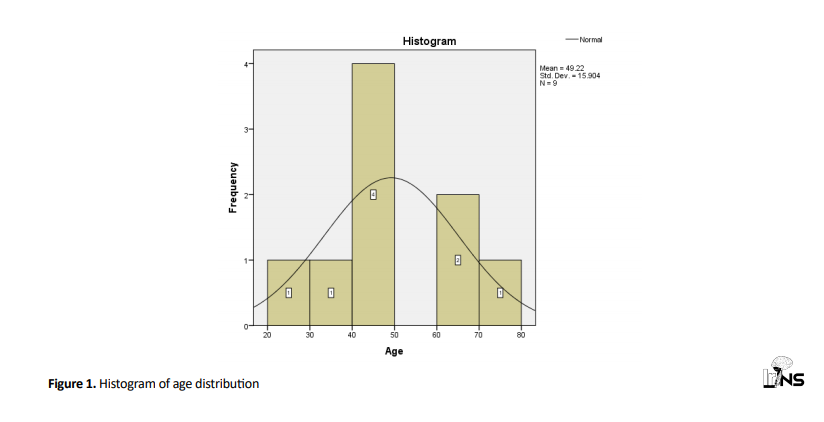
Totally 9 patients (aneurysm) were studied. Three of them were men and 6 patients were women. Patients’ age ranged from 39 to 78 years (Figure 1).
The clinical status of patients was assessed by the Hunt-Hess scale. Five cases were grade 1, 2 cases classified as grade 2, and 2 cases were in the grade 3 group. Among 9 aneurysms, 7 cases were about A.Com artery and 2 cases were in connection with MCA artery, having a size of 5 to 11 millimeters. Friedman test was applied to explore average latency change percentage in 1st, 2nd, and 3rd minutes, where results suggested that average latency change percentage in 1st, 2nd, and 3rd minutes were significantly different for 1st clips (P=0.050) (Figure 2).

For the 2nd temporary clips, the average latency change percentage in the 1st, 2nd, and 3rd minutes were analyzed. According to the results, the 2nd temporary clip was not significantly different for minutes 1, 2, and 3 (P=0.607) (Figure 3).

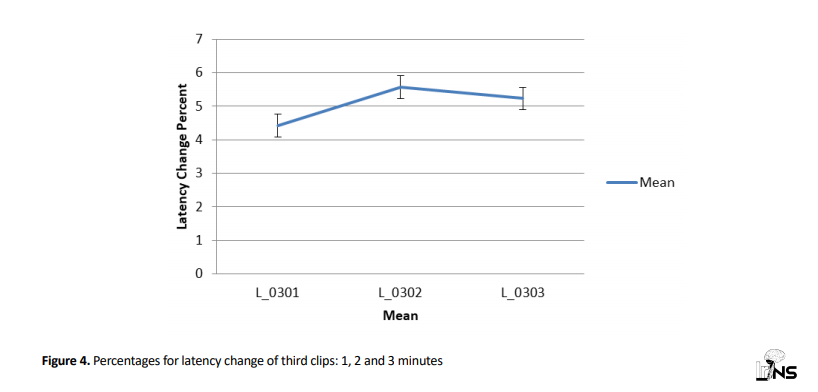
Moreover, the mean latency change percentage for 3rd clips in minutes 1, 2 and 3, suggested that mean values in minute 3 were not significantly different (P=0.641) (Figure 4).
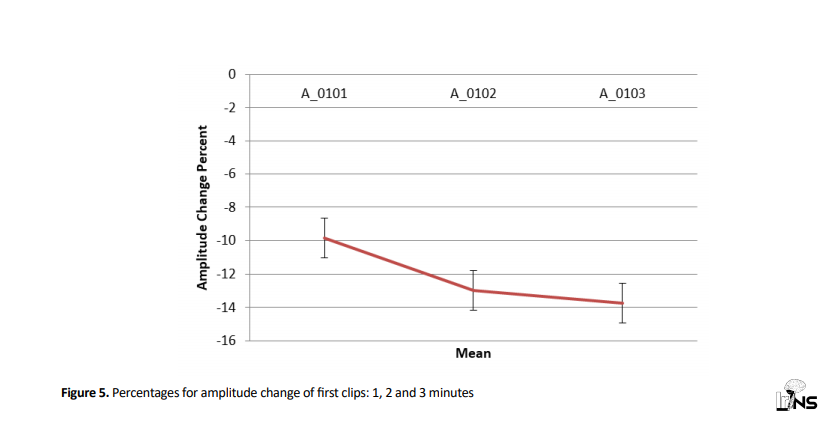
Employing Friedman test, amplitude change percentage for 1st clips in 1, 2, and 3 minutes were studied and results revealed that there was no significant difference in mean amplitude change percentage for 1st clips in 1, 2, and 3 minutes (P=0.276) (Figure 5).
Mean amplitude change percentages for 2nd clips in 1, 2, and 3 minutes were studied and results revealed that there was no significant difference for mean latency change percentages for 2nd clips in 1, 2, and 3 minutes (P=0.417) (Figure 6).

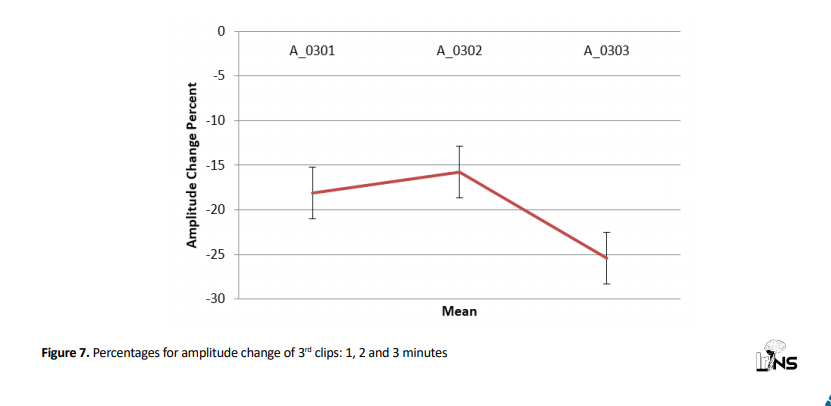
Analyzing average amplitude change in 3rd clips revealed that there was no significant difference between mean amplitude change percentages of 3rd clips in 1, 2, and 3 minutes (P=0.490) (Figure 7).
Finally, after surgery for all 9 patients, SSEP changes (as percentage values), the rates of decrease in amplitude percentage compared to the baseline value, were respectively 23%, 19%, 30%, 14%, and 10% for the rest of the cases. Post-op neurological deficit was not reported for any patient. In post-op delayed brain CT scans, there was no case of cortical or underlying tissue infarction in the territory of the clipped artery. Of all 9 patients, 1 case had delayed infraction in contralateral nucleus basalis, 1 case had infraction in the contralateral head of the caudate nucleus and 1 case of unilateral carotid infraction was observed.
4. Discussion
A large number of studies have been devoted to neuromonitoring in brain aneurysm surgery all over the world, but no study was done in Iran. In a study in Pittsburgh of USA (2016), 14 articles with a sample size of 2015 people were examined. The advantages and values of SSEP monitoring for predicting outcomes of surgeries conducted on aneurysm patients were discussed and it was revealed that this method had a high specificity of 84.5% and in patients with post-op neurological deficit, SSEP changes were 7 times more [14]. In another study in Korea (2016), 1208 patients who were under microsurgical clipping were studied. It was revealed that ischemic complications were 0.9% for patients who were under SSEP monitoring and 5.6% for those who were not monitored [15]. Another research in the USA (2012) studied 691 patients for whom clipping aneurysm surgery was done. Findings represented that SSEP monitoring values were more for predicting post-operative stroke in unruptured compared with ruptured aneurysms [16]. In all studies relating to the efficiency of SSEP monitoring in aneurysm clipping surgery, surgeons released the temporary clips just in presence of significant changes in neuromonitoring, and passing the time of clipping does not matter. Since the reliability of this method has not been approved for us and has never been registered in any neurosurgical reference, we ultimately released temporary clips in 3 minutes to reduce the risk of injury. In this period, we did not find any significant change in SSEP at all. According to statistical results of SSEP changes, either at amplitude reduction or latency increase, we finally reached the half amount threshold for significant changes (around -25% for amplitude and +5% for latency). It seems that the predictive value of ischemic injury for both factors are similar and we cannot prefer one to the others.
Although the insufficient number of studies may have caused a lack of statistically significant difference, results still have clinically significant differences and more studies need to be performed. Since all patients showed most SSEP changes in the 3rd minute of temporary clipping, we can conclude that using the temporary clips multiple times leads to better outcomes against increasing the time of vascular clipping and reduces the probability of ischemic injuries.
In this study, we attempted to gain stochastic time of significant changes in SSEP, which was unfortunately impossible despite using statistical formulas. SSEP monitoring limits to exploring functional somatosensory paths and sensory cortex and is not able to recognize ischemia in the internal capsule and neuronal pathway of speech, therefore neuromonitoring may seem normal in ischemic injuries in this area. It is advised to use direct cerebral oximetry with intraparenchymal brain probe with continuous monitoring of partial Oxygen Pressure of Brain Tissue (PBTO2).
5. Conclusion
According to the results of this study and comparing with similar researches in literature, neuromonitoring can be used as an index for examining tissue perfusion level of the brain and help to prevent accidental ischemic injuries of the brain followed by temporary clipping.
Ethical Considerations
Compliance with ethical guidelines
This trial was registered on the Iranian Website For Clinical Trials Registry as http://www.irct.ir:IRCT: 20171124037606N2.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Alireza Tabibkhooei, Maziar Azar and Asghar Aslaninia; Data collection: Alireza Tabibkhooei, Siamak Alizadeh and Asgahr Aslaninia; Data analysis and interpretation: Asghar Alaninia and Alireza Tabibkhooei; Drafting the article: Alireza Tabibkhooei and Asghar Aslaninia; Critically revising the article: Alireza Tabibkhooei and Asghar Aslaninia; Reviewing submitted version of manuscript: Alireza Tabibkhooei and Asghar Aslaninia; Approving the final version of the manuscript: Alireza Tabibkhooei, Maziar Azar and Asghar Aslaninia.
Conflict of interest
The authors declared no conflict of interest.
Different strategies can be applied to provide cytoprotection during cerebrovascular surgery. The most rational and direct approach to reduce ischemia is probably limiting the ischemic length. Temporary arterial obstruction for CEA (Carotid Endarterectomy) and aneurysm dissection, as well as permanent clipping, are now known as common and standard methods in surgeries. However, maintaining optimal obstruction time by emphasizing on anatomic level of obstruction and artery blockage area, plays an important role. Duration of focal brain ischemia which can safely be tracked (without any clinical consequence), varies for different situations and mainly depends on the vascular area [1-5]. Cerebrovascular brain incidents especially brain aneurysm rupture are known as a major cause of death and disability [6]. Despite all progressions, in emergency divisions, brain aneurysm is an important cause of demise and disability in Iran as well as other countries, which mostly leads to severe effects [7].
Treatment of intracranial aneurysms is progressing and several technical innovations have taken place especially in the recent 2 decades. Improvements in morbidity and mortality rate have apparently been achieved through better diagnosis, rapid recovery of ruptured aneurysm, and developments in managing clinical methods [8-10]. Using temporary clips is a standard method for brain aneurysm surgery and corresponding rupture or preventing aneurysm rupture during dissection [11]. However, employing temporary clips elevates the risk of hypoperfusion in the territory of arteries, and the resulting additional post-op deficit is still considered a terrible complication.
Neuromonitoring technique can theoretically be utilized during surgery to alarm for brain hypoperfusion and reduce these kinds of complications. Monitoring Somatosensory Evoked Potential (SSEP) and corresponding changes are used to identify cerebral ischemia and help to predict neuronal injuries during using temporary clips in brain aneurysm surgeries [12]. This approach is limited to integrated performance evaluation for somatosensory and cortex paths [13]. Motor Evoked Potential (MEP) monitoring provides more information about subcortical structures, motor cortex, and related paths and shows more sensitivity for recognizing insufficient bloodstream in MCA (Middle Cerebral Artery) and anterior choroidal artery, compared with SSEP monitoring. MEP monitoring may be restricted because of any interruption caused by patients’ head moving during surgery. There is a strong correlation between the decrease of brain bloodstream with the decrease of amplitude and increase of latency in SSEP. In spite of the many existing reports on SSEP conducted during brain aneurysm surgery, the proper time of locating a temporary clip is still a matter of question. The time of releasing vascular occlusions for restarting brain blood circulation varies from person to person and depends on arterial collateral arteries.
There is a common agreement on temporary vascular obstruction in aneurysm surgery for short and repeated clipping periods that leads to a higher level of safety and lower degrees of risks for a neurological defect after surgery, compared to long periods of blockage, but no deterministic data can verify this common agreement e.g., Christopher Wallace, the author of Youmans’ book, uses a technique routinely in microsurgical aneurysm surgery in which each temporary clipping lasts less than 3 minutes and there are at least 5 minutes as interval period between every 2 clippings.
2. Methods and Materials/Patients
This study was conducted as a clinical trial for candidate patients of anterior cerebral circulation aneurysm surgery during 2017-2018 in Rasul Akram Hospital, Tehran, Iran. In order to investigate the anatomical status of aneurysm and collateral arteries, medical imaging including brain angiography or CT scan angiography, and MR angiography was performed for all patients. SSEP monitoring was performed related to the median nerve in the contralateral wrist to examine the Middle Cerebral Artery (MCA) and posterior tibialis nerve in the contralateral ankle to examine the Anterior Cerebral Artery (ACA) during the surgery procedure. Incentive parameters with a power of 5 to 25 milliampere, corresponding duration of 0.2 milliseconds, and waves with a frequency of 3.3 Hz were registered. Before locating temporary clips, SSEP was extracted as a baseline from every patient and then recorded. Temporary clips were performed for each patient during dissection of the aneurysm 3 times on average and each clip was released after at most 3 minutes or in case of any significant change in monitoring. SSEP changes for any clip and at the end of any minute were registered in form of latency percentage increase and amplitude percentage decrease compared to baseline. After locating the permanent clip and at the end of the surgery, the final SSEP was registered to compare with any changes over the baseline. Critical significant SSEP changes were defined as an amplitude reduction of the cerebral evoked potential of greater than 50%, latency delay of greater than 10%.
All patients were admitted to ICU after surgery and were evaluated for any neurological deficit. Brain CT scan was immediately performed after surgery for all patients and control CT scans were done to identify delayed ischemic changes. Besides, delayed CT scan was performed as a 2-month follow-up plan.
3. Results
Totally 9 patients (aneurysm) were studied. Three of them were men and 6 patients were women. Patients’ age ranged from 39 to 78 years (Figure 1).

The clinical status of patients was assessed by the Hunt-Hess scale. Five cases were grade 1, 2 cases classified as grade 2, and 2 cases were in the grade 3 group. Among 9 aneurysms, 7 cases were about A.Com artery and 2 cases were in connection with MCA artery, having a size of 5 to 11 millimeters. Friedman test was applied to explore average latency change percentage in 1st, 2nd, and 3rd minutes, where results suggested that average latency change percentage in 1st, 2nd, and 3rd minutes were significantly different for 1st clips (P=0.050) (Figure 2).

For the 2nd temporary clips, the average latency change percentage in the 1st, 2nd, and 3rd minutes were analyzed. According to the results, the 2nd temporary clip was not significantly different for minutes 1, 2, and 3 (P=0.607) (Figure 3).

Moreover, the mean latency change percentage for 3rd clips in minutes 1, 2 and 3, suggested that mean values in minute 3 were not significantly different (P=0.641) (Figure 4).

Employing Friedman test, amplitude change percentage for 1st clips in 1, 2, and 3 minutes were studied and results revealed that there was no significant difference in mean amplitude change percentage for 1st clips in 1, 2, and 3 minutes (P=0.276) (Figure 5).

Mean amplitude change percentages for 2nd clips in 1, 2, and 3 minutes were studied and results revealed that there was no significant difference for mean latency change percentages for 2nd clips in 1, 2, and 3 minutes (P=0.417) (Figure 6).

Analyzing average amplitude change in 3rd clips revealed that there was no significant difference between mean amplitude change percentages of 3rd clips in 1, 2, and 3 minutes (P=0.490) (Figure 7).

Finally, after surgery for all 9 patients, SSEP changes (as percentage values), the rates of decrease in amplitude percentage compared to the baseline value, were respectively 23%, 19%, 30%, 14%, and 10% for the rest of the cases. Post-op neurological deficit was not reported for any patient. In post-op delayed brain CT scans, there was no case of cortical or underlying tissue infarction in the territory of the clipped artery. Of all 9 patients, 1 case had delayed infraction in contralateral nucleus basalis, 1 case had infraction in the contralateral head of the caudate nucleus and 1 case of unilateral carotid infraction was observed.
4. Discussion
A large number of studies have been devoted to neuromonitoring in brain aneurysm surgery all over the world, but no study was done in Iran. In a study in Pittsburgh of USA (2016), 14 articles with a sample size of 2015 people were examined. The advantages and values of SSEP monitoring for predicting outcomes of surgeries conducted on aneurysm patients were discussed and it was revealed that this method had a high specificity of 84.5% and in patients with post-op neurological deficit, SSEP changes were 7 times more [14]. In another study in Korea (2016), 1208 patients who were under microsurgical clipping were studied. It was revealed that ischemic complications were 0.9% for patients who were under SSEP monitoring and 5.6% for those who were not monitored [15]. Another research in the USA (2012) studied 691 patients for whom clipping aneurysm surgery was done. Findings represented that SSEP monitoring values were more for predicting post-operative stroke in unruptured compared with ruptured aneurysms [16]. In all studies relating to the efficiency of SSEP monitoring in aneurysm clipping surgery, surgeons released the temporary clips just in presence of significant changes in neuromonitoring, and passing the time of clipping does not matter. Since the reliability of this method has not been approved for us and has never been registered in any neurosurgical reference, we ultimately released temporary clips in 3 minutes to reduce the risk of injury. In this period, we did not find any significant change in SSEP at all. According to statistical results of SSEP changes, either at amplitude reduction or latency increase, we finally reached the half amount threshold for significant changes (around -25% for amplitude and +5% for latency). It seems that the predictive value of ischemic injury for both factors are similar and we cannot prefer one to the others.
Although the insufficient number of studies may have caused a lack of statistically significant difference, results still have clinically significant differences and more studies need to be performed. Since all patients showed most SSEP changes in the 3rd minute of temporary clipping, we can conclude that using the temporary clips multiple times leads to better outcomes against increasing the time of vascular clipping and reduces the probability of ischemic injuries.
In this study, we attempted to gain stochastic time of significant changes in SSEP, which was unfortunately impossible despite using statistical formulas. SSEP monitoring limits to exploring functional somatosensory paths and sensory cortex and is not able to recognize ischemia in the internal capsule and neuronal pathway of speech, therefore neuromonitoring may seem normal in ischemic injuries in this area. It is advised to use direct cerebral oximetry with intraparenchymal brain probe with continuous monitoring of partial Oxygen Pressure of Brain Tissue (PBTO2).
5. Conclusion
According to the results of this study and comparing with similar researches in literature, neuromonitoring can be used as an index for examining tissue perfusion level of the brain and help to prevent accidental ischemic injuries of the brain followed by temporary clipping.
Ethical Considerations
Compliance with ethical guidelines
This trial was registered on the Iranian Website For Clinical Trials Registry as http://www.irct.ir:IRCT: 20171124037606N2.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Alireza Tabibkhooei, Maziar Azar and Asghar Aslaninia; Data collection: Alireza Tabibkhooei, Siamak Alizadeh and Asgahr Aslaninia; Data analysis and interpretation: Asghar Alaninia and Alireza Tabibkhooei; Drafting the article: Alireza Tabibkhooei and Asghar Aslaninia; Critically revising the article: Alireza Tabibkhooei and Asghar Aslaninia; Reviewing submitted version of manuscript: Alireza Tabibkhooei and Asghar Aslaninia; Approving the final version of the manuscript: Alireza Tabibkhooei, Maziar Azar and Asghar Aslaninia.
Conflict of interest
The authors declared no conflict of interest.
- mittent reperfusion during temporal focal ischemia. Journal of Neurosurgery. 1992; 77(6):911-6. [DOI:10.3171/jns.1992.77.6.0911] [PMID]
- Symon L. Management of giant intracranial aneurysms. Acta Neurochirurgica. 1992; 116(2-4):107-18. [DOI:10.3171/jns.1992.77.6.0911]
- Jabre A, Symon L. Temporary vascular occlusion during aneurysm surgery. Surgical Neurology. 1987; 27(1):47-62. [DOI:10.3171/jns.1992.77.6.0911]
- Buchthal A, Belopavlovic M, Mooij JJ. Evoked potential monitoring and temporary clipping in cerebral aneurysm surgery. Acta Neurochirurgica. 1988; 93(1-2):28-36. [DOI:10.1007/BF01409899] [PMID]
- Batjer H, Samson D. Intraoperative aneurysmal rupture: Incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986; 18(6):701-7. [DOI:10.1097/00006123-198606000-00004] [PMID]
- Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms: A prospective randomized study. Stroke. 2000; 31(10):2369-77. [DOI:10.1161/01.STR.31.10.2369] [PMID]
- Mashhadinejad H, Samini F, Faraji M, Mashhadinejad A. [Brain aneurysms and the results of their surgery in Mashhad Ghaem Hospital during 1998-2007 (Persian)]. Iranian Journal of Surgery. 2012; 19(4). https://www.sid.ir/en/journal/ViewPaper.aspx?id=262214
- Molyneux A, Kerr R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002; 360(9342):1267-74. [DOI:10.1016/S0140-6736(02)11314-6]
- Cesarini KG, Hårdemark HG, Persson L. Improved survival after aneurysmal subarachnoid hemorrhage: Review of case management during a 12- year period. Journal of Neurosurgery. 1999; 90(4):664-72. [DOI:10.3171/jns.1999.90.4.0664] [PMID]
- Wermer MJ, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ, et al. Follow-up screening after subarachnoid haemorrhage: Frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005; 128(Pt 10):2421-9. [DOI:10.1093/brain/awh587] [PMID]
- Schick U, Döhnert J, Meyer JJ, Vitzthum HE. Effects of temporary clips on somatosensory evoked potentials in aneurysm surgery. Neurocritical Care. 2005; 2(2):141-9. [DOI:10.1385/NCC:2:2:141]
- Kashkoush AI, Jankowitz BT, Gardner P, Friedlander RM, Chang YF, Crammond DJ, et al. Somatosensory evoked potentials during temporary arterial occlusion for intracranial aneurysm surgery: Predictive value for perioperative stroke. World Neurosurgery. 2017; 104:442-51. [DOI:10.1016/j.wneu.2017.05.036] [PMID]
- Buchthal A, Belopavlovic M. Somatosensory evoked potentials in cerebral aneurysm surgery. European Journal of Anaesthesiology. 1992; 9(6):493-7. [PMID]
- Thirumala PD, Udesh R, Muralidharan A, Thiagarajan K, Crammond DJ, Chang YF, et al. Diagnostic value of somatosensory-Evoked potential monitoring during cerebral aneurysm clipping : A systematic review. World Neurosurgery. 2016; 89:672-80. [DOI:10.1016/j.wneu.2015.12.008] [PMID]
- Byoun HS, Bang JS, Oh CW, Kwon OK, Hwang G, Han JH, et al. The incidence of and risk factors for ischemic complications after microsurgical clipping of unruptured middle cerebral artery aneurysms and the efficacy of intraoperative monitoring of somatosensory evoked potentials: A retrospective study. Clinical Neurology and Neurosurgery. 2016; 151:128-35. [DOI:10.1016/j.clineuro.2016.10.008] [PMID]
- Wicks RT, Pradilla G, Raza SM, Hadelsberg U, Coon AL, Huang J, et al. Impact of changes in intraoperative somatosensory evoked potentials on stroke rates after clipping of intracranial aneurysms. Neurosurgery. 2012; 70(5):1114-24. [DOI:10.1227/NEU.0b013e31823f5cf7] [PMID]
Type of Study: Clinical Trial |
Subject:
Vascular Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






