Thu, Dec 25, 2025
Volume 1, Issue 4 (3-2016)
Iran J Neurosurg 2016, 1(4): 15-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Thiam A B, Okome Mezui E D, Ndoye N, Thioub M, Badiane S B. Endoscopic Treatment of Colloid Cysts of Third Ventricle: Study of Three Cases. Iran J Neurosurg 2016; 1 (4) :15-19
URL: http://irjns.org/article-1-26-en.html
URL: http://irjns.org/article-1-26-en.html
Alioune Badara Thiam1 
 , Elyse Denise Okome Mezui2
, Elyse Denise Okome Mezui2 
 , Ndaraw Ndoye1
, Ndaraw Ndoye1 
 , Mbaye Thioub3
, Mbaye Thioub3 
 , Seydou Boubacar Badiane *4
, Seydou Boubacar Badiane *4 



 , Elyse Denise Okome Mezui2
, Elyse Denise Okome Mezui2 
 , Ndaraw Ndoye1
, Ndaraw Ndoye1 
 , Mbaye Thioub3
, Mbaye Thioub3 
 , Seydou Boubacar Badiane *4
, Seydou Boubacar Badiane *4 


1- MD, Assisstant Professor of Neurosurgery, Neurosurgery Clinic, Fann National University Hospital, Dakar, Senegal, West Africa
2- MD, Fellowship of Neurosurgery, University of Montreal, Chief Assistant of Neurosurgery, Universite of Health Sciences of Gabon, West Africa
3- MD, Chief Assistant of Neurosurgery, Neurosurgery Clinic, Fann National University Hospital, Dakar, Senegal, West Africa
4- MD, Professor of Neurosurgery, Head of Neurosurgery Clinic, Fann National University Hospital, Dakar, Senegal, West Africa
2- MD, Fellowship of Neurosurgery, University of Montreal, Chief Assistant of Neurosurgery, Universite of Health Sciences of Gabon, West Africa
3- MD, Chief Assistant of Neurosurgery, Neurosurgery Clinic, Fann National University Hospital, Dakar, Senegal, West Africa
4- MD, Professor of Neurosurgery, Head of Neurosurgery Clinic, Fann National University Hospital, Dakar, Senegal, West Africa
Full Text [PDF 599 kb]
(4237 Downloads)
| Abstract (HTML) (8307 Views)
Full Text: (4549 Views)
Introduction
Colloid cyst of the third ventricle is found in 0.5-1% of intracranial tumors (1-3), and in 15-20% of intra-ventricular lesions (4). These are slow-growing benign lesions of endodermal origin. They are usually located on the roof of the third ventricle, near the interventricular foramen, that can lead to obstruction. Thus, they are responsible for an acute obstructive hydrocephalus. In fact, the disease can lead to a sudden death (2,5,6) or be diagnosed accidentally on imaging. Traditionally, surgical treatment of cyst by direct approach was performed by transcallosal or transcortical-transventricular approach (1,7,8). Also, CSF shunts with a ventriculo-peritoneal shunt valve have been placed as an alternative to the direct approach of the cyst in some centers for a long time (9). To reduce the risk of morbidity and mortality related to microsurgery and bypass valves, less invasive techniques have been developed over the years, including the simple puncture, stereotactic aspiration, and in the recent past years, cyst endoscopic aspiration. Today, many neurosurgical practices have developed resection of colloid cysts endoscopically, whether assisted by a guidance system or not (1,10,11). In our department, neuro-endoscopy is a new technique, introduced since 2010, its indications have long been limited to ventriculocisternostomy ways in the management of hydrocephalus (12). Over the years, they have been expanded including biopsy and excision of intraventricular tumors. We reported our early experience with the endoscopic treatment of colloid cysts in three cases.
Case Presentation
We conducted a retrospective study from January 2012 to December 2014 in which the medical records of patients with a colloid cyst operated at the Neurosurgical Clinic Fann endoscopically were analyzed. We used endoscopy type Karl Storz kit with a neuroendoscope with 2.7 mm in diameter, rigid optic 30 °, monopolar coagulation catheter, a biopsy forceps, an aspiration micro-catheter and micro scissors.
Surgical Technique
All patients were operated in the same manner and by the same surgeon. Under general anesthesia, they were put in the supine position. Their head were raised to about 30 ° and fixed on a headrest machine with the tape. Cefuroxime-based prophylactic antibiotic was prescribed in induction time and continued for 48 hours postoperatively. After vertical cutaneous incision of about three cm, a trepan hole was made two cm anterior to the coronal suture and 12 cm to nasal bridge or root and 2.5 cm to midline. After ventricular catheterization, the neuroendoscope connected to the camera with cold light source was introduced into the lateral ventricle. The irrigation system was set up immediately. Intraventricular anatomical route was visualized through which we progressed ahead of choroid plexus to foramen of Monro. At this stage, the cyst obstructing foramen of Monro was identified. The cyst wall was carefully cauterized, allowing retraction. The small arterioles that irrigated the cyst were also coagulated in order to limit bleeding during resection of the cyst. Once the coagulation of the wall was made, we conducted a puncture of the cyst which allowed decreasing its volume as well as the intra-cystic pressure to facilitate its removal from the other parts, and assess the viscosity of its contents. We proceeded subsequently to an opening of the cyst wall with micro-scissors and the resection was carried out gradually by fragmentation using micro-scissors and grasping forceps. Residues of the capsule were coagulated. The irrigation with Ringer solution continued throughout the procedure to avoid contamination of ventricular cavities by the contents of the cyst. The endoscope was withdrawn after verifying haemostasis and a trepan hole was occluded by putting a piece of surgicel.
OUR COMMENTS
Observation 1
The patient aged 47 with no particular history, was admitted on August 8, 2012 due to chronic intermittent headaches and positional gaits, lasting for 20 months along with gradual progression and brief loss of consciousness. The physical examination showed normal condition. Brain MRI revealed an oval lesion in hyper signal T1 and hypo signal T2 with thin walls, located on the supero-anterior part of the third ventricle in contact with the inter ventricular foramina. It was measured to be 15 mm in the transverse axis, 16 mm in anteroposterior and 13 mm in height, suggesting a colloid cyst, with an active biventricular hydrocephalus and engagement of cerebellum amygdala (Figure 1). In the emergency surgery, the cyst content was yellowish and gelatinous. The duration of surgery was 100 minutes. Due to continued headaches, cerebral MRI was performed on the second postoperative day, showing cystic residue with dimension of 10 mm × 10 mm × 8 mm. Thus, the second surgery was performed 14 days after the first surgery. The postoperative recovery course was desirable and the patient’s symptoms disappeared. However, the patient had a transient amnesia, and was discharged on the second day after the second surgery.
Colloid cyst of the third ventricle is found in 0.5-1% of intracranial tumors (1-3), and in 15-20% of intra-ventricular lesions (4). These are slow-growing benign lesions of endodermal origin. They are usually located on the roof of the third ventricle, near the interventricular foramen, that can lead to obstruction. Thus, they are responsible for an acute obstructive hydrocephalus. In fact, the disease can lead to a sudden death (2,5,6) or be diagnosed accidentally on imaging. Traditionally, surgical treatment of cyst by direct approach was performed by transcallosal or transcortical-transventricular approach (1,7,8). Also, CSF shunts with a ventriculo-peritoneal shunt valve have been placed as an alternative to the direct approach of the cyst in some centers for a long time (9). To reduce the risk of morbidity and mortality related to microsurgery and bypass valves, less invasive techniques have been developed over the years, including the simple puncture, stereotactic aspiration, and in the recent past years, cyst endoscopic aspiration. Today, many neurosurgical practices have developed resection of colloid cysts endoscopically, whether assisted by a guidance system or not (1,10,11). In our department, neuro-endoscopy is a new technique, introduced since 2010, its indications have long been limited to ventriculocisternostomy ways in the management of hydrocephalus (12). Over the years, they have been expanded including biopsy and excision of intraventricular tumors. We reported our early experience with the endoscopic treatment of colloid cysts in three cases.
Case Presentation
We conducted a retrospective study from January 2012 to December 2014 in which the medical records of patients with a colloid cyst operated at the Neurosurgical Clinic Fann endoscopically were analyzed. We used endoscopy type Karl Storz kit with a neuroendoscope with 2.7 mm in diameter, rigid optic 30 °, monopolar coagulation catheter, a biopsy forceps, an aspiration micro-catheter and micro scissors.
Surgical Technique
All patients were operated in the same manner and by the same surgeon. Under general anesthesia, they were put in the supine position. Their head were raised to about 30 ° and fixed on a headrest machine with the tape. Cefuroxime-based prophylactic antibiotic was prescribed in induction time and continued for 48 hours postoperatively. After vertical cutaneous incision of about three cm, a trepan hole was made two cm anterior to the coronal suture and 12 cm to nasal bridge or root and 2.5 cm to midline. After ventricular catheterization, the neuroendoscope connected to the camera with cold light source was introduced into the lateral ventricle. The irrigation system was set up immediately. Intraventricular anatomical route was visualized through which we progressed ahead of choroid plexus to foramen of Monro. At this stage, the cyst obstructing foramen of Monro was identified. The cyst wall was carefully cauterized, allowing retraction. The small arterioles that irrigated the cyst were also coagulated in order to limit bleeding during resection of the cyst. Once the coagulation of the wall was made, we conducted a puncture of the cyst which allowed decreasing its volume as well as the intra-cystic pressure to facilitate its removal from the other parts, and assess the viscosity of its contents. We proceeded subsequently to an opening of the cyst wall with micro-scissors and the resection was carried out gradually by fragmentation using micro-scissors and grasping forceps. Residues of the capsule were coagulated. The irrigation with Ringer solution continued throughout the procedure to avoid contamination of ventricular cavities by the contents of the cyst. The endoscope was withdrawn after verifying haemostasis and a trepan hole was occluded by putting a piece of surgicel.
OUR COMMENTS
Observation 1
The patient aged 47 with no particular history, was admitted on August 8, 2012 due to chronic intermittent headaches and positional gaits, lasting for 20 months along with gradual progression and brief loss of consciousness. The physical examination showed normal condition. Brain MRI revealed an oval lesion in hyper signal T1 and hypo signal T2 with thin walls, located on the supero-anterior part of the third ventricle in contact with the inter ventricular foramina. It was measured to be 15 mm in the transverse axis, 16 mm in anteroposterior and 13 mm in height, suggesting a colloid cyst, with an active biventricular hydrocephalus and engagement of cerebellum amygdala (Figure 1). In the emergency surgery, the cyst content was yellowish and gelatinous. The duration of surgery was 100 minutes. Due to continued headaches, cerebral MRI was performed on the second postoperative day, showing cystic residue with dimension of 10 mm × 10 mm × 8 mm. Thus, the second surgery was performed 14 days after the first surgery. The postoperative recovery course was desirable and the patient’s symptoms disappeared. However, the patient had a transient amnesia, and was discharged on the second day after the second surgery.
Observation 2
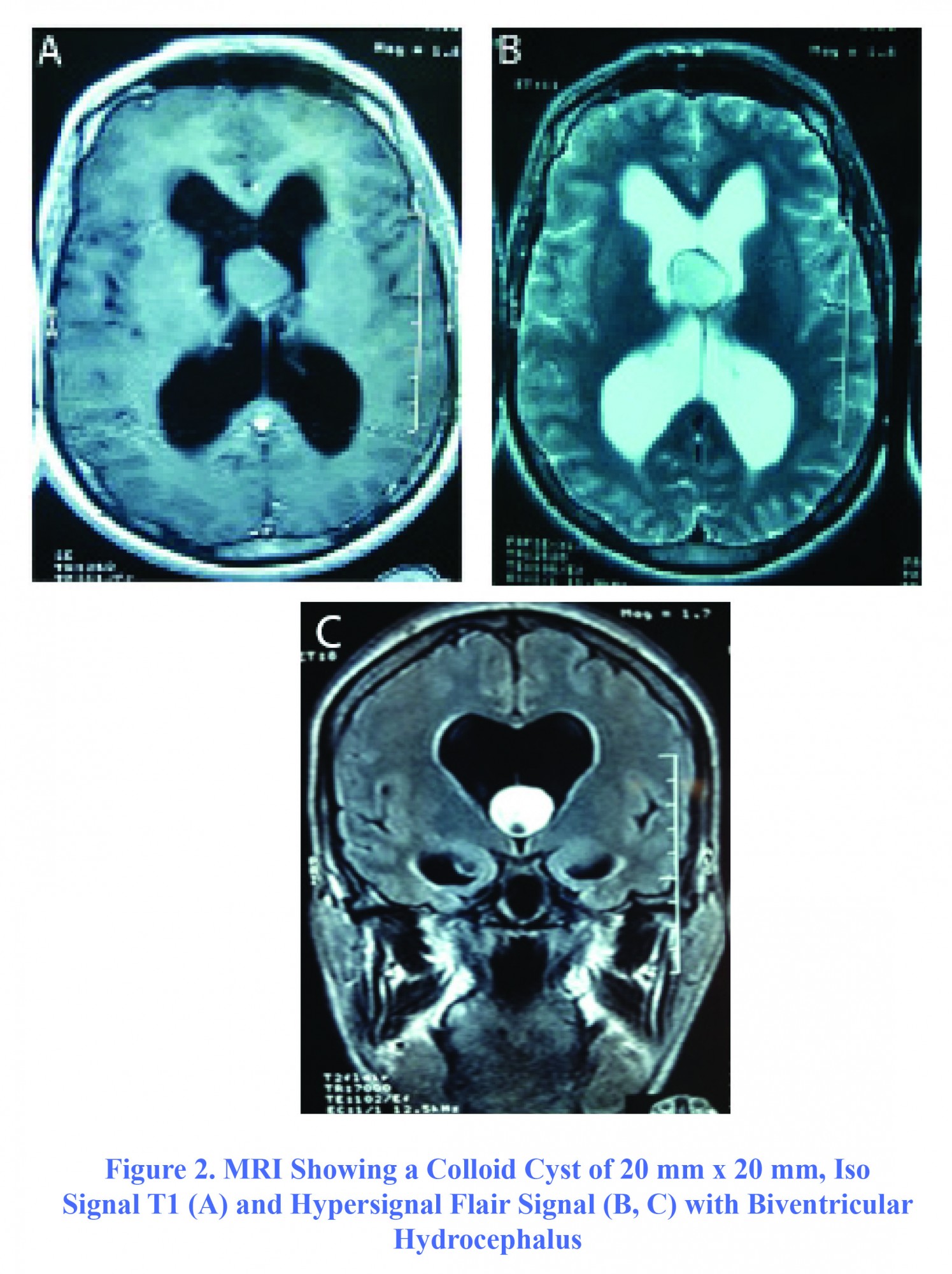
The second case, aged 35, was admitted to the ward on November 10, 2012 showing severe intracranial hypertension involving acute intense headache, vomiting, decreased bilateral visual acuity and cognitive disorder. All signs and symptoms were manifested for three days in a context of repeated seizure crisis in a patient with seizure history for 20 years. In the examination, the patient showed sleepiness with a GCS of 13/15 (E4V3M6), decreased bilateral visual acuity with bilateral papilledema on fundus. Brain MRI revealed a colloid cyst of third ventricle with a dimension of 20 mm × 20 mm hyper signal in T2 and flair and discreetly T1 hyper signal not enhanced by gadolinium, responsible for an active biventricular hydrocephalus (Figure 2). The surgery was performed urgently after admission. The content of the cyst was gelatinous. After unblocking the foramen of Monro, a ventriculocisternostomy was also performed. The duration of surgery was 80 minutes. The recovery was remarkable and symptoms were disappeared completely one day postoperatively. On the next day, the patient developed hyperthermia at 39° C, after which CSF analysis showed aseptic meningitis. Therefore, he received ceftriaxone. The outcome was favorable and the patient was discharged on the 13th day postoperatively. He was regularly monitored as outpatients, and his condition was good.
The second case, aged 35, was admitted to the ward on November 10, 2012 showing severe intracranial hypertension involving acute intense headache, vomiting, decreased bilateral visual acuity and cognitive disorder. All signs and symptoms were manifested for three days in a context of repeated seizure crisis in a patient with seizure history for 20 years. In the examination, the patient showed sleepiness with a GCS of 13/15 (E4V3M6), decreased bilateral visual acuity with bilateral papilledema on fundus. Brain MRI revealed a colloid cyst of third ventricle with a dimension of 20 mm × 20 mm hyper signal in T2 and flair and discreetly T1 hyper signal not enhanced by gadolinium, responsible for an active biventricular hydrocephalus (Figure 2). The surgery was performed urgently after admission. The content of the cyst was gelatinous. After unblocking the foramen of Monro, a ventriculocisternostomy was also performed. The duration of surgery was 80 minutes. The recovery was remarkable and symptoms were disappeared completely one day postoperatively. On the next day, the patient developed hyperthermia at 39° C, after which CSF analysis showed aseptic meningitis. Therefore, he received ceftriaxone. The outcome was favorable and the patient was discharged on the 13th day postoperatively. He was regularly monitored as outpatients, and his condition was good.
Observation 3
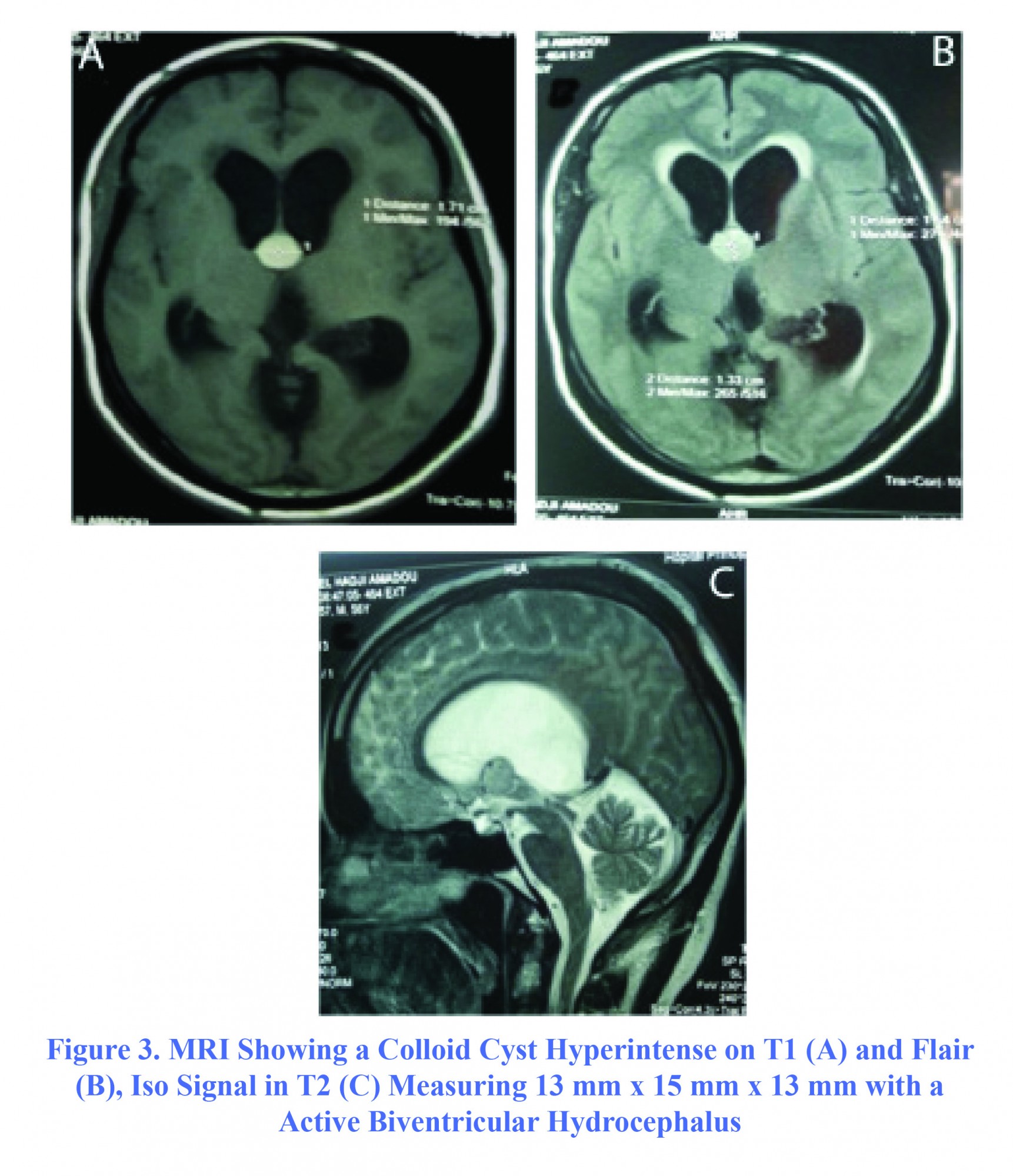
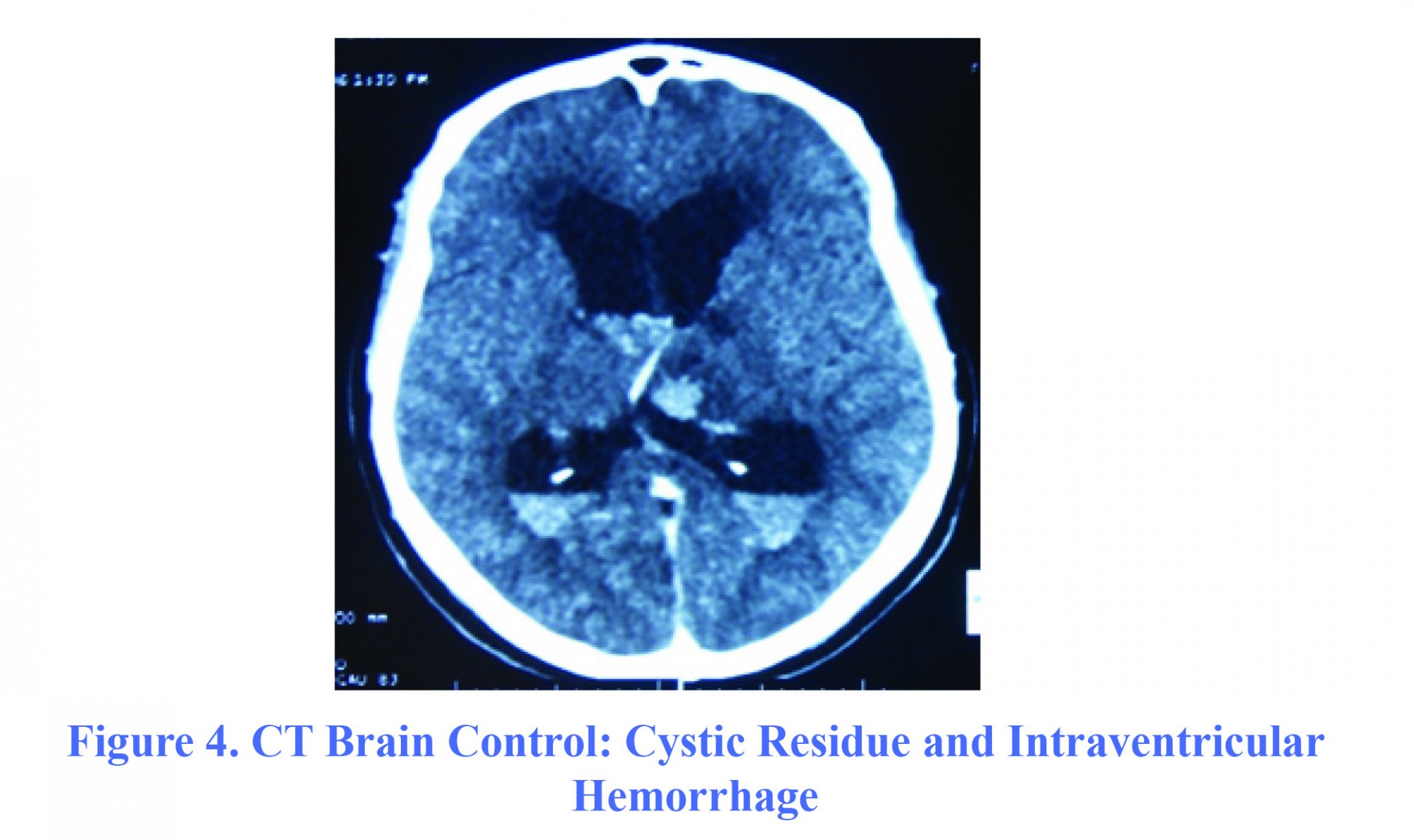
The patient, aged 53, with no particular disease, was admitted to the Neurosurgery ward on March 1, 2013 for progressive impairment of consciousness along with right hemicorporal motor weakness lasting for three weeks. On admission day, the patient had a GCS=6 (E2V1M3) with a cortical type of right pyramidal syndrome. Brain MRI showed a rounded mass in the third ventricle measuring 15 mm × 13 mm × 16 mm, in hypersignal T1 that was not enhanced after gadolinium injection, and was isosignal in T2. This mass was very close to pericallosal arteries, compressing the foramen of Monro and became the origin of active biventricular hydrocephalus (Figure 3). Thus, the operation was performed immediately. The content of the cyst was viscous and yellowish. During the procedure, the patient had a moderate intraventricular hemorrhage, and had been initially managed by abundant irrigation with Ringer's lactate solution until ventricular cavities become clear. The duration of surgery was 120 minutes. A gradual improvement in the patient’s consciousness with a GCS=10 was observed. A control cerebral scan showed a cystic residue associated with intra-ventricular hemorrhage (Figure 4). An external ventricular derivation (EVD) was placed on sixth day postoperatively, and neurological condition remained constant. The patient secondarily developed nosocomial bronchopneumopathy and a urinary infection caused by Acinetobacter. The patient died on 42nd day postoperatively due to septic shock in ICU.
The patient, aged 53, with no particular disease, was admitted to the Neurosurgery ward on March 1, 2013 for progressive impairment of consciousness along with right hemicorporal motor weakness lasting for three weeks. On admission day, the patient had a GCS=6 (E2V1M3) with a cortical type of right pyramidal syndrome. Brain MRI showed a rounded mass in the third ventricle measuring 15 mm × 13 mm × 16 mm, in hypersignal T1 that was not enhanced after gadolinium injection, and was isosignal in T2. This mass was very close to pericallosal arteries, compressing the foramen of Monro and became the origin of active biventricular hydrocephalus (Figure 3). Thus, the operation was performed immediately. The content of the cyst was viscous and yellowish. During the procedure, the patient had a moderate intraventricular hemorrhage, and had been initially managed by abundant irrigation with Ringer's lactate solution until ventricular cavities become clear. The duration of surgery was 120 minutes. A gradual improvement in the patient’s consciousness with a GCS=10 was observed. A control cerebral scan showed a cystic residue associated with intra-ventricular hemorrhage (Figure 4). An external ventricular derivation (EVD) was placed on sixth day postoperatively, and neurological condition remained constant. The patient secondarily developed nosocomial bronchopneumopathy and a urinary infection caused by Acinetobacter. The patient died on 42nd day postoperatively due to septic shock in ICU.
Clinical Data
The clinical manifestations of colloid cysts are nonspecific. However, the positional paroxysmal headache is the most common symptom and is rather specific (1-3,18). It is due to variation of the interventricular foramina obstruction by the cyst by a valve mechanism (15). Commonly, the disease is manifested as intracranial hypertension due to an acute obstructive hydrocephalus. Two patients showed intracranial hypertension with severe disturbance of consciousness (Glasgow score=6/15). Other clinical signs and symptoms were visual disturbances, decreased visual acuity, optic disc swelling, nausea, vomiting, dizziness, motor deficits and seizures. The risk of sudden death occurred as a complication of colloid cysts requiring surgical management of symptomatic cases and regular monitoring of cysts with a few symptoms or cases of accidental diagnosis. The other complication of colloid cyst is the intracystic hemorrhage. This is an extremely rare complication, which achieves an apoplexy of the cyst and abruptly increases the volume of the cyst and accounts for a rapid severe intracranial pressure (6,20).
Paraclinical Data
Neuroimaging including CT scan and MRI facilitates diagnosis. Usually there is a round lesion density or variable signal (3,18) associated with the viscosity and the concentration of cholesterol in cysts of varying sizes, sitting in the anterior superior part of the V3 that can be accompanied by hydrocephalus or not. The viscosity of the cyst can be analyzed initially preoperatively using proper imaging. It is known as predictive main factor in difficulty of endoscopic aspiration of the cyst (3,21). The hyperdense cyst is correlated with hyperviscosity (21). Thus, Carl El Khoury et al. in their study noted that 89% of hyperdense cysts on CT had difficulty in aspiration, while 31% of hypodense cysts underwent easy aspiration (3). The more the cyst became hyperdense, the more its aspiration was difficult. Carl El Khoury et al. in the same study showed that there was no correlation between the cyst signal to the brain MRI T1-weighted images and resection of the cyst difficulty. Conversely, if the cyst had a weak signal in T2-weighted sequence, resection of the cyst was difficult. Therefore, the lesions with a low signal on T2 will have difficult aspiration, reflecting a hyperviscosity of cystic content (3). All patients were diagnosed with a brain MRI. The lesion was hypersignal in T1 in all three patients, conversely, it was hyposignal in T2 in two patients, and it was observed that the lesion was hypersignal in T2 in one patient. Aspiration difficulty had not much difference in the cases. The limitation of small sample did not allow us to conclude the existence of a correlation between the perioperative signal of cyst and operation difficulty. The size of the cyst is variable up to five cm in diameter (3,16,18,22,23). In our study, the size of the cyst ranged from 13 mm to 20 mm at diagnosis time, and was associated with a biventricular active hydrocephalus due to inter-ventricular obstructive foramen in all three cases. Meanwhile, the researchers have shown that the size of the cyst influenced very little on the quality of its resection, and the easy removal of large cysts is related to the viscosity of the contents rather its size (2,3). Good aspiration of the cystic contents initially facilitates secondary resection of the capsule.
Therapeutic Aspects
The traditional treatment of colloid cysts has long been made by either transcallosal interhemispheric approach or transcortical transventricular one with or without a shunt in CSF. Before the introduction of the microscopic surgery, the postoperative mortality rate in these lesions ranged from 5.5% to 35%. From the advent of microsurgery and endoscopic surgery, the mortality rate significantly reduced to less than 2% (9). Micro-invasive techniques including endoscopic surgery are increasingly used for the treatment of colloid cysts. Although some studies have shown the limits of the endoscope, including the quality of excision of the cyst, the major interest remains in the reduction of the time and cost of the surgery (10,11) and mini-invasiveness (16) . In our study, the mean surgical time was 100 minutes with the extremes of 80-120 minutes. Our results were similar to those of Sribnick that found a mean of 82 minutes with the extremes of 43-212 minutes (14). The average mean duration of intervention was longer in the cohort of Wilson study (180.4 min), probably because of the technique developed by his team using a double instrumentation in order to achieve the fullest possible resection (22). The use of this technique allowed them to achieve a complete resection in 82% of cases. The duration of the intervention was generally reduced with the endoscopic technique unlike microsurgical resection (2,16). However, some factors may lengthen the endoscopic intervention, especially, an inexperienced surgeon, the occurrence of intraoperative complications such as intra- ventricular hemorrhage, requiring prolonged irrigation with a saline Ringer, and hyperviscosity of cyst requiring a much longer aspiration. Decq reported in his study that a case of endoscopic aspiration of the cyst required four hours of intervention on hyperviscosity of the cyst (23).
In our study, the surgical technique consisted of a coagulation of the cyst wall, followed by a content puncture aspiration and removal of the capsule by fragmentation using micro-scissors and grasping forceps that facilitated detachment of the capsule by small movements of traction and torsion exerted on the capsule. Ibanaz-Botella et al. described the same technique and devided it into four steps: coagulation, suction opening, separation and fragmentation (19). The choice of the first side is guided by the asymmetry of the ventricles, the lateralization of the cyst to a hole of Monro. All patients were discussed on the right side with the input site, point Kocher, because they all had a biventricular hydrocephalus. On the other hand, other researchers used a first anterolateral, located 7-8 cm behind the nasion and 5-7 cm from the midline. This allows a better first anterolateral approach angle of the attachment point of the colloid cyst V3 roof level, thus making easier dissection and thereby the complete excision of the cyst (2,14,22).
Several approaches were proposed to endoscopic resection of the cyst. Among them, the transventricular transforaminal approach is mostly used and it is the one we used in our study. The cyst was dealt with through the foramen of Monro. In the transventricular-transchoroidal approach, choroidal fissure is opened to access the V3. It is indicated for the case where the cyst is not sufficiently visible within the lateral ventricle with a small foramen of Monro. It is rarely described in the literature (19). During the surgery, some teams also performed the treatment of hydrocephalus that it depended on the case, by performing a ventriculostomy (VCS), a septostomy, a ventriculoperitoneal shunt or external ventricular shunt (2,18). In our study, we performed a preventive VCS after
The clinical manifestations of colloid cysts are nonspecific. However, the positional paroxysmal headache is the most common symptom and is rather specific (1-3,18). It is due to variation of the interventricular foramina obstruction by the cyst by a valve mechanism (15). Commonly, the disease is manifested as intracranial hypertension due to an acute obstructive hydrocephalus. Two patients showed intracranial hypertension with severe disturbance of consciousness (Glasgow score=6/15). Other clinical signs and symptoms were visual disturbances, decreased visual acuity, optic disc swelling, nausea, vomiting, dizziness, motor deficits and seizures. The risk of sudden death occurred as a complication of colloid cysts requiring surgical management of symptomatic cases and regular monitoring of cysts with a few symptoms or cases of accidental diagnosis. The other complication of colloid cyst is the intracystic hemorrhage. This is an extremely rare complication, which achieves an apoplexy of the cyst and abruptly increases the volume of the cyst and accounts for a rapid severe intracranial pressure (6,20).
Paraclinical Data
Neuroimaging including CT scan and MRI facilitates diagnosis. Usually there is a round lesion density or variable signal (3,18) associated with the viscosity and the concentration of cholesterol in cysts of varying sizes, sitting in the anterior superior part of the V3 that can be accompanied by hydrocephalus or not. The viscosity of the cyst can be analyzed initially preoperatively using proper imaging. It is known as predictive main factor in difficulty of endoscopic aspiration of the cyst (3,21). The hyperdense cyst is correlated with hyperviscosity (21). Thus, Carl El Khoury et al. in their study noted that 89% of hyperdense cysts on CT had difficulty in aspiration, while 31% of hypodense cysts underwent easy aspiration (3). The more the cyst became hyperdense, the more its aspiration was difficult. Carl El Khoury et al. in the same study showed that there was no correlation between the cyst signal to the brain MRI T1-weighted images and resection of the cyst difficulty. Conversely, if the cyst had a weak signal in T2-weighted sequence, resection of the cyst was difficult. Therefore, the lesions with a low signal on T2 will have difficult aspiration, reflecting a hyperviscosity of cystic content (3). All patients were diagnosed with a brain MRI. The lesion was hypersignal in T1 in all three patients, conversely, it was hyposignal in T2 in two patients, and it was observed that the lesion was hypersignal in T2 in one patient. Aspiration difficulty had not much difference in the cases. The limitation of small sample did not allow us to conclude the existence of a correlation between the perioperative signal of cyst and operation difficulty. The size of the cyst is variable up to five cm in diameter (3,16,18,22,23). In our study, the size of the cyst ranged from 13 mm to 20 mm at diagnosis time, and was associated with a biventricular active hydrocephalus due to inter-ventricular obstructive foramen in all three cases. Meanwhile, the researchers have shown that the size of the cyst influenced very little on the quality of its resection, and the easy removal of large cysts is related to the viscosity of the contents rather its size (2,3). Good aspiration of the cystic contents initially facilitates secondary resection of the capsule.
Therapeutic Aspects
The traditional treatment of colloid cysts has long been made by either transcallosal interhemispheric approach or transcortical transventricular one with or without a shunt in CSF. Before the introduction of the microscopic surgery, the postoperative mortality rate in these lesions ranged from 5.5% to 35%. From the advent of microsurgery and endoscopic surgery, the mortality rate significantly reduced to less than 2% (9). Micro-invasive techniques including endoscopic surgery are increasingly used for the treatment of colloid cysts. Although some studies have shown the limits of the endoscope, including the quality of excision of the cyst, the major interest remains in the reduction of the time and cost of the surgery (10,11) and mini-invasiveness (16) . In our study, the mean surgical time was 100 minutes with the extremes of 80-120 minutes. Our results were similar to those of Sribnick that found a mean of 82 minutes with the extremes of 43-212 minutes (14). The average mean duration of intervention was longer in the cohort of Wilson study (180.4 min), probably because of the technique developed by his team using a double instrumentation in order to achieve the fullest possible resection (22). The use of this technique allowed them to achieve a complete resection in 82% of cases. The duration of the intervention was generally reduced with the endoscopic technique unlike microsurgical resection (2,16). However, some factors may lengthen the endoscopic intervention, especially, an inexperienced surgeon, the occurrence of intraoperative complications such as intra- ventricular hemorrhage, requiring prolonged irrigation with a saline Ringer, and hyperviscosity of cyst requiring a much longer aspiration. Decq reported in his study that a case of endoscopic aspiration of the cyst required four hours of intervention on hyperviscosity of the cyst (23).
In our study, the surgical technique consisted of a coagulation of the cyst wall, followed by a content puncture aspiration and removal of the capsule by fragmentation using micro-scissors and grasping forceps that facilitated detachment of the capsule by small movements of traction and torsion exerted on the capsule. Ibanaz-Botella et al. described the same technique and devided it into four steps: coagulation, suction opening, separation and fragmentation (19). The choice of the first side is guided by the asymmetry of the ventricles, the lateralization of the cyst to a hole of Monro. All patients were discussed on the right side with the input site, point Kocher, because they all had a biventricular hydrocephalus. On the other hand, other researchers used a first anterolateral, located 7-8 cm behind the nasion and 5-7 cm from the midline. This allows a better first anterolateral approach angle of the attachment point of the colloid cyst V3 roof level, thus making easier dissection and thereby the complete excision of the cyst (2,14,22).
Several approaches were proposed to endoscopic resection of the cyst. Among them, the transventricular transforaminal approach is mostly used and it is the one we used in our study. The cyst was dealt with through the foramen of Monro. In the transventricular-transchoroidal approach, choroidal fissure is opened to access the V3. It is indicated for the case where the cyst is not sufficiently visible within the lateral ventricle with a small foramen of Monro. It is rarely described in the literature (19). During the surgery, some teams also performed the treatment of hydrocephalus that it depended on the case, by performing a ventriculostomy (VCS), a septostomy, a ventriculoperitoneal shunt or external ventricular shunt (2,18). In our study, we performed a preventive VCS after
excision of the cyst in one patient because its ventricles were very dilated with a significant trans-ependymal resorption. Ibanez in his study performed a VCS after resection of the cyst in two patients suspected of obstruction of the Sylvius aqueduct by a clot and conducted prophylactic septostomy in 11 patients who had univentricular hydrocephalus. The question about a VCS or additional septostomy resection of colloid cyst remains answerless. We believe that additional procedure as possible contribute to the rapid improvement of the clinical condition of the patient even though the excision of the cyst is contributing to the management of hydrocephalus. We observed a significant improvement of symptoms in our patient after 10 hours of surgery. This measure would also be beneficial in cases of partial resection, thereby preventing long-term cases of sudden death. External shunts are recommended in case of uncontrolled intraoperative bleeding or in the event of contamination of the ventricles by the debris and the contents to prevent cystic aseptic meningitis and obstruction of the ventricular cavities (1,2).
The incidents during surgery are mainly intraoperative bleeding when conducting the excision of the cyst (19). This happened in the case 3 in our study. We conducted an extensive irrigation intraoperatively, and an external ventricular shunt was put away after performing a control CT scan which showed an intra ventricular hemorrhage.
It would be advisable to perform coagulation of all arterioles supplying the cyst and its wall completely cauterized before making any attempt to puncture or excision. In some cases, the coagulation of the anterior septal vein was necessary without clinical complication (19). Mild bleeding is generally controlled by simply prolonged irrigation.
The quality of the resection can be judged by the neurosurgeon in situ and completed by postoperative control imaging. In all cases, the intraoperative resection was considered partial, and foramen of Monro permeability was realized as satisfactory, and a brain control scanner was performed in patient 3 after slow improvement of his consciousness postoperatively, and the patient 2 showed headache persistence by brain MRI scan. In both cases, these examinations have shown the existence of a residual cyst justifying further surgery only in the patient 2. Conversely, the patient 1 did not received a control imaging because of financial problems. Nevertheless, the clinical condition improved postoperatively. Endoscopic resection of colloid cyst was generally associated with a high rate cystic residue, and therefore a higher risk of recurrence compared to the microsurgical technique (10,16,24). Decq and Abdou reported an incomplete excision rate of 23% and 20%, respectively (10,23). Horn et al. observed higher levels, 48% (1). However, some have reported rates of complete or subtotal resection as 95.8-100% (19,24). The rate of complete resection is comparable to that found in a meta-analysis study involving microsurgical techniques, where the rate of complete resection was 96.8% (17). Endoscopic resection rates would be even better when it is coupled to the stereotaxy or neuronavigation (2). The quality of the endoscopic resection of cysts is variable from one study to another. In fact, it has been improved significantly in recent years, because the learning courses have already been held in some neurosurgical institutions, and also due to the improvement of neuroendoscopes and instruments (19).
The reoperation rate was also a function of the surgical technique. It was 0.38% and 3% for microsurgical and endoscopic techniques, respectively (22). One patient was re-operated due to the lack of clinical improvement after the first intervention and confirming the persistence of the cyst through control MRI. Boogarts reported a re-operation rate of 9.4% in his study (11), and Horn re-operated only two patients out of nine cases with residual cyst in his study (1). Despite a high rate of residual cyst observed, necessary re-operation rate remains low.
The postoperative complications in our study were as follows: one case showed intra-ventricular hemorrhage and meningitis, and one case suffered from memory disorders. These were the most frequently encountered complications in the endoscopic treatment of colloid cysts. The complication rate among patients undergoing endoscopy was generally lower than that in patients operated by microsurgery, with 10.5% against 16.3%, respectively. This was an advantage in the choice of endoscopic surgery. However, there was a higher rate of intra-ventricular hemorrhage with the endoscopy (2.8%), so that Sheikh suggested that it may require an external ventricular shunt. According to the comparative study, there was not a statistically significant difference between the two patient groups. Complication rate in patients undergoing microsurgery and endoscopy was 1.4% and 0.6%, respectively (17). One advantage of neuroendoscopy was to reduce the length of hospitalization (16). In our study, it was 13 and 17 days for the case 2 and 1, respectively. This difference was related to complications that occurred in these patients. However, two days after the second surgery, the case 1 was able to leave the hospital. The overall recurrence rate in endoscopic surgery varies 5-10% (16). The time to onset of recurrence has not been well defined yet, because it can be early or late. Some report a period of 10-15 years after surgery (2).
Due to a sudden death risk, a long-term radiological monitoring was necessary, not only to asymptomatic patients but also for patients whose excision was partial. This monitoring failed in our two survivor patients. Because of financial problems, they were not able to achieve remote interventional MRI, and because they lived in a neighboring country, we contacted them by phone. Ruth E and Coll recommended performing a brain MRI in the first month of the first, fifth and tenth years (2). Horn proposed to conduct MRI once in every three months after surgery, if the lesion was stable for four years, an MRI may be performed in sixth and 12th months and once in every two years (2).
Conclusion
The endoscopic treatment of colloid cysts is becoming widespread in neurosurgical practice because it is a reliable technique that offers many advantages (reduced surgery time, shorter hospital stay and also reduction in morbidity and mortality compared to microsurgery). In our practice, it is an up-to-date approach, and preliminary results are encouraging, thus, it is a promising technique for the treatment of colloid cysts in our department. However, education would be necessary, which remains an important factor in improving the quality of the endoscopic removal of cysts. The technique is associated with a significant recurrence rate, which may sometimes require re-interventions. Our greatest difficulty was the lack of long-term radiological monitoring of these patients, because the risk of sudden death should always be considered and long-term monitoring of patients is recommended.
Funding
None.
Conflicts of Interest
The authors have no conflicts of interest.
References
The incidents during surgery are mainly intraoperative bleeding when conducting the excision of the cyst (19). This happened in the case 3 in our study. We conducted an extensive irrigation intraoperatively, and an external ventricular shunt was put away after performing a control CT scan which showed an intra ventricular hemorrhage.
It would be advisable to perform coagulation of all arterioles supplying the cyst and its wall completely cauterized before making any attempt to puncture or excision. In some cases, the coagulation of the anterior septal vein was necessary without clinical complication (19). Mild bleeding is generally controlled by simply prolonged irrigation.
The quality of the resection can be judged by the neurosurgeon in situ and completed by postoperative control imaging. In all cases, the intraoperative resection was considered partial, and foramen of Monro permeability was realized as satisfactory, and a brain control scanner was performed in patient 3 after slow improvement of his consciousness postoperatively, and the patient 2 showed headache persistence by brain MRI scan. In both cases, these examinations have shown the existence of a residual cyst justifying further surgery only in the patient 2. Conversely, the patient 1 did not received a control imaging because of financial problems. Nevertheless, the clinical condition improved postoperatively. Endoscopic resection of colloid cyst was generally associated with a high rate cystic residue, and therefore a higher risk of recurrence compared to the microsurgical technique (10,16,24). Decq and Abdou reported an incomplete excision rate of 23% and 20%, respectively (10,23). Horn et al. observed higher levels, 48% (1). However, some have reported rates of complete or subtotal resection as 95.8-100% (19,24). The rate of complete resection is comparable to that found in a meta-analysis study involving microsurgical techniques, where the rate of complete resection was 96.8% (17). Endoscopic resection rates would be even better when it is coupled to the stereotaxy or neuronavigation (2). The quality of the endoscopic resection of cysts is variable from one study to another. In fact, it has been improved significantly in recent years, because the learning courses have already been held in some neurosurgical institutions, and also due to the improvement of neuroendoscopes and instruments (19).
The reoperation rate was also a function of the surgical technique. It was 0.38% and 3% for microsurgical and endoscopic techniques, respectively (22). One patient was re-operated due to the lack of clinical improvement after the first intervention and confirming the persistence of the cyst through control MRI. Boogarts reported a re-operation rate of 9.4% in his study (11), and Horn re-operated only two patients out of nine cases with residual cyst in his study (1). Despite a high rate of residual cyst observed, necessary re-operation rate remains low.
The postoperative complications in our study were as follows: one case showed intra-ventricular hemorrhage and meningitis, and one case suffered from memory disorders. These were the most frequently encountered complications in the endoscopic treatment of colloid cysts. The complication rate among patients undergoing endoscopy was generally lower than that in patients operated by microsurgery, with 10.5% against 16.3%, respectively. This was an advantage in the choice of endoscopic surgery. However, there was a higher rate of intra-ventricular hemorrhage with the endoscopy (2.8%), so that Sheikh suggested that it may require an external ventricular shunt. According to the comparative study, there was not a statistically significant difference between the two patient groups. Complication rate in patients undergoing microsurgery and endoscopy was 1.4% and 0.6%, respectively (17). One advantage of neuroendoscopy was to reduce the length of hospitalization (16). In our study, it was 13 and 17 days for the case 2 and 1, respectively. This difference was related to complications that occurred in these patients. However, two days after the second surgery, the case 1 was able to leave the hospital. The overall recurrence rate in endoscopic surgery varies 5-10% (16). The time to onset of recurrence has not been well defined yet, because it can be early or late. Some report a period of 10-15 years after surgery (2).
Due to a sudden death risk, a long-term radiological monitoring was necessary, not only to asymptomatic patients but also for patients whose excision was partial. This monitoring failed in our two survivor patients. Because of financial problems, they were not able to achieve remote interventional MRI, and because they lived in a neighboring country, we contacted them by phone. Ruth E and Coll recommended performing a brain MRI in the first month of the first, fifth and tenth years (2). Horn proposed to conduct MRI once in every three months after surgery, if the lesion was stable for four years, an MRI may be performed in sixth and 12th months and once in every two years (2).
Conclusion
The endoscopic treatment of colloid cysts is becoming widespread in neurosurgical practice because it is a reliable technique that offers many advantages (reduced surgery time, shorter hospital stay and also reduction in morbidity and mortality compared to microsurgery). In our practice, it is an up-to-date approach, and preliminary results are encouraging, thus, it is a promising technique for the treatment of colloid cysts in our department. However, education would be necessary, which remains an important factor in improving the quality of the endoscopic removal of cysts. The technique is associated with a significant recurrence rate, which may sometimes require re-interventions. Our greatest difficulty was the lack of long-term radiological monitoring of these patients, because the risk of sudden death should always be considered and long-term monitoring of patients is recommended.
Funding
None.
Conflicts of Interest
The authors have no conflicts of interest.
References
1. Horn EM, Feiz-Erfan I, Bristol RE, Lekovic GP, Goslar PW, Smith KA, et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60:613-618.
2. Bristol RE, Nakaji P, Smith KA. Endoscopic management of colloid cysts. Oper Tech Neurosurg. 2005;8:176-178.
3. Khoury CE, Brugières P, Decq P, Cosson-Stanescu R, Combes C, Ricolfi F et al. Colloid Cysts of the Third Ventricle: Are MR Imaging Patterns Predictive of Difficulty with Percutaneous Treatment? AJNR Am J Neuroradiol. 2000;21:489-492.
4. Algin O, Ozmen E, Arslan H. Radiologic manifestations of colloid cysts: A pictorial assay. Canadian Association of Radiologists Journal. 2013;64:56-60.
5. De Witt Hamer PC, Verstegen MJ, De Haan RJ, Vandertop WP, Thomeer RT, Mooij JJ et al. High risk of acute deterioration in patients harboring symptomatic colloid cysts of the third ventricle. J Neurosurg. 2002;96:1041–1045.
6. Tamura Y, Uesugi T,Tucker A, Ukita T, Tsuji M, Miyake H, et al. Hemorrhagic colloid cyst with intraventricular extension. J Neurosurg. 2013;118:498-501.
7. Gokalp HZ, Yuceer N, Arasil E, Erdogan A, Dincer C, Baskaya M. Colloid cyst of the third ventricle. Evaluation of 28 cases of colloid cyst of the third ventricle operated on by transcorticaltransventricular (25 cases) and transcallosal/transventricular (3 cases) approaches. Acta Neurochir. 1996;138:45-49.
8. Desai KI, Nadkarni TD, Muzumdar DP, Goel AH. Surgical management of colloid cyst of the third ventricle-A study of 105 cases. Surg Neurol. 2002;57:295-304.
9. Hernesniemi J, Leivo S. Management outcome in third ventricular colloid cysts in a defined population: a series of 40 patients treated mainly by transcallosal microsurgery. Surg Neurol. 1996;45:2-14.
10. Abdou MS, Cohen AR. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89:1062-1068.
11. Boogaarts HD, Decq P, Grotenhuis JA, Le Guérinel C, Nseir R, Jarraya B, et al. Long-term results of the neuroendoscopic management of colloid cysts of the third ventricle: a series of 90 cases. Neurosurgery. 2011;68:179-187.
12. Salem-Memou S, Thiam A B, Kpelao E, Mbaye M, Ba MC, Badiane SB. Traitement de l’hydrocéphalie de l’enfant par ventriculocisternostomie endoscopique au Sénégal. j. neuchi doi:2014;60(5):254-257.
13. Wallmann H. Eine colloid cysteim dritten Hirn ventrikel und ein Lipomin plexus Choroides. Virchows Arch Pathol Anat. 1858;14:385-388.
14. Sribnick EA, Dadashev VY, Miller BA, Hawkins S, Hadjipanayis CG. Neuroendoscopic colloid cyst resection: a case cohort with follow-up and patient satisfaction. World Neurosurg. 2014;81,3/4:584-593.
15. Taous A, Rouimi A. Kyste colloïde obstructive du troisième ventricule. Pan African Medical Journal. 2015;20:217.
16. Sampath R, Vannemreddy P, Nanda A. Microsurgical excision of colloid cyst with favorable cognitive outcomes and short operative time and hospital stay: operative techniques and analyses of outcomes with review of previous studies. Neurosurgery. 2010;66:368-375.
17. Sheikh AB, Mendelson ZS, LIU JK. Endoscopic versus microsurgical resection of colloid cyst: a systematic review and meta-analysis of 1278 patients. World Neurosurg. 2014;82(6):1187-1197.
18. Buchsbaum HW, Colton RP. Anterior third ventricle cysts in infancy. Case report. J Neurosurg. 1967;26:264-266.
19. Ibáñez-Botella G, Domínguez M, Ros B, De Miguel L, Márquez B, Arráez A. Endoscopic transchoroidal and transforaminal approaches for resection of third ventricular colloid cysts. Neurosurg Rev. 2014;37:227-234.
20. Carrasco R, Pascual JM, Medina-López D, Burdaspal-Moratilla A. Acute hemorrhage in a colloid cyst of the third ventricle: a rare cause of sudden deterioration. Surg Neurol Int. 2012;3:24.
21. Kondziolka D, Lunsford D. Stereotactic management of colloid cysts: factors predicting success. J Neurosurg. 1991;75:45-51.
22. Wilson DA, Fusco DJ, Wait SD, Nakaji P. Endoscopic resection of colloid cysts: use of a dual-instrument technique and an anterolateral approach. World Neurosurg. 2012;80:576-583.
Type of Study: Research |
Subject:
Gamma Knife Radiosurgery
References
1. Horn EM, Feiz-Erfan I, Bristol RE, Lekovic GP, Goslar PW, Smith KA, et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60:613-618. [DOI:10.1227/01.NEU.0000255409.61398.EA] [PMID]
2. Bristol RE, Nakaji P, Smith KA. Endoscopic management of colloid cysts. Oper Tech Neurosurg. 2005;8:176-178. [DOI:10.1053/j.otns.2005.07.009]
3. Khoury CE, Brugières P, Decq P, Cosson-Stanescu R, Combes C, Ricolfi F et al. Colloid Cysts of the Third Ventricle: Are MR Imaging Patterns Predictive of Difficulty with Percutaneous Treatment? AJNR Am J Neuroradiol. 2000;21:489-492. [PMID]
4. Algin O, Ozmen E, Arslan H. Radiologic manifestations of colloid cysts: A pictorial assay. Canadian Association of Radiologists Journal. 2013;64:56-60. [DOI:10.1016/j.carj.2011.12.011] [PMID]
5. De Witt Hamer PC, Verstegen MJ, De Haan RJ, Vandertop WP, Thomeer RT, Mooij JJ et al. High risk of acute deterioration in patients harboring symptomatic colloid cysts of the third ventricle. J Neurosurg. 2002;96:1041–1045. [DOI:10.3171/jns.2002.96.6.1041] [PMID]
6. Tamura Y, Uesugi T,Tucker A, Ukita T, Tsuji M, Miyake H, et al. Hemorrhagic colloid cyst with intraventricular extension. J Neurosurg. 2013;118:498-501. [DOI:10.3171/2012.10.JNS12793] [PMID]
7. Gokalp HZ, Yuceer N, Arasil E, Erdogan A, Dincer C, Baskaya M. Colloid cyst of the third ventricle. Evaluation of 28 cases of colloid cyst of the third ventricle operated on by transcorticaltransventricular (25 cases) and transcallosal/transventricular (3 cases) approaches. Acta Neurochir. 1996;138:45-49.
8. Desai KI, Nadkarni TD, Muzumdar DP, Goel AH. Surgical management of colloid cyst of the third ventricle-A study of 105 cases. Surg Neurol. 2002;57:295-304. [DOI:10.1016/S0090-3019(02)00701-2]
9. Hernesniemi J, Leivo S. Management outcome in third ventricular colloid cysts in a defined population: a series of 40 patients treated mainly by transcallosal microsurgery. Surg Neurol. 1996;45:2-14. [DOI:10.1016/0090-3019(95)00379-7]
10. Abdou MS, Cohen AR. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89:1062-1068. [DOI:10.3171/jns.1998.89.6.1062] [PMID]
11. Boogaarts HD, Decq P, Grotenhuis JA, Le Guérinel C, Nseir R, Jarraya B, et al. Long-term results of the neuroendoscopic management of colloid cysts of the third ventricle: a series of 90 cases. Neurosurgery. 2011;68:179-187. [DOI:10.1227/NEU.0b013e3181ffae71] [PMID]
12. Salem-Memou S, Thiam A B, Kpelao E, Mbaye M, Ba MC, Badiane SB. Traitement de l'hydrocéphalie de l'enfant par ventriculocisternostomie endoscopique au Sénégal. j. neuchi doi:2014;60(5):254-257.
13. Wallmann H. Eine colloid cysteim dritten Hirn ventrikel und ein Lipomin plexus Choroides. Virchows Arch Pathol Anat. 1858;14:385-388. [DOI:10.1007/BF01929335]
14. Sribnick EA, Dadashev VY, Miller BA, Hawkins S, Hadjipanayis CG. Neuroendoscopic colloid cyst resection: a case cohort with follow-up and patient satisfaction. World Neurosurg. 2014;81,3/4:584-593. [DOI:10.1016/j.wneu.2013.12.006] [PMID]
15. Taous A, Rouimi A. Kyste colloïde obstructive du troisième ventricule. Pan African Medical Journal. 2015;20:217. [DOI:10.11604/pamj.2015.20.217.6264] [PMID] [PMCID]
16. Sampath R, Vannemreddy P, Nanda A. Microsurgical excision of colloid cyst with favorable cognitive outcomes and short operative time and hospital stay: operative techniques and analyses of outcomes with review of previous studies. Neurosurgery. 2010;66:368-375. [DOI:10.1227/01.NEU.0000363858.17782.82] [PMID]
17. Sheikh AB, Mendelson ZS, LIU JK. Endoscopic versus microsurgical resection of colloid cyst: a systematic review and meta-analysis of 1278 patients. World Neurosurg. 2014;82(6):1187-1197. [DOI:10.1016/j.wneu.2014.06.024] [PMID]
18. Buchsbaum HW, Colton RP. Anterior third ventricle cysts in infancy. Case report. J Neurosurg. 1967;26:264-266. [DOI:10.3171/jns.1967.26.2.0264] [PMID]
19. Ibá-ez-Botella G, Domínguez M, Ros B, De Miguel L, Márquez B, Arráez A. Endoscopic transchoroidal and transforaminal approaches for resection of third ventricular colloid cysts. Neurosurg Rev. 2014;37:227-234. [DOI:10.1007/s10143-014-0529-7] [PMID]
20. Carrasco R, Pascual JM, Medina-López D, Burdaspal-Moratilla A. Acute hemorrhage in a colloid cyst of the third ventricle: a rare cause of sudden deterioration. Surg Neurol Int. 2012;3:24. [DOI:10.4103/2152-7806.92932] [PMID] [PMCID]
21. Kondziolka D, Lunsford D. Stereotactic management of colloid cysts: factors predicting success. J Neurosurg. 1991;75:45-51. [DOI:10.3171/jns.1991.75.1.0045] [PMID]
22. Wilson DA, Fusco DJ, Wait SD, Nakaji P. Endoscopic resection of colloid cysts: use of a dual-instrument technique and an anterolateral approach. World Neurosurg. 2012;80:576-583. [DOI:10.1016/j.wneu.2012.07.014] [PMID]
23. Decq P, Le Guerinel C, Brugières P, Djindjian M, Silva D, Kéravel Y et al. Endoscopic management of colloid cysts. Neurosurgery. 1988;42(6):1288-94. [DOI:10.1097/00006123-199806000-00051]
24. Hellwig D, Bauer BL, Schulte M, Gatscher S, Riegel T, Bertalanffy H. Neuroendoscopic treatment of colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52(3):525-531. [DOI:10.1227/01.NEU.0000047671.27057.55] [PMID]
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |