Wed, Jul 2, 2025
Volume 8 - Special Issue
Iran J Neurosurg 2022, 8 - Special Issue: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Javadi S A H, Mosallami Aghili S M, Javadi A M, Raminfard S. Methods to Improve Fiber Reconstruction at DTI-Based Tractography in the Area of Brain Tumor: Case Illustration and Literature Review. Iran J Neurosurg 2022; 8 : 2
URL: http://irjns.org/article-1-300-en.html
URL: http://irjns.org/article-1-300-en.html
Seyed Amir Hossein Javadi *1 

 , Seyed Morsal Mosallami Aghili2
, Seyed Morsal Mosallami Aghili2 

 , Amir Mohammad Javadi3
, Amir Mohammad Javadi3 

 , Samira Raminfard4
, Samira Raminfard4 




 , Seyed Morsal Mosallami Aghili2
, Seyed Morsal Mosallami Aghili2 

 , Amir Mohammad Javadi3
, Amir Mohammad Javadi3 

 , Samira Raminfard4
, Samira Raminfard4 


1- Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran. , javadi1978@yahoo.com
2- Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
3- Noor Ophthalmology Research Center, Noor Ophthalmology Hospital, Tehran, Iran.
4- Neuroimaging and Analysis Group, Research Center for Molecular and Cellular Imaging, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
3- Noor Ophthalmology Research Center, Noor Ophthalmology Hospital, Tehran, Iran.
4- Neuroimaging and Analysis Group, Research Center for Molecular and Cellular Imaging, Tehran University of Medical Sciences, Tehran, Iran.
Full Text [PDF 2652 kb]
(682 Downloads)
| Abstract (HTML) (2132 Views)
Full Text: (880 Views)
1. Introduction
iffusion Tensor Imaging (DTI) tractography is a valuable method used to determine the course, extent, and connectivity of white matter fiber tracts [1]. DTI-based tractography calculates fiber orientation by analyzing the main water diffusion trajectory or diffusion tensor [1]. Therefore, reconstructed fiber tracts are post-processed areas where a large portion of the nerve fibers are expected to be parallel to each other [2]. This modality could also help us visualize fiber tracts’ spatial relationship with brain lesions [1-6].
Several factors may interfere with the procedure of diffusion-based tractography, including a Signal-to-Noise Ratio (SNR), the threshold of anisotropy, tractography model and algorithm, the complexity of the fiber tract (e.g. fan-shaped Corticospinal Tract [CST] or crossing fibers), and selection of the Region of iInterest (ROIs). All the aforementioned variables become more complex than others in the setting of edema and tumor infiltration [7].
Anisotropy alterations in the white matter adjacent to the tumor can impact the visualization of trajectories, which is a potential limitation of tractography [3]. Due to the adjacent brain tumors, the accuracy and sensitivity of DTI tractography of fiber tracts are decreased, which can displace, infiltrate, or destroy the fiber tracts [7]. This occurs due to edema or infiltration of tumors [3]. The peritumoral edema can affect the local Fractional Anisotropy (FA). Anisotropy changes affect the arcuate fasciculus more than the corticospinal or optic tracts.
The current study aims to discuss several solutions to improve the procedure of fiber reconstruction adjacent to or within brain lesions, whether tumors or edema. Besides, illustrative cases are presented.
2. Methods and Materials/Patients
This article is a narrative review of methods that can improve fiber reconstruction in diffusion-based tractography in the area of brain tumors. To provide up-to-date information, we have briefly reviewed related articles. We retrieved the relevant articles from Google Scholar, Medline, and PubMed using the keywords “diffusion tensor imaging”, “brain mapping”, “brain neoplasms”, “diffusion-based tractography”, “fiber reconstruction”, “fractional anisotropy”, “probability density function”, “region of interest”, “signal-to-noise ratio”, “corticospinal tract”, “neuronavigation”, “q-ball” and “tractography”. Finally, the extracted papers were reviewed and critically analyzed.
3. Results
Technique 1: Very low fractional anisotropy (FA) application
The procedure of fiber tract reconstruction depends on the DTI-based tractography algorithm [2, 7, 8]. In the deterministic method, voxel fiber reconstruction regarding FA threshold is performed. However, the FA threshold is much lower than normal values in the tumor area (Figures 1 and 2) [7]. To detect real anatomic fibers around the tumor, it is necessary to alter the tracking parameters (decrease in FA threshold, change in fiber length range, and increase the angle threshold).


These changes lead to an almost real result of the tract shape adjacent to the tumor. The probabilistic techniques measure fiber probabilities via the distribution of potential orientations by constructing PDFs (probability density functions). Therefore, the fiber trajectory could even be traced in the area of very low FA thresholds, with undetectable diffusion directions [7]. However, this method has less power to identify the fiber tracts in patients with a brain tumor [9].
While it has been proposed that DTI tractography with an FA threshold of 0.2 to 0.3 can help prevent inappropriate inclusions (grey matter, cerebral spinal fluid), it can also impair their accurate visualization by underestimating their existence and increasing the proximity between them and the tumor [3].
Technique 2: Resampling techniques, Q-ball, and high angular resolution diffusion imaging (HARDI)
New methods, such as bootstrap, a nonparametric statistical technique of resampling, can estimate the course of fiber tracts. This technique provides nonparametric quantification of uncertainties; residual bootstrap is more accurate than resampling with repetition [7, 10]. To assess the uncertainty in multimodal q-ball reconstructions, the suggested residual bootstrap approach uses a spherical harmonic representation for High Angular Resolution Diffusion Imaging (HARDI) data [11].
Q-ball modeling
The probabilistic method measures several uncertainties and performs multiple estimations, which is more sensitive than the deterministic single estimation approach. However, random fiber connections should be detected and corrected with null fiber tracking [7].
The q-space imaging method quantifies diffusion function by applying the Fourier relationship in a model-independent method, which demands a large number of gradient directions [12].
HARDI is utilized to provide more information when reconstructing more complex areas, such as crossing fibers. Q-ball imaging reconstructs HARDI data to generate an Orientation Distribution Function (ODF) that can be utilized to detect the orientation of numerous fibers in complex regions [11].
Compression may cause delayed axonal transmission, demyelination, and axonal loss. When a tumor compresses the fiber tracts, the diffusivity increases parallel to the fiber tracts but decreases perpendicular to the fibers, resulting in increased FA [13]. Changes in FA will help detect fiber displacement or destruction.
While increased FA suggests compression and displacement of fiber tracts, decreased FA may indicate infiltration of fiber tracts and impaired tracking ability. Displacement due to compression results in higher density and tension of fiber tracts, which explains increased FA and reduced diffusivity. Besides, the tight fibers in a bundle indicate more homogeneity [13].
Residual bootstrap estimate of uncertainty
Using a HARDI acquisition, the residual bootstrap approach was utilized to determine the ODF uncertainty of a voxel. The main technique is to fit the HARDI acquisition to a spherical harmonic model and then use the model residuals to generate a large number of simulated HARDI data sets [10]. According to numerical simulations, the residual bootstrap accurately assesses the uncertainty in q-ball data.
In complex areas with more than one population of fiber tracts in a voxel, DTI cannot depict the architecture, and the FA is reduced to the level of gray matter and Cerebrospinal Fluid (CSF) [11, 13]. However, a high number of directions, such as HARDI, will be helpful in such situations but it increases the procedure time and thereby increases motion artifacts.
Spherical deconvolution and the diffusion orientation transform have been offered as alternatives to HARDI data for q-ball imaging. The residual bootstrap of the q-ball fiber tracking algorithm’s probabilistic nature allows evaluation of the technique’s findings. Many streamlines pass through voxels that are more likely to be in the white matter tract of interest [1].
The probabilistic q-ball fiber tracks revealed motor fiber connections to the superior and lateral parts of the motor cortex but not to the entire primary motor cortex. It has been reported that DTI fiber tracking is unable to show the motor tract in the lateral parts of the motor tract regulates the voluntary movement of the head and upper extremity [14].
As one example of clinical use, fiber tracking with the q-ball ODF has the capacity to more thoroughly outline the motor tract and assist in the preoperative planning of insular tumors. DTI fiber tracking is confined to regions with high anisotropy single fiber populations. Fibers from the corticospinal tract, superior longitudinal fasciculus, and interhemispheric callosal fibers connect and cross in the centrum semiovale.
Technique 3: Separate reconstruction of fiber tracts
To estimate the course of subcortical fiber tracts near subcortical brain tumors, ROIs can be selected outsidethis area, such as ROIs defined at the motor cortex and cerebral peduncle to reconstruct the corticospinal tract. One strategy is to define ROIs separately at each end to reconstruct fiber tracts separately at each end. Figure 3 shows a large glioma infiltrating CST. The subcortical course of CST through brain neoplasm cannot be reconstructed by selecting both ROIs; however, the remaining part was reconstructed by selecting ROI at the cerebral peduncle.

Technique 4: Functional magnetic resonance imaging (fmri)-based ROI selection
Tumors can make it impossible to reconstruct fiber tracts. Figure 4 shows the reappearance of the fiber tracts in a patient with hemiparesis that improved dramatically in the postoperative period; the ROIs were selected according to functional Magnetic Resonance Imaging (fMRI).

Brain edema and neoplasm infiltration may distort the normal anatomy of grey and white matter, especially the eloquent area and subcortical fiber tracts. One helpful strategy in the presence of a tumor is the selection of ROIs according to fMRI since the anatomical configuration is distorted by the neoplasm [13]. The selection of ROIs is critical in the corticospinal tract, while fanning of the fiber tracts necessitates the selection of at least two ROIs [8, 13]. Furthermore, fibers, such as Superior Longitudinal Fasciculus (SLF) are directionally heterogeneous even in the normal brain, and brain tumors prevent accurate ROI selection. The method, as mentioned above, defines functionally relevant fiber tracts.
Selection of ROIs in cases with brain lesions should be performed according to fMRI besides anatomic information. Furthermore, different algorithms may result in various trajectories, the probabilistic tractography algorithm reveals fiber connectivity that progresses into the gray matter [15].
Technique 5: Unprocessed images
Visualization of crossing fibers, such as optic chiasma is challenging, especially in the area of tumor involvement. Figure 5 shows a large pituitary adenoma with undetectable optic nerves; however, optic tracts could be visualized. Therefore, unprocessed images were used to reconstruct optic nerves. Processing includes several data corrections and deletions. Such information assists preoperative planning and correlates with the clinical situation.
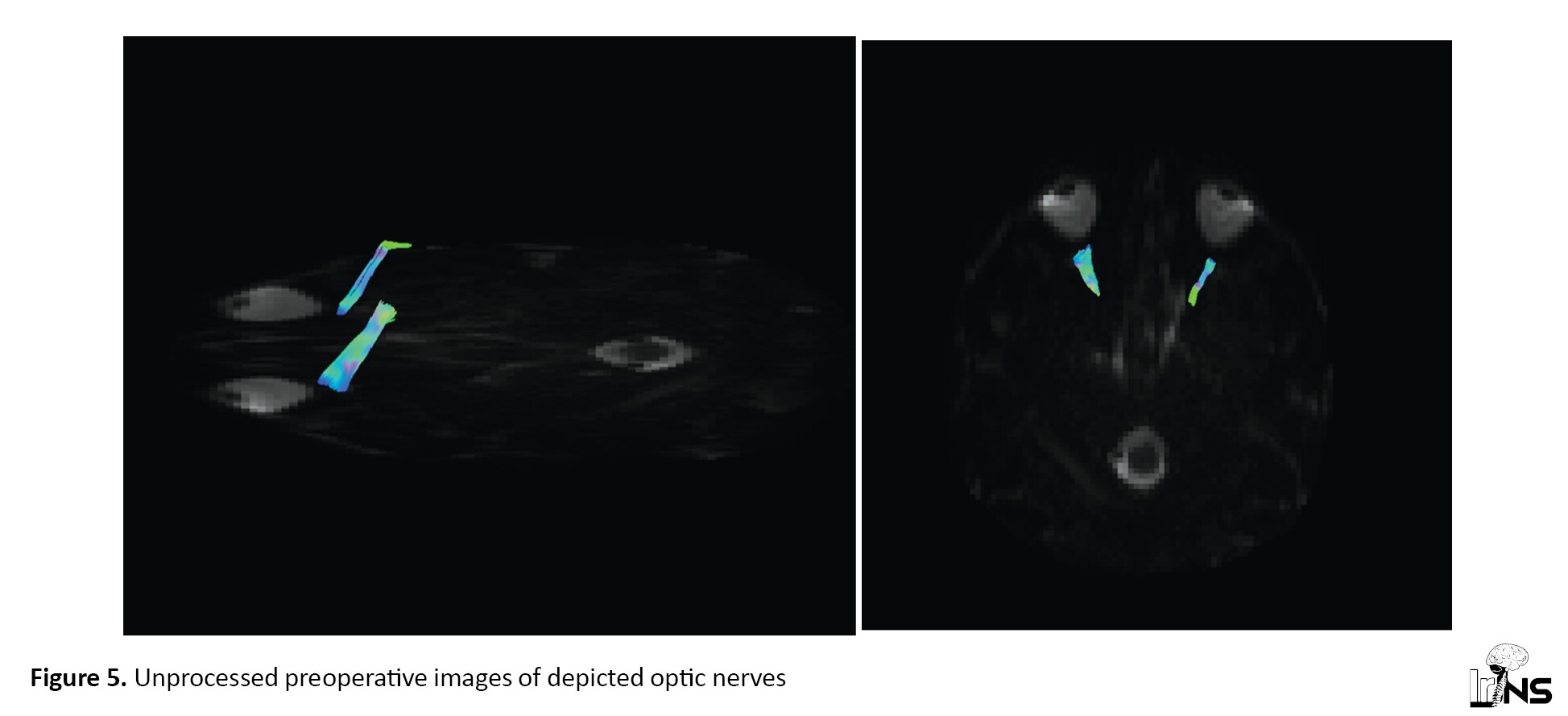
In voxels containing multiple fiber orientations, such as the optic chiasma, the tensor estimation model does not support identifying real connections [16]. Constrained Spherical Deconvolution (CSD) is the method that can overcome the problem with the diffusion tensor model in the crossing fibers. This model can also be applied to peri-tumoral fibers in the peri-tumoral edematous area [17].
As shown in Figure 6, In the setting of intraoperative MR, debulking tumor and decompression of chiasma result in optic nerve depiction. This confirms preoperative data regarding compressed optic nerves.

4. Discussion
The effect of edema is less detectable in low-grade tumors. Associated edema may affect local anisotropy in the presence of a brain tumor, and the bulk flow results in false fiber tracking. However, such effects can be visualized with fMRI [15]. However, successful tractography of such tracts has been reported.
Vasogenic edema causes increased diffusion as well as swelling of neural fibers in both the longitudinal and transverse directions. Anisotropy should be reduced as a result of these adjustments. Despite the presence of intact axonal structures in this location, if the anisotropy reduction was significant enough, it may no longer be able to detect sufficient diffusion directionality to reconstruct a fiber pathway [18].
Crossing fibers is a major limitation of fiber tracking. At the level of the centrum semiovale, the motor tracts of the brain should be fan-shaped. On the other hand, the fiber-tracking technique can only show fibers heading to the brain’s vertex.
The following conditions are deemed “discontinuous” (vs. “continuous”) for a tract’s trajectory:
1. They do not connect anatomically related sites.
2. Anisotropy-weighted directional color maps show any part of the tract discontinuously.
3. Despite a significant reduction of stopping criteria, fiber-tracking terminates at discontinuity [1].
DTI tractography is highly sensitive to noise and artifacts. Distortions may be caused by several reasons, including significant and real tract alteration (such as actual tract injury from the lesion or surgery or acute tract deviations caused by the lesion), errors in the tensor field from image noise or artifacts, or partial volume averaging between intersecting and neighboring fiber tracts [1, 6].
Overall, DTI tractography has low sensitivity for estimating the extent of the corticospinal tract near tumors; however, it can accurately estimate the course of the fiber tract [7, 8, 14].
Several variables affect diffusion tractography, involving diffusion modeling techniques (DTI, q-ball, diffusion spectrum imaging, and spherical deconvolution) and algorithm/approach (deterministic and stochastic streamlines, non-streamline, and global tracking) [7].
5. Conclusion
The application of tractography techniques, including very low FA, resampling techniques, separated ROIs, selection of ROI by fMRI, and unprocessed images can improve fiber reconstruction in the presence of brain tumor or edema.
Ethical Considerations
Compliance with ethical guidelines
Written consent was obtained from patients to publish their corresponding radiological images.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Seyed Amir Hossein Javadi; Data collection, Data analysis and interpretatio: Seyed Amir Hossein Javadi, Amir Mohammad Javadi, Samira Raminfar; Drafting, Critically revising the article: All suthors; Reviewing submitted version of manuscript: Seyed Amir Hossein Javadi, Seyed Morsal Mosallami Aghili; Approving the final version of the manuscript: Seyed Amir Hossein Javadi, Seyed Morsal Mosallami Aghili.
Conflict of interest
The authors declared no conflict of interest.
References
iffusion Tensor Imaging (DTI) tractography is a valuable method used to determine the course, extent, and connectivity of white matter fiber tracts [1]. DTI-based tractography calculates fiber orientation by analyzing the main water diffusion trajectory or diffusion tensor [1]. Therefore, reconstructed fiber tracts are post-processed areas where a large portion of the nerve fibers are expected to be parallel to each other [2]. This modality could also help us visualize fiber tracts’ spatial relationship with brain lesions [1-6].
Several factors may interfere with the procedure of diffusion-based tractography, including a Signal-to-Noise Ratio (SNR), the threshold of anisotropy, tractography model and algorithm, the complexity of the fiber tract (e.g. fan-shaped Corticospinal Tract [CST] or crossing fibers), and selection of the Region of iInterest (ROIs). All the aforementioned variables become more complex than others in the setting of edema and tumor infiltration [7].
Anisotropy alterations in the white matter adjacent to the tumor can impact the visualization of trajectories, which is a potential limitation of tractography [3]. Due to the adjacent brain tumors, the accuracy and sensitivity of DTI tractography of fiber tracts are decreased, which can displace, infiltrate, or destroy the fiber tracts [7]. This occurs due to edema or infiltration of tumors [3]. The peritumoral edema can affect the local Fractional Anisotropy (FA). Anisotropy changes affect the arcuate fasciculus more than the corticospinal or optic tracts.
The current study aims to discuss several solutions to improve the procedure of fiber reconstruction adjacent to or within brain lesions, whether tumors or edema. Besides, illustrative cases are presented.
2. Methods and Materials/Patients
This article is a narrative review of methods that can improve fiber reconstruction in diffusion-based tractography in the area of brain tumors. To provide up-to-date information, we have briefly reviewed related articles. We retrieved the relevant articles from Google Scholar, Medline, and PubMed using the keywords “diffusion tensor imaging”, “brain mapping”, “brain neoplasms”, “diffusion-based tractography”, “fiber reconstruction”, “fractional anisotropy”, “probability density function”, “region of interest”, “signal-to-noise ratio”, “corticospinal tract”, “neuronavigation”, “q-ball” and “tractography”. Finally, the extracted papers were reviewed and critically analyzed.
3. Results
Technique 1: Very low fractional anisotropy (FA) application
The procedure of fiber tract reconstruction depends on the DTI-based tractography algorithm [2, 7, 8]. In the deterministic method, voxel fiber reconstruction regarding FA threshold is performed. However, the FA threshold is much lower than normal values in the tumor area (Figures 1 and 2) [7]. To detect real anatomic fibers around the tumor, it is necessary to alter the tracking parameters (decrease in FA threshold, change in fiber length range, and increase the angle threshold).


These changes lead to an almost real result of the tract shape adjacent to the tumor. The probabilistic techniques measure fiber probabilities via the distribution of potential orientations by constructing PDFs (probability density functions). Therefore, the fiber trajectory could even be traced in the area of very low FA thresholds, with undetectable diffusion directions [7]. However, this method has less power to identify the fiber tracts in patients with a brain tumor [9].
While it has been proposed that DTI tractography with an FA threshold of 0.2 to 0.3 can help prevent inappropriate inclusions (grey matter, cerebral spinal fluid), it can also impair their accurate visualization by underestimating their existence and increasing the proximity between them and the tumor [3].
Technique 2: Resampling techniques, Q-ball, and high angular resolution diffusion imaging (HARDI)
New methods, such as bootstrap, a nonparametric statistical technique of resampling, can estimate the course of fiber tracts. This technique provides nonparametric quantification of uncertainties; residual bootstrap is more accurate than resampling with repetition [7, 10]. To assess the uncertainty in multimodal q-ball reconstructions, the suggested residual bootstrap approach uses a spherical harmonic representation for High Angular Resolution Diffusion Imaging (HARDI) data [11].
Q-ball modeling
The probabilistic method measures several uncertainties and performs multiple estimations, which is more sensitive than the deterministic single estimation approach. However, random fiber connections should be detected and corrected with null fiber tracking [7].
The q-space imaging method quantifies diffusion function by applying the Fourier relationship in a model-independent method, which demands a large number of gradient directions [12].
HARDI is utilized to provide more information when reconstructing more complex areas, such as crossing fibers. Q-ball imaging reconstructs HARDI data to generate an Orientation Distribution Function (ODF) that can be utilized to detect the orientation of numerous fibers in complex regions [11].
Compression may cause delayed axonal transmission, demyelination, and axonal loss. When a tumor compresses the fiber tracts, the diffusivity increases parallel to the fiber tracts but decreases perpendicular to the fibers, resulting in increased FA [13]. Changes in FA will help detect fiber displacement or destruction.
While increased FA suggests compression and displacement of fiber tracts, decreased FA may indicate infiltration of fiber tracts and impaired tracking ability. Displacement due to compression results in higher density and tension of fiber tracts, which explains increased FA and reduced diffusivity. Besides, the tight fibers in a bundle indicate more homogeneity [13].
Residual bootstrap estimate of uncertainty
Using a HARDI acquisition, the residual bootstrap approach was utilized to determine the ODF uncertainty of a voxel. The main technique is to fit the HARDI acquisition to a spherical harmonic model and then use the model residuals to generate a large number of simulated HARDI data sets [10]. According to numerical simulations, the residual bootstrap accurately assesses the uncertainty in q-ball data.
In complex areas with more than one population of fiber tracts in a voxel, DTI cannot depict the architecture, and the FA is reduced to the level of gray matter and Cerebrospinal Fluid (CSF) [11, 13]. However, a high number of directions, such as HARDI, will be helpful in such situations but it increases the procedure time and thereby increases motion artifacts.
Spherical deconvolution and the diffusion orientation transform have been offered as alternatives to HARDI data for q-ball imaging. The residual bootstrap of the q-ball fiber tracking algorithm’s probabilistic nature allows evaluation of the technique’s findings. Many streamlines pass through voxels that are more likely to be in the white matter tract of interest [1].
The probabilistic q-ball fiber tracks revealed motor fiber connections to the superior and lateral parts of the motor cortex but not to the entire primary motor cortex. It has been reported that DTI fiber tracking is unable to show the motor tract in the lateral parts of the motor tract regulates the voluntary movement of the head and upper extremity [14].
As one example of clinical use, fiber tracking with the q-ball ODF has the capacity to more thoroughly outline the motor tract and assist in the preoperative planning of insular tumors. DTI fiber tracking is confined to regions with high anisotropy single fiber populations. Fibers from the corticospinal tract, superior longitudinal fasciculus, and interhemispheric callosal fibers connect and cross in the centrum semiovale.
Technique 3: Separate reconstruction of fiber tracts
To estimate the course of subcortical fiber tracts near subcortical brain tumors, ROIs can be selected outsidethis area, such as ROIs defined at the motor cortex and cerebral peduncle to reconstruct the corticospinal tract. One strategy is to define ROIs separately at each end to reconstruct fiber tracts separately at each end. Figure 3 shows a large glioma infiltrating CST. The subcortical course of CST through brain neoplasm cannot be reconstructed by selecting both ROIs; however, the remaining part was reconstructed by selecting ROI at the cerebral peduncle.

Technique 4: Functional magnetic resonance imaging (fmri)-based ROI selection
Tumors can make it impossible to reconstruct fiber tracts. Figure 4 shows the reappearance of the fiber tracts in a patient with hemiparesis that improved dramatically in the postoperative period; the ROIs were selected according to functional Magnetic Resonance Imaging (fMRI).

Brain edema and neoplasm infiltration may distort the normal anatomy of grey and white matter, especially the eloquent area and subcortical fiber tracts. One helpful strategy in the presence of a tumor is the selection of ROIs according to fMRI since the anatomical configuration is distorted by the neoplasm [13]. The selection of ROIs is critical in the corticospinal tract, while fanning of the fiber tracts necessitates the selection of at least two ROIs [8, 13]. Furthermore, fibers, such as Superior Longitudinal Fasciculus (SLF) are directionally heterogeneous even in the normal brain, and brain tumors prevent accurate ROI selection. The method, as mentioned above, defines functionally relevant fiber tracts.
Selection of ROIs in cases with brain lesions should be performed according to fMRI besides anatomic information. Furthermore, different algorithms may result in various trajectories, the probabilistic tractography algorithm reveals fiber connectivity that progresses into the gray matter [15].
Technique 5: Unprocessed images
Visualization of crossing fibers, such as optic chiasma is challenging, especially in the area of tumor involvement. Figure 5 shows a large pituitary adenoma with undetectable optic nerves; however, optic tracts could be visualized. Therefore, unprocessed images were used to reconstruct optic nerves. Processing includes several data corrections and deletions. Such information assists preoperative planning and correlates with the clinical situation.

In voxels containing multiple fiber orientations, such as the optic chiasma, the tensor estimation model does not support identifying real connections [16]. Constrained Spherical Deconvolution (CSD) is the method that can overcome the problem with the diffusion tensor model in the crossing fibers. This model can also be applied to peri-tumoral fibers in the peri-tumoral edematous area [17].
As shown in Figure 6, In the setting of intraoperative MR, debulking tumor and decompression of chiasma result in optic nerve depiction. This confirms preoperative data regarding compressed optic nerves.

4. Discussion
The effect of edema is less detectable in low-grade tumors. Associated edema may affect local anisotropy in the presence of a brain tumor, and the bulk flow results in false fiber tracking. However, such effects can be visualized with fMRI [15]. However, successful tractography of such tracts has been reported.
Vasogenic edema causes increased diffusion as well as swelling of neural fibers in both the longitudinal and transverse directions. Anisotropy should be reduced as a result of these adjustments. Despite the presence of intact axonal structures in this location, if the anisotropy reduction was significant enough, it may no longer be able to detect sufficient diffusion directionality to reconstruct a fiber pathway [18].
Crossing fibers is a major limitation of fiber tracking. At the level of the centrum semiovale, the motor tracts of the brain should be fan-shaped. On the other hand, the fiber-tracking technique can only show fibers heading to the brain’s vertex.
The following conditions are deemed “discontinuous” (vs. “continuous”) for a tract’s trajectory:
1. They do not connect anatomically related sites.
2. Anisotropy-weighted directional color maps show any part of the tract discontinuously.
3. Despite a significant reduction of stopping criteria, fiber-tracking terminates at discontinuity [1].
DTI tractography is highly sensitive to noise and artifacts. Distortions may be caused by several reasons, including significant and real tract alteration (such as actual tract injury from the lesion or surgery or acute tract deviations caused by the lesion), errors in the tensor field from image noise or artifacts, or partial volume averaging between intersecting and neighboring fiber tracts [1, 6].
Overall, DTI tractography has low sensitivity for estimating the extent of the corticospinal tract near tumors; however, it can accurately estimate the course of the fiber tract [7, 8, 14].
Several variables affect diffusion tractography, involving diffusion modeling techniques (DTI, q-ball, diffusion spectrum imaging, and spherical deconvolution) and algorithm/approach (deterministic and stochastic streamlines, non-streamline, and global tracking) [7].
5. Conclusion
The application of tractography techniques, including very low FA, resampling techniques, separated ROIs, selection of ROI by fMRI, and unprocessed images can improve fiber reconstruction in the presence of brain tumor or edema.
Ethical Considerations
Compliance with ethical guidelines
Written consent was obtained from patients to publish their corresponding radiological images.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Seyed Amir Hossein Javadi; Data collection, Data analysis and interpretatio: Seyed Amir Hossein Javadi, Amir Mohammad Javadi, Samira Raminfar; Drafting, Critically revising the article: All suthors; Reviewing submitted version of manuscript: Seyed Amir Hossein Javadi, Seyed Morsal Mosallami Aghili; Approving the final version of the manuscript: Seyed Amir Hossein Javadi, Seyed Morsal Mosallami Aghili.
Conflict of interest
The authors declared no conflict of interest.
References
- Lazar M, Alexander A, Thottakara P, Badie B, Field A. White matter reorganization after surgical resection of brain tumors and vascular malformations. American Journal of Neuroradiology. 2006; 27(6):1258-71. [PMCID]
- Bauer MH, Kuhnt D, Barbieri S, Klein J, Becker A, Freisleben B, et al. Reconstruction of white matter tracts via repeated deterministic streamline tracking-initial experience. Plos one. 2013; 8(5):e63082. [DOI:10.1371/journal.pone.0063082] [PMID] [PMCID]
- Romano A, D’Andrea G, Minniti G, Mastronardi L, Ferrante L, Fantozzi L, et al. Pre-surgical planning and MR-tractography utility in brain tumour resection. European radiology. 2009; 19(12):2798-808. [DOI:10.1007/s00330-009-1483-6] [PMID]
- Vassal F, Schneider F, Nuti C. Intraoperative use of diffusion tensor imaging-based tractography for resection of gliomas located near the pyramidal tract: Comparison with subcortical stimulation mapping and contribution to surgical outcomes. British Journal of Neuroradiology. 2013; 27(5):668-75. [DOI:10.3109/02688697.2013.771730] [PMID]
- Berman J. Diffusion MR tractography as a tool for surgical planning. Magnetic resonance imaging clinics of North America. 2009; 17(2):205-14. [DOI:10.1016/j.mric.2009.02.002] [PMID]
- D’Andrea G, Angelini A, Romano A, Di Lauro A, Sessa G, Bozzao A, et al. Intraoperative DTI and brain mapping for surgery of neoplasm of the motor cortex and the corticospinal tract: Our protocol and series in BrainSUITE. Neurosurgical Review. 2012; 35(3):401-12. [DOI:10.1007/s10143-012-0373-6] [PMID]
- Mandelli ML, Berger MS, Bucci M, Berman JI, Amirbekian B, Henry RG. Quantifying accuracy and precision of diffusion MR tractography of the corticospinal tract in brain tumors: Clinical article. Journal of Neurosurgery. 2014; 121(2):349-58. [DOI:10.3171/2014.4.JNS131160] [PMID]
- Bucci M, Mandelli ML, Berman JI, Amirbekian B, Nguyen C, Berger MS, et al. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. NeuroImage: Clinical. 2013; 3:361-8. [DOI:10.1016/j.nicl.2013.08.008] [PMID] [PMCID]
- Jenabi M, Peck K, Young R, Brennan N, Holodny A. Identification of the corticobulbar tracts of the tongue and face using deterministic and probabilistic DTI fiber tracking in patients with brain tumor. American Journal of Neuroradiology. 2015; 36(11):2036-41. [DOI:10.3174/ajnr.A4430] [PMID] [PMCID]
- Chung H-W, Chou M-C, Chen C-Y. Principles and limitations of computational algorithms in clinical diffusion tensor MR tractography. American Journal of Neuroradiology. 2011; 32(1):3-13. [DOI:10.3174/ajnr.A2041] [PMID] [PMCID]
- Berman JI, Chung S, Mukherjee P, Hess CP, Han ET, Henry RG. Probabilistic streamline q-ball tractography using the residual bootstrap. NeuroImage. 2008; 39(1):215-22. [DOI:10.1016/j.neuroimage.2007.08.021] [PMID]
- Qazi AA, Radmanesh A, O’Donnell L, Kindlmann G, Peled S, Whalen S, et al. Resolving crossings in the corticospinal tract by two-tensor streamline tractography: Method and clinical assessment using fMRI. NeuroImage. 2009; 47(S 2):T98-106. [DOI:10.1016/j.neuroimage.2008.06.034] [PMID] [PMCID]
- Schonberg T, Pianka P, Hendler T, Pasternak O, Assaf Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. NeuroImage. 2006; 30(4):1100-11. [DOI:10.1016/j.neuroimage.2005.11.015] [PMID]
- Javadi SA, Nabavi A, Giordano M, Faghihzadeh E, Samii A. Evaluation of diffusion tensor imaging-based tractography of the corticospinal tract: A correlative study with intraoperative magnetic resonance imaging and direct electrical subcortical stimulation. Neurosurgery. 2017; 80(2):287-99. [DOI:10.1227/NEU.0000000000001347] [PMID]
- Kinoshita M, Yamada K, Hashimoto N, Kato A, Izumoto S, Baba T, et al. Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage. 2005; 25(2):424-9. [DOI:10.1016/j.neuroimage.2004.07.076] [PMID]
- Tournier J-D, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P. Resolving crossing fibres using constrained spherical deconvolution: Validation using diffusion-weighted imaging phantom data. NeuroImage. 2008; 42(2):617-25. [DOI:10.1016/j.neuroimage.2008.05.002] [PMID]
- Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007; 35(4):1459-72. [DOI:10.1016/j.neuroimage.2007.02.016] [PMID]
- Yu CS, Li KC, Xuan Y, Ji XM, Qin W. Diffusion tensor tractography in patients with cerebral tumors: A helpful technique for neurosurgical planning and postoperative assessment. European Journal of Radiology. 2005; 56(2):197-204. [DOI:10.1016/j.ejrad.2005.04.010] [PMID]
Type of Study: Review |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



