Sat, Jul 5, 2025
Volume 8 - Special Issue
Iran J Neurosurg 2022, 8 - Special Issue: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirahmadi Eraghi M, Javadi S A H, Mortazavi M, Mosallami Aghili S M, Pestei S K. Application of 5-ALA Fluorescence-guided Resection in Patients Suffering From High-grade Gliomas: A Report of 30 Consecutive Cases and a Literature Review. Iran J Neurosurg 2022; 8 : 6
URL: http://irjns.org/article-1-308-en.html
URL: http://irjns.org/article-1-308-en.html
Mohammad Mirahmadi Eraghi1 

 , Seyed Amir Hossein Javadi *2
, Seyed Amir Hossein Javadi *2 

 , Martin Mortazavi3
, Martin Mortazavi3 

 , Seyed Morsal Mosallami Aghili4
, Seyed Morsal Mosallami Aghili4 

 , Seyed Khalil Pestei5
, Seyed Khalil Pestei5 




 , Seyed Amir Hossein Javadi *2
, Seyed Amir Hossein Javadi *2 

 , Martin Mortazavi3
, Martin Mortazavi3 

 , Seyed Morsal Mosallami Aghili4
, Seyed Morsal Mosallami Aghili4 

 , Seyed Khalil Pestei5
, Seyed Khalil Pestei5 


1- School of Medicine, Qeshm International Branch, Islamic Azad University, Qeshm, Iran AND Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
2- Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, IranAND Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran ,javadi1978@yahoo.com
3- California Institute of Neuroscience, Los Angeles, Ca, USA
4- Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
5- Department of Anesthesiology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
2- Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, IranAND Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran ,
3- California Institute of Neuroscience, Los Angeles, Ca, USA
4- Department of Neurosurgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
5- Department of Anesthesiology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
Keywords: 5-aminolevulinic acid (5-ALA), Fluorescence-guided surgery, High-grade glioma, Resection, Case series
Full Text [PDF 1243 kb]
(874 Downloads)
| Abstract (HTML) (2426 Views)
Full Text: (1540 Views)
1. Introduction
Gliomas are responsible for nearly 80% of all primary malignant brain tumors contributing to approximately 18,000 new diagnoses and 13,000 fatalities annually in the US [1]. Glioma surgery aims to maximize the extent of the tumor’s resection leading to a higher level of progression-free survival (PFS) rates, overall survival (OS), and patient quality of life (QOL) [2]. Strong evidence determines the extent of resection (EOR) as a significant predictor of promising OS, PFS, and patient quality of life (QOL) in patients with high-grade gliomas (HGG) [3, 4, 5, 6, 7].
Current technologies in this quest include 5-aminolevulinic acid (5-ALA)-induced fluorescence image-guided surgery, fluorescein image-guided surgery (FIGS), intraoperative ultrasound (IU), and intraoperative magnetic resonance imaging (iMRI). The application of the aforementioned evolving methods is expanding due to their capability to circumvent the brain shift amid the resection of HGG lesions, thereby maintaining the integrity of uninvolved brain tissue intraoperatively and providing subsequent maximal safe surgical resection for HGG suffers [6]. Conventionally, the tumor territory is identified by the gadolinium-enhancing region observed on MRI sequences. HGGs impair the integrity of the blood-brain barrier (BBB), leading to extravascular leakage of gadolinium-based contrast agents (GBCA). On the one hand, an appreciable amount of malignancy cells commonly infiltrate the radiologically enhancing region, compromising the specificity of gadolinium-based contrast agents (GBCA) where the BBB has not yet been disrupted. On the other hand, gross total resection (GTR) is only feasible in a low percentage of suffers [8, 9]. Accordingly, establishing a definitive intraoperative method to distinguish neoplastic tissue from normal brain parenchyma remains challenging during the surgery.
Recently, fluorescence-guided procedures have gained increasing attention [5, 10, 11]. Multiple fluorescent biomarkers have been demonstrated to possess promising outcomes regarding residual tumor identification and intraoperative navigation [5]. 5-ALA, which represents the only fluorescent therapeutic agent currently authorized by the US Food and Drug Administration (FDA) to be employed in HGG resection [12], was firstly used by Stummer in fluorescence-guided surgery of glioma patients in 1998 [11]. 5-ALA, as the intermediate precursor of heme biosynthesis, is metabolized to protoporphyrin IX (PpIX). It has been well established either in vitro or in vivo that abundant 5-ALA yields selective accumulation of PpIX in malignant glioma, whereby we can visualize a bright pink or red fluorescence region after being provoked by 370-440 nm wavelength light under the appropriate filter [5, 10, 12, 13]. Despite the auspicious outcomes of the above-mentioned agent in the context of complete resection rate and the 6- month PFS in patients suffering glioma, some serious drawbacks must be considered, e.g. costing approximately $1124 for each application, the necessity of drug administration orally 2.5 to 3.5 hours before anesthesia induction, and the necessity of prohibiting the direct exposure to strong room light or sunlight in the1st 24 hours of using due to the probability of skin sensitization and drug’s phototoxicity [9, 10, 12].
Here, a comprehensive literature review regarding patients with HGGs and quantifying the effect of 5-ALA guided surgery was established. Further attempts are warranted to establish the efficacy of this technique and to resolve and quantify the effect.
2. Case Presentation
In this retrospective study, 30 cases of HGG were included and operated on using oral 5-ALA. Functional MRI was performed during preoperative planning with bilateral blood oxygen level-dependent (BOLD) analysis to identify functional areas, esp. the motor cortex. Preoperative diffusion tensor imaging (DTI) tractography of bilateral corticospinal tract (CST), arcuate fasciculus, and optic radiation was done with iPlan® cranial 3.0 software (Brainlab, Feldkrichen, Germany) to evaluate the local anisotropy and illustrate the associated white matter structures. Prospective cortical depth-dependent motion, correction smoothing, and eddy current corrections were established to provide maximal quality of the images. Corticospinal fiber tracking was determined by selecting a predetermined precentral gyrus and ipsilateral cerebral peduncle. Contrast-enhanced T1 weighted (T1W) and T2 weighted (T2W) MRI were applied to obtain the target and volumetric evaluation of the core cellular part of contrast-enhancing and minimal/no contrast enhancement gliomas, respectively. Also, fluid-attenuated inversion recovery (FLAIR) images were considered to analyze the peripheral infiltrative area of the contrast-enhancing lesions. Through atlas data, manually object creation, segmentation of the anatomic structures, and tumor lesion was performed with or without automatic segmentation. The intraoperative navigation system was used to import the data. Reconstructed 3D images were installed on the navigation system. Oral 5-ALA was ingested with a dose of 20 mg/kg 4 hours prior to operation. All patients underwent general anesthesia (GA) with intraoperative neuro-monitoring. Neuro-navigation system was applied along with DESS and neuromonitoring, including motor-evoked potentials (MEPs). Central sulcus was detected with phase reversal of somatosensory evoked potential (SEP) since all of the tumors involved the motor area and CST. Tumor resection was continued until the wall of the resection cavity had reached the area of CST.
Under the microscope, borders of resection were assessed for 5-ALA using blue light and a filter. Tumors colored in pink/red with 5-ALA were resected and compared with neuro-navigation data in defining the borders of the tumor. The new samples were sent for pathology to confirm tumor involvement,
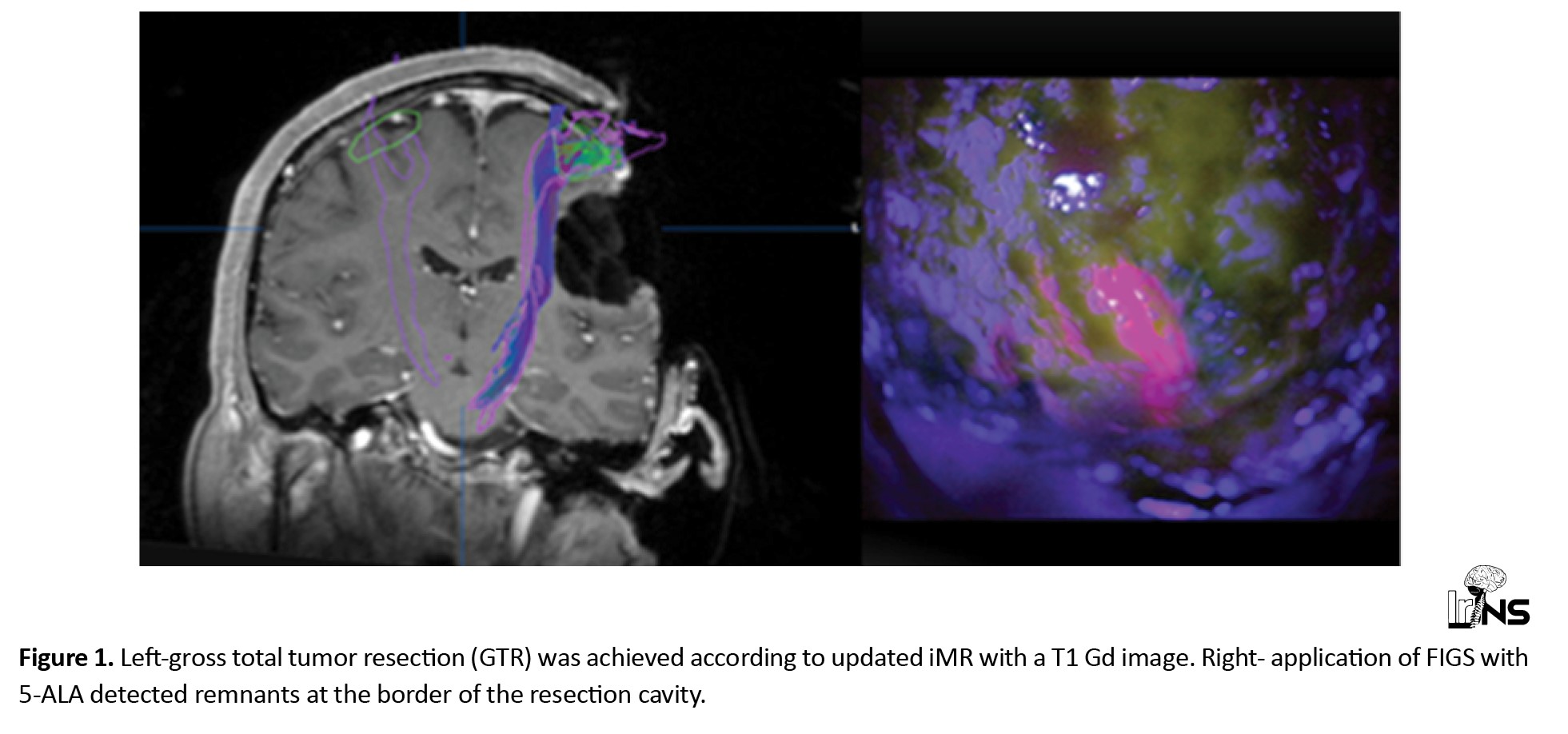
See further details in our previous publication, which coincided with the current settings [14] (Figure 1). Pathologies included 21 patients with Glioblastoma (GBM) (70%) and 9 patients with anaplastic astrocytoma (30%). Total gross removal was achieved at 90% (n=27). The use of 5-ALA increased the EOR by 40% (n=12) regarding T1 gadolinium (Gd) MR. Pathological assessment of resected parts with 5-ALA confirmed tumor infiltration. Subtotal removal was possible in 10% due to tumor invasion in the pyramidal tract. The mean preoperative volume of the tumor, according to T1 Gd MR and FLAIR images, was 16.8 cm3 and 47.6 cm3, respectively. (Range 8-105 cm3). The mean volume of tumor in FLAIR images was three times more than T1 Gd MRI, which indicated infiltrating part. Preoperative neurological status included 11 patients with motor paresis, 4 with seizures, and 15 intact.
All patients improved postoperatively regarding motor deficits and or seizures. No new permanent neurological deficits were detected in 3 months follow-up.
3. Discussion
HGG stands for grades III-IV World Health Organization (WHO) classification system of intra-axial central nervous system (CNS) lesions with an annual incidence rate ranging from 3.3- to 6 per 100 000 individuals [15, 16]. Pathologically, GBM and anaplastic astrocytoma were positive in 70%, and 30% of the suffers, respectively.
According to the Stupp protocol, the recommended standard of care for HGGs consists of GTR, temozolomide, and radiation therapy [11]. Due to the presence of BBB, further challenges are imposed to establish a successful therapeutic approach compared to other systemic malignancies. Surgical resection with accompanying concomitant/adjuvant chemoradiotherapy remains the cornerstone for the management; however, it is accompanied by a high mortality rate and a median survival period of merely 16 months [4, 17].
Emerging uses of 5-aminolevulinic acid (5-ALA)
Evolved intraoperative technologies are emerging to ensure maximal safe surgical resection in HGGs [7]. In this instance, fluorescein sodium-guided surgery, ALA-FIGS, folliculin (FLCN)-FIGS, intraoperative MRI (IoMRI), and intraoperative ultrasound (IoUS) are the leading alternatives to fulfill the aforementioned necessity [10, 12, 15, 18, 19, 20, 21, 22, 23, 24]. 5-ALA represents the most robustly studied fluorescent emerging tool in managing malignant gliomas to maximize tumor resection [11, 21]. The 1st application of well-tolerated fluorescence-guided neurosurgery with 5-ALA was reported in 1998 by Stummer and collaborators [25]. Following the initial promising results, they triggered the 1st randomized controlled multicenter phase III trial examining the effect of 5-ALA fluorescence-guided resection on the PFS and the EOR in patients with HGGs [26]. Gradually, it gained more attention annually and, in turn, led to the approval of 5-ALA to be utilized for HGG resection by the European :union: and FDA as an assistant intraoperative optical neuro-imaging agent in 2007 and 2017, respectively [27].
The greater the extent of total resection, the much longer the overall survival of the patients
Recent literature trends corroborate the significant positive correlation of GTR on preferable PFS, OS, and lower tumor recurrence rate in those harboring HGGs [10, 12, 28]. However, precisely total resection of HGGs is still challenging due to the Infiltration characteristics of such lesions. Several visualization techniques effectively fulfill the need for maximized EOR [11]. Concerning observational and clinical trials, data regarding how much supplementary improvement in EOR was achieved via 5-ALA vary across studies, ranging from 25% to 94% in GTR [29]. In phase III randomized control trial (RCT), Stummer and collaborators found a complete resection rate of 65% and 36% in those undergoing 5-ALA aided resection and those operated under a standard white light source, respectively. Maximal resection rate of 100% is suggested for GTR >90% and 93% for GTR >98% [30].
Above mentioned findings were compared to the results of a recent meta-analysis conducted by Gandhi and collaborators in the 998 HGG suffers undergoing 5-ALA aided resection, which was accompanied by approximately 76% and 77% in the rate of GTR in the cohort of all HGG suffers and in the subgroup of those suffering primary HGG, respectively [31]. These varying resections’ rates and the causality of such disparity must be further inspected. The current series confirms the improvement of EOR concerning T1Gd MR in HGGs using FIGS. Moreover, due to the invasive characteristics of tumors in the pyramidal tract, the subtotal removal rate was 10%.
As GTR is regarded as the most significant independent prognostic index of survival in HGG suffers, these promising results raise concerns regarding the utilization of fluorescence-guided resection surgery to achieve the optimal management of such lesions [31].
By examining in vitro or in vivo investigations, an increase in the establishment of 5-ALA has been observed as a feasible intraoperative photodynamic therapeutic choice for residual tumors [32, 33, 34, 35].
Early in vivo investigations have reflected promising outcomes. As 5-ALA does not carry cytotoxic effects through systemic administration and reflects a favorable adverse effect profile compared to other photosensitizer therapies, it can potentially serve beyond its role as the diagnostic agent for aggressive gliomas. Of note, 5-ALA and similar optical agents provide diagnostic markers and should not be employed as a means of interventional therapeutic intentions [31].
Combining 5-ALA with intraoperative technological modalities is significantly correlated with higher survival when using 5-ALA with each intraoperative i-CT, iMRI, ioUS, or contrast-enhanced ultrasound (CEUS) compared with the surgery solely with 5-ALA [21].
Tsugu and collaborators acknowledged the benefits of iMRI in those harboring negative fluorescence, raising the GTR from 0% to 55.6% [36]. Nevertheless, no considerable resection benefit with the adjunctive utilization of iMRI in those representing strong PpIX fluorescence emission was observed. Barbagallo and collaborators found no statistically significant benefit of intraoperative 5-ALA florescent utilization in conjunction with i-CT rather than 5-ALA alone in the context of the PFS and OS [37].
Conversely, based on a retrospective study conducted by Roder and collaborators, complete resection of the contrast-enhancing tumor rates with iMRI in conjunction with 5- ALA rather than 5-ALA alone was reported to be 74% and 45%, respectively [38]. In another study by Yamada and collaborators, including 99 consecutive surgical cases suffering malignant glioma executed with 5- ALA–guided surgery with accompanying iMRI, a mean resection rate of 95% was measured, highlighting the fact that 5-ALA yields marginal residual tumor identification beyond its radiological boundaries on iMRI [39]. Additionally, a recent systematic review conducted by Coburger and collaborators supported a superior resection rate of tumors’ margins beyond its radiological contrast enhancement following the simultaneous utilization of iMRI amid the 5-ALA-guided surgery [40]. Appreciable drawbacks of iMRI, including highly demanded facilities as well as prolonged surgical time, should be considered. In the current study, the mean volume of tumor 3D FLAIR images was three folds more than T1GdMRI (47.6 cm3 vs 16.8 cm3). Pepa and collaborators evaluate the simultaneous utilization of intraoperative 5-ALA with CEUS implying a higher EOR following resection solely with 5-ALA, CEUS, and conventional surgical modalities [41]. Neidert and collaborators declared that ioUS used in conjunction with 5-ALA yields a higher OS than surgery solely with 5-ALA [42].
The underlying mechanism whereby 5-ALA mainly targets malignant cells remains poorly understood [19]. 5-ALA represents a natural precursor used by mammalian cells which are converted to PpIX within the cellular heme biosynthetic pathway [22]. In malignant cells, the ferrochelatase (FeC) enzyme is used to convert the PpIX into the final product heme by adding Fe yielding the rate-limiting step in the biosynthesis of heme [43]. Hence, excess ALA either through injection or enteral administration provides more accumulated untransformed PpIX along with FeC deficiency [22]. To provide more insight into the causality of the increased amount of PpIX, the activities of FeC, ALA-dehydratase, and porphobilinogen deaminase were simultaneously assessed. Consequently, the activation of the aforementioned enzymes was negatively correlated with the mitochondrial amount of PpIX in the tumor cells [44].
Intracellular accumulated PpIX highly absorbs light between 375 and 440 nm, resulting in a high affinity for HGGs’ cells by reflecting observable intra-operative fluorescence with a peak wavelength at 635 nm (ranging from 620 to 710 nm) under an appropriate optical microscopic filter [11, 45]. Central core areas of infiltrated lesions are identified by fluoresce called bright ‘red’, with a margin of lighter ‘pink’ fluorescence in the surrounding area [45]. Harada and collaborators declared that PpIX possesses a very strong absorbance, i.e. the “Soret band” and four absorbance bands, i.e. “Q bands”, at roughly 405 nm, and 480- 650 nm wavelengths, respectively. Lesions harboring exceed PpIX are prone to emit the robust red fluorescence band at a wavelength of 635 nm following excitation by a violet-blue light at approximately 405 nm [46]. Although PpIX is synthesized in normal tissues, fluorescence cannot macroscopically be measured because the insignificant amount of accumulated PpIX enables the surgeons to make a proper differentiation between malignant lesions and normal brain tissues. In other words, tissues reflecting the red fluorescence contain a considerable amount of PpIX and indicate probable tumor tissues, while others without the red fluorescence contain few amounts of PpIX and consequently indicate the normal tissues [44]. Accordingly, a filter-fitted microscope capable of exciting PpIX ranging from 375 to 410 nm and illustrating red emission in a spectrum between 620 and 710 nm should be used [47].
Positive predictive value (PPV)
A large body of literature highlights the excellent diagnostic accuracy of 5-ALA-guided resection. In a prospective, phase II trial, Nabavi, and collaborators investigated biopsies rose from normal or abnormal areas under white light and estimated a positive predictive value (PPV) of 97.2% for abnormal fluorescing biopsies [48]. This rate was higher for tissues with robust fluorescence (91.7%) than weak fluorescence (82.4%), highlighting that the adjuvant therapeutic regime and the recent surgical scar did not compromise the diagnosis of a 5-ALA-mediated malignancy in the recurrent cases. A case report elucidated the beneficial outcome of intra-operative 5-ALA induced fluorescence-guided surgery in a patient receiving bevacizumab (an angiogenesis inhibitor) that impaired lesson imaging via MRI [49]. The negative predictive value attributed to 5-ALA-associated fluorescence in primary surgery ranges from 40% to 60% [50].
In comparing PFS and OS, PFS provides a more favorable primary study endpoint regarding survival analysis due to consideration of non-study-associated therapies affecting survival, narrow follow-up time, and noting the multiple reasons for death [31]. 5-ALA fluorescence-guided has been depicted to be effective and safe with minimal adverse effects improving the PFS rate and overall tumor resection compared to the lack thereof [9, 21, 26, 31, 51, 52]. In this regard, Hansen and collaborators made a comparison between 5- ALA-guided and fluorescein-guided resection of HGGs, in which PFS was significantly established at 8.7 months and 9.2 months, respectively [53].
In a phase III RCT of 322 HGG patients conducted by Stummer and collaborators, 5-ALA guided resection was well demonstrated to be associated with a higher complete resection of the contrast-enhancing lesion (65% versus 36%, P<0.0001), and a higher 6-month PFS (41.0% versus 21.1%, P=0.0003) [26]. However, OS was not significantly different between the aforementioned groups. In a retrospective study of patients suffering from glioblastoma receiving 5-ALA guided surgery, patients who had no residual tissue fluorescence demonstrated higher median OS than residual fluorescence (27 months versus 17.5 months, P=0.015) [55].
Lacroix and collaborators reported a significant survival of 13 months with resection of >98% and 8.8 months with resection of <98% of the tumor’s mass [28]. On the contrary, Barbagallo and collaborators found no significant correlation between the PFS and OS of 5-ALA guided resection v.s 5-ALA + intraoperative CT [37].
Overall, PFS and OS of the 5-ALA guided surgery in HGGs were longer compared to the white light control. According to the latest systematic review conducted by Eatz and collaborators, among all studies that directly compared the efficacy of 5-ALA with the control group, 5-ALA was associated with a greater PFS and OS in 88.4% and 67.5% of suffers, respectively [21].
Almost 10% of biopsies do not fluoresce in patients suffering from glioma infiltration [51]. The amount of malignant volume and subsequent histopathological grade will, in turn, affect the process of intra-operative fluorescence observation ranging from 95.4% to 24.1%–26.3% in World Health Organization (WHO) grade IV glioblastomas and grade I/II gliomas, respectively [55].
Given biological factors as well as different study designs, the wide variability of sensitivities and specificities in terms of 5-ALA guided surgery for gliomas patients has been reported, including sensitivities ranging from 21% to 95% and specificities ranging from 53% to 100%, respectively [47].
Timing
Despite the extensive use of 5-ALA, robust data regarding the best timing of preoperative administration are lacking [29]. According to the recent literature trend, 5-ALA may have a longer time window of detection, as previously described range of 2-4 hours [56]. In a prospective investigation of 201 tumor samples in 68 patients, Kaneko and collaborators described that maximal 5-ALA fluorescence intensity occurred at 7–8 hours after administration, in which strong fluorescence peaked 8–9 hours prior to the weak fluorescence [57]. Despite former animal investigations suggesting earlier fluorescence peaks, we have evidence supporting that the longer the latency time, the stronger fluorescence will occur in HGG lesions. According to a retrospective study of 16 patients receiving 5-ALA fluorescence 4 hours before the anesthesia induction, a significant amount of intraoperative 5-ALA was detected up to approximately 28 hours after the ingestion, without 5-ALA-associated toxicity [29].
The estimated peak of PpIX in the plasma and the skin was almost 8 hours [58], and 6.5-9.8 hours, respectively [58]. Consistent with the above-mentioned evidence, Kaneko and collaborators investigated 68 patients (201 samples) and concluded that the subsequent peak intensity might occur 7–8 hours after 5-ALA fluorescents administration. Therefore, they recommended that 5-ALA should be received earlier than what was previously suggested by FDA, particularly 4–5 hours before the anesthesia administration [59].
Microscopic
Employing a filter-fitted microscope corresponds with more accuracy in tumor identification and subsequent maximal resection [60, 61]. Richter and collaborators have integrated the utilization of spectrometric probes into their established routine of fluorescence-guided resection. They hereby indicate that PpIX was observed in 67% of the tumor marginal zone where no detectable fluorescence exists under the microscope [62]. Valdés and collaborators evaluate the use of a highly sensitive spectrally intraoperative probe, emending the distorting effects of tissue-associated optical properties. The accuracy of the tumor detection with the aid of the aforementioned probes was significantly higher compared to the lack thereof (87% versus 66%) [63]. Conventional fluorescent imaging possesses a rate of 47% in sensitivity of PpIX fluorescence, while quantitative fluorescence improves this rate up to 84% [64].
Handheld spectroscopic methods identify lower levels of PpIX fluorescence where lower tumor cell density exists. Handheld spectroscopic methods identify lower levels of PpIX fluorescence where lower tumor cell density exists [19, 47]. It represents an accuracy of 88%, a sensitivity of 72%, and a specificity of 95% [60]. Despite promising results, further translational investigations are necessary to introduce clinical tools with a narrower threshold of 5-ALA fluorescence detection.
Improving visualization by headlamps during fluorescence image-guided surgery (FIGS)
PpIX fluorescence provided by the headlamp visualization yields a sharper contrast between infiltrated and normal brain tissue. Since hemoglobin maximally absorbs the light in the fluorescent spectroscopy, whereby leads to obfuscating the fluorescence detection. Hence, this novel modality is especially suitable when the cavity is occupied by blood [64].
Potential pitfalls and drawbacks of 5-aminolevulinic acid (5-ALA)
Recent decades have witnessed the increase in the modern armamentarium to provide maximal safe resection, each with prominent advantages and drawbacks [47].
5-ALA is looked upon in an expensive manner imposing roughly $1115 USD on the patient [16]. The Zeiss OPMI-Pentero-800 microscope using a 400 nm blue filter does not offer an affordable choice, especially in developing countries or low-resource situations. Lovato and collaborators introduced a more affordable device with a total cost of approximately $380 USD, using 3D printing, with light filters enabling surgeons to fit them on any microscope and alternate between the fluorescent mode and white light [65]. The most common post-surgical neurological complications consist of motor, language, and visual. Aphasia, hemiparesis, seizures, hemianopsia, as well as, hemorrhages were also reported [21].
Photobleaching is regarded as the degradation of the PpIX fluorescence signal following prolonged light exposure, which is directly associated with the duration of exposure and also intensity of the microscope light [66]. It can alternate accurate detection or quantification of pathologies along with low fluorescence signal strength [67]. Moreover, 5-ALA-guided surgery demands intermittent switching between the white light and the blue light and different modes of the operating microscope. It also prolongs the surgery in which the higher subsequent pharmaceutical costs are possible.
Although it can be hypothesized that higher complication of 5-ALA-guided surgery in managing patients suffering HGG resection is more expected compared to white light control, the significant correlation between 5-ALA-guided surgery and more subsequent complications remains inconclusive and disparate [21]. Concerning potential complication rates of each approach based on the published studies, 5-ALA reflected a better and worse outcome than white light in 454 patients (42.2%) and 251 patients (23.3%), respectively. Hence no statistical correlation was observed between white light and 5-ALA in these 372 patients [21]. Leaving behind the remnant 5-ALA fluorescing lesions was performed in several surgeries to prohibit triggering neurological deficits. For instance, Chan and collaborators left minimal residual 5-ALA fluorescence behind in their three patients to prohibit post-surgical complications [68]. Puppa and collaborators stopped surgery in 26% of cases to avoid neurological deficits [30]; Feigl and collaborators halted 24% of their surgeries, leaving residual 5-ALA fluorescence behind to prohibit subsequent manifestations [69]. Jacquesson and collaborators also left 5-ALA fluorescence behind to prohibit subsequent deficits in 31.8% of their patients [70].
The greater extent of resection via 5-aminolevulinic acid (5-ALA) Fluorescence-guided surgery (FGS), the more neurological deterioration Is expected
According to two recent RCTs, neurological adverse events (AEs) were represented in 42.8% and 44.5% of the intervention arm (7.0% grade III-IV) and control arm (5.2% grade III-IV), respectively. Significant neurological AEs were represented in 12.4% and 11.6% of the intervention and control arms, respectively. The number of patients with deteriorated National Institutes of Health Stroke Scale was higher in the intervention arm compared to baseline at 48 hours (26.2% vs 14.5%). In contrast, 5-ALA group represented higher scale on postoperative day 7, post-operative week 6 and post-operative month 3, (20.5% vs. 10.7%), (17.1% vs. 11.3%), and (19.6% vs. 18.6%), respectively [71].
4. Conclusion
5-ALA FGS has yielded a favorable adjunctive therapeutic choice for HGGs in modern neurosurgical care. A large body of evidence has recently witnessed excellent PPV and negligible Net present value (NPV). When a robust signal is provided, it brings much confidence for surgeons to cut the normal-appearing glioma lesions under white light. However, the subsequent risk-benefit profile remains a hot matter for databases.
Limitations
The current retrospective study suffers from several drawbacks, including a small number of consecutive cases as well as a narrow spectrum of following up. Further prospective high evidence-based trials are warranted to address a firm conclusion on the efficacy of 5-ALA fluorescence-guided surgery (FGS) in managing high-grade glioma.
Ethical Considerations
Compliance with ethical guidelines
Written informed consent was obtained from the patients for the publication of their clinical details and clinical images.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Mohammad Mirahmadi Eraghi and Seyed Amir Hossein Javadi; Data collection: Martin Mortazavi; Data analysis and interpretation: Seyed Amir Hossein Javadi; Drafting the article: All authors; Critically revising the article: Mohammad Mirahmadi Eraghi and Seyed Khalil Pestei; Reviewing submitted version of manuscript: Mohammad Mirahmadi Eraghi, Martin Mortazavi, Seyed Khalil Pestei; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Gliomas are responsible for nearly 80% of all primary malignant brain tumors contributing to approximately 18,000 new diagnoses and 13,000 fatalities annually in the US [1]. Glioma surgery aims to maximize the extent of the tumor’s resection leading to a higher level of progression-free survival (PFS) rates, overall survival (OS), and patient quality of life (QOL) [2]. Strong evidence determines the extent of resection (EOR) as a significant predictor of promising OS, PFS, and patient quality of life (QOL) in patients with high-grade gliomas (HGG) [3, 4, 5, 6, 7].
Current technologies in this quest include 5-aminolevulinic acid (5-ALA)-induced fluorescence image-guided surgery, fluorescein image-guided surgery (FIGS), intraoperative ultrasound (IU), and intraoperative magnetic resonance imaging (iMRI). The application of the aforementioned evolving methods is expanding due to their capability to circumvent the brain shift amid the resection of HGG lesions, thereby maintaining the integrity of uninvolved brain tissue intraoperatively and providing subsequent maximal safe surgical resection for HGG suffers [6]. Conventionally, the tumor territory is identified by the gadolinium-enhancing region observed on MRI sequences. HGGs impair the integrity of the blood-brain barrier (BBB), leading to extravascular leakage of gadolinium-based contrast agents (GBCA). On the one hand, an appreciable amount of malignancy cells commonly infiltrate the radiologically enhancing region, compromising the specificity of gadolinium-based contrast agents (GBCA) where the BBB has not yet been disrupted. On the other hand, gross total resection (GTR) is only feasible in a low percentage of suffers [8, 9]. Accordingly, establishing a definitive intraoperative method to distinguish neoplastic tissue from normal brain parenchyma remains challenging during the surgery.
Recently, fluorescence-guided procedures have gained increasing attention [5, 10, 11]. Multiple fluorescent biomarkers have been demonstrated to possess promising outcomes regarding residual tumor identification and intraoperative navigation [5]. 5-ALA, which represents the only fluorescent therapeutic agent currently authorized by the US Food and Drug Administration (FDA) to be employed in HGG resection [12], was firstly used by Stummer in fluorescence-guided surgery of glioma patients in 1998 [11]. 5-ALA, as the intermediate precursor of heme biosynthesis, is metabolized to protoporphyrin IX (PpIX). It has been well established either in vitro or in vivo that abundant 5-ALA yields selective accumulation of PpIX in malignant glioma, whereby we can visualize a bright pink or red fluorescence region after being provoked by 370-440 nm wavelength light under the appropriate filter [5, 10, 12, 13]. Despite the auspicious outcomes of the above-mentioned agent in the context of complete resection rate and the 6- month PFS in patients suffering glioma, some serious drawbacks must be considered, e.g. costing approximately $1124 for each application, the necessity of drug administration orally 2.5 to 3.5 hours before anesthesia induction, and the necessity of prohibiting the direct exposure to strong room light or sunlight in the1st 24 hours of using due to the probability of skin sensitization and drug’s phototoxicity [9, 10, 12].
Here, a comprehensive literature review regarding patients with HGGs and quantifying the effect of 5-ALA guided surgery was established. Further attempts are warranted to establish the efficacy of this technique and to resolve and quantify the effect.
2. Case Presentation
In this retrospective study, 30 cases of HGG were included and operated on using oral 5-ALA. Functional MRI was performed during preoperative planning with bilateral blood oxygen level-dependent (BOLD) analysis to identify functional areas, esp. the motor cortex. Preoperative diffusion tensor imaging (DTI) tractography of bilateral corticospinal tract (CST), arcuate fasciculus, and optic radiation was done with iPlan® cranial 3.0 software (Brainlab, Feldkrichen, Germany) to evaluate the local anisotropy and illustrate the associated white matter structures. Prospective cortical depth-dependent motion, correction smoothing, and eddy current corrections were established to provide maximal quality of the images. Corticospinal fiber tracking was determined by selecting a predetermined precentral gyrus and ipsilateral cerebral peduncle. Contrast-enhanced T1 weighted (T1W) and T2 weighted (T2W) MRI were applied to obtain the target and volumetric evaluation of the core cellular part of contrast-enhancing and minimal/no contrast enhancement gliomas, respectively. Also, fluid-attenuated inversion recovery (FLAIR) images were considered to analyze the peripheral infiltrative area of the contrast-enhancing lesions. Through atlas data, manually object creation, segmentation of the anatomic structures, and tumor lesion was performed with or without automatic segmentation. The intraoperative navigation system was used to import the data. Reconstructed 3D images were installed on the navigation system. Oral 5-ALA was ingested with a dose of 20 mg/kg 4 hours prior to operation. All patients underwent general anesthesia (GA) with intraoperative neuro-monitoring. Neuro-navigation system was applied along with DESS and neuromonitoring, including motor-evoked potentials (MEPs). Central sulcus was detected with phase reversal of somatosensory evoked potential (SEP) since all of the tumors involved the motor area and CST. Tumor resection was continued until the wall of the resection cavity had reached the area of CST.
Under the microscope, borders of resection were assessed for 5-ALA using blue light and a filter. Tumors colored in pink/red with 5-ALA were resected and compared with neuro-navigation data in defining the borders of the tumor. The new samples were sent for pathology to confirm tumor involvement,
See further details in our previous publication, which coincided with the current settings [14] (Figure 1). Pathologies included 21 patients with Glioblastoma (GBM) (70%) and 9 patients with anaplastic astrocytoma (30%). Total gross removal was achieved at 90% (n=27). The use of 5-ALA increased the EOR by 40% (n=12) regarding T1 gadolinium (Gd) MR. Pathological assessment of resected parts with 5-ALA confirmed tumor infiltration. Subtotal removal was possible in 10% due to tumor invasion in the pyramidal tract. The mean preoperative volume of the tumor, according to T1 Gd MR and FLAIR images, was 16.8 cm3 and 47.6 cm3, respectively. (Range 8-105 cm3). The mean volume of tumor in FLAIR images was three times more than T1 Gd MRI, which indicated infiltrating part. Preoperative neurological status included 11 patients with motor paresis, 4 with seizures, and 15 intact.
All patients improved postoperatively regarding motor deficits and or seizures. No new permanent neurological deficits were detected in 3 months follow-up.
3. Discussion
HGG stands for grades III-IV World Health Organization (WHO) classification system of intra-axial central nervous system (CNS) lesions with an annual incidence rate ranging from 3.3- to 6 per 100 000 individuals [15, 16]. Pathologically, GBM and anaplastic astrocytoma were positive in 70%, and 30% of the suffers, respectively.
According to the Stupp protocol, the recommended standard of care for HGGs consists of GTR, temozolomide, and radiation therapy [11]. Due to the presence of BBB, further challenges are imposed to establish a successful therapeutic approach compared to other systemic malignancies. Surgical resection with accompanying concomitant/adjuvant chemoradiotherapy remains the cornerstone for the management; however, it is accompanied by a high mortality rate and a median survival period of merely 16 months [4, 17].
Emerging uses of 5-aminolevulinic acid (5-ALA)
Evolved intraoperative technologies are emerging to ensure maximal safe surgical resection in HGGs [7]. In this instance, fluorescein sodium-guided surgery, ALA-FIGS, folliculin (FLCN)-FIGS, intraoperative MRI (IoMRI), and intraoperative ultrasound (IoUS) are the leading alternatives to fulfill the aforementioned necessity [10, 12, 15, 18, 19, 20, 21, 22, 23, 24]. 5-ALA represents the most robustly studied fluorescent emerging tool in managing malignant gliomas to maximize tumor resection [11, 21]. The 1st application of well-tolerated fluorescence-guided neurosurgery with 5-ALA was reported in 1998 by Stummer and collaborators [25]. Following the initial promising results, they triggered the 1st randomized controlled multicenter phase III trial examining the effect of 5-ALA fluorescence-guided resection on the PFS and the EOR in patients with HGGs [26]. Gradually, it gained more attention annually and, in turn, led to the approval of 5-ALA to be utilized for HGG resection by the European :union: and FDA as an assistant intraoperative optical neuro-imaging agent in 2007 and 2017, respectively [27].
The greater the extent of total resection, the much longer the overall survival of the patients
Recent literature trends corroborate the significant positive correlation of GTR on preferable PFS, OS, and lower tumor recurrence rate in those harboring HGGs [10, 12, 28]. However, precisely total resection of HGGs is still challenging due to the Infiltration characteristics of such lesions. Several visualization techniques effectively fulfill the need for maximized EOR [11]. Concerning observational and clinical trials, data regarding how much supplementary improvement in EOR was achieved via 5-ALA vary across studies, ranging from 25% to 94% in GTR [29]. In phase III randomized control trial (RCT), Stummer and collaborators found a complete resection rate of 65% and 36% in those undergoing 5-ALA aided resection and those operated under a standard white light source, respectively. Maximal resection rate of 100% is suggested for GTR >90% and 93% for GTR >98% [30].
Above mentioned findings were compared to the results of a recent meta-analysis conducted by Gandhi and collaborators in the 998 HGG suffers undergoing 5-ALA aided resection, which was accompanied by approximately 76% and 77% in the rate of GTR in the cohort of all HGG suffers and in the subgroup of those suffering primary HGG, respectively [31]. These varying resections’ rates and the causality of such disparity must be further inspected. The current series confirms the improvement of EOR concerning T1Gd MR in HGGs using FIGS. Moreover, due to the invasive characteristics of tumors in the pyramidal tract, the subtotal removal rate was 10%.
As GTR is regarded as the most significant independent prognostic index of survival in HGG suffers, these promising results raise concerns regarding the utilization of fluorescence-guided resection surgery to achieve the optimal management of such lesions [31].
By examining in vitro or in vivo investigations, an increase in the establishment of 5-ALA has been observed as a feasible intraoperative photodynamic therapeutic choice for residual tumors [32, 33, 34, 35].
Early in vivo investigations have reflected promising outcomes. As 5-ALA does not carry cytotoxic effects through systemic administration and reflects a favorable adverse effect profile compared to other photosensitizer therapies, it can potentially serve beyond its role as the diagnostic agent for aggressive gliomas. Of note, 5-ALA and similar optical agents provide diagnostic markers and should not be employed as a means of interventional therapeutic intentions [31].
Combining 5-ALA with intraoperative technological modalities is significantly correlated with higher survival when using 5-ALA with each intraoperative i-CT, iMRI, ioUS, or contrast-enhanced ultrasound (CEUS) compared with the surgery solely with 5-ALA [21].
Tsugu and collaborators acknowledged the benefits of iMRI in those harboring negative fluorescence, raising the GTR from 0% to 55.6% [36]. Nevertheless, no considerable resection benefit with the adjunctive utilization of iMRI in those representing strong PpIX fluorescence emission was observed. Barbagallo and collaborators found no statistically significant benefit of intraoperative 5-ALA florescent utilization in conjunction with i-CT rather than 5-ALA alone in the context of the PFS and OS [37].
Conversely, based on a retrospective study conducted by Roder and collaborators, complete resection of the contrast-enhancing tumor rates with iMRI in conjunction with 5- ALA rather than 5-ALA alone was reported to be 74% and 45%, respectively [38]. In another study by Yamada and collaborators, including 99 consecutive surgical cases suffering malignant glioma executed with 5- ALA–guided surgery with accompanying iMRI, a mean resection rate of 95% was measured, highlighting the fact that 5-ALA yields marginal residual tumor identification beyond its radiological boundaries on iMRI [39]. Additionally, a recent systematic review conducted by Coburger and collaborators supported a superior resection rate of tumors’ margins beyond its radiological contrast enhancement following the simultaneous utilization of iMRI amid the 5-ALA-guided surgery [40]. Appreciable drawbacks of iMRI, including highly demanded facilities as well as prolonged surgical time, should be considered. In the current study, the mean volume of tumor 3D FLAIR images was three folds more than T1GdMRI (47.6 cm3 vs 16.8 cm3). Pepa and collaborators evaluate the simultaneous utilization of intraoperative 5-ALA with CEUS implying a higher EOR following resection solely with 5-ALA, CEUS, and conventional surgical modalities [41]. Neidert and collaborators declared that ioUS used in conjunction with 5-ALA yields a higher OS than surgery solely with 5-ALA [42].
The underlying mechanism whereby 5-ALA mainly targets malignant cells remains poorly understood [19]. 5-ALA represents a natural precursor used by mammalian cells which are converted to PpIX within the cellular heme biosynthetic pathway [22]. In malignant cells, the ferrochelatase (FeC) enzyme is used to convert the PpIX into the final product heme by adding Fe yielding the rate-limiting step in the biosynthesis of heme [43]. Hence, excess ALA either through injection or enteral administration provides more accumulated untransformed PpIX along with FeC deficiency [22]. To provide more insight into the causality of the increased amount of PpIX, the activities of FeC, ALA-dehydratase, and porphobilinogen deaminase were simultaneously assessed. Consequently, the activation of the aforementioned enzymes was negatively correlated with the mitochondrial amount of PpIX in the tumor cells [44].
Intracellular accumulated PpIX highly absorbs light between 375 and 440 nm, resulting in a high affinity for HGGs’ cells by reflecting observable intra-operative fluorescence with a peak wavelength at 635 nm (ranging from 620 to 710 nm) under an appropriate optical microscopic filter [11, 45]. Central core areas of infiltrated lesions are identified by fluoresce called bright ‘red’, with a margin of lighter ‘pink’ fluorescence in the surrounding area [45]. Harada and collaborators declared that PpIX possesses a very strong absorbance, i.e. the “Soret band” and four absorbance bands, i.e. “Q bands”, at roughly 405 nm, and 480- 650 nm wavelengths, respectively. Lesions harboring exceed PpIX are prone to emit the robust red fluorescence band at a wavelength of 635 nm following excitation by a violet-blue light at approximately 405 nm [46]. Although PpIX is synthesized in normal tissues, fluorescence cannot macroscopically be measured because the insignificant amount of accumulated PpIX enables the surgeons to make a proper differentiation between malignant lesions and normal brain tissues. In other words, tissues reflecting the red fluorescence contain a considerable amount of PpIX and indicate probable tumor tissues, while others without the red fluorescence contain few amounts of PpIX and consequently indicate the normal tissues [44]. Accordingly, a filter-fitted microscope capable of exciting PpIX ranging from 375 to 410 nm and illustrating red emission in a spectrum between 620 and 710 nm should be used [47].
Positive predictive value (PPV)
A large body of literature highlights the excellent diagnostic accuracy of 5-ALA-guided resection. In a prospective, phase II trial, Nabavi, and collaborators investigated biopsies rose from normal or abnormal areas under white light and estimated a positive predictive value (PPV) of 97.2% for abnormal fluorescing biopsies [48]. This rate was higher for tissues with robust fluorescence (91.7%) than weak fluorescence (82.4%), highlighting that the adjuvant therapeutic regime and the recent surgical scar did not compromise the diagnosis of a 5-ALA-mediated malignancy in the recurrent cases. A case report elucidated the beneficial outcome of intra-operative 5-ALA induced fluorescence-guided surgery in a patient receiving bevacizumab (an angiogenesis inhibitor) that impaired lesson imaging via MRI [49]. The negative predictive value attributed to 5-ALA-associated fluorescence in primary surgery ranges from 40% to 60% [50].
In comparing PFS and OS, PFS provides a more favorable primary study endpoint regarding survival analysis due to consideration of non-study-associated therapies affecting survival, narrow follow-up time, and noting the multiple reasons for death [31]. 5-ALA fluorescence-guided has been depicted to be effective and safe with minimal adverse effects improving the PFS rate and overall tumor resection compared to the lack thereof [9, 21, 26, 31, 51, 52]. In this regard, Hansen and collaborators made a comparison between 5- ALA-guided and fluorescein-guided resection of HGGs, in which PFS was significantly established at 8.7 months and 9.2 months, respectively [53].
In a phase III RCT of 322 HGG patients conducted by Stummer and collaborators, 5-ALA guided resection was well demonstrated to be associated with a higher complete resection of the contrast-enhancing lesion (65% versus 36%, P<0.0001), and a higher 6-month PFS (41.0% versus 21.1%, P=0.0003) [26]. However, OS was not significantly different between the aforementioned groups. In a retrospective study of patients suffering from glioblastoma receiving 5-ALA guided surgery, patients who had no residual tissue fluorescence demonstrated higher median OS than residual fluorescence (27 months versus 17.5 months, P=0.015) [55].
Lacroix and collaborators reported a significant survival of 13 months with resection of >98% and 8.8 months with resection of <98% of the tumor’s mass [28]. On the contrary, Barbagallo and collaborators found no significant correlation between the PFS and OS of 5-ALA guided resection v.s 5-ALA + intraoperative CT [37].
Overall, PFS and OS of the 5-ALA guided surgery in HGGs were longer compared to the white light control. According to the latest systematic review conducted by Eatz and collaborators, among all studies that directly compared the efficacy of 5-ALA with the control group, 5-ALA was associated with a greater PFS and OS in 88.4% and 67.5% of suffers, respectively [21].
Almost 10% of biopsies do not fluoresce in patients suffering from glioma infiltration [51]. The amount of malignant volume and subsequent histopathological grade will, in turn, affect the process of intra-operative fluorescence observation ranging from 95.4% to 24.1%–26.3% in World Health Organization (WHO) grade IV glioblastomas and grade I/II gliomas, respectively [55].
Given biological factors as well as different study designs, the wide variability of sensitivities and specificities in terms of 5-ALA guided surgery for gliomas patients has been reported, including sensitivities ranging from 21% to 95% and specificities ranging from 53% to 100%, respectively [47].
Timing
Despite the extensive use of 5-ALA, robust data regarding the best timing of preoperative administration are lacking [29]. According to the recent literature trend, 5-ALA may have a longer time window of detection, as previously described range of 2-4 hours [56]. In a prospective investigation of 201 tumor samples in 68 patients, Kaneko and collaborators described that maximal 5-ALA fluorescence intensity occurred at 7–8 hours after administration, in which strong fluorescence peaked 8–9 hours prior to the weak fluorescence [57]. Despite former animal investigations suggesting earlier fluorescence peaks, we have evidence supporting that the longer the latency time, the stronger fluorescence will occur in HGG lesions. According to a retrospective study of 16 patients receiving 5-ALA fluorescence 4 hours before the anesthesia induction, a significant amount of intraoperative 5-ALA was detected up to approximately 28 hours after the ingestion, without 5-ALA-associated toxicity [29].
The estimated peak of PpIX in the plasma and the skin was almost 8 hours [58], and 6.5-9.8 hours, respectively [58]. Consistent with the above-mentioned evidence, Kaneko and collaborators investigated 68 patients (201 samples) and concluded that the subsequent peak intensity might occur 7–8 hours after 5-ALA fluorescents administration. Therefore, they recommended that 5-ALA should be received earlier than what was previously suggested by FDA, particularly 4–5 hours before the anesthesia administration [59].
Microscopic
Employing a filter-fitted microscope corresponds with more accuracy in tumor identification and subsequent maximal resection [60, 61]. Richter and collaborators have integrated the utilization of spectrometric probes into their established routine of fluorescence-guided resection. They hereby indicate that PpIX was observed in 67% of the tumor marginal zone where no detectable fluorescence exists under the microscope [62]. Valdés and collaborators evaluate the use of a highly sensitive spectrally intraoperative probe, emending the distorting effects of tissue-associated optical properties. The accuracy of the tumor detection with the aid of the aforementioned probes was significantly higher compared to the lack thereof (87% versus 66%) [63]. Conventional fluorescent imaging possesses a rate of 47% in sensitivity of PpIX fluorescence, while quantitative fluorescence improves this rate up to 84% [64].
Handheld spectroscopic methods identify lower levels of PpIX fluorescence where lower tumor cell density exists. Handheld spectroscopic methods identify lower levels of PpIX fluorescence where lower tumor cell density exists [19, 47]. It represents an accuracy of 88%, a sensitivity of 72%, and a specificity of 95% [60]. Despite promising results, further translational investigations are necessary to introduce clinical tools with a narrower threshold of 5-ALA fluorescence detection.
Improving visualization by headlamps during fluorescence image-guided surgery (FIGS)
PpIX fluorescence provided by the headlamp visualization yields a sharper contrast between infiltrated and normal brain tissue. Since hemoglobin maximally absorbs the light in the fluorescent spectroscopy, whereby leads to obfuscating the fluorescence detection. Hence, this novel modality is especially suitable when the cavity is occupied by blood [64].
Potential pitfalls and drawbacks of 5-aminolevulinic acid (5-ALA)
Recent decades have witnessed the increase in the modern armamentarium to provide maximal safe resection, each with prominent advantages and drawbacks [47].
5-ALA is looked upon in an expensive manner imposing roughly $1115 USD on the patient [16]. The Zeiss OPMI-Pentero-800 microscope using a 400 nm blue filter does not offer an affordable choice, especially in developing countries or low-resource situations. Lovato and collaborators introduced a more affordable device with a total cost of approximately $380 USD, using 3D printing, with light filters enabling surgeons to fit them on any microscope and alternate between the fluorescent mode and white light [65]. The most common post-surgical neurological complications consist of motor, language, and visual. Aphasia, hemiparesis, seizures, hemianopsia, as well as, hemorrhages were also reported [21].
Photobleaching is regarded as the degradation of the PpIX fluorescence signal following prolonged light exposure, which is directly associated with the duration of exposure and also intensity of the microscope light [66]. It can alternate accurate detection or quantification of pathologies along with low fluorescence signal strength [67]. Moreover, 5-ALA-guided surgery demands intermittent switching between the white light and the blue light and different modes of the operating microscope. It also prolongs the surgery in which the higher subsequent pharmaceutical costs are possible.
Although it can be hypothesized that higher complication of 5-ALA-guided surgery in managing patients suffering HGG resection is more expected compared to white light control, the significant correlation between 5-ALA-guided surgery and more subsequent complications remains inconclusive and disparate [21]. Concerning potential complication rates of each approach based on the published studies, 5-ALA reflected a better and worse outcome than white light in 454 patients (42.2%) and 251 patients (23.3%), respectively. Hence no statistical correlation was observed between white light and 5-ALA in these 372 patients [21]. Leaving behind the remnant 5-ALA fluorescing lesions was performed in several surgeries to prohibit triggering neurological deficits. For instance, Chan and collaborators left minimal residual 5-ALA fluorescence behind in their three patients to prohibit post-surgical complications [68]. Puppa and collaborators stopped surgery in 26% of cases to avoid neurological deficits [30]; Feigl and collaborators halted 24% of their surgeries, leaving residual 5-ALA fluorescence behind to prohibit subsequent manifestations [69]. Jacquesson and collaborators also left 5-ALA fluorescence behind to prohibit subsequent deficits in 31.8% of their patients [70].
The greater extent of resection via 5-aminolevulinic acid (5-ALA) Fluorescence-guided surgery (FGS), the more neurological deterioration Is expected
According to two recent RCTs, neurological adverse events (AEs) were represented in 42.8% and 44.5% of the intervention arm (7.0% grade III-IV) and control arm (5.2% grade III-IV), respectively. Significant neurological AEs were represented in 12.4% and 11.6% of the intervention and control arms, respectively. The number of patients with deteriorated National Institutes of Health Stroke Scale was higher in the intervention arm compared to baseline at 48 hours (26.2% vs 14.5%). In contrast, 5-ALA group represented higher scale on postoperative day 7, post-operative week 6 and post-operative month 3, (20.5% vs. 10.7%), (17.1% vs. 11.3%), and (19.6% vs. 18.6%), respectively [71].
4. Conclusion
5-ALA FGS has yielded a favorable adjunctive therapeutic choice for HGGs in modern neurosurgical care. A large body of evidence has recently witnessed excellent PPV and negligible Net present value (NPV). When a robust signal is provided, it brings much confidence for surgeons to cut the normal-appearing glioma lesions under white light. However, the subsequent risk-benefit profile remains a hot matter for databases.
Limitations
The current retrospective study suffers from several drawbacks, including a small number of consecutive cases as well as a narrow spectrum of following up. Further prospective high evidence-based trials are warranted to address a firm conclusion on the efficacy of 5-ALA fluorescence-guided surgery (FGS) in managing high-grade glioma.
Ethical Considerations
Compliance with ethical guidelines
Written informed consent was obtained from the patients for the publication of their clinical details and clinical images.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception and design: Mohammad Mirahmadi Eraghi and Seyed Amir Hossein Javadi; Data collection: Martin Mortazavi; Data analysis and interpretation: Seyed Amir Hossein Javadi; Drafting the article: All authors; Critically revising the article: Mohammad Mirahmadi Eraghi and Seyed Khalil Pestei; Reviewing submitted version of manuscript: Mohammad Mirahmadi Eraghi, Martin Mortazavi, Seyed Khalil Pestei; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nature Clinical Practice Neurology. 2006; 2(9):494-503. [DOI:10.1038/ncpneuro0289] [PMID]
- Luzzi S, Lucifero AG, Martinelli A, Del Maestro M, Savioli G, Simoncelli A, et al. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurgical Focus. 2021; 51(2):E5. [DOI:10.3171/2021.5.FOCUS21185] [PMID]
- Li Y, Rey-Dios R, Roberts DW, Valdés PA, Cohen-Gadol AA. Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurgery. 2014; 82(1-2):175-85. [DOI:10.1016/j.wneu.2013.06.014] [PMID]
- Acerbi F, Broggi M, Schebesch KM, Höhne J, Cavallo C, De Laurentis C, et al. Fluorescein-guided surgery for resection of high-grade gliomas: A multicentric prospective phase II study (FLUOGLIO). Clinical Cancer Research. 2018; 24(1):52-61. [DOI:10.1158/1078-0432.CCR-17-1184] [PMID]
- Su X, Huang QF, Chen HL, Chen J. Fluorescence-guided resection of high-grade gliomas: A systematic review and meta-analysis. Photodiagnosis and Photodynamic Therapy. 2014; 11(4):451-8. [DOI:10.1016/j.pdpdt.2014.08.001] [PMID]
- Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; A comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis and Photodynamic Therapy. 2016; 16:35-43. [DOI:10.1016/j.pdpdt.2016.07.012] [PMID]
- Javadi SA, Khan ZH. An overview on the management of cerebral glioma of highly eloquent areas. Journal of Neurosurgical Sciences. 2019; 63(2):103-5. [DOI:10.23736/S0390-5616.18.04640-4] [PMID]
- Palmieri G, Cofano F, Salvati LF, Monticelli M, Zeppa P, Perna GD, et al. Fluorescence-guided surgery for high-grade gliomas: State of the art and new perspectives. Technology in Cancer Research & Treatment. 2021; 20:15330338211021605. [DOI:10.1177/15330338211021605] [PMID] [PMCID]
- Acerbi F, Cavallo C, Broggi M, Cordella R, Anghileri E, Eoli M, et al. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurgical Review. 2014; 37(4):547-57. [DOI:10.1007/s10143-014-0546-6] [PMID]
- Zhang N, Tian H, Huang D, Meng X, Guo W, Wang C, et al. Sodium fluorescein-guided resection under the yellow 560 nm surgical microscope filter in malignant gliomas: Our first 38 cases experience. BioMed Research International. 2017; 2017. [DOI:10.1155/2017/7865747] [PMID] [PMCID]
- Schupper AJ, Rao M, Mohammadi N, Baron R, Lee JY, Acerbi F, et al. Fluorescence-guided surgery: A review on timing and use in brain tumor surgery. Frontiers in Neurology. 2021; 12:682151. [DOI:10.3389/fneur.2021.682151] [PMID] [PMCID]
- Smith EJ, Gohil K, Thompson CM, Naik A, Hassaneen W. Fluorescein-guided resection of high grade gliomas: A meta-analysis. World Neurosurgery. 2021; 155:181-8.e7. [DOI:10.1016/j.wneu.2021.08.126] [PMID]
- Elliott JT, Wirth DJ, Davis SC, Olson JD, Simmons NE, Ryken TC, et al. Improving the usability of 5-aminolevulinic acid fluorescence-guided surgery by adding an optimized secondary light source. World Neurosurgery. 2021; 149:195-203.e4. [DOI:10.1016/j.wneu.2021.01.042] [PMID]
- Javadi SA, Nabavi A, Giordano M, Faghihzadeh E, Samii A. Evaluation of diffusion tensor imaging-based tractography of the corticospinal tract: A correlative study with intraoperative magnetic resonance imaging and direct electrical subcortical stimulation. Neurosurgery. 2017; 80(2):287-99. [DOI:10.1227/NEU.0000000000001347] [PMID]
- Catapano G, Sgulò FG, Seneca V, Lepore G, Columbano L, di Nuzzo G. Fluorescein-guided surgery for high-grade glioma resection: An intraoperative contrast-enhancer. World Neurosurgery. 2017; 104:239-47. [DOI:10.1016/j.wneu.2017.05.022] [PMID]
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: A state of the science review. Neuro-Oncology. 2014; 16(7):896-913. [DOI:10.1093/neuonc/nou087] [PMID] [PMCID]
- Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, et al. Survival and low-grade glioma: The emergence of genetic information. Neurosurgical Focus. 2015; 38(1):E6. [DOI:10.3171/2014.10.FOCUS12367] [PMID] [PMCID]
- Della Puppa A, De Pellegrin S, d’Avella E, Gioffrè G, Rossetto M, Gerardi A, et al. 5-Aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochirurgica. 2013; 155(6):965-72. [DOI:10.1007/s00701-013-1660-x] [PMID]
- Sun R, Cuthbert H, Watts C. Fluorescence-guided surgery in the surgical treatment of gliomas: Past, present and future. Cancers. 2021; 13(14):3508. [DOI:10.3390/cancers13143508] [PMID] [PMCID]
- Haider SA, Lim S, Kalkanis SN, Lee IY. The impact of 5-aminolevulinic acid on extent of resection in newly diagnosed high grade gliomas: A systematic review and single institutional experience. Journal of Neuro-Oncology. 2019; 141(3):507-15. [DOI:10.1007/s11060-018-03061-3] [PMID]
- Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME Intraoperative 5-ala fluorescence-guided resection of high- grade glioma leads to greater extent of resection with better outcomes: A systematic review. Journal of Neuro-Oncolo gy. 2022; 156(2):233-56. [DOI:10.1007/s11060-021-03901-9] [PMID]
- Kaneko S, Eljamel MS. Fluorescence image-guided neurosurgery. Future Oncology. 2017; 13(26):2341-8. [DOI:10.2217/fon-2017-0194] [PMID]
- Schebesch K-M, Proescholdt M, Höhne J, Hohenberger C, Hansen E, Riemenschneider MJ, et al. Sodium fluorescein-guided resection under the yellow 560 nm surgical microscope filter in malignant brain tumor surgery-a feasibility study. Acta Neurochirurgica. 2013; 155(4):693-9. [DOI:10.1007/s00701-013-1643-y] [PMID]
- Hong J, Chen B, Yao X, Yang Y. Outcome comparisons of high-grade glioma resection with or without fluorescein sodium-guidance. Current Problems in Cancer. 2019; 43(3):236-44. [DOI:10.1016/j.currproblcancer.2018.07.007] [PMID]
- Claire IC, Eva H, Kalman K, Harley SS, Hironobu S, Kazumi L, et al. Composite silent corticotroph pituitary adenoma with interspersed adrenocortical cells: Case report. Neurosurgery. 1998; 42(3):650-4. [DOI:10.1097/00006123-199803000-00039] [PMID]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. The lancet Oncology. 2006; 7(5):392-401. [DOI:10.1016/S1470-2045(06)70665-9]
- Kiesel B, Freund J, Reichert D, Wadiura L, Erkkilae MT, Woehrer A, et al. 5-ALA in suspected low-grade gliomas: Current role, limitations, and new approaches. Frontiers in Oncology. 2021; 11:699301. [DOI:10.3389/fonc.2021.699301] [PMID] [PMCID]
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. Journal of Neurosurgery. 2001; 95(2):190-8. [DOI:10.3171/jns.2001.95.2.0190] [PMID]
- Maragkos GA, Schüpper AJ, Lakomkin N, Sideras P, Price G, Baron R, et al. Fluorescence-guided high-grade glioma surgery more than four hours after 5-aminolevulinic acid administration. Frontiers in Neurology. 2021; 12:258. [DOI:10.3389/fneur.2021.644804] [PMID] [PMCID]
- Puppa AD, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M. 5-Aminolevulinic acid fluorescence in high grade glioma surgery: Surgical outcome, intraoperative findings, and fluorescence patterns. BioMed Research International. 2014; 2014:232561. [DOI:10.1155/2014/232561] [PMID] [PMCID]
- Gandhi S, Tayebi Meybodi A, Belykh E, Cavallo C, Zhao X, Syed MP, et al. Survival outcomes among patients with high-grade glioma treated with 5-aminolevulinic acid-guided surgery: A systematic review and meta-analysis. Frontiers in Oncology. 2019; 9:620. [DOI:10.3389/fonc.2019.00620] [PMID] [PMCID]
- Fujishiro T, Nonoguchi N, Pavliukov M, Ohmura N, Kawabata S, Park Y, et al. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis and Photodynamic Therapy. 2018; 24:58-68. [DOI:10.1016/j.pdpdt.2018.07.004] [PMID]
- Leroy HA, Vermandel M, Vignion-Dewalle AS, Leroux B, Maurage CA, Duhamel A, et al. Interstitial photodynamic therapy and glioblastoma: Light fractionation in a preclinical model. Lasers in Surgery and Medicine. 2017; 49(5):506-15. [DOI:10.1002/lsm.22620] [PMID]
- Yamamoto T, Ishikawa E, Miki S, Sakamoto N, Zaboronok A, Matsuda M, et al. Photodynamic diagnosis using 5-aminolevulinic acid in 41 biopsies for primary central nervous system lymphoma. Photochemistry and Photobiology. 2015; 91(6):1452-7. [DOI:10.1111/php.12510] [PMID]
- Shimizu K, Nitta M, Komori T, Maruyama T, Yasuda T, Fujii Y, et al. Intraoperative photodynamic diagnosis using talaporfin sodium simultaneously applied for photodynamic therapy against malignant glioma: A prospective clinical study. Frontiers in Neurology. 2018; 9:24. [DOI:10.3389/fneur.2018.00024] [PMID] [PMCID]
- Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, Yoshiyama M, et al. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurgery. 2011; 76(1-2):120-7. [DOI:10.1016/j.wneu.2011.02.005] [PMID]
- Barbagallo GM, Palmucci S, Visocchi M, Paratore S, Attinà G, Sortino G, et al. Portable intraoperative computed tomography scan in image-guided surgery for brain high-grade gliomas: Analysis of technical feasibility and impact on extent of tumor resection. Operative Neurosurgery. 2016; 12(1):19-30. [DOI:10.1227/NEU.0000000000001112] [PMID]
- Roder C, Bisdas S, Ebner FH, Honegger J, Nägele T, Ernemann U, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ala-assisted surgery. European Journal of Surgical Oncology (EJSO). 2014; 40(3):297-304. [DOI:10.1016/j.ejso.2013.11.022] [PMID]
- Yamada S, Muragaki Y, Maruyama T, Komori T, Okada Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clinical Neurology and Neurosurgery. 2015; 130:134-9. [DOI:10.1016/j.clineuro.2015.01.005] [PMID]
- Coburger J, Wirtz CR. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: A systematic review. Journal of Neuro-Oncology. 2019; 141(3):533-46. [DOI:10.1007/s11060-018-03052-4] [PMID]
- Della Pepa GM, Ius T, La Rocca G, Gaudino S, Isola M, Pignotti F, et al. 5-Aminolevulinic acid and contrast-enhanced ultrasound: The combination of the two techniques to optimize the extent of resection in glioblastoma surgery. Neurosurgery. 2020; 86(6):E529-40. [DOI:10.1093/neuros/nyaa037] [PMID]
- Neidert MC, Hostettler IC, Burkhardt J-K, Mohme M, Held U, Kofmehl R, et al. The influence of intraoperative resection control modalities on survival following gross total resection of glioblastoma. Neurosurgical Review. 2016; 39(3):401-9. [DOI:10.1007/s10143-015-0698-z] [PMID]
- Kostron H, Kaneko S, Stepp H, Eljamel S. Photodynamic medicine in neurosurgery: Biochemical, technical and clinical aspects. In: Kostron H, Hasan T, editors. Photodynamic medicine: From bench to clinic. Cambridge: Royal Society of Chemistry; 2016. [DOI:10.1039/9781782626824-00353]
- Kondo M. [Methods of determination of porphyrins and their precursors--introduction of analytical methods for porphyrin metabolites (Japanese)]. Nihon Rinsho. 1995; 53(6):1357-63. [PMID]
- Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998; 42(3):518-25. [DOI:10.1097/00006123-199803000-00017] [PMID]
- Harada K, Ohmori S, Kim Y, Tomokuni K. [Metabolic fate of porphyrin and its precursors in porphyria and porphyrinuria (Japanese)]. Nihon Rinsho. 1995; 53(6):1349-56. [PMID]
- Orillac C, Stummer W, Orringer DA. Fluorescence guidance and intraoperative adjuvants to maximize extent of resection. Neurosurgery. 2021; 89(5):727-36. [DOI:10.1093/neuros/nyaa475] [PMID] [PMCID]
- Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase ii study. Neurosurgery. 2009; 65(6):1070-6; Discussion 6-7. [DOI:10.1227/01.NEU.0000360128.03597.C7] [PMID]
- Wachter D, Kallenberg K, Wrede A, Schulz-Schaeffer W, Behm T, Rohde V. Fluorescence-guided operation in recurrent glioblastoma multiforme treated with bevacizumab-fluorescence of the noncontrast enhancing tumor tissue? Journal of Neurological Surgery Part A: Central European Neurosurgery. 2012; 73(06):401-6. [DOI:10.1055/s-0032-1304810] [PMID]
- Chohan MO, Berger MS. 5-Aminolevulinic acid fluorescence guided surgery for recurrent high-grade gliomas. Journal of Neuro-Oncology. 2019; 141(3):517-22. [DOI:10.1007/s11060-018-2956-8] [PMID]
- Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ala-induced porphyrins: A prospective study in 52 consecutive patients. Journal of Neurosurgery. 2000; 93(6):1003-13. [DOI:10.3171/jns.2000.93.6.1003] [PMID]
- Karthik L, Kumar G, Keswani T, Bhattacharyya A, Chandar SS, Bhaskara Rao K. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. Plos one. 2014; 9(3):e90972. [DOI:10.1371/journal.pone.0090972] [PMID] [PMCID]
- Hansen RW, Pedersen CB, Halle B, Korshoej AR, Schulz MK, Kristensen BW, et al. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: A single-center retrospective study. Journal of Neurosurgery. 2019; 133(5):1324-31. [DOI:10.3171/2019.6.JNS191531] [PMID]
- Aldave G, Tejada S, Pay E, Marigil M, Bejarano B, Idoate MA, et al. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery. 2013; 72(6):915-21. [DOI:10.1227/NEU.0b013e31828c3974] [PMID]
- Ji SY, Kim JW, Park C-K. Experience profiling of fluorescence-guided surgery I: Gliomas. Brain Tumor Research and Treatment. 2019; 7(2):98-104. [DOI:10.14791/btrt.2019.7.e38] [PMID] [PMCID]
- Stummer W, Stepp H, Wiestler OD, Pichlmeier U. Randomized, prospective double-blinded study comparing 3 different doses of 5-aminolevulinic acid for fluorescence-guided resections of malignant gliomas. Neurosurgery. 2017; 81(2):230-9. [DOI:10.1093/neuros/nyx074] [PMID] [PMCID]
- Dorner TE, Tröstl A, Womastek I, Groman E. Predictors of short-term success in smoking cessation in relation to attendance at a smoking cessation program. Nicotine & Tobacco Research. 2011; 13(11):1068-75. [DOI:10.1093/ntr/ntr179] [PMID]
- Rick K, Sroka R, Stepp H, Kriegmair M, Huber R, Jacob K, et al. Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood. Journal of Photochemistry and Photobiology B: Biology. 1997; 40(3):313-9. [DOI:10.1016/S1011-1344(97)00076-6] [PMID]
- Kaneko S, Molina ES, Sporns P, Schipmann S, Black D, Stummer W. Fluorescence real-time kinetics of protoporphyrin ix after 5-ala administration in low-grade glioma. Journal of Neurosurgery. 2021; 1(aop):1-7. [DOI:10.3171/2020.10.JNS202881] [PMID]
- Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, et al. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014; 74(3):310-9. [DOI:10.1227/NEU.0000000000000267] [PMID] [PMCID]
- Acerbi F, Broggi M, Eoli M, Anghileri E, Cavallo C, Boffano C, et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurgical Focus. 2014; 36(2):E5. [DOI:10.3171/2013.11.FOCUS13487] [PMID]
- Richter JC, Haj-Hosseini N, Hallbeck M, Wårdell K. Combination of hand-held probe and microscopy for fluorescence guided surgery in the brain tumor marginal zone. Photodiagnosis and Photodynamic Therapy. 2017; 18:185-92. [DOI:10.1016/j.pdpdt.2017.01.188] [PMID]
- Valdés PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, et al. Quantitative fluorescence in intracranial tumor: Implications for ala-induced ppix as an intraoperative biomarker. Journal of Neurosurgery. 2011; 115(1):11-7. [DOI:10.3171/2011.2.JNS101451] [PMID] [PMCID]
- Henderson Jr F, Belykh E, Ramos AD. Qualitative head-to-head comparison of headlamp and microscope for visualizing 5-ala fluorescence during resection of glioblastoma. Neurosurgical Focus Video. 2022; 6(1):V7. [DOI:10.3171/2021.10.FOCVID21181]
- Erdman CM, Christie C, Iqbal MO, Mazzola CA, Tomycz L. The utilization of sodium fluorescein in pediatric brain stem gliomas: A case report and review of the literature. Child’s Nervous System. 2021; 37(5):1753-8. [DOI:10.1007/s00381-020-04857-3] [PMID]
- Belykh E, Miller EJ, Patel AA, Bozkurt B, Yağmurlu K, Robinson TR, et al. Optical characterization of neurosurgical operating microscopes: Quantitative fluorescence and assessment of ppix photobleaching. Scientific Reports. 2018; 8(1):12543. [DOI:10.1038/s41598-018-30247-6] [PMID] [PMCID]
- Gol S, Pena RN, Rothschild MF, Tor M, Estany J. A polymorphism in the fatty acid desaturase-2 gene is associated with the arachidonic acid metabolism in pigs. Scientific Reports. 2018; 8(1):14336. [DOI:10.1038/s41598-018-32710-w] [PMID] [PMCID]
- Chan DTM, Sonia HY-P, Poon WS. 5-Aminolevulinic acid fluorescence guided resection of malignant glioma: Hong Kong experience. Asian Journal of Surgery. 2018; 41(5):467-72. [DOI:10.1016/j.asjsur.2017.06.004] [PMID]
- Feigl GC, Ritz R, Moraes M, Klein J, Ramina K, Gharabaghi A, et al. Resection of malignant brain tumors in eloquent cortical areas: A new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. Journal of Neurosurgery. 2010; 113(2):352-7. [DOI:10.3171/2009.10.JNS09447] [PMID]
- Jacquesson T, Ducray F, Maucort-Boulch D, Armoiry X, Louis-Tisserand G, Mbaye M, et al. [Surgery of high-grade gliomas guided by fluorescence: A retrospective study of 22 patients (French)]. Neurochirurgie. 2013; 59(1):9-16. [DOI:10.1016/j.neuchi.2012.07.002] [PMID]
- Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database of Systematic Reviews. 2018; 1(1):CD012788. [DOI:10.1002/14651858.CD012788.pub2] [PMID] [PMCID]
Type of Study: Case Series |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |