Tue, Feb 3, 2026
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 1-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kargar Jahromi H, Khorram A, Atashpoor S, Jalali M, Kargar Jahromi Z, Mohammadi F, et al . The Effect of Hydroalcoholic Extract of Salep on Pentylenetetrazole-Induced Seizure in Adult Male Rats. Iran J Neurosurg 2023; 9 : 1
URL: http://irjns.org/article-1-332-en.html
URL: http://irjns.org/article-1-332-en.html
Hossein Kargar Jahromi1 

 , Arman Khorram2
, Arman Khorram2 

 , Shekofeh Atashpoor3
, Shekofeh Atashpoor3 

 , Maryam Jalali4
, Maryam Jalali4 

 , Zahra Kargar Jahromi1
, Zahra Kargar Jahromi1 

 , Fatemeh Mohammadi5
, Fatemeh Mohammadi5 

 , Mohammad Amin Serpoosh5
, Mohammad Amin Serpoosh5 

 , Najmeh Sadeghi *6
, Najmeh Sadeghi *6 




 , Arman Khorram2
, Arman Khorram2 

 , Shekofeh Atashpoor3
, Shekofeh Atashpoor3 

 , Maryam Jalali4
, Maryam Jalali4 

 , Zahra Kargar Jahromi1
, Zahra Kargar Jahromi1 

 , Fatemeh Mohammadi5
, Fatemeh Mohammadi5 

 , Mohammad Amin Serpoosh5
, Mohammad Amin Serpoosh5 

 , Najmeh Sadeghi *6
, Najmeh Sadeghi *6 


1- Research Center for Non-Communicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran
2- Student Research Committee, Jahrom University of Medical Sciences, Jahrom, Iran
3- Department of Pharmacology, Faculty of Medicine, Jahrom University of Medical Sciences, Jahrom, Iran
4- Research Center for Social Determinants of Health, Jahrom University of Medical Sciences, Jahrom, Iran
5- Department of Basic Sciences, Sirjan School of Medical Sciences, Sirjan, Iran
6- Department of Basic Sciences, Sirjan School of Medical Sciences, Sirjan, Iran ,najmehsa@yahoo.com
2- Student Research Committee, Jahrom University of Medical Sciences, Jahrom, Iran
3- Department of Pharmacology, Faculty of Medicine, Jahrom University of Medical Sciences, Jahrom, Iran
4- Research Center for Social Determinants of Health, Jahrom University of Medical Sciences, Jahrom, Iran
5- Department of Basic Sciences, Sirjan School of Medical Sciences, Sirjan, Iran
6- Department of Basic Sciences, Sirjan School of Medical Sciences, Sirjan, Iran ,
Full Text [PDF 1204 kb]
(1582 Downloads)
| Abstract (HTML) (3647 Views)
Full Text: (1506 Views)
1. Introduction
Drugs
PTZ was purchased from Sigma-Aldrich Co. The drugs were dissolved in a saline solution (0.9%) and injected intraperitoneally (i.p.) in a volume of 1 mL/kg of the rat’s body weight.
Statistical analysis
SPSS software, version 25 was applied for statistical analysis. Data are shown as Mean±SEM. P<0.05 is considered significant. One-way analysis of variance (ANOVA) with the Duncan post hoc test was used to compare the groups.
3. Results
Effect of Salep extract on latency of tonic seizures in PTZ-induced rats
The Salep extract manifested dose-dependent effects against PTZ-induced seizures. The latency of tonic seizure (LTS) can reduce convulsing animals in the control group. Pretreatment with extract (160 and 320 mg/kg) significantly increased latency and attenuated PTZ-induced seizures (Figure 1).

Histograms represent Mean±SEM for eight animals. Different letters showed P<0.05, versus the PTZ group by ANOVA with Duncan’s test.
onset and the latency of clonic seizure (LCS)
The extract at doses of 80, 160, and 320 mg/kg can significantly alter the latency for clonic seizures compared with PTZ-treated rats (Figure 2). Histograms represent Mean±SEM for eight animals. Different letters showed P<0.05, versus the PTZ group by ANOVA with Duncan’s test.
.png)
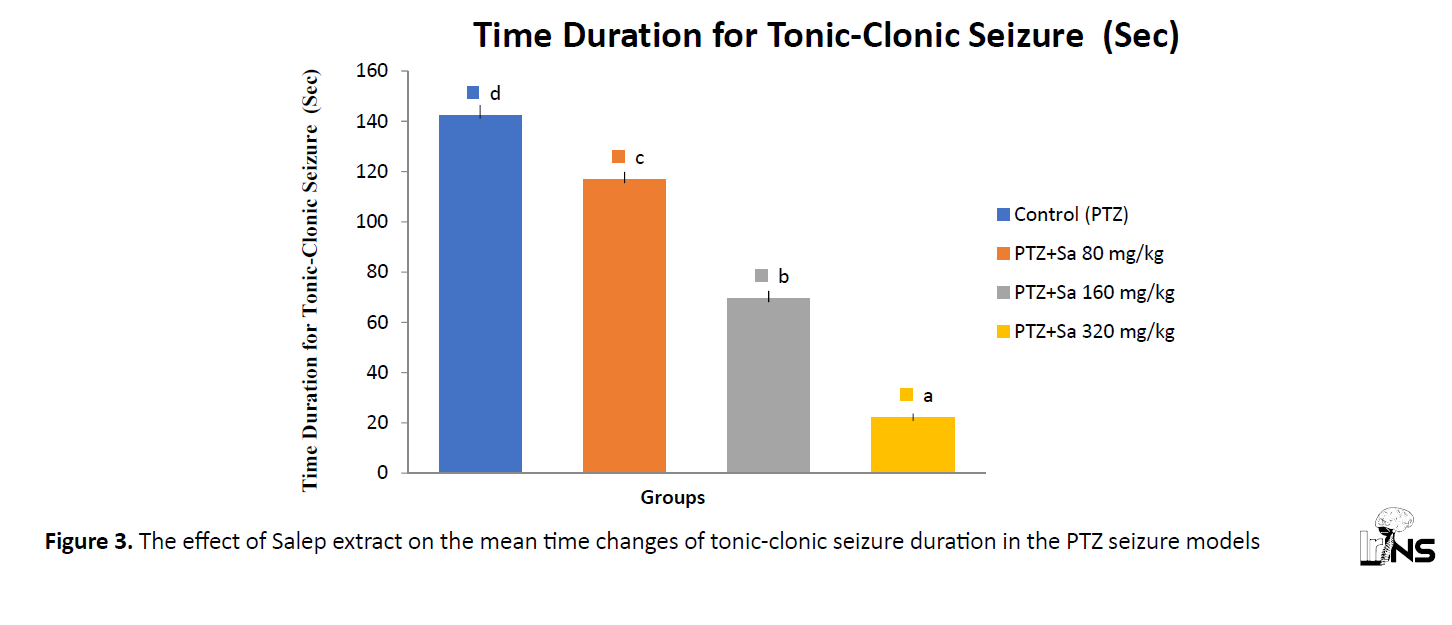
The onset and duration of tonic-clonic seizure time (DTCS)
The PTZ injection increased the onset of tonic-clonic seizure time which was significantly attenuated by extract (80, 160, 320 mg/kg) (Figure 3). Data represent Mean±SEM (n=8). Different letters showed a significant difference, (P<0.05) compared with the PTZ-injected group according to Duncan’s test.
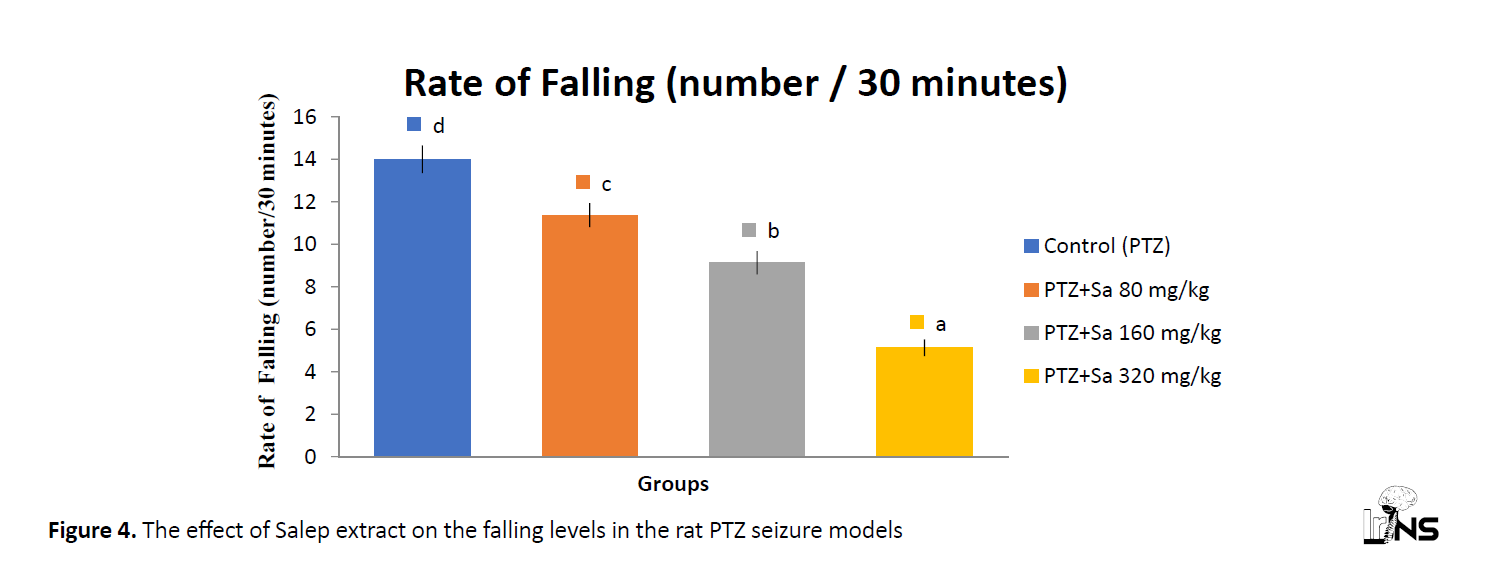
Evaluation of the (balance-falling) position
The PTZ-treated rats showed a reduction in falls. Alternatively, it was significantly increased in the PTZ-induced group (Figure 4). Data represent Mean±SEM (n=8), and different letters showed P<0.05 compared with the control.
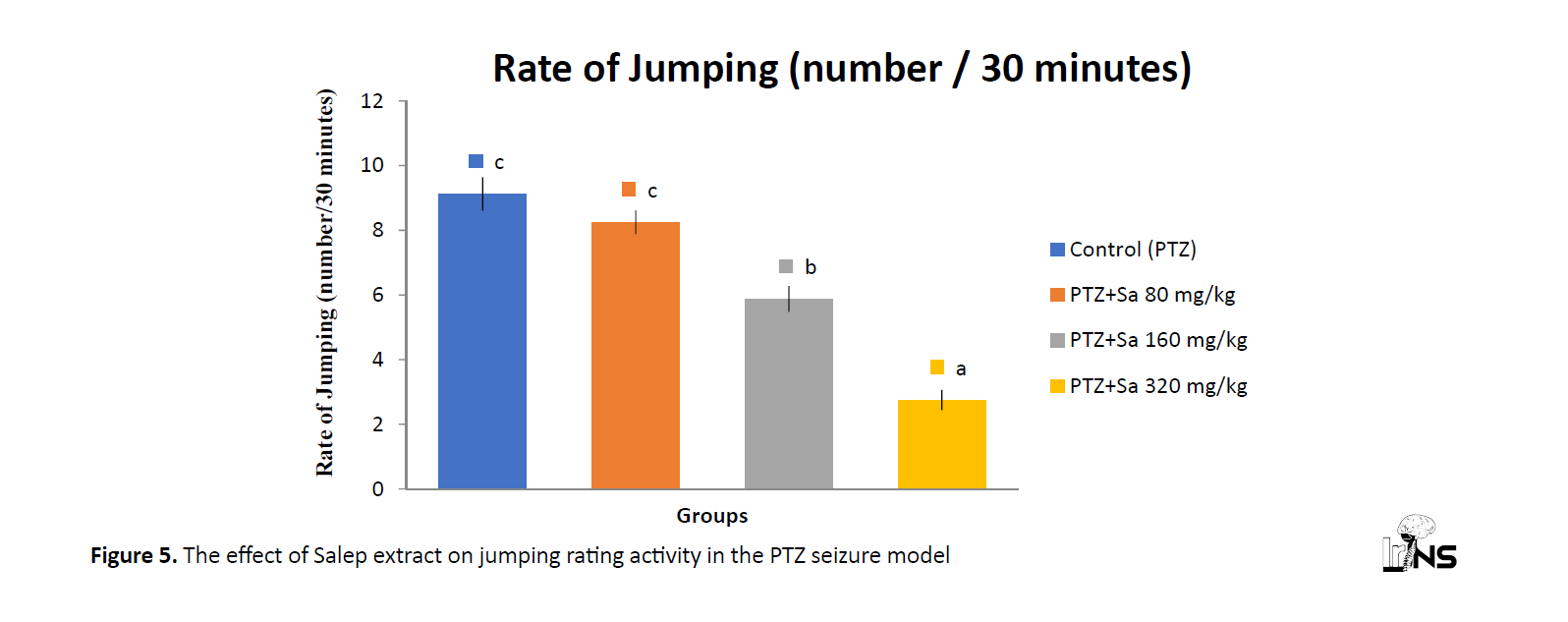
The effect of Salep extract on jumping activities in the PTZ-treated animals
The rate of jumping activities increased following the PTZ administration. However, the extract (160 and 320 mg/kg) can significantly prevent the effect of PTZ on jumping activities (Figure 5). Data represent Mean±SEM (n=8). Different letters showed P<0.05 compared with the control non-treated group according to Duncan’s test.
Time changes of seizure duration (Mean±SD)
According to the obtained results of Salep extract, an increase in duration times (min) of seizure was observed. The reduction was observed after pretreatment with Salep (Figure 6). The means in each column, which have at least one common letter, do not have any significant difference, according to Duncan’s test.
.png)
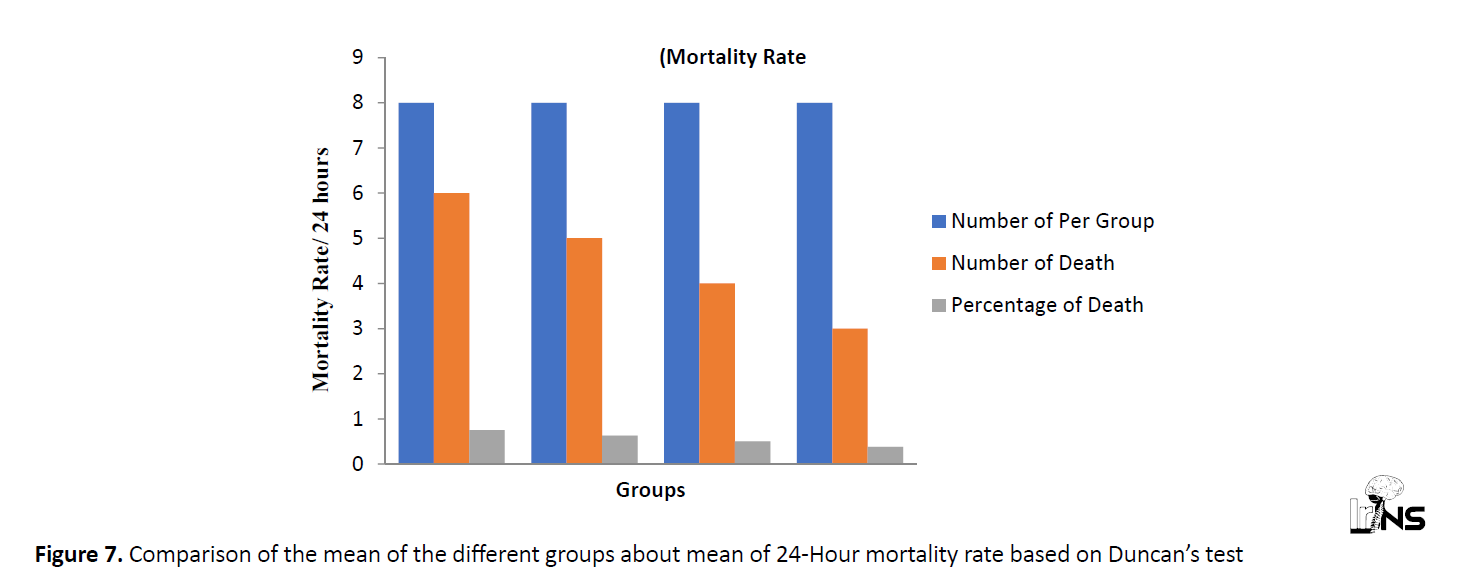
The effect of Salep extract on mortality rate in the PTZ seizure model
Compared with the control group, all groups receiving Salep extract represented a reduction in mortality rate. The highest effect belonged to the concentration of 320 mg/kg (Figure 7). The averages in each column, which have at least one common letter, did not have any significant difference, according to Duncan’s test.
4. Discussion
The results of the present study showed that Salep extract has beneficial properties for seizure. Salep extract delayed the occurrence of tonic-clonic seizure and decreased the duration of the tonic-colonial phase and the total time of the seizure. It also decreased the rats’ mortality rate up to one day after inducing a seizure and decreased the number of jumps and falls in rats. Decreasing the duration of seizure after injection of Salep extract was one of the crucial findings in this study.
Oxygen free radicals appear to be involved in epilepsy induction and post-seizure neurological disorders. It had previously been indicated that PTZ induced epileptic seizures via oxidative stress by decreasing antioxidant defense systems and increasing lipid peroxidation in erythrocytes, the liver, and the brain. Decreasing oxidative stress and preventing the activity of antioxidant enzymes leads to reducing the death of brain cells during seizure [21]. Therefore, Salep extract can play a role in protecting neurons against oxidative stress and preventing their death, through its antioxidant properties.
nan fiber, nitrogenous substances, starch, protein, sugar, hydroxybenzaldehyde, ferulic acid, quercetin, dao cholesterol, garlic, and steroids [6]. Some of these components have antioxidant activity.
Previous studies indicated that quercetin is vital as an anti-carcinogenic, anti-inflammatory, antiviral, and antioxidant agent [17, 18]. For example, quercetin administration can decrease histological aspects of acute inflammation in animals by inhibiting the release of chemokine and lipid peroxidation and increasing antioxidant enzyme activity [19]. Quercetin also shows a neuro-protective function in several central nervous system disorders, such as seizures and Huntington’s disease [20, 21] (ameliorating effect of quercetin on epilepsy by inhibition of inflammation in glial cells).
On the other hand, a study on the antioxidant properties of Selinum vaginatum (Edgew) Cl has shown that ferulic acid in this plant contains phenolic compounds and is used in traditional medicine to treat some nervous system diseases, including epilepsy [22]. The rate of gluferent species [23]. Glucomannan polysaccharide acts as an analgesic component in animal models of acute and chronic pain due to its antioxidant and anti-inflammatory properties [24]. According to the researchers’ report, the analgesic effects of glucomannan are associated with the inhibition of inflammatory reactions as well as the inhibition of neurotransmitters involved in the pain pathway, such as glutamate and N-methyl-D-aspartate (NMDA) [25]. In a study of the compounds of Satureja khuzestanica Jamzad, it is shown that this plant contains Cirsilineol, which is a derivative of flavonoids and has antioxidant, anti-inflammatory, and anti-diabetic effects [26].
5. Conclusion
The present study confirms that the induction of epilepsy by PTZ leads to a decrease in seizure onset delay and an increase in the duration of tonic-colonic seizures and the number of falling, jumping, and total seizure times. Salep extract appears to have positive effects on these impairments. Though possible mechanisms of this process were not evaluated in the current study, according to previous studies, it seems that the protective effects of Salep extract are probably due to its antioxidant effect which requires further studies to determine its exact mechanisms in the future.
Ethical Considerations
Compliance with ethical guidelines
In this study, all ethical issues regarding working with laboratory animals were considered. The research was approved by the Research Ethics Committee of Jahrom University of Medical Sciences (No.: IR.JUMS.REC.1395.151).
Funding
This research was supported by the Research Affairs of Jahrom University of Medical Sciences (Grant No.: jums-151).
Authors' contributions
Conceptualization and study design: Hossein Kargar Jahromi; Data collection: Shekoufeh Atashpour and Maryam Jalali; Data analysis and interpretation: Arman Khurram; Writing the original draft, review, editing and critically revising: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the research assistants of Jahrom University of Medical Sciences, the personnel, and the manager of the research center for their great help.
References
Epilepsy is a neurological and common disorder characterized by unpredictable and periodic seizures. About 1%-2% of the world’s population experience seizures as the second most common cause of neurological diseases [1]. The prevalence of epilepsy is also estimated at 1%-3% of the total population in Iran [2].
Epileptic patients do not respond to one-drug treatment and multi-drug therapy has many side effects. In addition, 30% of patients with recurrent seizures are resistant to anticonvulsant medications [3]. Therefore, it is necessary to find some anti-epileptic medications with lower side effects. For many years, herbal medications were used to treat diseases [4], such as pulmonary fibrosis, epilepsy, and others due to their therapeutic aspects and lower side effects [4, 5].
One of the useful plants for the treatment of disease is the Salep plant which contains compounds, including nitrogenous substances, starch, protein, sugar, hydroxybenzaldehyde, ferulic acid, quercetin, daucosterol, cirsilineol, steroids as well as glucomannan [6-8]. Previous studies have shown that the hydroalcoholic extract of root-tubers of Salep (Dactylorhiza maculata) increases total antioxidant capacity (TAC) while decreasing lipid peroxidation indicators, such as malondialdehyde and total oxidant capacity (TOC) in the rat [9]. Pentylenetetrazole (PTZ) as an antagonist of the γ-aminobutyric acid type A (GABAA) receptor induces epilepsy [10, 11].
Epilepsy is characterized by an increase in neuronal excitability, which increases energy consumption at the synapses and increases oxidative stress [12, 13]. Neuronal discharges in epilepsy consume a lot of energy, therefore inducing oxidative stress.
Oxidative stress resulting from a deficit in peroxides and antioxidants balance leads to the accumulation of toxic free radicals, therefore it can damage lipids, proteins, or nucleic acids and finally results in mutation and cell death [3]. Previous studies also indicated that treatment with anti-epileptic drugs had no significant difference in the methyl-D-aspartate level [14].
On the other hand, recent studies investigated that the increase of internal antioxidants and the prescription of external antioxidants such as lipoic acid, maxidol, tocopherol, melatonin, resveratrol, and vitamins C and E have a protective effect against some damages induced by epilepsy on the brain [15, 16].
Previous studies on animal models of epilepsy showed an increase in oxidative stress in the epileptic rats, and antioxidant effects of Salep hydroalcoholic extract. Therefore, the present study assessed the effect of Salep hydroalcoholic extract on symptoms of epilepsy induced by PTZ in male rats.
2. Materials and Methods
Sampling
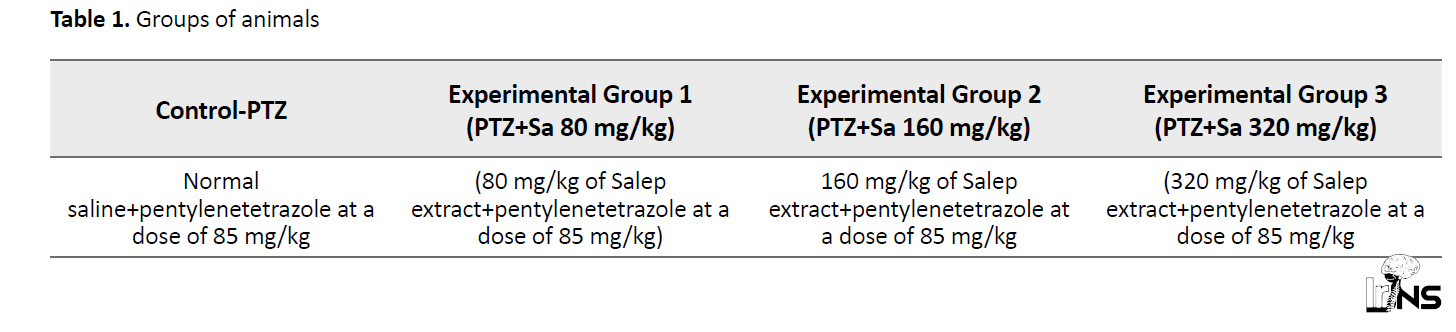
In this experimental study, 32 male Wistar rats with a mean weight of 19010 g were randomly divided into 4 groups of 8 according to Table 1.
These animals were taken from the Laboratory Animals Reproduction and Breeding Center in Jahrom University of Medical Sciences, Jahrom, Fars.
Rats were kept in the animal breeding room of Jahrom University of Medical Sciences for one week to adapt to the condition. Light-dark cycle consisted of 12 hours of lightness and 12 hours of darkness and ambient humidity was about 50% to 55%. The temperature was 23± 2°C. The prescribed concentration of Salep was specified in the amounts of 80, 160, and 320 mg per kg of body weight, according to previous articles and a pilot project [9].
In Vivo treatments
Different concentrations of Salep extract in a volume of 0.5 cc were injected intraperitoneally only once. In the evaluation process of seizure, a solvent or extract was injected intraperitoneally. After 30 minutes, 85 mg/kg of pentylenetetrazol was injected intraperitoneally into the animal. Rats were transferred to a separate cage immediately after the injection, and within 30 minutes of observation (onset time of a clonic seizure, onset time of a tonic seizure, duration of tonic-clonic seizure, total time of seizure, balance-falling position) and jumping during seizure up to 30 minutes. The mortality rate of rats in each group was assessed up to 24 hours later. The seizure stage was determined using the definitions given for each seizure below [17]:
Tonic phase: Severe stiffness of the muscles and stretching of arms and legs to the sides.
Clonic phase: A short period of seizure with twisting movements of the head and neck and movements of the hands and intense jumps and turns.
Tonic-clonic phase: Generalized seizures with sudden tonic spasms and stable epilepsy positions with very short jumps.
Seizure duration: Duration of seizure from the beginning to the end of seizure after PTZ injection.
Falling: Losing rats’ balance due to the seizure and lying on one side of the body.
Jumping: Sudden jumps higher than 20 cm above the ground.
Mortality: This quantity of seizure is discrete and recorded after observation. If the seizure is excessive, some rats die.
Salep extract preparation
We combined 100 grams of fresh and chopped root of Salep plant with 500 cc of ethanol 96 degrees and then put the mixture in a rotary machine for 24 hours at room temperature to achieve a homogeneous mixture. Then, the filtered solution was centrifuged at 3000 rpm for 5 minutes to ensure the separation of suspended particles in the plant. The obtained solution was placed in ambient conditions for 48 hours to convert into a solid, dry, alcohol-free extract. The obtained dry extract was dissolved in distilled water [9].
Determination of extract purification
Phenol determination
First, 1 mL of phenol solution was combined with 200 µL Salep extract. Then maintained in a dark place for 6 minutes. After that, 2 mL of Naco3 (7%) was added and kept for 120 minutes. The solution absorbance was measured at a wavelength of 765 nm. Finally, the total phenolic amount of extract was calculated using the standard curve of gallic acid and reported based on mg/(GAE)4/g. [18] (Table 2).
.png)
Flavonoid determination
The total flavonoid measurement was calculated based on the method of Dharmadasa et al. and reported using the quercetin standard curve as mg(QE)5/g of extract [19] (Table 2).
Anthocyanidin determination
Epileptic patients do not respond to one-drug treatment and multi-drug therapy has many side effects. In addition, 30% of patients with recurrent seizures are resistant to anticonvulsant medications [3]. Therefore, it is necessary to find some anti-epileptic medications with lower side effects. For many years, herbal medications were used to treat diseases [4], such as pulmonary fibrosis, epilepsy, and others due to their therapeutic aspects and lower side effects [4, 5].
One of the useful plants for the treatment of disease is the Salep plant which contains compounds, including nitrogenous substances, starch, protein, sugar, hydroxybenzaldehyde, ferulic acid, quercetin, daucosterol, cirsilineol, steroids as well as glucomannan [6-8]. Previous studies have shown that the hydroalcoholic extract of root-tubers of Salep (Dactylorhiza maculata) increases total antioxidant capacity (TAC) while decreasing lipid peroxidation indicators, such as malondialdehyde and total oxidant capacity (TOC) in the rat [9]. Pentylenetetrazole (PTZ) as an antagonist of the γ-aminobutyric acid type A (GABAA) receptor induces epilepsy [10, 11].
Epilepsy is characterized by an increase in neuronal excitability, which increases energy consumption at the synapses and increases oxidative stress [12, 13]. Neuronal discharges in epilepsy consume a lot of energy, therefore inducing oxidative stress.
Oxidative stress resulting from a deficit in peroxides and antioxidants balance leads to the accumulation of toxic free radicals, therefore it can damage lipids, proteins, or nucleic acids and finally results in mutation and cell death [3]. Previous studies also indicated that treatment with anti-epileptic drugs had no significant difference in the methyl-D-aspartate level [14].
On the other hand, recent studies investigated that the increase of internal antioxidants and the prescription of external antioxidants such as lipoic acid, maxidol, tocopherol, melatonin, resveratrol, and vitamins C and E have a protective effect against some damages induced by epilepsy on the brain [15, 16].
Previous studies on animal models of epilepsy showed an increase in oxidative stress in the epileptic rats, and antioxidant effects of Salep hydroalcoholic extract. Therefore, the present study assessed the effect of Salep hydroalcoholic extract on symptoms of epilepsy induced by PTZ in male rats.
2. Materials and Methods
Sampling
In this experimental study, 32 male Wistar rats with a mean weight of 19010 g were randomly divided into 4 groups of 8 according to Table 1.

These animals were taken from the Laboratory Animals Reproduction and Breeding Center in Jahrom University of Medical Sciences, Jahrom, Fars.
Rats were kept in the animal breeding room of Jahrom University of Medical Sciences for one week to adapt to the condition. Light-dark cycle consisted of 12 hours of lightness and 12 hours of darkness and ambient humidity was about 50% to 55%. The temperature was 23± 2°C. The prescribed concentration of Salep was specified in the amounts of 80, 160, and 320 mg per kg of body weight, according to previous articles and a pilot project [9].
In Vivo treatments
Different concentrations of Salep extract in a volume of 0.5 cc were injected intraperitoneally only once. In the evaluation process of seizure, a solvent or extract was injected intraperitoneally. After 30 minutes, 85 mg/kg of pentylenetetrazol was injected intraperitoneally into the animal. Rats were transferred to a separate cage immediately after the injection, and within 30 minutes of observation (onset time of a clonic seizure, onset time of a tonic seizure, duration of tonic-clonic seizure, total time of seizure, balance-falling position) and jumping during seizure up to 30 minutes. The mortality rate of rats in each group was assessed up to 24 hours later. The seizure stage was determined using the definitions given for each seizure below [17]:
Tonic phase: Severe stiffness of the muscles and stretching of arms and legs to the sides.
Clonic phase: A short period of seizure with twisting movements of the head and neck and movements of the hands and intense jumps and turns.
Tonic-clonic phase: Generalized seizures with sudden tonic spasms and stable epilepsy positions with very short jumps.
Seizure duration: Duration of seizure from the beginning to the end of seizure after PTZ injection.
Falling: Losing rats’ balance due to the seizure and lying on one side of the body.
Jumping: Sudden jumps higher than 20 cm above the ground.
Mortality: This quantity of seizure is discrete and recorded after observation. If the seizure is excessive, some rats die.
Salep extract preparation
We combined 100 grams of fresh and chopped root of Salep plant with 500 cc of ethanol 96 degrees and then put the mixture in a rotary machine for 24 hours at room temperature to achieve a homogeneous mixture. Then, the filtered solution was centrifuged at 3000 rpm for 5 minutes to ensure the separation of suspended particles in the plant. The obtained solution was placed in ambient conditions for 48 hours to convert into a solid, dry, alcohol-free extract. The obtained dry extract was dissolved in distilled water [9].
Determination of extract purification
Phenol determination
First, 1 mL of phenol solution was combined with 200 µL Salep extract. Then maintained in a dark place for 6 minutes. After that, 2 mL of Naco3 (7%) was added and kept for 120 minutes. The solution absorbance was measured at a wavelength of 765 nm. Finally, the total phenolic amount of extract was calculated using the standard curve of gallic acid and reported based on mg/(GAE)4/g. [18] (Table 2).
.png)
Flavonoid determination
The total flavonoid measurement was calculated based on the method of Dharmadasa et al. and reported using the quercetin standard curve as mg(QE)5/g of extract [19] (Table 2).
Anthocyanidin determination
The measurement of anthocyanidin was done according to the Tali Gene Pars kit and its concentration was reported based on mg/L in the extract (Table 2).
The antioxidant activity of the extract was assessed using reactive oxygen species (ROS) inhibitory method called 2,2-diphenyl-1-picryl-hydroxyl-hydrate (DPPH). First 1 mL of Salep extract was combined with 3 mL of methanolic solution of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH). Then it was kept in a dark and warm place for 30 minutes. The absorbance of the solution was read at a wavelength of 517 nm. Methanol was also used as a blank sample [20] (Table 2). Finally, the inhibitory activity was calculated based on the Equation 1:
1. % Inhibitory activity=[(Acontrol-Asample)/Aconntrol]×100
The antioxidant activity of the extract was assessed using reactive oxygen species (ROS) inhibitory method called 2,2-diphenyl-1-picryl-hydroxyl-hydrate (DPPH). First 1 mL of Salep extract was combined with 3 mL of methanolic solution of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH). Then it was kept in a dark and warm place for 30 minutes. The absorbance of the solution was read at a wavelength of 517 nm. Methanol was also used as a blank sample [20] (Table 2). Finally, the inhibitory activity was calculated based on the Equation 1:
1. % Inhibitory activity=[(Acontrol-Asample)/Aconntrol]×100
Drugs
PTZ was purchased from Sigma-Aldrich Co. The drugs were dissolved in a saline solution (0.9%) and injected intraperitoneally (i.p.) in a volume of 1 mL/kg of the rat’s body weight.
Statistical analysis
SPSS software, version 25 was applied for statistical analysis. Data are shown as Mean±SEM. P<0.05 is considered significant. One-way analysis of variance (ANOVA) with the Duncan post hoc test was used to compare the groups.
3. Results
Effect of Salep extract on latency of tonic seizures in PTZ-induced rats
The Salep extract manifested dose-dependent effects against PTZ-induced seizures. The latency of tonic seizure (LTS) can reduce convulsing animals in the control group. Pretreatment with extract (160 and 320 mg/kg) significantly increased latency and attenuated PTZ-induced seizures (Figure 1).

Histograms represent Mean±SEM for eight animals. Different letters showed P<0.05, versus the PTZ group by ANOVA with Duncan’s test.
onset and the latency of clonic seizure (LCS)
The extract at doses of 80, 160, and 320 mg/kg can significantly alter the latency for clonic seizures compared with PTZ-treated rats (Figure 2). Histograms represent Mean±SEM for eight animals. Different letters showed P<0.05, versus the PTZ group by ANOVA with Duncan’s test.
.png)
The onset and duration of tonic-clonic seizure time (DTCS)
The PTZ injection increased the onset of tonic-clonic seizure time which was significantly attenuated by extract (80, 160, 320 mg/kg) (Figure 3). Data represent Mean±SEM (n=8). Different letters showed a significant difference, (P<0.05) compared with the PTZ-injected group according to Duncan’s test.

Evaluation of the (balance-falling) position
The PTZ-treated rats showed a reduction in falls. Alternatively, it was significantly increased in the PTZ-induced group (Figure 4). Data represent Mean±SEM (n=8), and different letters showed P<0.05 compared with the control.

The effect of Salep extract on jumping activities in the PTZ-treated animals
The rate of jumping activities increased following the PTZ administration. However, the extract (160 and 320 mg/kg) can significantly prevent the effect of PTZ on jumping activities (Figure 5). Data represent Mean±SEM (n=8). Different letters showed P<0.05 compared with the control non-treated group according to Duncan’s test.

Time changes of seizure duration (Mean±SD)
According to the obtained results of Salep extract, an increase in duration times (min) of seizure was observed. The reduction was observed after pretreatment with Salep (Figure 6). The means in each column, which have at least one common letter, do not have any significant difference, according to Duncan’s test.
.png)
The effect of Salep extract on mortality rate in the PTZ seizure model
Compared with the control group, all groups receiving Salep extract represented a reduction in mortality rate. The highest effect belonged to the concentration of 320 mg/kg (Figure 7). The averages in each column, which have at least one common letter, did not have any significant difference, according to Duncan’s test.

4. Discussion
The results of the present study showed that Salep extract has beneficial properties for seizure. Salep extract delayed the occurrence of tonic-clonic seizure and decreased the duration of the tonic-colonial phase and the total time of the seizure. It also decreased the rats’ mortality rate up to one day after inducing a seizure and decreased the number of jumps and falls in rats. Decreasing the duration of seizure after injection of Salep extract was one of the crucial findings in this study.
Oxygen free radicals appear to be involved in epilepsy induction and post-seizure neurological disorders. It had previously been indicated that PTZ induced epileptic seizures via oxidative stress by decreasing antioxidant defense systems and increasing lipid peroxidation in erythrocytes, the liver, and the brain. Decreasing oxidative stress and preventing the activity of antioxidant enzymes leads to reducing the death of brain cells during seizure [21]. Therefore, Salep extract can play a role in protecting neurons against oxidative stress and preventing their death, through its antioxidant properties.
nan fiber, nitrogenous substances, starch, protein, sugar, hydroxybenzaldehyde, ferulic acid, quercetin, dao cholesterol, garlic, and steroids [6]. Some of these components have antioxidant activity.
Previous studies indicated that quercetin is vital as an anti-carcinogenic, anti-inflammatory, antiviral, and antioxidant agent [17, 18]. For example, quercetin administration can decrease histological aspects of acute inflammation in animals by inhibiting the release of chemokine and lipid peroxidation and increasing antioxidant enzyme activity [19]. Quercetin also shows a neuro-protective function in several central nervous system disorders, such as seizures and Huntington’s disease [20, 21] (ameliorating effect of quercetin on epilepsy by inhibition of inflammation in glial cells).
On the other hand, a study on the antioxidant properties of Selinum vaginatum (Edgew) Cl has shown that ferulic acid in this plant contains phenolic compounds and is used in traditional medicine to treat some nervous system diseases, including epilepsy [22]. The rate of gluferent species [23]. Glucomannan polysaccharide acts as an analgesic component in animal models of acute and chronic pain due to its antioxidant and anti-inflammatory properties [24]. According to the researchers’ report, the analgesic effects of glucomannan are associated with the inhibition of inflammatory reactions as well as the inhibition of neurotransmitters involved in the pain pathway, such as glutamate and N-methyl-D-aspartate (NMDA) [25]. In a study of the compounds of Satureja khuzestanica Jamzad, it is shown that this plant contains Cirsilineol, which is a derivative of flavonoids and has antioxidant, anti-inflammatory, and anti-diabetic effects [26].
5. Conclusion
The present study confirms that the induction of epilepsy by PTZ leads to a decrease in seizure onset delay and an increase in the duration of tonic-colonic seizures and the number of falling, jumping, and total seizure times. Salep extract appears to have positive effects on these impairments. Though possible mechanisms of this process were not evaluated in the current study, according to previous studies, it seems that the protective effects of Salep extract are probably due to its antioxidant effect which requires further studies to determine its exact mechanisms in the future.
Ethical Considerations
Compliance with ethical guidelines
In this study, all ethical issues regarding working with laboratory animals were considered. The research was approved by the Research Ethics Committee of Jahrom University of Medical Sciences (No.: IR.JUMS.REC.1395.151).
Funding
This research was supported by the Research Affairs of Jahrom University of Medical Sciences (Grant No.: jums-151).
Authors' contributions
Conceptualization and study design: Hossein Kargar Jahromi; Data collection: Shekoufeh Atashpour and Maryam Jalali; Data analysis and interpretation: Arman Khurram; Writing the original draft, review, editing and critically revising: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the research assistants of Jahrom University of Medical Sciences, the personnel, and the manager of the research center for their great help.
References
- Wright J, Pickard N, Whitfield A, Hakin N. A population-based study of the prevalence, clinical characteristics and effect of ethnicity in epilepsy. Seizure. 2000; 9(5):309-13. [DOI:10.1053/seiz.2000.0422] [PMID]
- Ashktorab T, Yadollahi S, Zayery F. [The correlation between self-management behaviors and drug adherence among people with epilepsy in Iran Epilepsy Association (Persian)]. Scientific Journal of Hamadan Nursing & Midwifery Faculty. 2013; 21(2):5-15. [Link]
- Grewal GK, Kukal S, Kanojia N, Saso L, Kukreti S, Kukreti R. Effect of oxidative stress on ABC transporters: Contribution to epilepsy pharmacoresistance. Molecules. 2017; 22(3):365. [DOI:10.3390/molecules22030365] [PMID] [PMCID]
- Ghaderkhani S, Moloudi M, Izadpanah E, Mohammadi R, Rostami A, Khom P, et al. [Effect of hydroalcoholic extract of cinnamomum on strychnine-induced seizure in mice (Persian)]. Journal of Isfahan Medical School. 2014; 32(299):1388-95. [Link]
- Hashemi A, Mohajel A, Sadeghi MR, Faramarzi A, Delazar A, Rezazadeh H. [Study of the methanolic extract of Peganum seeds on convulsion induced by Strychnine in Swiss mice (Persian)]. Pharmaceutical Sciences. 2009; 15(3):257-62. [Link]
- Cozzolino S, Widmer A. Orchid diversity: An evolutionary consequence of deception? Trends in Ecology & Evolution. 2005; 20(9):487-94. [DOI:10.1016/j.tree.2005.06.004] [PMID]
- Grieve M. A modern herbal: The medicinal, culinary, cosmetic and economic properties, cultivation and folk-lore of herbs, grasses, fungi, shrubs, & trees with all their modern scientific uses. North Chelmsford: Courier Corporation; 1971. [Link]
- Lumaga MB, Cozzolino S, Kocyan A. Exine micromorphology of Orchidinae (Orchidoideae, Orchidaceae): Phylogenetic constraints or ecological influences? Annals of Botany. 2006; 98(1):237-44. [DOI:10.1093/aob/mcl094] [PMID] [PMCID]
- Pourahmad M, Jahromi HK, Jahromi ZK. Protective effect of salep on liver. Hepatitis Monthly. 2015; 15(4):e28137. [DOI:10.5812/hepatmon.15(4)2015.28137] [PMID]
- Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant γ-aminobutyric acid type A (GABAA) receptors: Mechanism and site of action. Journal of Pharmacology and Experimental Therapeutics. 2001; 298(3):986-95. [Link]
- Namvaran-Abbasabad A, Tavakkoli Ghazani F. [The effect of Salvia officinalis hydroalcoholic extract on PTZ-induced seizure threshold in Vincristine injected mice (Persian)]. Journal of Shahrekord University of Medical Sciences. 2012; 13 (6):47-55. [Link]
- Mares J, Stopka P, Nohejlova K, Rokyta R. Oxidative stress induced by epileptic seizure and its attenuation by melatonin. Physiological Research. 2013; 62 (Suppl 1):S67-74. [DOI:10.33549/physiolres.932576] [PMID]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology. 2009; 7(1):65-74. [DOI:10.2174/157015909787602823] [PMID] [PMCID]
- Menon B, Ramalingam K, Kumar RV. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure. 2012; 21(10):780-4. [DOI:10.1016/j.seizure.2012.09.003] [PMID]
- Schmidt D, Loscher W. Drug resistance in epilepsy: Putative neurobiologic and clinical mechanisms. Epilepsia. 2005; 46(6):858-77. [DOI:10.1111/j.1528-1167.2005.54904.x] [PMID]
- van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 2005; 46(10):1569-80. [DOI:10.1111/j.1528-1167.2005.00250.x] [PMID]
- Azhdari-Zarmehri H, Naderi F, Erami E, Mohammad-Zadeh M. [Effects of Salvia Sahendica hydroalcoholic extract on PTZinduced seizure in male mice (Persian)]. Koomesh. 2013; 14(4):497-504. [Link]
- Alizadeh Behbahani B, Falah F, Lavi Arab F, Vasiee M, Tabatabaee Yazdi F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evidence-Based Complementary and Alternative Medicine. 2020; 2020:5190603. [DOI:10.1155/2020/5190603] [PMID] [PMCID]
- Dharmadasa R, Abeysinghe D, Dissanayake D, Abeywardhane K, Fernando N. Leaf essential oil composition, antioxidant activity, total phenolic content and total flavonoid content of Pimenta dioica (L.) Merr (Myrtaceae): A superior quality spice grown in Sri Lanka. Universal Journal of Agricultural Research. 2015; 3(2):49-52. [Link]
- Behbahani BA, Noshad M, Falah F. Cumin essential oil: Phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microbial Pathogenesis. 2019; 136:103716. [DOI:10.1016/j.micpath.2019.103716] [PMID]
- Velíšek L. Models of generalized seizures in freely moving animals. Reference Module in Neuroscience and Biobehavioral Psychology. 2016. [DOI:10.1016/B978-0-12-809324-5.00129-2]
- Pandey MM, Katara A, Pandey G, Rastogi S, Rawat AK. An important Indian traditional drug of ayurveda jatamansi and its substitute bhootkeshi: Chemical profiling and antioxidant activity. Evidence-Based Complementary and Alternative Medicine. 2013; 2013:142517. [DOI:10.1155/2013/142517] [PMID] [PMCID]
- Tekinşen KK, Güner A. Chemical composition and physicochemical properties of tubera salep produced from some Orchidaceae species. Food Chemistry. 2010; 121(2):468-71. [DOI:10.1016/j.foodchem.2009.12.066]
- Kargar Jahromi H, Solhjoo E, Hasannezhad A, Pourahmadi M, Sahraei R, Atashpour Sh. [Comparison of aqueous extract of Orchid's root (Dactylorhiza maculate) with ibuprofen on pain sensory thresholds using the formalin test in adult male rats (Persian)]. Journal of Jahrom University of Medical Sciences. 2020; 18(1):15-22. [Link]
- Córdova MM, Martins DF, Silva MD, Baggio CH, Carbonero ER, Ruthes AC, et al. Polysaccharide glucomannan isolated from Heterodermia obscurata attenuates acute and chronic pain in mice. Carbohydrate Polymers. 2013; 92(2):2058-64. [DOI:10.1016/j.carbpol.2012.11.041] [PMID]
- Malmir M, Encarnação S, Sousa D, Serrano R, Gohari A, Silva O. Phytochemical characterization, antioxidant and α-and β-glucosidase inhibitory potential of Satureja khuzestanica leaf traditional herbal preparations. Planta Medica. 2014; 80(16):P2B19. [DOI:10.1055/s-0034-1394896]
Type of Study: Research |
Subject:
Neuroscience
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



