Fri, May 3, 2024
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
V. Krylov V, V. Grigorieva E, A. Polunina N, A. Lukyanchikov V, A. Dalibaldyan V. Estimating Hemodynamic Significant Deformations of Brachiocephalic Arteries Using CT Perfusion. Iran J Neurosurg 2023; 9 : 7
URL: http://irjns.org/article-1-342-en.html
URL: http://irjns.org/article-1-342-en.html
Vladimir V. Krylov1 

 , Elena V. Grigorieva2
, Elena V. Grigorieva2 

 , Natalia A. Polunina *
, Natalia A. Polunina * 

 3, Viktor A. Lukyanchikov4
3, Viktor A. Lukyanchikov4 

 , Vagan A. Dalibaldyan5
, Vagan A. Dalibaldyan5 




 , Elena V. Grigorieva2
, Elena V. Grigorieva2 

 , Natalia A. Polunina *
, Natalia A. Polunina * 

 3, Viktor A. Lukyanchikov4
3, Viktor A. Lukyanchikov4 

 , Vagan A. Dalibaldyan5
, Vagan A. Dalibaldyan5 


1- Department of Fundamental Neurosurgery, Faculty of Postgraduate Education, Federal State Autonomous Educational Institution for Higher Education «Pirogov Russian National Research Medical University» Ministry of Health of the Russian Federation, Moscow, Russian Federation AND Department of Neurosurgery, N.V. Sklifosovsky Emergency Care Institute, Moscow, Russian Federation
2- Department of Clinical Medical Center, Faculty of General Medicine, Federal State Budgetary Educational Institution of Higher Education A.I. Evdokimova Moscow State University of Medicine and Dentistry, Moscow, Russian Federation3. Department of Neurosurgery and Neurointensive Care, Faculty of General Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation
3- Department of Clinical Medical Center, Faculty of General Medicine, Federal State Budgetary Educational Institution of Higher Education A.I. Evdokimova Moscow State University of Medicine and Dentistry, Moscow, Russian Federation3. Department of Neurosurgery and Neurointensive Care, Faculty of General Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation , npolunina82@yandex.ru
4- Department of Fundamental Neurosurgery, Faculty of Postgraduate Education, Federal State Autonomous Educational Institution for Higher Education «Pirogov Russian National Research Medical University» Ministry of Health of the Russian Federation, Moscow, Russian Federation AND Uniclinic Group of Companies LLC, Moscow, Russian Federation
5- Department of Neurosurgery and Neurointensive Care, Faculty of General Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation AND Department of Neurosurgery, N.V. Sklifosovsky Emergency Care Institute, Moscow, Russian Federation
2- Department of Clinical Medical Center, Faculty of General Medicine, Federal State Budgetary Educational Institution of Higher Education A.I. Evdokimova Moscow State University of Medicine and Dentistry, Moscow, Russian Federation3. Department of Neurosurgery and Neurointensive Care, Faculty of General Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation
3- Department of Clinical Medical Center, Faculty of General Medicine, Federal State Budgetary Educational Institution of Higher Education A.I. Evdokimova Moscow State University of Medicine and Dentistry, Moscow, Russian Federation3. Department of Neurosurgery and Neurointensive Care, Faculty of General Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation , npolunina82@yandex.ru
4- Department of Fundamental Neurosurgery, Faculty of Postgraduate Education, Federal State Autonomous Educational Institution for Higher Education «Pirogov Russian National Research Medical University» Ministry of Health of the Russian Federation, Moscow, Russian Federation AND Uniclinic Group of Companies LLC, Moscow, Russian Federation
5- Department of Neurosurgery and Neurointensive Care, Faculty of General Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation AND Department of Neurosurgery, N.V. Sklifosovsky Emergency Care Institute, Moscow, Russian Federation
Full Text [PDF 4305 kb]
(302 Downloads)
| Abstract (HTML) (1074 Views)
Full Text: (430 Views)
1. Introduction
In the modern structure of morbidity and mortality, acute cerebrovascular accidents occupy a leading position [1]. Up to 80% of ischemic strokes occur against the background of atherosclerotic lesions of the brachiocephalic arteries [1, 2]. Therefore, the diagnosis and treatment of occlusive-stenotic diseases of the internal carotid arteries (ICA) have become an increasingly important issue in the last decade. Modern researchers consider pathological deformities of the carotid arteries as one of the factors contributing to the development of acute cerebral ischemia, although a clear relationship between these pathologies has not been proven yet [1, 3]. The hemodynamic significance of the ICA tortuosity is determined by the morphological features of the deformities and is confirmed when local hemodynamic disorders are detected during a duplex study [1-3]. However, it remains unclear whether these local changes in the blood flow are important for the formation of cerebral perfusion deficit, which ultimately affects the volume and prevalence of acute and chronic ischemic changes in the brain.
This paper assesses the hemodynamic significance of various types of tortuosity of the ICA, including against the background of atherosclerosis, according to computed tomography (CT) angiography, and CT perfusion, to determine concomitant disorders of cerebral microcirculation.
2. Methods Materials/Patients
From January 1, 2015, until December 31, 2021, a total of 64 patients (42 women, 22 men) in the age range of 31 to 75 years were examined by a neurologist and a neurosurgeon. Pathological deformities of the ICA were revealed in 110 arteries. All patients underwent duplex color mapping of the brachiocephalic arteries with an examination by a neurologist and consultation with a neurosurgeon. In all patients, according to the preliminary examination, pathological deformities of one or both ICAs were diagnosed, often in combination with ICA stenosis of varying severity. The detection of pathological deformity of the ICA became the basis for referring patients to a multimodal CT study, which included CT of the brain, CT angiography of the brachycephalic arteries, and CT perfusion of the brain for a comprehensive assessment of the hemodynamic significance of previously diagnosed tortuosity. The study was performed on a 128-slice CT scanner Somatom Definition Flash (Siemens). A native CT scan of the brain was performed with a slice reconstruction of 0.6 mm to assess the structure of the brain parenchyma and identify the volume and localization of postischemic gliotic changes.
CT angiography of the brachiocephalic arteries was performed from the level of the aortic arch with the capture of the main vessels of the neck and the base of the arterial circle of the brain (circle of Willis) with a slice thickness of 4 mm, followed by reconstruction at 0.6 mm, against the background of intravenous injection of 60 mL of an iodine-containing contrast agent at a rate of 4.5-5.5 mL/s, with a 2-s delay. During post-processing, 2D maximum intensity projection (2 DMIP) and 3D reconstructions were obtained at the Leonardo workstation (Siemens), followed by the subtraction of bone structures for a more accurate assessment of the morphological features of the carotid arteries. The revealed deformations were characterized according to the Weibel-fields classification as tortuosity (C- and S-shaped) and looping. According to the CT angiography data, the authors considered looping (coiling) and C- and S-shaped deformities of the ICA with kinks or twists at an angle of fewer than 60 degrees (kinking) to be hemodynamically significant, with signs of an increase in the linear velocity of blood flow at the level of tortuosity, according to the results of preliminary duplex scanning more than 170 cm/s [1, 3]. In the presence of concomitant stenosis of the ampullary part of the ICA, the degree of narrowing of the artery was determined according to the criteria of the European carotid surgery trial. More than 50% of stenoses were considered hemodynamically significant [1].
CT perfusion of the brain was performed in a single block 8 cm thick, which made it possible to cover almost the entire volume of the brain, including the cerebral cortex and the stem structures. During CT perfusion, 35 mL of iodine-containing contrast agent was injected at a rate of 5.5–6 mL/s with a 6-s delay. All CT perfusions were performed at systolic blood pressure not higher than 160 mmHg and stable heart rate. Post-processing of CT perfusion data was performed using the Neuro VPCT software (Siemens Healthineers). The main indicators of perfusion were determined as follows: Cerebral blood flow (CBF), volumetric blood flow rate (mL/100 g/min); cerebral blood volume (CBV): mL/100 g; Mean transit time (MTT): s. To quantify the CT perfusion data, we determined the absolute and Mean values of the main perfusion parameters in similar areas of the cortex of both cerebral hemispheres (768 areas in total) in the frontal, temporal, and parietal-occipital areas. When calculating CT perfusion parameters, focal white matter changes and postischemic cysts were not included in the measurements. Normal indicators of CT perfusion were considered to be CBF of at least 39 mL/100 g/min (average 55–65 mL/100 g/min), CBV of at least 1.5 mL/100 g (normally 2-3 mL/100 g), MTT of no more than 6 s (normally 3-4.5 s), taking into account the data of the manufacturer and previous studies. A decrease in CBF to 39 mL/100 g/min or less in stable blood pressure was considered a neurologically significant perfusion deficiency; according to M. Wintermark et al., the presence of neurological focal symptoms was proved when CBF decreased below this value [4].
Six patients (9.4% of cases) with hemodynamically significant deformity of the ICA underwent a study with a stress test (oral intake of acetazolamide) and repeated CT perfusion 2 h after taking the drug. A decrease in the average perfusion values in comparison with the initial data on the side of hypoperfusion was regarded as a manifestation of the “steal” syndrome (with a normal increase in CBF while taking acetazolamide by 15% to 40%, which was considered a sign of intact autoregulation of cerebral blood flow.
All studies were performed in low-dose adaptation mode as follows: For CT perfusion, scanning was performed at 80 kV, 120 mAs. The total effective dose of multimodal CT for each patient was 8.5 mSv.
The data of all patients were used in statistical analysis to determine the correlation of the side of deformities and stenoses of the ICA, areas of hypoperfusion, and gliosis changes in the brain. The STATISTICA software, version 7.0 for Windows was used for statistical processing (StatSoftInc., USA). The normality of data distribution was tested using the Shapiro-Wilk test. When comparing groups on qualitative grounds, the one-sided Fisher’s exact test and the Pearson Chi-square test were used. Differences were considered statistically significant at P<0.05.
3. Results
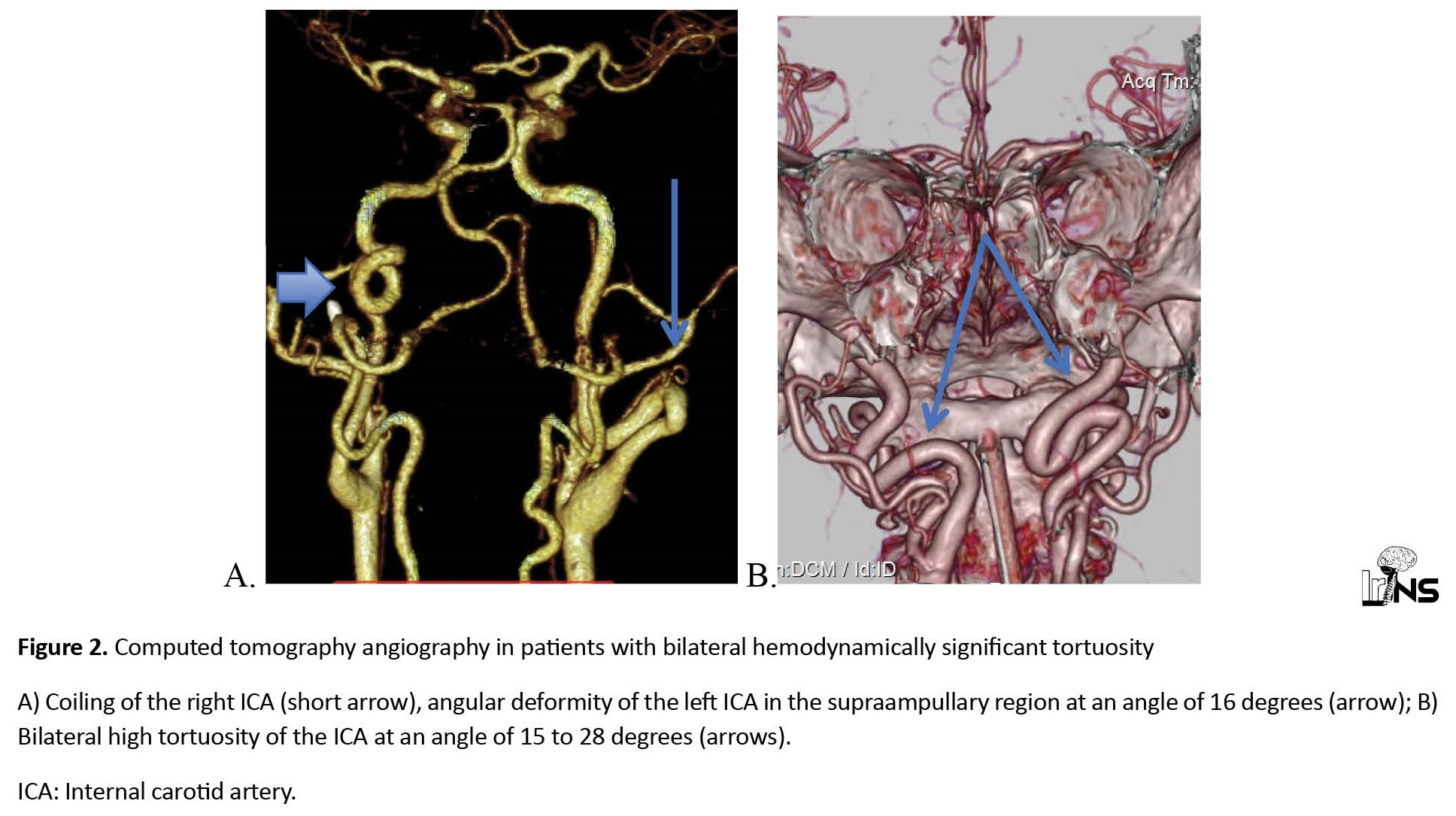
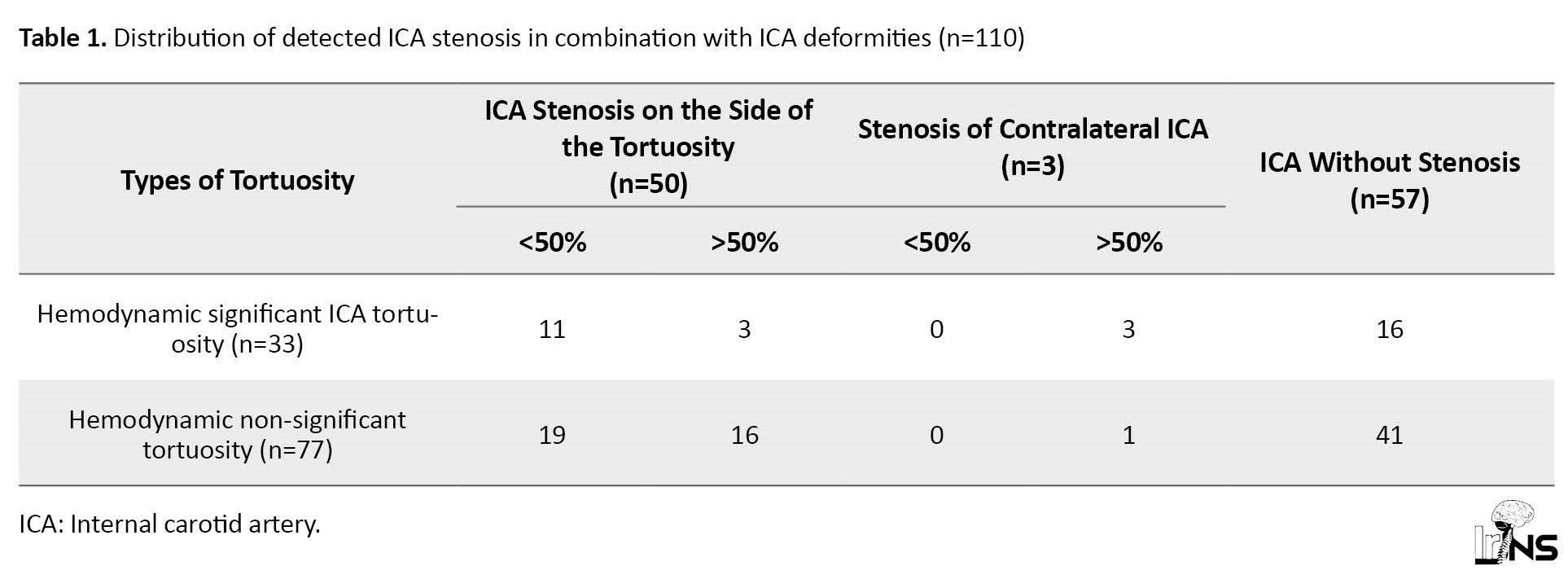
According to the CT angiography, 64 patients were diagnosed with deformities of 110 arteries, of which 18(28.1%) patients had unilateral tortuosity and 46(71.9%) patients had bilateral tortuosity. Hemodynamically significant deformities by duplex color mapping were detected in 33 cases (Figure 1) in 27 patients (30% of all deformities or 42% of patients), of which 6 patients were bilateral (Figure 2). Unilateral kinking was diagnosed in 18 patients (in 42% of cases on the right) and unilateral coiling in 3 patients (in 33% of cases on the right). The proportion of different variants of crimp in the study group is shown in Figure 3. In 54 cases (49% of all detected tortuosity), the deformities were accompanied by stenosis of the ampullary part of the ICA of varying degrees (from 35% to occlusion). Hemodynamic significant stenoses (more than 50%) were detected in 23 cases (42.6% of all stenoses), of which in 20 cases they were on the side of the ICA tortuosity and in 3 cases of patients with hemodynamic significant tortuosity (Figure 4). The distribution of arterial deformities, concomitant stenoses of the ICA, and their localization are presented in Table 1.
During the study, perfusion disorders were detected in 23(35.9%) out of 64 patients. Native CT revealed focal white matter changes of a vascular nature and postischemic cysts with perifocal gliosis of various sizes in only 14 patients (23.3% of cases). In the vast majority of cases (75% of all perfusion disorders), hypoperfusion was diagnosed on the side corresponding to the maximum degree of ICA stenosis, regardless of the location of the tortuosity (Figure 5). The depth and extent of hypoperfusion depended on the degree of stenosis.
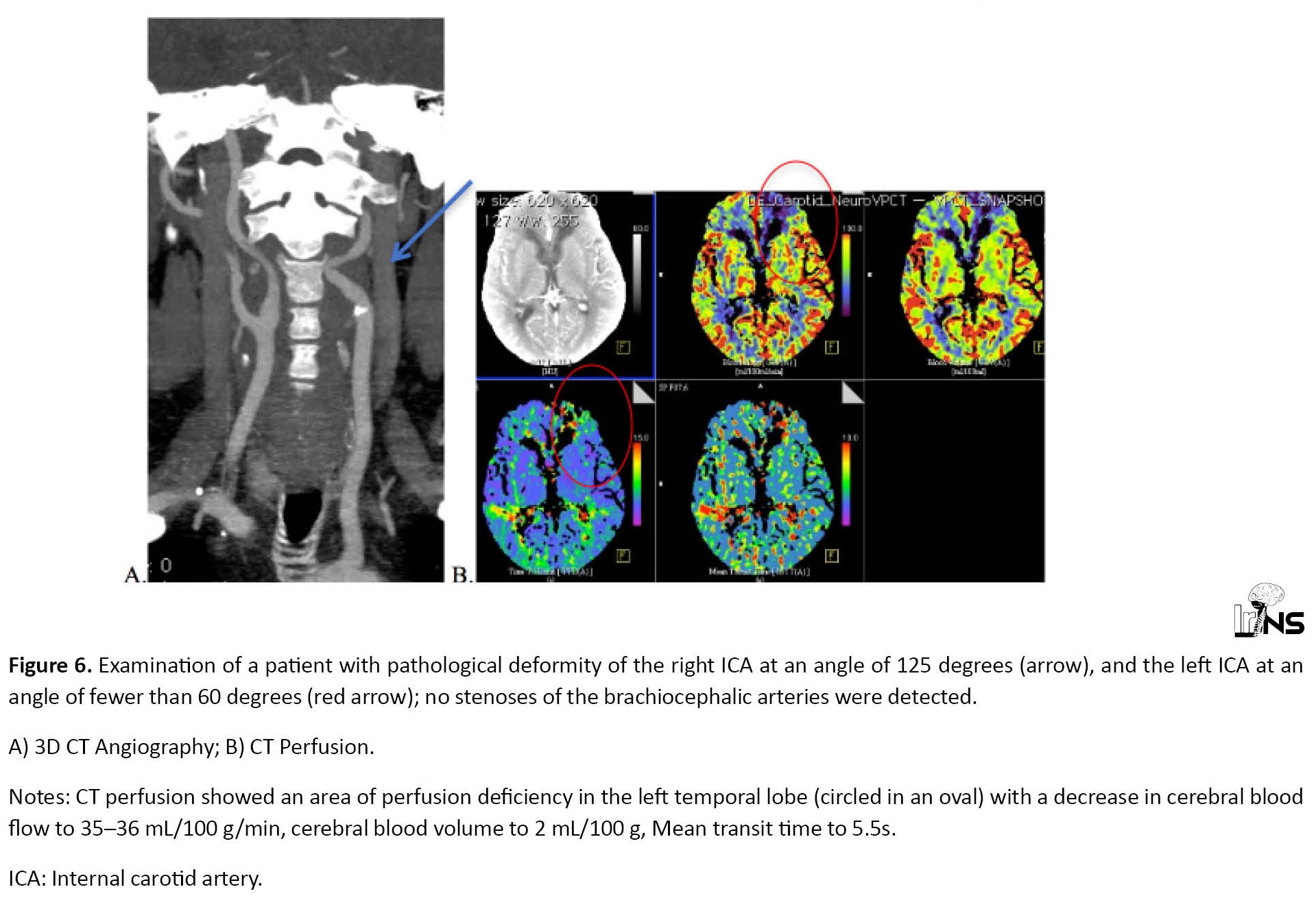
The zones of neurologically significant hypoperfusion were revealed (with a decrease in CBF to 32-35 mL/100 g/min; CBV is symmetrical and reaches 2-2.5 mL/100 g; MTT up to 5-6s) in 5 patients (7.8% of all cases) with hemodynamic significant ICA deformity, but without concomitant stenosis (Figure 6). Focal changes in the density of the brain parenchyma were not diagnosed in these cases. The difference in the Mean CBV values for similar areas of the cerebral hemispheres in these patients did not exceed 2%, which indicates compensation for the perfusion deficit by collateral blood flow.
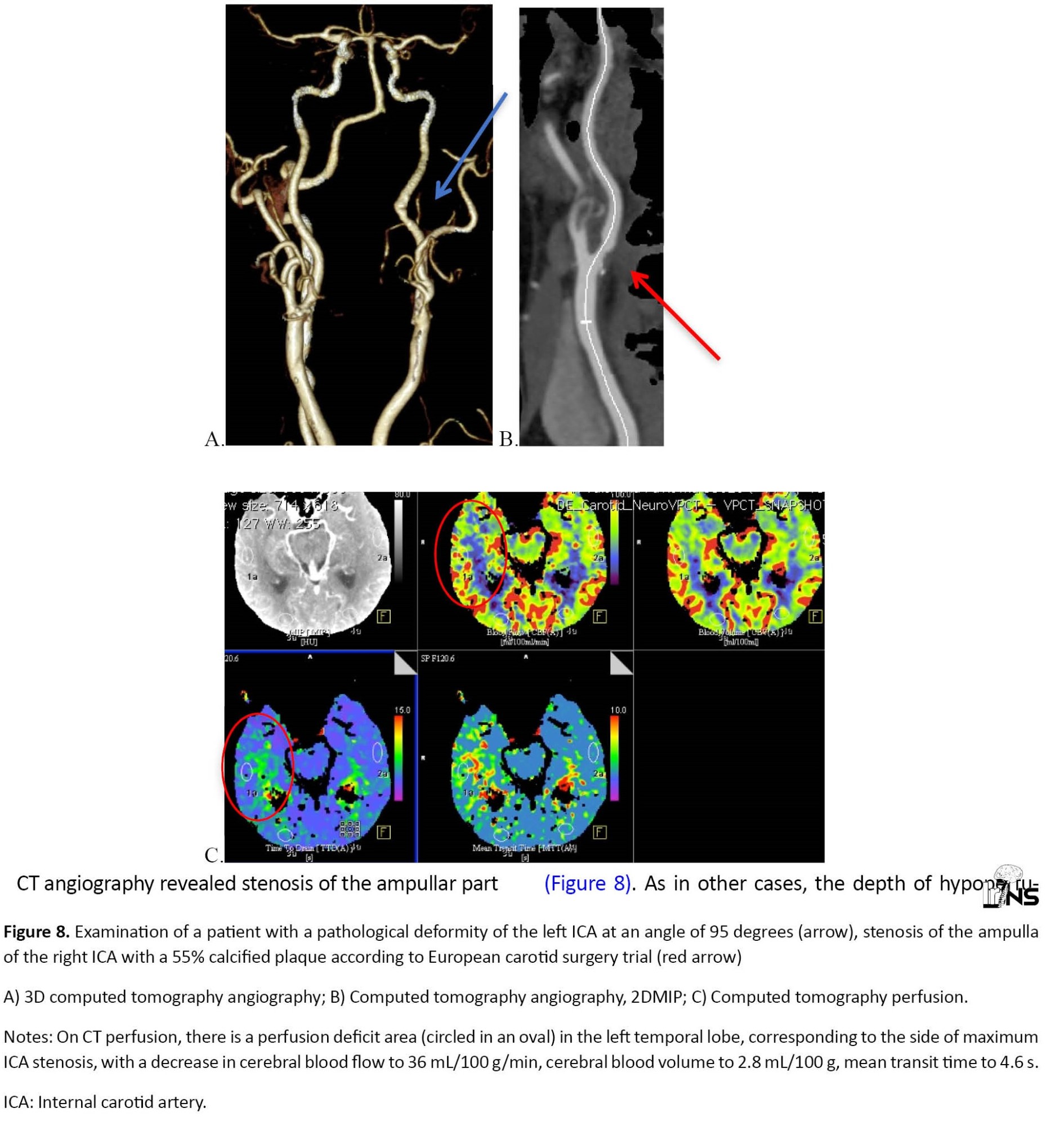
CT angiography revealed stenosis of the ampullar part of the ICA on the side of the tortuosity in 49 cases, the degree of stenosis ranged from 35% to 82% according to European carotid surgery trial. However, only 3 patients of this group with hemodynamic significant tortuosity (5.7% of all detected stenoses) had hypoperfusion in the ICA basin on the side of deformity with concomitant ICA stenosis of more than 50% (Figure 7). At the same time, in 3 patients with various variants of hemodynamic significant tortuosity and stenosis of the opposite ICA, more than 50% hypoperfusion was found on the side of stenosis, rather than arterial deformity (Figure 8). As in other cases, the depth of hypoperfusion varied depending on the degree of stenosis, the maximum decrease in CBF on the side of the deformity reached 46 mL/100 g/min, and the CBV values were symmetrical, indicating active collateral blood flow.
The distribution of the perfusion deficits depending on the hemodynamic significance of tortuosity and concomitant stenosis of the ICA is presented in Table 2.

In the group of hemodynamically insignificant tortuosity, 11 out of 12 cases of hypoperfusion corresponded to the side of ICA stenosis. In 6 patients with hemodynamic significant deformity of the ICA, the study was performed with a stress test, while taking 1 g of acetazolamide orally, with repeated CT perfusion after 2 h. In 3 patients out of this group, critical stenoses and occlusions of the contralateral ICA were diagnosed, the “steal” zones in these cases corresponded to the side of occlusions, not the pathological deformities (Figure 9).
4. Discussion
Cerebrovascular pathology remains one of the most common causes of death and disability both in the Russian Federation and worldwide. Stenosis and deformity of extracranial arteries play a significant role in the development of this disorder [1, 2]. Large randomized studies in recent years have proven the relationship between the degree of narrowing the ICA and the risk of ischemic stroke which gave rise to an increase in surgical activity in the treatment of patients with occlusive-stenotic diseases of the carotid arteries [1, 2, 5].
However, if the effectiveness of surgical treatment of ICA stenoses is rarely questioned, the tactics of treating carotid artery deformities, especially the ones that are not accompanied by atherosclerosis, still raise several questions. The prevalence of ICA deformities ranges from 12% to 43%, and among patients with symptoms of cerebral insufficiency, it is from 4% to 17% [1, 3]. It remains unclear whether ICA tortuosity is congenital or occurs in response to changes in local hemodynamics against the background of atherosclerosis [6]. Tortuosity detected in childhood and young age is a consequence of a violation of the embryogenesis of the aortic arch and those diagnosed in old age and against the background of hypertension are acquired [3]. Most researchers suggest that the disease has a multifactorial etiology as follows: Hypertension, atherosclerosis, connective tissue dysplasia, and nonspecific aortoarteritis [6, 7, 8, 9]. At the same time, according to DelCorso (1998), Ballota (2005), and Beigelman (2010), other deformities of the carotid arteries (kinking and coiling) occur in the population regardless of age, may not be associated with atherosclerotic lesions. Therefore, they must be surgically corrected in the presence of neurological symptoms (transient ischemic attack or history of ischemic stroke) [10, 11, 12]. The study by Saba and Mallarini (2010) on the example of 50 patients with ICA kinking or coiling proved the existence of a correlation between the presence of neurological symptoms and ICA kinking, while coiling was asymptomatic in all cases [13]. Ilic et al. (2011) described a case of transient ischemic attack in a patient with isolated kinking when turning their head [14]. According to the authors of national guidelines for the treatment of patients with brachiocephalic artery disease, 16% to 56% of patients with pathological deformities of the ICA suffer from a persistent or transient neurological deficit, regardless of the presence of atherosclerosis and hypertension. The natural course of ICA deformities, according to the same source, leads to the development of disorders of cerebral hemodynamics. Thus, if the criteria for hemodynamic significance are met, the presence of tortuosity is an indication for surgery even in asymptomatic patients [1].
Nevertheless, there is a theory of an alternative mechanism for the development of deformities against the background of hypertensive macroangiopathy, in which the formation of arterial tortuosity can be considered an adaptation mechanism that smooths out the rise in blood pressure; therefore, it does not require surgical correction. A total of 60% to 85% have concomitant arterial hypertension [1]. In the study by Saba and Mallarini, the correlation between the degree of ICA stenosis and the presence of neurological symptoms was more significant than between ICA kinking and neurological disorders, which is partly confirmed by the results of this study [13].
In our study, the number of hemodynamic significant tortuosity detected in combination with and without ICA stenosis was almost equal (14 and 16 cases, respectively). Similarly, in the group of patients with hemodynamic non-significant tortuosity, in 35 cases (45.5% of the group), ICA deformity was combined with stenosis, and in 53.2%, narrowing of the ICA lumen on the side of the tortuosity was not diagnosed. Considering an arbitrary sample of patients by sex and age, the data do not allow us to judge with confidence the congenital or acquired nature of the occurrence of tortuosity.
To answer the question about the etiology and mechanism of the development of blood flow disorders in pathological deformities of the ICA, it is necessary to conduct a large randomized study, similar to those that helped determine the tactics of treatment in patients with stenosis and occlusion of the brachiocephalic arteries. It is not the problem of etiology that is fundamental, but an accurate and comprehensive assessment of the hemodynamic significance of the revealed tortuosity, which allows, given the historical data, to determine the indications for surgery in each specific case. In the national guidelines for the management of patients with diseases of the brachiocephalic arteries, the criteria for the hemodynamic significance of ICA tortuosity are determined according to duplex scanning data and are based on an increase in the maximum linear blood flow velocity in the deformity zone and the presence of turbulent blood flow in the ICA lumen [1].
According to Naylor et al. (2018), the indication for surgery is also precisely the local hemodynamic significance, which is determined by ultrasound data (duplex scanning and transcranial Doppler sonography) [12]. Several authors state the need for an additional assessment of disorders of the functional state of the brain in tortuosity of the carotid arteries using neurophysiological techniques [1, 3]. The indication for surgery is also precisely the local hemodynamic significance, which is determined by ultrasound data (duplex scanning and transcranial doppler sonography) [12]. Several authors state the need for an additional assessment of disorders of the functional state of the brain in tortuosity of the carotid arteries using neurophysiological techniques [1, 3].
However, the hemodynamic significance of deformities at the level of the extracranial part of the brachiocephalic arteries depends on the local disturbance of blood flow at the level of tortuosity and on many intracranial factors that were not previously taken into account, and above all, on the state of cerebral autoregulation and the quality of the collateral blood flow of the brain, capable of compensating for changes in velocity and pressure in the lumen of the cervical ICA. Ultrasound methods, including transcranial dopplerography, can only give an indirect idea of the state of the collateral blood flow of the brain; the data of these methods are less stable and reliable and depend on the qualifications of the operator and the characteristics of the patient’s anatomy. Thus, it would be more logical to study the effect of ICA deformities on cerebral microcirculation using perfusion techniques, including CT perfusion.
The advantage of using CT perfusion in a multimodal CT study is the possibility of an independent assessment of the perfusion parameters of individual brain regions due to the presence of a direct relationship between the flow of contrasted blood into the vessel in the selected region and the calculated perfusion parameters that do not require comparison with the opposite hemisphere or healthy part of the brain, which is especially valuable in case of widespread atherosclerosis or stenosis of the opposite ICA. Combined use with CT angiography provides information about the anatomical features of the carotid arteries. Conducting CT perfusion with a thickness of weakly 8 cm in the present study allowed us to assess peripheral blood flow disorders in almost the entire volume of the cerebral hemispheres, and several studies with functional load revealed the state of the cerebrovascular reserve.
In the group of hemodynamically insignificant tortuosity (77 cases), the proportion of detected perfusion deficits was low (15.6%), and in almost all cases (91.7%), peripheral blood flow disturbances corresponded to the side and degree of ICA stenosis, the most extensive hypoperfusion zones were noted in patients with stenosis of more than 70%. Only in 1 case out of 12, a neurologically insignificant perfusion deficit was detected in a patient with hemodynamically insignificant tortuosity.
On the contrary, in patients with hemodynamically significant deformities of the ICA (kinking and coiling; 33 cases), perfusion disorders were noted in 24.2% of cases. However, only in 5 cases (15.1%) hypoperfusion was diagnosed in the territory of the tortuous ICA without arterial stenosis; in all these cases, the decrease in CBF with unchanged CBV corresponded to neurologically insignificant disorders compensated by collateral blood flow. The most profound perfusion disorders (CBF up to 25–36 mL/100 g/min), corresponding to chronic cerebral ischemia and capable of influencing the development of neurological symptoms were found only in the combination of kinking and ICA stenosis of more than 50% (3 cases, or 9% of the group). At the same time, in 3 cases, narrowing of the opposite ICA by more than 50% caused no less severe perfusion disorders on the side of stenosis rather than tortuosity. Therefore, analyzing the data of both groups as a whole, it can be argued that only in 6 cases out of 110(5.5%), ICA deformities in the absence of arterial lumen narrowing were accompanied by impaired cerebral perfusion, in all cases compensated by collateral blood flow.
This statement is also consistent with the data of statistical analysis, showing a high correlation between the combination of tortuosity, ICA stenosis, and hypoperfusion on the side of stenosis (P<0.003), and at the same time, the absence of a significant relationship between ICA tortuosity of any nature and cerebral perfusion disorders on the side of tortuosity. Moreover, studies with functional load, albeit on a small number of observations, revealed the absence of perfusion deficits on the kinking side of the ICA and the presence of the “steal” syndrome only on the side of the occlusion of the ICA.
5. Conclusion
The obtained data of our study suggest that the criteria for the hemodynamic significance of pathological deformities of the ICA should be revised, considering the new possibilities of radiological diagnosis in assessing the effect of carotid artery tortuosity on cerebral hemodynamics. Decisions on surgical correction of ICA tortuosity without concomitant stenosis in each specific case should be made only by considering proven violations of autoregulation of cerebral blood flow, including in the presence of cerebral perfusion deficiency.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Local Ethics Committee of the State University of Medicine and Dentistry named after A.I. Evdokimov. The authors obtained written informed consent from all patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Vladimir Krylov and Elena Grigorieva; Data collection: Elena Grigorieva, Viktor Lukyanchikov, Natalia Polunina and Vagan Dalibaldyan; Data analysis and interpretation: Elena Grigorieva, Viktor Lukyanchikov; Drafting: Elena Grigorieva, Natalia Polunina; Critical revision ans final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
In the modern structure of morbidity and mortality, acute cerebrovascular accidents occupy a leading position [1]. Up to 80% of ischemic strokes occur against the background of atherosclerotic lesions of the brachiocephalic arteries [1, 2]. Therefore, the diagnosis and treatment of occlusive-stenotic diseases of the internal carotid arteries (ICA) have become an increasingly important issue in the last decade. Modern researchers consider pathological deformities of the carotid arteries as one of the factors contributing to the development of acute cerebral ischemia, although a clear relationship between these pathologies has not been proven yet [1, 3]. The hemodynamic significance of the ICA tortuosity is determined by the morphological features of the deformities and is confirmed when local hemodynamic disorders are detected during a duplex study [1-3]. However, it remains unclear whether these local changes in the blood flow are important for the formation of cerebral perfusion deficit, which ultimately affects the volume and prevalence of acute and chronic ischemic changes in the brain.
This paper assesses the hemodynamic significance of various types of tortuosity of the ICA, including against the background of atherosclerosis, according to computed tomography (CT) angiography, and CT perfusion, to determine concomitant disorders of cerebral microcirculation.
2. Methods Materials/Patients
From January 1, 2015, until December 31, 2021, a total of 64 patients (42 women, 22 men) in the age range of 31 to 75 years were examined by a neurologist and a neurosurgeon. Pathological deformities of the ICA were revealed in 110 arteries. All patients underwent duplex color mapping of the brachiocephalic arteries with an examination by a neurologist and consultation with a neurosurgeon. In all patients, according to the preliminary examination, pathological deformities of one or both ICAs were diagnosed, often in combination with ICA stenosis of varying severity. The detection of pathological deformity of the ICA became the basis for referring patients to a multimodal CT study, which included CT of the brain, CT angiography of the brachycephalic arteries, and CT perfusion of the brain for a comprehensive assessment of the hemodynamic significance of previously diagnosed tortuosity. The study was performed on a 128-slice CT scanner Somatom Definition Flash (Siemens). A native CT scan of the brain was performed with a slice reconstruction of 0.6 mm to assess the structure of the brain parenchyma and identify the volume and localization of postischemic gliotic changes.
CT angiography of the brachiocephalic arteries was performed from the level of the aortic arch with the capture of the main vessels of the neck and the base of the arterial circle of the brain (circle of Willis) with a slice thickness of 4 mm, followed by reconstruction at 0.6 mm, against the background of intravenous injection of 60 mL of an iodine-containing contrast agent at a rate of 4.5-5.5 mL/s, with a 2-s delay. During post-processing, 2D maximum intensity projection (2 DMIP) and 3D reconstructions were obtained at the Leonardo workstation (Siemens), followed by the subtraction of bone structures for a more accurate assessment of the morphological features of the carotid arteries. The revealed deformations were characterized according to the Weibel-fields classification as tortuosity (C- and S-shaped) and looping. According to the CT angiography data, the authors considered looping (coiling) and C- and S-shaped deformities of the ICA with kinks or twists at an angle of fewer than 60 degrees (kinking) to be hemodynamically significant, with signs of an increase in the linear velocity of blood flow at the level of tortuosity, according to the results of preliminary duplex scanning more than 170 cm/s [1, 3]. In the presence of concomitant stenosis of the ampullary part of the ICA, the degree of narrowing of the artery was determined according to the criteria of the European carotid surgery trial. More than 50% of stenoses were considered hemodynamically significant [1].
CT perfusion of the brain was performed in a single block 8 cm thick, which made it possible to cover almost the entire volume of the brain, including the cerebral cortex and the stem structures. During CT perfusion, 35 mL of iodine-containing contrast agent was injected at a rate of 5.5–6 mL/s with a 6-s delay. All CT perfusions were performed at systolic blood pressure not higher than 160 mmHg and stable heart rate. Post-processing of CT perfusion data was performed using the Neuro VPCT software (Siemens Healthineers). The main indicators of perfusion were determined as follows: Cerebral blood flow (CBF), volumetric blood flow rate (mL/100 g/min); cerebral blood volume (CBV): mL/100 g; Mean transit time (MTT): s. To quantify the CT perfusion data, we determined the absolute and Mean values of the main perfusion parameters in similar areas of the cortex of both cerebral hemispheres (768 areas in total) in the frontal, temporal, and parietal-occipital areas. When calculating CT perfusion parameters, focal white matter changes and postischemic cysts were not included in the measurements. Normal indicators of CT perfusion were considered to be CBF of at least 39 mL/100 g/min (average 55–65 mL/100 g/min), CBV of at least 1.5 mL/100 g (normally 2-3 mL/100 g), MTT of no more than 6 s (normally 3-4.5 s), taking into account the data of the manufacturer and previous studies. A decrease in CBF to 39 mL/100 g/min or less in stable blood pressure was considered a neurologically significant perfusion deficiency; according to M. Wintermark et al., the presence of neurological focal symptoms was proved when CBF decreased below this value [4].
Six patients (9.4% of cases) with hemodynamically significant deformity of the ICA underwent a study with a stress test (oral intake of acetazolamide) and repeated CT perfusion 2 h after taking the drug. A decrease in the average perfusion values in comparison with the initial data on the side of hypoperfusion was regarded as a manifestation of the “steal” syndrome (with a normal increase in CBF while taking acetazolamide by 15% to 40%, which was considered a sign of intact autoregulation of cerebral blood flow.
All studies were performed in low-dose adaptation mode as follows: For CT perfusion, scanning was performed at 80 kV, 120 mAs. The total effective dose of multimodal CT for each patient was 8.5 mSv.
The data of all patients were used in statistical analysis to determine the correlation of the side of deformities and stenoses of the ICA, areas of hypoperfusion, and gliosis changes in the brain. The STATISTICA software, version 7.0 for Windows was used for statistical processing (StatSoftInc., USA). The normality of data distribution was tested using the Shapiro-Wilk test. When comparing groups on qualitative grounds, the one-sided Fisher’s exact test and the Pearson Chi-square test were used. Differences were considered statistically significant at P<0.05.
3. Results
According to the CT angiography, 64 patients were diagnosed with deformities of 110 arteries, of which 18(28.1%) patients had unilateral tortuosity and 46(71.9%) patients had bilateral tortuosity. Hemodynamically significant deformities by duplex color mapping were detected in 33 cases (Figure 1) in 27 patients (30% of all deformities or 42% of patients), of which 6 patients were bilateral (Figure 2). Unilateral kinking was diagnosed in 18 patients (in 42% of cases on the right) and unilateral coiling in 3 patients (in 33% of cases on the right). The proportion of different variants of crimp in the study group is shown in Figure 3. In 54 cases (49% of all detected tortuosity), the deformities were accompanied by stenosis of the ampullary part of the ICA of varying degrees (from 35% to occlusion). Hemodynamic significant stenoses (more than 50%) were detected in 23 cases (42.6% of all stenoses), of which in 20 cases they were on the side of the ICA tortuosity and in 3 cases of patients with hemodynamic significant tortuosity (Figure 4). The distribution of arterial deformities, concomitant stenoses of the ICA, and their localization are presented in Table 1.

During the study, perfusion disorders were detected in 23(35.9%) out of 64 patients. Native CT revealed focal white matter changes of a vascular nature and postischemic cysts with perifocal gliosis of various sizes in only 14 patients (23.3% of cases). In the vast majority of cases (75% of all perfusion disorders), hypoperfusion was diagnosed on the side corresponding to the maximum degree of ICA stenosis, regardless of the location of the tortuosity (Figure 5). The depth and extent of hypoperfusion depended on the degree of stenosis.
The zones of neurologically significant hypoperfusion were revealed (with a decrease in CBF to 32-35 mL/100 g/min; CBV is symmetrical and reaches 2-2.5 mL/100 g; MTT up to 5-6s) in 5 patients (7.8% of all cases) with hemodynamic significant ICA deformity, but without concomitant stenosis (Figure 6). Focal changes in the density of the brain parenchyma were not diagnosed in these cases. The difference in the Mean CBV values for similar areas of the cerebral hemispheres in these patients did not exceed 2%, which indicates compensation for the perfusion deficit by collateral blood flow.
CT angiography revealed stenosis of the ampullar part of the ICA on the side of the tortuosity in 49 cases, the degree of stenosis ranged from 35% to 82% according to European carotid surgery trial. However, only 3 patients of this group with hemodynamic significant tortuosity (5.7% of all detected stenoses) had hypoperfusion in the ICA basin on the side of deformity with concomitant ICA stenosis of more than 50% (Figure 7). At the same time, in 3 patients with various variants of hemodynamic significant tortuosity and stenosis of the opposite ICA, more than 50% hypoperfusion was found on the side of stenosis, rather than arterial deformity (Figure 8). As in other cases, the depth of hypoperfusion varied depending on the degree of stenosis, the maximum decrease in CBF on the side of the deformity reached 46 mL/100 g/min, and the CBV values were symmetrical, indicating active collateral blood flow.
The distribution of the perfusion deficits depending on the hemodynamic significance of tortuosity and concomitant stenosis of the ICA is presented in Table 2.

In the group of hemodynamically insignificant tortuosity, 11 out of 12 cases of hypoperfusion corresponded to the side of ICA stenosis. In 6 patients with hemodynamic significant deformity of the ICA, the study was performed with a stress test, while taking 1 g of acetazolamide orally, with repeated CT perfusion after 2 h. In 3 patients out of this group, critical stenoses and occlusions of the contralateral ICA were diagnosed, the “steal” zones in these cases corresponded to the side of occlusions, not the pathological deformities (Figure 9).
4. Discussion
Cerebrovascular pathology remains one of the most common causes of death and disability both in the Russian Federation and worldwide. Stenosis and deformity of extracranial arteries play a significant role in the development of this disorder [1, 2]. Large randomized studies in recent years have proven the relationship between the degree of narrowing the ICA and the risk of ischemic stroke which gave rise to an increase in surgical activity in the treatment of patients with occlusive-stenotic diseases of the carotid arteries [1, 2, 5].
However, if the effectiveness of surgical treatment of ICA stenoses is rarely questioned, the tactics of treating carotid artery deformities, especially the ones that are not accompanied by atherosclerosis, still raise several questions. The prevalence of ICA deformities ranges from 12% to 43%, and among patients with symptoms of cerebral insufficiency, it is from 4% to 17% [1, 3]. It remains unclear whether ICA tortuosity is congenital or occurs in response to changes in local hemodynamics against the background of atherosclerosis [6]. Tortuosity detected in childhood and young age is a consequence of a violation of the embryogenesis of the aortic arch and those diagnosed in old age and against the background of hypertension are acquired [3]. Most researchers suggest that the disease has a multifactorial etiology as follows: Hypertension, atherosclerosis, connective tissue dysplasia, and nonspecific aortoarteritis [6, 7, 8, 9]. At the same time, according to DelCorso (1998), Ballota (2005), and Beigelman (2010), other deformities of the carotid arteries (kinking and coiling) occur in the population regardless of age, may not be associated with atherosclerotic lesions. Therefore, they must be surgically corrected in the presence of neurological symptoms (transient ischemic attack or history of ischemic stroke) [10, 11, 12]. The study by Saba and Mallarini (2010) on the example of 50 patients with ICA kinking or coiling proved the existence of a correlation between the presence of neurological symptoms and ICA kinking, while coiling was asymptomatic in all cases [13]. Ilic et al. (2011) described a case of transient ischemic attack in a patient with isolated kinking when turning their head [14]. According to the authors of national guidelines for the treatment of patients with brachiocephalic artery disease, 16% to 56% of patients with pathological deformities of the ICA suffer from a persistent or transient neurological deficit, regardless of the presence of atherosclerosis and hypertension. The natural course of ICA deformities, according to the same source, leads to the development of disorders of cerebral hemodynamics. Thus, if the criteria for hemodynamic significance are met, the presence of tortuosity is an indication for surgery even in asymptomatic patients [1].
Nevertheless, there is a theory of an alternative mechanism for the development of deformities against the background of hypertensive macroangiopathy, in which the formation of arterial tortuosity can be considered an adaptation mechanism that smooths out the rise in blood pressure; therefore, it does not require surgical correction. A total of 60% to 85% have concomitant arterial hypertension [1]. In the study by Saba and Mallarini, the correlation between the degree of ICA stenosis and the presence of neurological symptoms was more significant than between ICA kinking and neurological disorders, which is partly confirmed by the results of this study [13].
In our study, the number of hemodynamic significant tortuosity detected in combination with and without ICA stenosis was almost equal (14 and 16 cases, respectively). Similarly, in the group of patients with hemodynamic non-significant tortuosity, in 35 cases (45.5% of the group), ICA deformity was combined with stenosis, and in 53.2%, narrowing of the ICA lumen on the side of the tortuosity was not diagnosed. Considering an arbitrary sample of patients by sex and age, the data do not allow us to judge with confidence the congenital or acquired nature of the occurrence of tortuosity.
To answer the question about the etiology and mechanism of the development of blood flow disorders in pathological deformities of the ICA, it is necessary to conduct a large randomized study, similar to those that helped determine the tactics of treatment in patients with stenosis and occlusion of the brachiocephalic arteries. It is not the problem of etiology that is fundamental, but an accurate and comprehensive assessment of the hemodynamic significance of the revealed tortuosity, which allows, given the historical data, to determine the indications for surgery in each specific case. In the national guidelines for the management of patients with diseases of the brachiocephalic arteries, the criteria for the hemodynamic significance of ICA tortuosity are determined according to duplex scanning data and are based on an increase in the maximum linear blood flow velocity in the deformity zone and the presence of turbulent blood flow in the ICA lumen [1].
According to Naylor et al. (2018), the indication for surgery is also precisely the local hemodynamic significance, which is determined by ultrasound data (duplex scanning and transcranial Doppler sonography) [12]. Several authors state the need for an additional assessment of disorders of the functional state of the brain in tortuosity of the carotid arteries using neurophysiological techniques [1, 3]. The indication for surgery is also precisely the local hemodynamic significance, which is determined by ultrasound data (duplex scanning and transcranial doppler sonography) [12]. Several authors state the need for an additional assessment of disorders of the functional state of the brain in tortuosity of the carotid arteries using neurophysiological techniques [1, 3].
However, the hemodynamic significance of deformities at the level of the extracranial part of the brachiocephalic arteries depends on the local disturbance of blood flow at the level of tortuosity and on many intracranial factors that were not previously taken into account, and above all, on the state of cerebral autoregulation and the quality of the collateral blood flow of the brain, capable of compensating for changes in velocity and pressure in the lumen of the cervical ICA. Ultrasound methods, including transcranial dopplerography, can only give an indirect idea of the state of the collateral blood flow of the brain; the data of these methods are less stable and reliable and depend on the qualifications of the operator and the characteristics of the patient’s anatomy. Thus, it would be more logical to study the effect of ICA deformities on cerebral microcirculation using perfusion techniques, including CT perfusion.
The advantage of using CT perfusion in a multimodal CT study is the possibility of an independent assessment of the perfusion parameters of individual brain regions due to the presence of a direct relationship between the flow of contrasted blood into the vessel in the selected region and the calculated perfusion parameters that do not require comparison with the opposite hemisphere or healthy part of the brain, which is especially valuable in case of widespread atherosclerosis or stenosis of the opposite ICA. Combined use with CT angiography provides information about the anatomical features of the carotid arteries. Conducting CT perfusion with a thickness of weakly 8 cm in the present study allowed us to assess peripheral blood flow disorders in almost the entire volume of the cerebral hemispheres, and several studies with functional load revealed the state of the cerebrovascular reserve.
In the group of hemodynamically insignificant tortuosity (77 cases), the proportion of detected perfusion deficits was low (15.6%), and in almost all cases (91.7%), peripheral blood flow disturbances corresponded to the side and degree of ICA stenosis, the most extensive hypoperfusion zones were noted in patients with stenosis of more than 70%. Only in 1 case out of 12, a neurologically insignificant perfusion deficit was detected in a patient with hemodynamically insignificant tortuosity.
On the contrary, in patients with hemodynamically significant deformities of the ICA (kinking and coiling; 33 cases), perfusion disorders were noted in 24.2% of cases. However, only in 5 cases (15.1%) hypoperfusion was diagnosed in the territory of the tortuous ICA without arterial stenosis; in all these cases, the decrease in CBF with unchanged CBV corresponded to neurologically insignificant disorders compensated by collateral blood flow. The most profound perfusion disorders (CBF up to 25–36 mL/100 g/min), corresponding to chronic cerebral ischemia and capable of influencing the development of neurological symptoms were found only in the combination of kinking and ICA stenosis of more than 50% (3 cases, or 9% of the group). At the same time, in 3 cases, narrowing of the opposite ICA by more than 50% caused no less severe perfusion disorders on the side of stenosis rather than tortuosity. Therefore, analyzing the data of both groups as a whole, it can be argued that only in 6 cases out of 110(5.5%), ICA deformities in the absence of arterial lumen narrowing were accompanied by impaired cerebral perfusion, in all cases compensated by collateral blood flow.
This statement is also consistent with the data of statistical analysis, showing a high correlation between the combination of tortuosity, ICA stenosis, and hypoperfusion on the side of stenosis (P<0.003), and at the same time, the absence of a significant relationship between ICA tortuosity of any nature and cerebral perfusion disorders on the side of tortuosity. Moreover, studies with functional load, albeit on a small number of observations, revealed the absence of perfusion deficits on the kinking side of the ICA and the presence of the “steal” syndrome only on the side of the occlusion of the ICA.
5. Conclusion
The obtained data of our study suggest that the criteria for the hemodynamic significance of pathological deformities of the ICA should be revised, considering the new possibilities of radiological diagnosis in assessing the effect of carotid artery tortuosity on cerebral hemodynamics. Decisions on surgical correction of ICA tortuosity without concomitant stenosis in each specific case should be made only by considering proven violations of autoregulation of cerebral blood flow, including in the presence of cerebral perfusion deficiency.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Local Ethics Committee of the State University of Medicine and Dentistry named after A.I. Evdokimov. The authors obtained written informed consent from all patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Vladimir Krylov and Elena Grigorieva; Data collection: Elena Grigorieva, Viktor Lukyanchikov, Natalia Polunina and Vagan Dalibaldyan; Data analysis and interpretation: Elena Grigorieva, Viktor Lukyanchikov; Drafting: Elena Grigorieva, Natalia Polunina; Critical revision ans final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Bokeria LA. [National guidelines for the management of patients with brachiocephalic artery disease (Russian)]. Angiology and Vascular Surgery. 2013; 2:1-45. [Link]
- Pokrovskiĭ AV, Beloiartsev DF, Timina IE, Adyrkhaev ZA. Clinical manifestations and diagnosis of pathological deformation of the internal carotid artery. Angiology and Vascular Surgery. 2011; 17(3):7-18. [Link]
- Saba L, Sanfillipo R, Suri JS, Cademartiri F, Corrias G, Mannelli L, et al. Does carotid artery tortuosity play a role in stroke? Canadian Association of Radiologists Journal. 2021; 72(4):789-96. [DOI:10.1177/0846537121991057] [PMID]
- Wintermark M, Sesay M, Barbier E, Borbély K, Dillon WP, Eastwood JD, et al. Comparative overview of brain perfusion imaging techniques. Stroke. 2005; 36(9):e83-99. [DOI:10.1161/01.STR.0000177884.72657.8b] [PMID]
- Krylov VV, DashyanVG, LemenevVL. Surgical treatment of patients with bilateral occlusive-stenotic lesions of the brachiocephalic arteries. Neurosurgery. 2014; 4:16-25. [Link]
- Sethi SS, Lau JF, Godbold J, Gustavson S, Olin JW. The S curve: A novel morphological finding in the internal carotid artery in patients with fibromuscular dysplasia. Vascular Medicine. 2014; 19(5):356-62. [DOI:10.1177/1358863X14547122] [PMID]
- Cvetko E. Concurrence of bilateral kinking of the extracranial part of the internal carotid artery with coiling and tortuosity of the external carotid artery - a case report. Romanian Journal of Morphology and Embryology. 2014; 55(2):433-5. [Link]
- Lemenev AL, Siluyanova AS, ShamshilinAA, Akhmetov VV. The choice of the method of vascular reconstruction in patients with pathological tortuosity of the internal carotid artery. Neurosurgery. 2014; 3:42-9. [Link]
- Siluyanova AS, Shamshilin AA, Barmina TG, Lemenev VL. The reconstructive operations at patients with combined tortuosity of common and internal carotid arteries. Russian Journal of Neurosurgery. 2015(2):39-44. [Link]
- Del Corso L, Moruzzo D, Conte B, Agelli M, Romanelli AM, Pastine F, et al., Tortuosity, kinking and coiling of the carotid artery: Expression of atherosclerosis or aging? Angiology. 1998; 49(5):361-71. [DOI:10.1177/000331979804900505] [PMID]
- Yu J, Qu L, Xu B, Wang S, Li C, Xu X, et al. Current understanding of dolichoarteriopathies of the internal carotid artery: A review. International Journal of Medical Sciences. 2017; 14(8):772-84. [DOI:10.7150/ijms.19229] [PMID] [PMCID]
- Naylor AR , Ricco JB. Management of atherosclerotic carotid and vertebral artery disease: 2017 Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular & Endovascular Surgery. 2018; 55(6):3-81. [DOI:10.1016/j.ejvs.2018.03.023] [PMID]
- Saba L, Mallarini G. Correlation between kinking and coiling of the carotid arteries as assessed using MDCTA with symptoms and degree of stenosis. Clinal Radiology. 2010; 65(9):729-34 [DOI:10.1016/j.crad.2010.04.015] [PMID]
- Ilić M, Tanasković S, Ilijevski N, Radak Đ. [Acute reversible ischaemic neurological deficit induced by internal carotid artery kinking - Case report (serbian)]. Srpski Arhiv Za Celokupno Lekarstvo. 2011; 139(1-2):92-4. [DOI:10.2298/SARH1102092I] [PMID]
Type of Study: Research |
Subject:
Vascular Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |