Sat, Jul 5, 2025
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 99-106 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Naseri Z, Mamoudi F, Abdolmaleki A, Soluki M. Neuroprotective Potential of Trans-Anethole Following Crush Injury of the Sciatic Nerve in Rats. Iran J Neurosurg 2023; 9 : 13
URL: http://irjns.org/article-1-350-en.html
URL: http://irjns.org/article-1-350-en.html
1- Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran
2- Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran ,f.mahmoudi@uma.ac.ir
3- Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran AND Department of Engineering Sciences, Faculty of Advanced Technologies, University of Mohaghegh Ardabili, Namin, Iran
2- Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran ,
3- Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran AND Department of Engineering Sciences, Faculty of Advanced Technologies, University of Mohaghegh Ardabili, Namin, Iran
Full Text [PDF 2140 kb]
(690 Downloads)
| Abstract (HTML) (2221 Views)
Full Text: (864 Views)
1. Introduction
2. Methods and Materials/Patients
Chemical and animals
Surgical procedures
Animals were deeply anesthetized by an intraperitoneal injection of a mixture of ketamine/xylazine. First, the hair on the thigh of the right leg was shaved. Then by splitting the muscle layer with a surgical blade, the sciatic nerve became visible. Afterward, a piece of the nerve above the trifurcation of the sciatic nerve was compressed for 20 s with locking forceps. After disinfection with betadine (10% povidone-iodine). Then, the cut tissue was sutured. After recovery, each rat was kept separately in special cages. To reduce the pain in rats undergoing surgery, all rats were treated with buprenorphine (1 mg/kg) for two days after surgery. All operations were performed under sterile conditions [13].
Treatment and animal group
Rats were grouped (n=7). The control and negative control groups received saline. The other two groups were injected with 125 mg/kg and 250 mg/kg trans-anethole intraperitoneally for one week [14, 15].
Motor recovery evaluation
All groups were subjected to motor evaluation using “walking track” tests once every two weeks on the day before surgery and one week after surgery until the eighth week. This quantitative method to analyze the function of the hind limbs by examining the footprints is known as the sciatic function index (SFI) [16]. In the implementation of this test, the soles of both feet of the rat were stained with ink. The animal was allowed to move, then its gait was checked. Finally, after recording three suitable footprints, the paper containing the rat’s footprints was analyzed. SFI of all the rats was calculated using Bain’s formula. The value of SFI varies between -100 and zero (the SFI of a healthy and normal nerve is zero) where -100 indicates a general disorder. Therefore, the closer the number is to zero, the better the nerve recovery will be [17, 18].
Sensory recovery evaluation
Evaluation of sensory recovery was carried out using the “hot plate” test once every two weeks on the day before surgery and one week after surgery until the eighth week. To perform this test, the operated leg of the rats was placed standing on the metal surface of the hot plate device, which was heated to a temperature of 54±1. The response of the rats was determined by lifting the leg from the surface of the hot plate. The duration of the reaction was recorded in seconds using a stopwatch. To avoid tissue damage, the test cutoff time was 10 s [19].
Histomorphometric analysis
For histomorphometric analysis, a piece of the injured nerve was isolated. Then it was fixed in 2.5% glutaraldehyde. Then secondary fixation was done with 1% osmium tetroxide. After dehydrating the tissue samples in ethanol, the samples were embedded in resin. Then they were stained with toluidine blue (1%) to prepare semi-thin sections (1 μm). Finally, they were evaluated using a light microscope. The samples were randomly selected and measured using ImageJ software to measure the main morphological parameters, including the number of myelinated fibers, myelin sheath thickness, and fiber diameter [20].
Histological evaluation of gastrocnemius muscle
Sampling was done from both legs to examine the histological changes in the gastrocnemius muscle. For evaluation, 10 μm transverse sections were prepared from the middle part of the muscle ventricle. Then Masson’s trichrome staining was used according to standard methods. The dimensions and number of muscle fibers and the presence of fibrous connective tissue between muscle fibers were examined using a light microscope [20].
Gastrocnemius muscle mass
At the end of the studies, the gastrocnemius muscles of the right and left legs were isolated in all groups and weighed using a digital Yamato scale (China). Finally, the gastrocnemius muscle weight ratio for each rat was calculated based on the ratio of the weight of the operated leg muscle to the intact leg muscle [21].
Statistical methods
Data were analyzed using SPSS software, version 23 and one-way analysis of variance (ANOVA) to evaluate the difference between groups. Tukey’s test was used to check the significant difference between groups. P<0.05 were considered as the minimum level of significance. All data were expressed as Mean±SEM.
3. Results
Motor recovery evaluation
The values of SFI in all the tested groups decreased significantly in the second week, which indicated a motor disorder in rats (P<0.05). During the fourth, sixth, and eighth weeks, the SFI in the TA-treated groups increased compared with the negative control group, but the increase was not statistically significant (Figure 1).
.png)
Sensory recovery evaluation
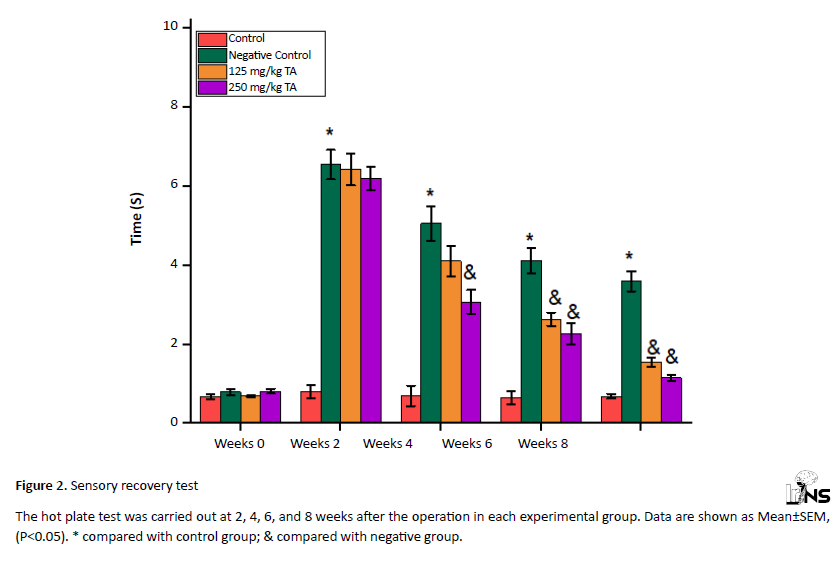
All the tested groups showed a significant drop in sensory function in the second week after surgery (P<0.05). But gradually, in the fourth, sixth, and eighth weeks, recovery in sensory function was observed in all groups. In total, the groups treated with TA had a greater recovery in sensory function than the negative control group, and this rate was higher in the high dose (P<0.05) (Figure 2).
Histomorphometric analysis
Analysis of these results manifested that in the groups treated with TA compared with the negative control group, at the end of the eighth week after surgery, the morphometric parameters increased significantly (Table 1 and Figure 3).
.png)

The results of the morphometric examination also revealed that the number of myelinated fibers in all groups was higher than the control. The increase in the number of fibers in the group receiving 250 mg/kg of TA was significant compared with the negative control (P<0.05). The thickness of the myelin sheath in the negative control group and in the group receiving 125 mg/kg was significantly reduced compared with the control group. No significant difference was observed in the thickness of the myelin sheath in the group receiving 250 mg/kg compared with the controls. The increase in the thickness of the myelin sheath in the group receiving 250 mg/kg of TA was significant compared with the negative control.
Examination of muscle histology
The gastrocnemius muscle sections were stained with Masson’s trichrome to evaluate the amount of muscle atrophy. Then, the samples were examined by a light microscope. The results presented that in the groups treated with TA compared with the negative control group, the rate of muscular dystrophy was lower and the rate of muscle recovery was higher. Furthermore, more fibrous tissue was formed in the negative control group than in other groups, which indicated more muscular dystrophy. In the group receiving a higher dose of TA (250 mg/kg), more muscle recovery was observed (Figure 4).

Investigation of gastrocnemius muscle mass
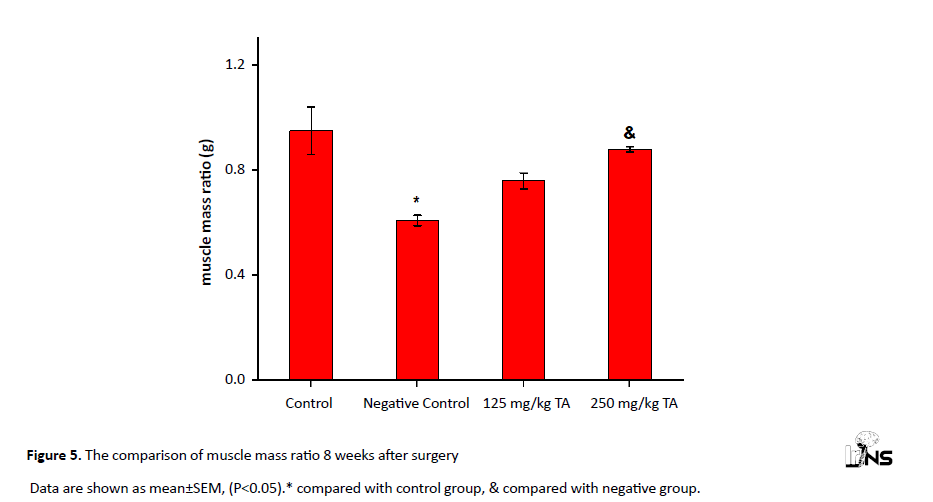
It was found that the ratio of muscle mass in negative control rats had a significant decrease compared with the controls. The ratio of muscle mass in rats treated with 250 mg/kg of TA was significantly higher than that of the negative group (P<0.05). However, the increase in muscle mass in rats receiving 125 mg/kg TA was not significant compared with the negative control group (Figure 5).
4. Discussion
Peripheral nerve injury (PNI) is a common disease that leads to sensory impairment and reduced motor function in the injured patient. About one-third of all PNIs show incomplete nerve recovery and poor functional outcomes, including the loss or partial recovery of motor, sensory function, chronic pain, muscle atrophy, and severe weakness. As a result, loss of normal organ function may result in lifelong complications and permanent disability [13]. Following the nervous system injuries, a series of events, such as increased oxidative stress, inflammation, and the spread of damage occur, which can lead to damage to mitochondria, protein degradation, and cell apoptosis. Therefore, any method that can protect nerve cells from these damages can be effective in improving the recovery process [22, 23].
Ryu et al. reported that TA protects cortical neurons against glucose/oxygenation by activating the N-methyl-D-aspartate (NMDA) receptors and reducing free radicals. TA also reduces mitochondrial membrane depolarization. Hence, the neuroprotective effect of TA can be due to its ability to inhibit excitotoxicity, oxidative stress, and mitochondrial dysfunction [24].
PNI causes the production of reactive oxygen species and nitric oxide in axotomized neurons. Schwann cells and macrophages in the injured nerve express pro-inflammatory molecules, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), transforming growth factor beta (TGF-β) and nitric oxide synthases [25]. The anti-inflammatory effect of TA can also be another crucial factor in improving sensory/motor function in this study. Currently, it is known that the anti-inflammatory properties of Anethole are due to the modulation of ion channel function, reducing histamine and serotonin, and pro-inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) and nitric oxide [9]. It seems that one of the vital factors that played a role in improving the healing process in this research is the antioxidant, anti-oxidative stress property of TA, its ability to separate free radicals, and inhibition of pro-inflammatory factors [6].
Other research discovered that TA reduces the production of reactive oxygen species. This phytochemical compound reduces the expression of phosphoprotein p53, caspase-3, caspase-9, Bcl-2-associated protein X (BAX), and polymerase [11]. Therefore, in this research, it is assumed that trans-anethole can improve sciatic neurons by reducing the production of reactive oxygen species and inhibiting the apoptosis pathway.
5. Conclusion
The results specified that a one-week injection of TA into rats caused a faster recovery. Improvement was observed in the group that received a higher drug dose (250 mg/kg) than the other group (125 mg/kg). It seems that the concentration of 125 mg/kg TA was not enough to improve sensory and motor performance. The results of our study confirmed the neuroprotective effects of TA as a new drug. However, further studies are recommended to confirm our findings.
Ethical Considerations
Compliance with ethical guidelines
Ethical principles in all stages of the research were carried out by the University of Mohaghegh Ardabili, Iran after obtaining the Code of Laboratory Ethics with reference number IR.UMA.REC.1400.031.
Funding
This research was financially supported by the University of Mohaghegh Ardabili, Iran.
Authors' contributions
Conception and design: Arash Abdolmaleki; Data collection: Zahra Naseri, Milad Soluki; Data analysis and interpretation: Fariba Mahmoudi; Drafting the article: Zahra Naseri; Critically revising the article: All authors; Reviewing the submitted version of the manuscript: All authors; Approving the final version of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are grateful to the University of Mohaghegh Ardabili for supporting this research.
References
Restoring the damaged nervous system is considered a big challenge due to its complex physiological system and limited regeneration capacity [1]. The nerve fibers of the severed nerve are regenerated by themselves to a limited extent and with the formation of scar tissue, but many injuries require nerve regeneration and surgery [2]. Many clinical researches and experiments are being carried out to provide further information for the use of plants in the prevention and treatment of diseases [3]. Anise is a flowering plant in the family Apiaceae. Its seeds contain essential oil and protein. More than 90% of the main ingredients of essential oil is Anethole, which is considered an estrogenic active agent [4, 5]. Anethole, as a phenylpropanoid with the chemical formula “C10H12O”, has antioxidant, anti-inflammatory, analgesic, antimicrobial, and anticancer effects. Studies have shown that the antioxidant effect of Anethole is due to its ability to separate free radicals [6, 7]. Previous studies have reported the antioxidant effects of trans-anethole (TA) and its function in improving some diseases [8, 9]. In some studies, the therapeutic effect of trans-anethole on central nervous system disease has been observed [10]. According to a previous report, trans-anethole suppresses the growth and proliferation of human prostate cancer cells through cell cycle arrest and apoptosis [11]. Recent studies have proposed that trans-anethole leads to inhibiting the production or release of nitric oxide (NO) and prostaglandins (PGE2) levels [12]. Therefore, due to its broad properties, especially the neuroprotective properties of trans-anethole, in this research, the effects of trans-anethole on the injury of the sciatic nerve were investigated in rats.
2. Methods and Materials/Patients
Chemical and animals
Trans-anethole was purchased from Sigma Aldrich. A total of 28 male Wistar rats weighing 250 to 300 g were used. The rats were kept in special rat cages with free access to water and enough food, a suitable temperature of 22±2°C, and a 12 h light/12 h dark cycle. All experiments were conducted between 8:00 AM and 12:00 PM. Ethical principles in all stages of the research were carried out by the University of Mohaghegh Ardabili, Iran (Code: IR.UMA.REC.1400.031).
Surgical procedures
Animals were deeply anesthetized by an intraperitoneal injection of a mixture of ketamine/xylazine. First, the hair on the thigh of the right leg was shaved. Then by splitting the muscle layer with a surgical blade, the sciatic nerve became visible. Afterward, a piece of the nerve above the trifurcation of the sciatic nerve was compressed for 20 s with locking forceps. After disinfection with betadine (10% povidone-iodine). Then, the cut tissue was sutured. After recovery, each rat was kept separately in special cages. To reduce the pain in rats undergoing surgery, all rats were treated with buprenorphine (1 mg/kg) for two days after surgery. All operations were performed under sterile conditions [13].
Treatment and animal group
Rats were grouped (n=7). The control and negative control groups received saline. The other two groups were injected with 125 mg/kg and 250 mg/kg trans-anethole intraperitoneally for one week [14, 15].
Motor recovery evaluation
All groups were subjected to motor evaluation using “walking track” tests once every two weeks on the day before surgery and one week after surgery until the eighth week. This quantitative method to analyze the function of the hind limbs by examining the footprints is known as the sciatic function index (SFI) [16]. In the implementation of this test, the soles of both feet of the rat were stained with ink. The animal was allowed to move, then its gait was checked. Finally, after recording three suitable footprints, the paper containing the rat’s footprints was analyzed. SFI of all the rats was calculated using Bain’s formula. The value of SFI varies between -100 and zero (the SFI of a healthy and normal nerve is zero) where -100 indicates a general disorder. Therefore, the closer the number is to zero, the better the nerve recovery will be [17, 18].
Sensory recovery evaluation
Evaluation of sensory recovery was carried out using the “hot plate” test once every two weeks on the day before surgery and one week after surgery until the eighth week. To perform this test, the operated leg of the rats was placed standing on the metal surface of the hot plate device, which was heated to a temperature of 54±1. The response of the rats was determined by lifting the leg from the surface of the hot plate. The duration of the reaction was recorded in seconds using a stopwatch. To avoid tissue damage, the test cutoff time was 10 s [19].
Histomorphometric analysis
For histomorphometric analysis, a piece of the injured nerve was isolated. Then it was fixed in 2.5% glutaraldehyde. Then secondary fixation was done with 1% osmium tetroxide. After dehydrating the tissue samples in ethanol, the samples were embedded in resin. Then they were stained with toluidine blue (1%) to prepare semi-thin sections (1 μm). Finally, they were evaluated using a light microscope. The samples were randomly selected and measured using ImageJ software to measure the main morphological parameters, including the number of myelinated fibers, myelin sheath thickness, and fiber diameter [20].
Histological evaluation of gastrocnemius muscle
Sampling was done from both legs to examine the histological changes in the gastrocnemius muscle. For evaluation, 10 μm transverse sections were prepared from the middle part of the muscle ventricle. Then Masson’s trichrome staining was used according to standard methods. The dimensions and number of muscle fibers and the presence of fibrous connective tissue between muscle fibers were examined using a light microscope [20].
Gastrocnemius muscle mass
At the end of the studies, the gastrocnemius muscles of the right and left legs were isolated in all groups and weighed using a digital Yamato scale (China). Finally, the gastrocnemius muscle weight ratio for each rat was calculated based on the ratio of the weight of the operated leg muscle to the intact leg muscle [21].
Statistical methods
Data were analyzed using SPSS software, version 23 and one-way analysis of variance (ANOVA) to evaluate the difference between groups. Tukey’s test was used to check the significant difference between groups. P<0.05 were considered as the minimum level of significance. All data were expressed as Mean±SEM.
3. Results
Motor recovery evaluation
The values of SFI in all the tested groups decreased significantly in the second week, which indicated a motor disorder in rats (P<0.05). During the fourth, sixth, and eighth weeks, the SFI in the TA-treated groups increased compared with the negative control group, but the increase was not statistically significant (Figure 1).
.png)
Sensory recovery evaluation
All the tested groups showed a significant drop in sensory function in the second week after surgery (P<0.05). But gradually, in the fourth, sixth, and eighth weeks, recovery in sensory function was observed in all groups. In total, the groups treated with TA had a greater recovery in sensory function than the negative control group, and this rate was higher in the high dose (P<0.05) (Figure 2).

Histomorphometric analysis
Analysis of these results manifested that in the groups treated with TA compared with the negative control group, at the end of the eighth week after surgery, the morphometric parameters increased significantly (Table 1 and Figure 3).
.png)

The results of the morphometric examination also revealed that the number of myelinated fibers in all groups was higher than the control. The increase in the number of fibers in the group receiving 250 mg/kg of TA was significant compared with the negative control (P<0.05). The thickness of the myelin sheath in the negative control group and in the group receiving 125 mg/kg was significantly reduced compared with the control group. No significant difference was observed in the thickness of the myelin sheath in the group receiving 250 mg/kg compared with the controls. The increase in the thickness of the myelin sheath in the group receiving 250 mg/kg of TA was significant compared with the negative control.
Examination of muscle histology
The gastrocnemius muscle sections were stained with Masson’s trichrome to evaluate the amount of muscle atrophy. Then, the samples were examined by a light microscope. The results presented that in the groups treated with TA compared with the negative control group, the rate of muscular dystrophy was lower and the rate of muscle recovery was higher. Furthermore, more fibrous tissue was formed in the negative control group than in other groups, which indicated more muscular dystrophy. In the group receiving a higher dose of TA (250 mg/kg), more muscle recovery was observed (Figure 4).

Investigation of gastrocnemius muscle mass
It was found that the ratio of muscle mass in negative control rats had a significant decrease compared with the controls. The ratio of muscle mass in rats treated with 250 mg/kg of TA was significantly higher than that of the negative group (P<0.05). However, the increase in muscle mass in rats receiving 125 mg/kg TA was not significant compared with the negative control group (Figure 5).

4. Discussion
Peripheral nerve injury (PNI) is a common disease that leads to sensory impairment and reduced motor function in the injured patient. About one-third of all PNIs show incomplete nerve recovery and poor functional outcomes, including the loss or partial recovery of motor, sensory function, chronic pain, muscle atrophy, and severe weakness. As a result, loss of normal organ function may result in lifelong complications and permanent disability [13]. Following the nervous system injuries, a series of events, such as increased oxidative stress, inflammation, and the spread of damage occur, which can lead to damage to mitochondria, protein degradation, and cell apoptosis. Therefore, any method that can protect nerve cells from these damages can be effective in improving the recovery process [22, 23].
Ryu et al. reported that TA protects cortical neurons against glucose/oxygenation by activating the N-methyl-D-aspartate (NMDA) receptors and reducing free radicals. TA also reduces mitochondrial membrane depolarization. Hence, the neuroprotective effect of TA can be due to its ability to inhibit excitotoxicity, oxidative stress, and mitochondrial dysfunction [24].
PNI causes the production of reactive oxygen species and nitric oxide in axotomized neurons. Schwann cells and macrophages in the injured nerve express pro-inflammatory molecules, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), transforming growth factor beta (TGF-β) and nitric oxide synthases [25]. The anti-inflammatory effect of TA can also be another crucial factor in improving sensory/motor function in this study. Currently, it is known that the anti-inflammatory properties of Anethole are due to the modulation of ion channel function, reducing histamine and serotonin, and pro-inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) and nitric oxide [9]. It seems that one of the vital factors that played a role in improving the healing process in this research is the antioxidant, anti-oxidative stress property of TA, its ability to separate free radicals, and inhibition of pro-inflammatory factors [6].
Other research discovered that TA reduces the production of reactive oxygen species. This phytochemical compound reduces the expression of phosphoprotein p53, caspase-3, caspase-9, Bcl-2-associated protein X (BAX), and polymerase [11]. Therefore, in this research, it is assumed that trans-anethole can improve sciatic neurons by reducing the production of reactive oxygen species and inhibiting the apoptosis pathway.
5. Conclusion
The results specified that a one-week injection of TA into rats caused a faster recovery. Improvement was observed in the group that received a higher drug dose (250 mg/kg) than the other group (125 mg/kg). It seems that the concentration of 125 mg/kg TA was not enough to improve sensory and motor performance. The results of our study confirmed the neuroprotective effects of TA as a new drug. However, further studies are recommended to confirm our findings.
Ethical Considerations
Compliance with ethical guidelines
Ethical principles in all stages of the research were carried out by the University of Mohaghegh Ardabili, Iran after obtaining the Code of Laboratory Ethics with reference number IR.UMA.REC.1400.031.
Funding
This research was financially supported by the University of Mohaghegh Ardabili, Iran.
Authors' contributions
Conception and design: Arash Abdolmaleki; Data collection: Zahra Naseri, Milad Soluki; Data analysis and interpretation: Fariba Mahmoudi; Drafting the article: Zahra Naseri; Critically revising the article: All authors; Reviewing the submitted version of the manuscript: All authors; Approving the final version of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are grateful to the University of Mohaghegh Ardabili for supporting this research.
References
- Boni R, Ali A, Shavandi A, Clarkson AN.Current and novel polymeric biomaterials for neural tissue engineering. Journal of Biomedical Science. 218; 25(1):90. [DOI:10.1186/s12929-018-0491-8] [PMID] [PMCID]
- Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Research International. 2014; 2014:698256.[DOI:10.1155/2014/698256] [PMID] [PMCID]
- Ilijeva R, Buchbauer G. Biological properties of some volatile phenylpropanoids. Natural Product Communications. 2016; 11(10):1619-29. [DOI:10.1177/1934578X1601101041]
- Helal E, Abd-El-Aziz M, Ahmed SS. Effect of Anise (Pimpinella Anisum L.) as phytoestrogen on some sex hormones and biochemical parameters. The Egyptian Journal of Hospital Medicine. 2019; 75(1):1918-22. [DOI:10.21608/ejhm.2019.29114]
- Kang NH, Mukherjee S, Min T, Kang SC, Yun JW. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie. 2018; 151:1-13. [DOI:10.1016/j.biochi.2018.05.009] [PMID]
- Ritter AM, Ames FQ, Otani F, de Oliveira RM, Cuman RK, Bersani-Amado CA. Effects of anethole in nociception experimental models. Evidence-Based Complementary and Alternative Medicine: eCAM. 2014; 2014:345829.[DOI:10.1155/2014/345829] [PMID] [PMCID]
- Yu C, Zhang J, Li Q, Xiang X, Yang Z, Wang T. Effects of trans-anethole supplementation on serum lipid metabolism parameters, carcass characteristics, meat quality, fatty acid and amino acid profiles of breast muscle in broiler chickens. Poultry Science. 2021; 100(12):101484 [DOI:10.1016/j.psj.2021.101484] [PMID] [PMCID]
- Seo E, Kang P, Seol GH. Trans-anethole prevents hypertension induced by chronic exposure to both restraint stress and nicotine in rats. Biomedicine & Pharmacotherapy. 2018; 102:249-53. [DOI:10.1016/j.biopha.2018.03.081] [PMID]
- Kim KY, Lee HS, Seol GH. Anti-inflammatory effects of trans-anethole in a mouse model of chronic obstructive pulmonary disease. Biomedicine & pharmacotherapy. 2017; 91:925-30. [DOI:10.1016/j.biopha.2017.05.032] [PMID]
- da Guedes E, Ribeiro LR, Carneiro CA, Santos AMF, Brito Monteiro Á, de Andrade HHN, et al. Anticonvulsant activity of trans-anethole in mice. BioMed Research International. 2022; 2022:9902905. [DOI:10.1155/2022/9902905] [PMID] [PMCID]
- Pandit K, Kaur S, Kumar A, Bhardwaj R, Kaur S. Trans-anethole abrogates cell proliferation and induces apoptosis through the mitochondrial-mediated pathway in human osteosarcoma cells. Nutrition and Cancer. 2021; 73(9):1727-45. [DOI:10.1080/01635581.2020.1803927] [PMID]
- Domiciano TP, Dalalio MM, Silva EL, Ritter AM, Estevão-Silva CF, Ramos FS, et al. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn-Schmiedeberg's Archives of Pharmacology. 2013; 386(4):331-8 [DOI:10.1007/s00210-012-0820-5] [PMID]
- Wang B, Zhang G, Yang M, Liu N, Li YX, Ma H, et al. Neuroprotective effect of anethole against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurochemical Research. 2018; 43(12):2404-22 [DOI:10.1007/s11064-018-2668-7] [PMID]
- Ritter AM, Domiciano TP, Verri WA, Zarpelon AC, da Silva LG, Barbosa CP, et al. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology. 2013; 21(2):187-97. [PMID]
- Vastegani SM, Khoshnam SE, Mansouri E, Hajipour S, Ghafouri S, Bakhtiari N, et al. Neuroprotective effect of anethole against rotenone induced non-motor deficits and oxidative stress in rat model of Parkinson’s disease. Behavioural Brain Research. 2023; 437:114100. [DOI:10.1016/j.bbr.2022.114100] [PMID]
- Varejão AS, Meek MF, Ferreira AJ, Patrício JA, Cabrita AM. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. Journal of Neuroscience Methods. 2001; 108(1):1-9. [DOI:10.1016/S0165-0270(01)00378-8] [PMID]
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal and posterior tibial nerve lesions in the rat. Plastic and Reconstructive Surgery. 1989; 83(1):129-38. [PMID]
- Soluki M, Mahmoudi F, Abdolmaleki A, Asadi A, Sabahi Namini A. Cerium oxide nanoparticles as a new neuroprotective agent to promote functional recovery in a rat model of sciatic nerve crush injury. British Journal of Neurosurgery. 2020; 26:1-6. [DOI:10.1080/02688697.2020.1864292] [PMID]
- Gunn A, Bobeck EN, Weber C, Morgan MM. The influence of non-nociceptive factors on hot-plate latency in rats. The Journal of Pain. 2011; 12(2):222-7. [DOI:10.1016/j.jpain.2010.06.011] [PMID] [PMCID]
- Azimpour M, Mahmoudi F, Abdolmaleki A, Bayrami A. Thyroxine accelerates functional recovery in a rat model of sciatic nerve crush. Turkish Neurosurgery. 2022; 32(2):298-304. [DOI:10.5137/1019-5149.JTN.34966-21.4] [PMID]
- Magaz A, Faroni A, Gough JE, Reid AJ, Li X, Blaker JJ. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Advanced Healthcare Materials. 2018; 7(23):e1800308. [DOI:10.1002/adhm.201800308] [PMID]
- Khalkhal E, Razzaghi M, Rostami-Nejad M, Rezaei-Tavirani M, Heidari Beigvand H, Rezaei Tavirani M. Evaluation of laser effects on the human body after laser therapy. Journal of Lasers in Medical Sciences. 2020; 11(1):91-7. [DOI:10.15171/jlms.2020.15] [PMID] [PMCID]
- Ghayour MB, Abdolmaleki A, Behnam-Rassouli M.The effect of Riluzole on functional recovery of locomotion in the rat sciatic nerve crush model. European Journal of Trauma and Emergency Surgery: Official Publication of the European Trauma Society. 2017; 43(5):691-9 [DOI:10.1007/s00068-016-0691-4] [PMID]
- Ryu S, Seol GH, Park H, Choi IY. Trans-anethole protects cortical neuronal cells against oxygen-glucose deprivation/reoxygenation. Neurological sciences. 2014; 35(10):1541-7. [DOI:10.1007/s10072-014-1791-8] [PMID]
- Lanza C, Raimondo S, Vergani L, Catena N, Sénès F, Tos P, et al. Expression of antioxidant molecules after peripheral nerve injury and regeneration. Journal of Neuroscience Research. 2012; 90(4):842-8. [DOI:10.1002/jnr.22778] [PMID]
Type of Study: Research |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






