Tue, Jul 15, 2025
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 113-118 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mortazavi S A, Digaleh H, Saffar H, Ebrahimi H, Kazemi H, Madreseh E. Smoking and Brain Neoplasm: An Immunohistochemical Data Evaluating Caspase-3 and MMP-2 in Rat Brain. Iran J Neurosurg 2023; 9 : 15
URL: http://irjns.org/article-1-353-en.html
URL: http://irjns.org/article-1-353-en.html
Seyed Abolghasem Mortazavi1 

 , Hadi Digaleh *2
, Hadi Digaleh *2 

 , Hiva Saffar3
, Hiva Saffar3 

 , Hannan Ebrahimi1
, Hannan Ebrahimi1 

 , Hossein Kazemi1
, Hossein Kazemi1 

 , Elham Madreseh4
, Elham Madreseh4 




 , Hadi Digaleh *2
, Hadi Digaleh *2 

 , Hiva Saffar3
, Hiva Saffar3 

 , Hannan Ebrahimi1
, Hannan Ebrahimi1 

 , Hossein Kazemi1
, Hossein Kazemi1 

 , Elham Madreseh4
, Elham Madreseh4 


1- Department of Neurosurgery, Sina Hospital, Tehran, Iran
2- Department of Neurosurgery, Sina Hospital, Tehran, Iran ,hadi.hd71@gmail.com
3- Department of Pathology, Shariati Hospital, Tehran, Iran
4- Department of Epidemiology and Biostatistics, School of Health, Tehran University of Medical Sciences, Tehran, Iran
2- Department of Neurosurgery, Sina Hospital, Tehran, Iran ,
3- Department of Pathology, Shariati Hospital, Tehran, Iran
4- Department of Epidemiology and Biostatistics, School of Health, Tehran University of Medical Sciences, Tehran, Iran
Full Text [PDF 733 kb]
(583 Downloads)
| Abstract (HTML) (2327 Views)
Full Text: (881 Views)
1. Introduction
Smoking has been extensively studied for its tumorigenicity, including brain tumors [1]. Smoking and nicotine have controversial associations with brain tumor incidence, especially glial tumors, the most common neoplastic brain lesions in adults [2, 3]. Smoking and cigarette use have potential links with caspase-3 activity, which has been reported in the bronchiolar epithelium and umbilical vein endothelial cells [4, 5]. Among signaling cascades introduced in glioma inception, caspase-3 activation has recently gained attention due to its dual role in pro- and anti-tumoral activities [6, 7]. In this manner, caspase-3 activation in the glioma environment has been discussed as proangiogenic signaling [6].
Matrix metalloproteinases (MMPs) are engaged in tumor-induced vasculogenesis and demonstrated in downstream signaling of caspase-3 as AKT/P-AKT/cleaved-caspase-3/MMP-2/MMP-9 signaling cascade [8]. In this manner, several MMPs have been discussed in correlation with smoking, in which MMP-2 and MMP-9 have been studied extensively [9]. MMP-2 plays an important role in glioma invasiveness and may have prognostic potential [10]. Furthermore, MMP-2 is also discussed as a proangiogenic factor in malignant glioma environments [11].
Here, we aimed to survey immunohistochemical assay to find out whether smoking has an expressional impact on the level of caspase-3 and MMP-2 in rat brain tissue. The caspase-3/MMP-2 link induces tumor proliferation and invasiveness; however, this functional interaction should be evaluated in intact, nontumoral tissue. We also examined electronic smoking in another specific rat group, other than the non-smoking control group, due to its diminished proto-oncogenic activity and tobacco substitution [12].
2. Materials and Methods/Patients
Animals and smoke exposure
Thirty male and 30 female Wistar rats (4-8 weeks old, weighing 200±20 g) were adopted from the Faculty of Pharmacy, Tehran University of Medical Sciences. They were housed in standard cages. Environmental conditions were controlled temperature (22±2°C), humidity, and a 12 h light/dark cycle (light on 07:00–19:00). Food and water were provided ad libitum. The sample size was calculated with continuous endpoints at a power of 80% (α=0.05), which determined a minimum of 10 for each group. The rats were divided into two groups: treatment and control groups. The treatment group consisted of cigarettes and electronic cigarettes, with 10 rats in each subgroup. Subgroup 1 included cigarette smoke male/female groups; subgroup 2 had electronic cigarette smoke male/female groups, and control male/female groups. To adapt to the environment, these animals were kept at the Urology Research Center animal house for two weeks before the experiment to prevent possible environmental changes. All procedures and animal euthanasia were under the guidelines of the ethical committee for the use and care of laboratory animals at the Tehran University of Medical Sciences, Tehran, Iran [13].
The control group was exposed to sham air, and the treatment groups were exposed to cigarette or e-cigarette smoke entering the box through a suction device. Also, after the smoke enters the box, the smoke gradually escapes through a special sponge chimney placed on the box; each period of exposure of mice to cigarette smoke or e-cigarettes lasts 10 minutes. These 10 minutes were performed five times daily (Teague TE10 smoke exposure system). In these chambers, animals (n=10 per chamber) were exposed to tobacco smoke, and each cigarette contained 0.6 mg of nicotine. The samples were exposed to cigarette smoke or e-cigarettes for 50 minutes a day. In the e-smoking group, animals were in contact with electronic cigarette aerosols yielded by an electronic cigarette (1.8 Ω single-coil, Mini Protank 2, KangerTech, Shenzhen, China). E-cigarettes are loaded with a solution of analytical grade propylene glycol, glycerol, and nicotine (free bases) procured from Sigma Aldrich. The solution combined 70:30 PG.: VG. by volume like available commercial liquids. The nicotine concentration was 0.6 mg/mL nicotine. The whole liquid composition was similar to commercially available products. The machine activates a pump during each puff that forces lab air into the air inlet ports of the ECIG. Afterward, aerosol exits the Ecig mouthpiece and enters the whole-body exposure chamber. A 4-s duration puff production of volume 116.7 mL every 10 s was programmed 5 times a day for 120 consecutive days.
Tissue collection
Animals were injected with 100 mg/kg of Zoletil® as general anesthesia and decapitated according to the Ministerial guidelines. Brains were collected, washed carefully, and immediately frozen in liquid nitrogen. After euthanasia, the brain was removed and placed in a steel mold (Matrix model-A.S.I.–Instruments-C.B.M. -2000C USA) for performing coronal sections. From coronal sections were obtained 3-μm thick sections and embedded in paraffin.
Immunohistochemical staining
Representative paraffin blocks were selected for immunohistochemical study. Three-micrometer sections were deparaffinized with xylene and alcohol and then rehydrated. The slides were immersed in 3% hydrogen peroxide for 15 min. Antigen retrieval was done at an appropriate pH for each marker. The protein block reagent was applied to each sample for 10 min. Subsequently, sections were incubated with the first antibody for 1 h (Table 1), washed, and then incubated with the second antibody (Medaysis Meda View TM Two-step polymer-AP Anti-mouse & rabbit system) for 20 min.
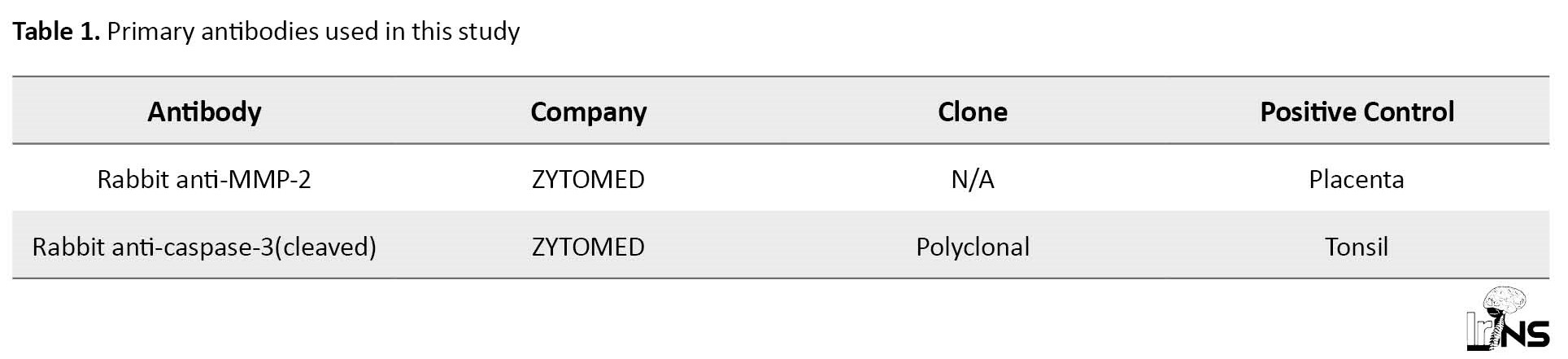
Finally, the antigens were detected by using chromogen.
For protein expression analysis, two fields were selected at 400x magnification, with a greater concentration of positive cells or marked (areas of “hot spots”) for each protein under study. A semi-quantitative scoring method (scores 1 to 3) based on the intensity of neurons’ staining was used to evaluate immunopositivity.
Data analysis
The categorical variables were described as n (%). After assessing the interaction effect using GEE ordinal logistic regression model, the odds ratio (95% CI) for sex and intervention groups were calculated for MMP-2 and caspase-3 separately using ordinal logistic models. All analyses at P<0.05 were considered statistically significant. The data were analyzed, blinded to experimental groups, using the IBM SPSS software, version 22.0 and R 4.1.1.
3. Results
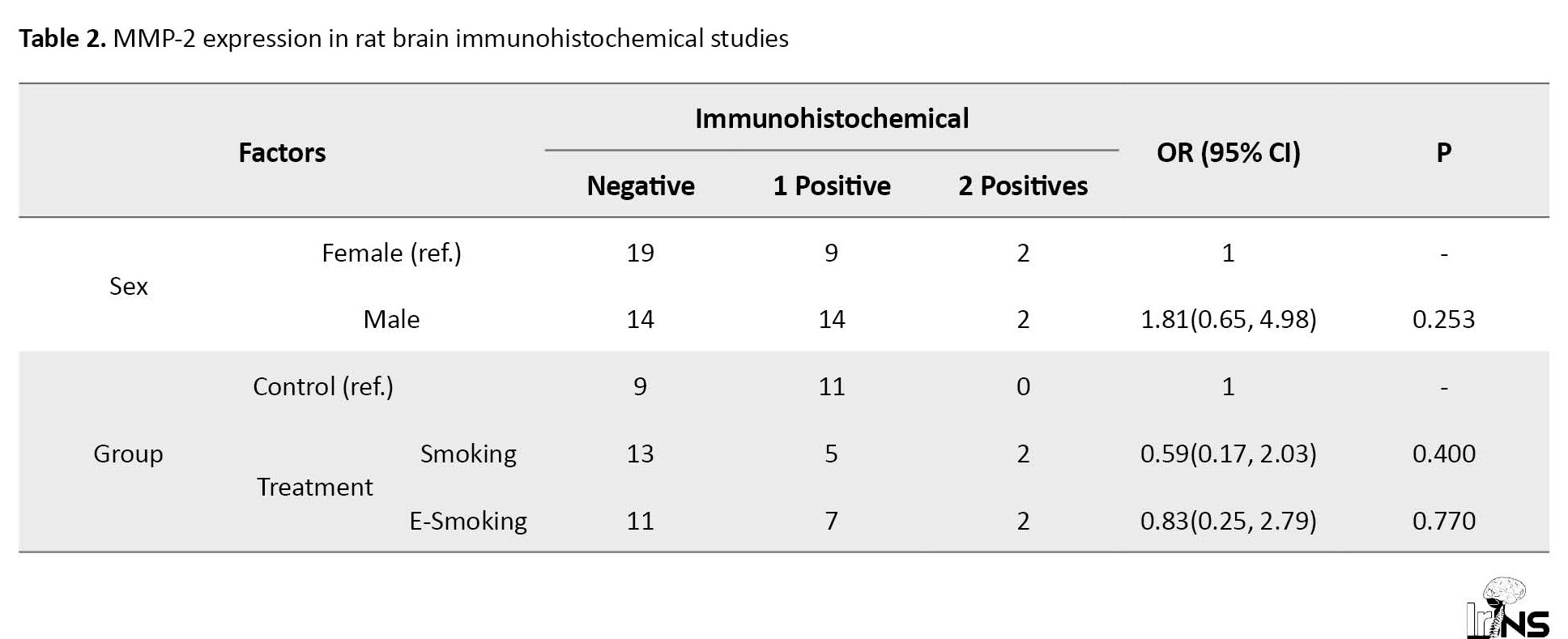
The whole brain’s coronal sections reacted immunohistochemically to caspase-3 and MMP-2 in each group, demonstrating 1+, 2+, and negative expressions (Tables 2 and 3).

Although there was mild induction of caspase-3 in female rats exposed to electronic smoking, only male rats in the cigarette smoking group revealed a significant increase in protein expression due to immunohistochemical analysis.
In this manner, the expression of MMP-2 was not significantly different between none of the studied groups. Here, we also observed a mild, but not statistically significant increase in MMP-2 expression in female rats exposed to electronic smoking and male rats exposed to cigarette smoking. These data suggest an important inductive cigarette smoking activity on caspase-3 and not MMP-2 expression.
4. Discussion
In this brief study, we observed a significant correlation between increased caspase-3 expression and smoking in rat brain tissue, although this effect was not detected in rats exposed to electronic smoking. This correlation was further significant if male and female rats were independently analyzed. On the other hand, MMP-2 expression level on immunohistochemical extracts is not significantly correlated with either smoking or electronic smoking in treated rats.
Caspase-3 is a potent programmed cell death activator and has been studied extensively in the role of anti-tumor activity [14]. Interestingly, another aspect of caspase-3 activity has been revealed in recent years. Tumor repopulation and in response to caspase-3 downstream inflammatory molecules are shown following glioma treatment [15]. From another point of view, the caspase-3/MMP-2 pathway, promoting tumor angiogenesis and invasion, is pro-tumor signaling reported in lung cancer cells [8]. Our data suggest an increased expression of caspase-3 in rat brains, but not MMP-2, in response to cigarette smoking. According to the data, caspase-3 activation following smoking has been reported in various tumoral and non-tumoral tissues. In contrast, caspase-3 inhibition by glioma tumor cells is also displayed in tumor growth [7]. This should be noted that the level of caspase-3 activation and or expression may dictate the fate of downstream signaling in the tumoral microenvironment [16].
In the search for cross-talk of MMP-2 and brain glial tumors, MMP-2 expression has been discussed as a prognostic factor in astrocytoma [10] and despite aggressive treatment, patients often experience recurrence. Survival decreases with increasing tumor grade, and especially patients with grade IV glioblastoma have poor prognosis due to the aggressive character of this tumor. Matrix metalloproteinase-2 (MMP-2). Our data is a limited demonstration of caspase-3 as an upstream activator of MMP-2, which may significantly affect tumoral angiogenesis, although this effect can be minimal in non-tumoral tissue. It is intriguing to speculate that MMP-2 and other MMPs have many upstream signaling inhibitors/activators in normal and tumoral contexts [17].
5. Conclusion
These data suggest that cigarette smoking is an important activator of caspase-3 in rat brain tissue with limited pro-tumoral signaling impact in normal tissue. However, it can further augment invasiveness and repopulation in tumoral tissue.
Ethical Considerations
Compliance with ethical guidelines
All procedures were performed under the guidelines of the Ethics Committee for the use and care of laboratory animals at the Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SINAHOSPITAL.REC.1399.019).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
Writing the main draft, conception, and design: Seyed Abolghasem Mortazavi, Hadi Digaleh, Hiva Saffar; Editing the manuscript thoroughly: Hadi Digaleh, Hiva Saffar; Data collection: Hannan Ebrahimi, Hossein Kazemi; Data analysis and interpretation: Elham Madreseh; Reviewing the submitted version of the manuscript, critically revising and drafting the article, and approval of the final version: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Neurosurgery Department of Sina Hospital and Pathology Department of Shariati Hospital, Tehran.
References
Smoking has been extensively studied for its tumorigenicity, including brain tumors [1]. Smoking and nicotine have controversial associations with brain tumor incidence, especially glial tumors, the most common neoplastic brain lesions in adults [2, 3]. Smoking and cigarette use have potential links with caspase-3 activity, which has been reported in the bronchiolar epithelium and umbilical vein endothelial cells [4, 5]. Among signaling cascades introduced in glioma inception, caspase-3 activation has recently gained attention due to its dual role in pro- and anti-tumoral activities [6, 7]. In this manner, caspase-3 activation in the glioma environment has been discussed as proangiogenic signaling [6].
Matrix metalloproteinases (MMPs) are engaged in tumor-induced vasculogenesis and demonstrated in downstream signaling of caspase-3 as AKT/P-AKT/cleaved-caspase-3/MMP-2/MMP-9 signaling cascade [8]. In this manner, several MMPs have been discussed in correlation with smoking, in which MMP-2 and MMP-9 have been studied extensively [9]. MMP-2 plays an important role in glioma invasiveness and may have prognostic potential [10]. Furthermore, MMP-2 is also discussed as a proangiogenic factor in malignant glioma environments [11].
Here, we aimed to survey immunohistochemical assay to find out whether smoking has an expressional impact on the level of caspase-3 and MMP-2 in rat brain tissue. The caspase-3/MMP-2 link induces tumor proliferation and invasiveness; however, this functional interaction should be evaluated in intact, nontumoral tissue. We also examined electronic smoking in another specific rat group, other than the non-smoking control group, due to its diminished proto-oncogenic activity and tobacco substitution [12].
2. Materials and Methods/Patients
Animals and smoke exposure
Thirty male and 30 female Wistar rats (4-8 weeks old, weighing 200±20 g) were adopted from the Faculty of Pharmacy, Tehran University of Medical Sciences. They were housed in standard cages. Environmental conditions were controlled temperature (22±2°C), humidity, and a 12 h light/dark cycle (light on 07:00–19:00). Food and water were provided ad libitum. The sample size was calculated with continuous endpoints at a power of 80% (α=0.05), which determined a minimum of 10 for each group. The rats were divided into two groups: treatment and control groups. The treatment group consisted of cigarettes and electronic cigarettes, with 10 rats in each subgroup. Subgroup 1 included cigarette smoke male/female groups; subgroup 2 had electronic cigarette smoke male/female groups, and control male/female groups. To adapt to the environment, these animals were kept at the Urology Research Center animal house for two weeks before the experiment to prevent possible environmental changes. All procedures and animal euthanasia were under the guidelines of the ethical committee for the use and care of laboratory animals at the Tehran University of Medical Sciences, Tehran, Iran [13].
The control group was exposed to sham air, and the treatment groups were exposed to cigarette or e-cigarette smoke entering the box through a suction device. Also, after the smoke enters the box, the smoke gradually escapes through a special sponge chimney placed on the box; each period of exposure of mice to cigarette smoke or e-cigarettes lasts 10 minutes. These 10 minutes were performed five times daily (Teague TE10 smoke exposure system). In these chambers, animals (n=10 per chamber) were exposed to tobacco smoke, and each cigarette contained 0.6 mg of nicotine. The samples were exposed to cigarette smoke or e-cigarettes for 50 minutes a day. In the e-smoking group, animals were in contact with electronic cigarette aerosols yielded by an electronic cigarette (1.8 Ω single-coil, Mini Protank 2, KangerTech, Shenzhen, China). E-cigarettes are loaded with a solution of analytical grade propylene glycol, glycerol, and nicotine (free bases) procured from Sigma Aldrich. The solution combined 70:30 PG.: VG. by volume like available commercial liquids. The nicotine concentration was 0.6 mg/mL nicotine. The whole liquid composition was similar to commercially available products. The machine activates a pump during each puff that forces lab air into the air inlet ports of the ECIG. Afterward, aerosol exits the Ecig mouthpiece and enters the whole-body exposure chamber. A 4-s duration puff production of volume 116.7 mL every 10 s was programmed 5 times a day for 120 consecutive days.
Tissue collection
Animals were injected with 100 mg/kg of Zoletil® as general anesthesia and decapitated according to the Ministerial guidelines. Brains were collected, washed carefully, and immediately frozen in liquid nitrogen. After euthanasia, the brain was removed and placed in a steel mold (Matrix model-A.S.I.–Instruments-C.B.M. -2000C USA) for performing coronal sections. From coronal sections were obtained 3-μm thick sections and embedded in paraffin.
Immunohistochemical staining
Representative paraffin blocks were selected for immunohistochemical study. Three-micrometer sections were deparaffinized with xylene and alcohol and then rehydrated. The slides were immersed in 3% hydrogen peroxide for 15 min. Antigen retrieval was done at an appropriate pH for each marker. The protein block reagent was applied to each sample for 10 min. Subsequently, sections were incubated with the first antibody for 1 h (Table 1), washed, and then incubated with the second antibody (Medaysis Meda View TM Two-step polymer-AP Anti-mouse & rabbit system) for 20 min.

Finally, the antigens were detected by using chromogen.
For protein expression analysis, two fields were selected at 400x magnification, with a greater concentration of positive cells or marked (areas of “hot spots”) for each protein under study. A semi-quantitative scoring method (scores 1 to 3) based on the intensity of neurons’ staining was used to evaluate immunopositivity.
Data analysis
The categorical variables were described as n (%). After assessing the interaction effect using GEE ordinal logistic regression model, the odds ratio (95% CI) for sex and intervention groups were calculated for MMP-2 and caspase-3 separately using ordinal logistic models. All analyses at P<0.05 were considered statistically significant. The data were analyzed, blinded to experimental groups, using the IBM SPSS software, version 22.0 and R 4.1.1.
3. Results
The whole brain’s coronal sections reacted immunohistochemically to caspase-3 and MMP-2 in each group, demonstrating 1+, 2+, and negative expressions (Tables 2 and 3).


Although there was mild induction of caspase-3 in female rats exposed to electronic smoking, only male rats in the cigarette smoking group revealed a significant increase in protein expression due to immunohistochemical analysis.
In this manner, the expression of MMP-2 was not significantly different between none of the studied groups. Here, we also observed a mild, but not statistically significant increase in MMP-2 expression in female rats exposed to electronic smoking and male rats exposed to cigarette smoking. These data suggest an important inductive cigarette smoking activity on caspase-3 and not MMP-2 expression.
4. Discussion
In this brief study, we observed a significant correlation between increased caspase-3 expression and smoking in rat brain tissue, although this effect was not detected in rats exposed to electronic smoking. This correlation was further significant if male and female rats were independently analyzed. On the other hand, MMP-2 expression level on immunohistochemical extracts is not significantly correlated with either smoking or electronic smoking in treated rats.
Caspase-3 is a potent programmed cell death activator and has been studied extensively in the role of anti-tumor activity [14]. Interestingly, another aspect of caspase-3 activity has been revealed in recent years. Tumor repopulation and in response to caspase-3 downstream inflammatory molecules are shown following glioma treatment [15]. From another point of view, the caspase-3/MMP-2 pathway, promoting tumor angiogenesis and invasion, is pro-tumor signaling reported in lung cancer cells [8]. Our data suggest an increased expression of caspase-3 in rat brains, but not MMP-2, in response to cigarette smoking. According to the data, caspase-3 activation following smoking has been reported in various tumoral and non-tumoral tissues. In contrast, caspase-3 inhibition by glioma tumor cells is also displayed in tumor growth [7]. This should be noted that the level of caspase-3 activation and or expression may dictate the fate of downstream signaling in the tumoral microenvironment [16].
In the search for cross-talk of MMP-2 and brain glial tumors, MMP-2 expression has been discussed as a prognostic factor in astrocytoma [10] and despite aggressive treatment, patients often experience recurrence. Survival decreases with increasing tumor grade, and especially patients with grade IV glioblastoma have poor prognosis due to the aggressive character of this tumor. Matrix metalloproteinase-2 (MMP-2). Our data is a limited demonstration of caspase-3 as an upstream activator of MMP-2, which may significantly affect tumoral angiogenesis, although this effect can be minimal in non-tumoral tissue. It is intriguing to speculate that MMP-2 and other MMPs have many upstream signaling inhibitors/activators in normal and tumoral contexts [17].
5. Conclusion
These data suggest that cigarette smoking is an important activator of caspase-3 in rat brain tissue with limited pro-tumoral signaling impact in normal tissue. However, it can further augment invasiveness and repopulation in tumoral tissue.
Ethical Considerations
Compliance with ethical guidelines
All procedures were performed under the guidelines of the Ethics Committee for the use and care of laboratory animals at the Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SINAHOSPITAL.REC.1399.019).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
Writing the main draft, conception, and design: Seyed Abolghasem Mortazavi, Hadi Digaleh, Hiva Saffar; Editing the manuscript thoroughly: Hadi Digaleh, Hiva Saffar; Data collection: Hannan Ebrahimi, Hossein Kazemi; Data analysis and interpretation: Elham Madreseh; Reviewing the submitted version of the manuscript, critically revising and drafting the article, and approval of the final version: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Neurosurgery Department of Sina Hospital and Pathology Department of Shariati Hospital, Tehran.
References
- McConnell DD, Carr SB, Litofsky NS. Potential effects of nicotine on glioblastoma and chemoradiotherapy: A review. Expert Review of Neurotherapeutics. 2019; 19(6):545-55. [DOI:10.1080/14737175.2019.1617701] [PMID]
- Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of cigarette smoking and adult glioma: Dosage, duration, and latency. Neuro-Oncology. 2007; 9(3):326-34. [DOI:10.1215/15228517-2007-005] [PMID] [PMCID]
- Hou L, Jiang J, Liu B, Han W, Wu Y, Zou X, et al. Smoking and adult glioma: A population-based case-control study in China. Neuro-Oncology. 2016; 18(1):105-13. [DOI:10.1093/neuonc/nov146] [PMID] [PMCID]
- Chiappara G, Gjomarkaj M, Sciarrino S, Vitulo P, Pipitone L, Pace E. Altered expression of p21, activated caspase-3, and PCNA in bronchiolar epithelium of smokers with and without chronic obstructive pulmonary disease. Experimental Lung Research. 2014; 40(7):343-53. [DOI:10.3109/01902148.2014.928836] [PMID]
- Wang J, Wilcken DEL, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Molecular Genetics and Metabolism. 2001; 72(1):82-8. [DOI:10.1006/mgme.2000.3115] [PMID]
- Feng X, Yu Y, He S, Cheng J, Gong Y, Zhang Z, et al. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Letters. 2017; 385:12-20. [DOI:10.1016/j.canlet.2016.10.042] [PMID] [PMCID]
- Shen X, Burguillos MA, Osman AM, Frijhoff J, Carrillo-Jiménez A, Kanatani S, et al. Glioma-induced inhibition of caspase-3 in microglia promotes a tumor-supportive phenotype. Nature Immunology. 2016; 17(11):1282-90. [DOI:10.1038/ni.3545] [PMID]
- Li H, Zhao J, Jia X, Zhang Y, Du Y, Li H, et al. miR-21 promotes growth, invasion and migration of lung cancer cells by AKT/P-AKT/cleaved-caspase 3/MMP-2/MMP-9 signaling pathway. International Journal of Clinical and Experimental Pathology. 2020; 13(4):692-700. [PMID]
- Perlstein TS, Lee RT. Smoking, metalloproteinases, and vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006; 26(2):250-6. [DOI:10.1161/01.ATV.0000199268.27395.4f] [PMID]
- Ramachandran RK, Sørensen MD, Aaberg-Jessen C, Hermansen SK, Kristensen BW. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. Plos One. 2017; 12(2):e0172234. [DOI:10.1371/journal.pone.0172234] [PMID] [PMCID]
- Liu X, Shen S, Zhu L, Su R, Zheng J, Ruan X, et al. SRSF10 inhibits biogenesis of circ-ATXN1 to regulate glioma angiogenesis via miR-526b-3p/MMP2 pathway. Journal of Experimental & Clinical Cancer Research. 2020; 39(1):1-17. [DOI:10.1186/s13046-020-01625-8]
- Rahman SK, Kawatra P, Aiyappa C, Shivamurthy MC. E-Cigarette: An AID to smoking cessation? World Journal of Pharmaceutical Research. 2016; 5(9):1648-56. [Link]
- Ahmadi-Noorbakhsh S, Mirabzadeh Ardakani E, Sadighi J, Aldavood SJ, Farajli Abbasi M, Farzad-Mohajeri S, et al. Guideline for the care and use of laboratory animals in Iran. Lab Animal. 2021; 50(11):303-5. [DOI:10.1038/s41684-021-00871-3] [PMID]
- Floyd DH, Zhang Y, Dey BK, Kefas B, Breit H, Marks K, et al. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS One. 2014; 9(5):e96239. [DOI:10.1371/journal.pone.0096239] [PMID] [PMCID]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nature Medicine. 2011; 17(7):860-6. [DOI:10.1038/nm.2385] [PMID] [PMCID]
- Liu PF, Hu YC, Kang BH, Tseng YK, Wu PC, Liang CC, et al. Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. Plos One. 2017; 12(7):e0180620. [DOI:10.1371/journal.pone.0180620] [PMID] [PMCID]
- Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation-and metastasis-sustaining neovasculature. Matrix Biology. 2015; 44-46:94-112. [DOI:10.1016/j.matbio.2015.04.004] [PMID] [PMCID]
Type of Study: Research |
Subject:
Neuroscience
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



