Wed, Jul 16, 2025
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 119-126 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Thavara B D, Parambil R M, Muriyil P V, Mangla V, Jose B V, Sasi P K. Analysis of Nonvestibular Cranial Nerve Schwannomas. Iran J Neurosurg 2023; 9 : 16
URL: http://irjns.org/article-1-354-en.html
URL: http://irjns.org/article-1-354-en.html
Binoy Damodar Thavara1 

 , Rajeev Mandaka Parambil *2
, Rajeev Mandaka Parambil *2 

 , Pavithran Vadakkam Muriyil1
, Pavithran Vadakkam Muriyil1 

 , Vishal Mangla1
, Vishal Mangla1 

 , Byjo Valiyaveetil Jose3
, Byjo Valiyaveetil Jose3 

 , Prem kumar Sasi1
, Prem kumar Sasi1 




 , Rajeev Mandaka Parambil *2
, Rajeev Mandaka Parambil *2 

 , Pavithran Vadakkam Muriyil1
, Pavithran Vadakkam Muriyil1 

 , Vishal Mangla1
, Vishal Mangla1 

 , Byjo Valiyaveetil Jose3
, Byjo Valiyaveetil Jose3 

 , Prem kumar Sasi1
, Prem kumar Sasi1 


1- Department of Neurosurgery, Government Medical College, Kozhikode, Kerala State, India
2- Department of Neurosurgery, Government Medical College, Kozhikode, Kerala State, India ,drrajeevmp@gmail.com
3- Department of Neurosurgery, Government Medical College, Kozhikode, Kerala State, India.
2- Department of Neurosurgery, Government Medical College, Kozhikode, Kerala State, India ,
3- Department of Neurosurgery, Government Medical College, Kozhikode, Kerala State, India.
Keywords: Cranial Nerve, Magnetic Resonance Imaging, Nonvestibular Cranial Nerve Schwannoma, Retrosigmoid suboccipital approach
Full Text [PDF 1087 kb]
(849 Downloads)
| Abstract (HTML) (2091 Views)
Full Text: (675 Views)
1. Introduction
Abenign nerve sheath tumor occurring along the cranial nerve (CN) is called CN schwannoma [1]. Non-vestibular cranial nerve schwannomas (NVCNS) represent only 5%-10% of CN schwannomas [2]. The most common NVCNS are trigeminal and jugular foramen schwannomas [3]. They are either detected incidentally or present with loss of function of the affected nerve. The treatment options include observation, total excision, subtotal removal, and radiation treatment. The ideal treatment for the symptomatic patient is the total excision of the tumor [1]. Due to tumor location, and extent and to reduce morbidity, it is not possible to achieve a gross total resection (GTR) in all cases. In such cases, subtotal resection followed by radiation is the alternative treatment [2]. Postoperative complications consisted of meningitis, cerebrospinal fluid (CSF) leaks, vasospasm, hemiparesis, and new onset partial CN deficits [4].
The authors analyzed their experience in the surgically treated cases of the NVCNS. These lesions are surrounded by vital structures, such as cranial nerves and brain stem. Operating such cases is challenging and associated with morbidity. Previous experience in performing skull base surgery helps in decision-making in the extent of surgical resection. This study was conducted to analyze the clinical profile, distribution, and surgical outcome of the NVCNS.
2. Methods and Materials/Patients
This is a retrospective observational study to review the medical records of the patients with NVCNS operated between January 2007 and December 2021. This study was conducted at the Department of Neurosurgery, Government Medical College, Kozhikode, India. Institutional Ethics Committee clearance was obtained for the study. The clinical profile of the patients was analyzed. Radiological diagnosis was done using computed tomography (CT) scan or magnetic resonance imaging (MRI) scan. The size of the tumor was measured in CT/MRI scan. All the cases are histopathologically confirmed as schwannomas. VIIIth CN (Vestibulocochlear nerve) schwannomas and conservatively managed NVCNS were excluded from the study. When it was difficult to differentiate the origin of the tumor as IXth or Xth or XIth cranial nerve, they were called lower cranial nerve (LCN) schwannomas in this study. This study was conducted to analyze the clinical profile, distribution, and surgical outcome of the NVCNS.
Patient’s age, sex, diagnosis, clinical features, Glasgow Coma Scale (GCS), CT/MRI finding, tumor size, types of NVCNS, CN involved, side of involvement, tumor location (middle cranial fossa, posterior cranial fossa, neck or cervical canal), presence of hydrocephalus, surgical approaches, the principle of tumor removal, the extent of resection, histopathology, postoperative complications and duration of stay in the hospital after surgery were analyzed. Statistical analysis was performed using Epi info software.
3. Results
A total of 25 patients existed with NVCNSs. Ten patients (40%) had trigeminal schwannomas, 3(12%) facial nerve schwannomas, 9 (36%) LCN schwannomas, 1 (4%) vagal schwannoma in neck and 2(8%) hypoglossal schwannomas. Among these, 4 recurrent cases (1 trigeminal, 1 facial, and 2 LCNs) existed. Eight patients (32%) were male and 17(68%) were female. The age of the patients ranged from 13 to 62 years (Mean±SD: 39±14 years). Twelve (48%) tumors were on the right side and 13(52%) were on the left side. Table 1 presents the clinical features and structures involved.

Twenty-four patients had Glasgow Coma Scale (GCS) of 15/15 during admission. One patient had decreased consciousness during admission, which improved in the postoperative period. The smallest tumor was vagal schwannoma in the neck measuring 4.6×1.3 cm and the largest was trigeminal schwannoma measuring 7.8×5.3 cm. Six patients had hydrocephalus in which 3 underwent ventriculo-peritoneal shunt in the preoperative period. Table 2 presents the locations of the tumors in the CT/MRI scan. One vagal and one hypoglossal schwannoma were located in the neck. One LCN schwannoma was located in the posterior cranial fossa (PCF) extending to the neck. Two LCN schwannomas were located in PCF extending to the upper cervical canal. All the patients underwent surgery.

Table 3 presents the surgical approaches. During surgery, the authors used a cavitron ultrasonic surgical aspirator (CUSA) as a main tool to remove the tumor in the skull base region. It helped to remove the tumor without manipulation or damage to the surrounding structures. Initially, the tumor capsule was opened and internal debulking was done till the capsule becomes thin. Then capsule was reflected without damaging the surrounding structures. Removal of part of the tumor near the brainstem was the most difficult part of the surgery. In difficult cases, part of the tumor was left behind considering the benign nature of the lesion. Achieving hemostasis was challenging and enough time was spent on this at the end of the tumor removal. Haemostasis was achieved using bipolar electrocautery and absorbable hemostatic agents. Valsalva maneuver was used to check if any bleeding points were remaining. The dura was sutured watertight and pericranium was used for dural closure in some cases.

Thirteen patients (52%) underwent GTR, 7(28%) underwent near-total resection (NTR) and 5(20%) underwent subtotal resection (STR). Table 4 presents the extent of resection. All tumors were large (the smallest tumor size is 4.6×1.3 cm). Hence, GTR was not attempted in some cases to decrease the complications. The authors believe that experience in skull base neurosurgery can help in better surgical results.

All the patients improved in the postoperative period. Complications developed in the postoperative period (Table 5) were managed with conservative treatment. The authors believe that complications can be decreased by avoiding injury to the vital surrounding structures, such as the brainstem, by achieving proper hemostasis at the end of the surgery, watertight dural closure, and by waxing air sinuses. The duration of stay in the hospital after surgery was 7 to 46 days (Mean±SD: 16±10 days).

Illustrative case
A 42-year-old male presented with headache, intermittent difficulty in speech, and left-sided submandibular neck swelling of 8 months duration. MRI of the neck revealed a well-defined strongly enhancing lesion measuring 4.6×1.3 cm in the left carotid space at the C2 level causing compression of the cervical internal carotid artery (ICA) with proximity to the jugular foramen and compressing the internal jugular vein (Figure 1).

The patient underwent surgery with a submandibular transcervical approach. A vascular surgeon’s help was sought due to its proximity to carotid and jugular vessels. Intraoperatively digastric muscle was divided. Hypoglossal nerve, carotid bifurcation and internal jugular vein were identified and preserved. The carotid sheath was opened and a solid cystic encapsulated greyish-white lesion arising from the left vagus nerve was defined (Figure 2). The vagus nerve was not separable from the tumor. Excision of the tumor was done. Histopathology confirmed it as vagal nerve schwannoma. Postoperatively patient developed a change in voice and uvula deviation to the right side which partially improved in the follow-up period.
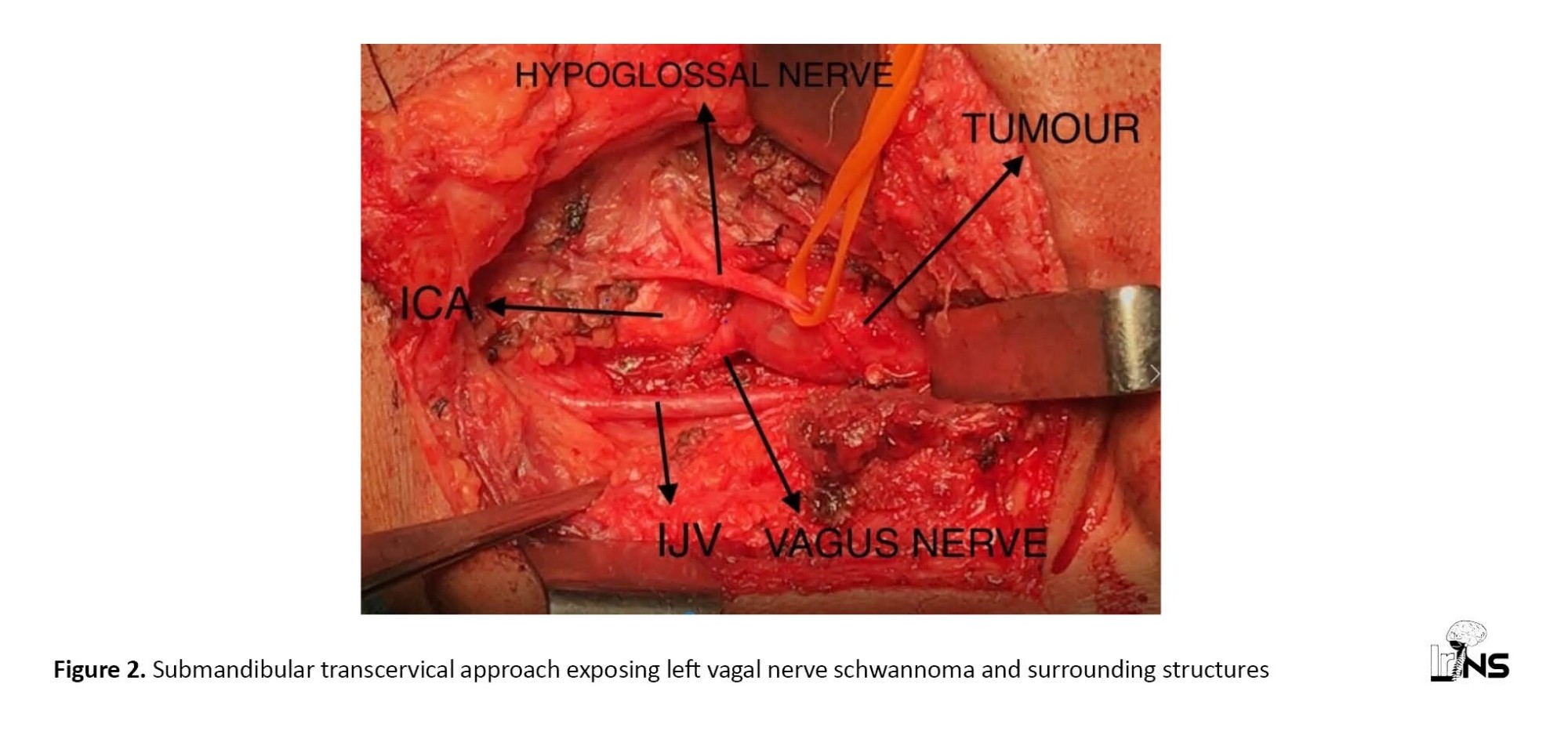
Experience in upper cervical neck dissection is important for operating such cases. Neurosurgeons can expose vagal nerve schwannoma with the help of a vascular surgeon given its proximity to carotid and jugular vessels. Anesthetists should be aware of the cardiac effect of the vagus nerve during surgery. Care should be taken to avoid injury to the hypoglossal nerve, carotid bifurcation, carotid vessels, and internal jugular vein. If possible, the vagus nerve should be preserved. But in the author’s case, the tumor was large and the vagus nerve was not separable and hence divided.
4. Discussion
Schwannoma is World Health Organization (WHO) grade I tumor. It occurs due to the loss of function of the Merlin gene [5]. The schwannomas form a well-demarcated, eccentric mass and deflect the nerve [1]. NVCNS are relatively rare tumors [3]. Chowdhury et al. presented their experience in 30 cases of NVCNS. The most common tumor was trigeminal schwannoma followed by glossopharyngeal+/ vagus nerve schwannoma. They achieved total resection of the tumor in 80% of cases. Postoperatively 25 cases improved, 3 cases developed new deficits and 2 patients died. They concluded that the surgical approach to these tumors is associated with significant morbidity. But correct decision-making and surgical planning can result in good outcomes [6]. In the author’s study, the most common tumors are trigeminal and LCN schwannomas. They achieved total resection in only 52% of cases, but all the patients improved in the postoperative period without any mortality. Since these are benign lesions, the authors believe that postoperative clinical improvement along with decreased complications should be the goal of the surgery.
Safavi-Abbasi et al. noted that larger tumors were treated with microsurgery or combined microsurgery and radiosurgery. Tumors with a mean tumor volume of 5.38±3.23 cc received microsurgical treatment alone. Postoperative complications include CSF leak, hydrocephalus, and meningitis. New CN deficits were identified in the 14.3% microsurgical group. Other complications include new hearing deficits, new facial numbness, transient hoarseness, and mild dysphagia [3]. Facial nerve palsy and chest infection were the most common complications in the author’s cases.
Sarma et al. reported their experience in 26 trigeminal nerve schwannomas. The mean tumor equivalent diameter (TED) was 2.52 cm. Trigeminal nerve dysfunction was the most common presenting symptom. The other CNs involved in trigeminal schwannomas are VI, II, III, IV, VII, and VIII. Twenty of these patients underwent surgery via an anterolateral craniotomy with extradural or intradural approach. The remaining six patients underwent surgery via a lateral intradural approach (partial labyrinthectomy and petrous apicectomy) or other transpetrosal approaches [4]. Our author reported 10 cases of trigeminal schwannomas. The smallest tumor measured 3.9 x 4.1 cm and the largest measured 7.8 x 5.3 cm. Five patients underwent surgery by temporal craniotomy and another 5 by retrosigmoid suboccipital approach.
Sarma et al. reported that the main presenting complaint in facial nerve schwannoma was facial nerve weakness. Other CNs involved are VIII, IX, and X. The mean tumor equivalent diameter (TED) was 0.99 cm. The tumors located at the geniculate segment of the facial nerve underwent a middle fossa and transmastoid approach. The tumors in the cerebellopontine angle underwent a retrosigmoid approach. Lower cranial nerve schwannomas occupy the jugular foramen area. Glossopharyngeal nerve schwannomas presented with CNs IX, VIII, VI, and V impairment or cerebellar impairment. Cisternal tumors underwent a retrosigmoid approach. Tumors extending into the jugular foramen underwent a retrosigmoid and transjugular approach. The vagal nerve schwannoma underwent retrosigmoid approach and the accessory nerve schwannoma underwent an extreme lateral approach. Hypoglossal nerve schwannomas presented with weakness of CNs IX, X, and XII. Complete tumor excision was achieved in all cases. Cisternal tumors underwent an extreme lateral and partial transcondylar approach [4]. In the author’s study, 2 patients underwent temporal craniotomy and 1 patient underwent retrosigmoid suboccipital approach in facial nerve schwannoma. The retrosigmoid, suboccipital approach was used in 8 cases, and the far lateral approach in 1 case of LCN schwannomas. Vagal schwannoma underwent a submandibular transcervical approach. The retrosigmoid suboccipital approach and submandibular transcervical approach were used in each case of hypoglossal schwannoma. The authors believe that the selection of surgical approaches depends on the location of the tumor and the surgeon’s preference.
Cervical vagal schwannoma is a rare, benign pathology with non-specific symptoms. It is 1.5 times more common in women with a median age of 44 years. The surgical decision is taken by balancing risks and benefits. For benign lesions, intracapsular enucleation gives better results in terms of preserving nerve function [7].
Hypoglossal schwannomas occur rarely and account for 5% of non-vestibular schwannomas. Due to its negative effects on delicate surrounding structures, treatment consists of the surgical excision of the lesion. An intracranial lesion with extracranial extension is the most common type of lesion. Isolated extracranial lesions are rare [8].
In the author’s study, NVCNS are more common in women. Findings of cerebellar involvement, VIIIth CN involvement, and headache are the most common clinical features. All the tumors are large in the author’s study. Thirteen (52%) patients underwent GTR of tumors. The retrosigmoid suboccipital approach is the most commonly used surgical approach for trigeminal and LCN schwannoma. Postoperative complications were managed conservatively with good results. All the patients recovered with good surgical outcomes.
5. Conclusion
NVCNS are more common in women. Trigeminal and lower cranial nerve schwannomas are the most common NVCNS. The retrosigmoid suboccipital approach is the most commonly used surgical approach for tumors located in the posterior cranial fossa. Facial nerve palsy is the most common cranial nerve palsy occurring in the postoperative period. Since NVCNS are benign lesions, postoperative clinical improvement along with decreasing the complications should be the goal of the surgery. Hence, though gross total resection is the most commonly achieved extent of resection, near-total or subtotal resection can be done wherever GTR is not possible.
Ethical Considerations
Compliance with ethical guidelines
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Institutional ethical committee (institutional review board) permission was obtained for the study from Government Medical College, Kozhikode, India (GMCKKD/RP 2022/IEC/29). This is a retrospective study. Individual patient consent was not obtained from all the patients. Consent was obtained from the patient whose clinical details are described in the illustrative case.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception or design of the work: Rajeev Mandaka Parambil, Binoy Damodar Thavara; Data collection: Binoy Damodar Thavara, Vishal Mangla; Data analysis and interpretation: Pavithran Vadakkam Muriyil, Byjo Valiyaveetil Jose; Drafting the article: All authors; Critical revision of the article: All authors; Approving the final version of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The study was supported by the Department of Neurosurgery, Government Medical College, Kozhikode, India.
References
Abenign nerve sheath tumor occurring along the cranial nerve (CN) is called CN schwannoma [1]. Non-vestibular cranial nerve schwannomas (NVCNS) represent only 5%-10% of CN schwannomas [2]. The most common NVCNS are trigeminal and jugular foramen schwannomas [3]. They are either detected incidentally or present with loss of function of the affected nerve. The treatment options include observation, total excision, subtotal removal, and radiation treatment. The ideal treatment for the symptomatic patient is the total excision of the tumor [1]. Due to tumor location, and extent and to reduce morbidity, it is not possible to achieve a gross total resection (GTR) in all cases. In such cases, subtotal resection followed by radiation is the alternative treatment [2]. Postoperative complications consisted of meningitis, cerebrospinal fluid (CSF) leaks, vasospasm, hemiparesis, and new onset partial CN deficits [4].
The authors analyzed their experience in the surgically treated cases of the NVCNS. These lesions are surrounded by vital structures, such as cranial nerves and brain stem. Operating such cases is challenging and associated with morbidity. Previous experience in performing skull base surgery helps in decision-making in the extent of surgical resection. This study was conducted to analyze the clinical profile, distribution, and surgical outcome of the NVCNS.
2. Methods and Materials/Patients
This is a retrospective observational study to review the medical records of the patients with NVCNS operated between January 2007 and December 2021. This study was conducted at the Department of Neurosurgery, Government Medical College, Kozhikode, India. Institutional Ethics Committee clearance was obtained for the study. The clinical profile of the patients was analyzed. Radiological diagnosis was done using computed tomography (CT) scan or magnetic resonance imaging (MRI) scan. The size of the tumor was measured in CT/MRI scan. All the cases are histopathologically confirmed as schwannomas. VIIIth CN (Vestibulocochlear nerve) schwannomas and conservatively managed NVCNS were excluded from the study. When it was difficult to differentiate the origin of the tumor as IXth or Xth or XIth cranial nerve, they were called lower cranial nerve (LCN) schwannomas in this study. This study was conducted to analyze the clinical profile, distribution, and surgical outcome of the NVCNS.
Patient’s age, sex, diagnosis, clinical features, Glasgow Coma Scale (GCS), CT/MRI finding, tumor size, types of NVCNS, CN involved, side of involvement, tumor location (middle cranial fossa, posterior cranial fossa, neck or cervical canal), presence of hydrocephalus, surgical approaches, the principle of tumor removal, the extent of resection, histopathology, postoperative complications and duration of stay in the hospital after surgery were analyzed. Statistical analysis was performed using Epi info software.
3. Results
A total of 25 patients existed with NVCNSs. Ten patients (40%) had trigeminal schwannomas, 3(12%) facial nerve schwannomas, 9 (36%) LCN schwannomas, 1 (4%) vagal schwannoma in neck and 2(8%) hypoglossal schwannomas. Among these, 4 recurrent cases (1 trigeminal, 1 facial, and 2 LCNs) existed. Eight patients (32%) were male and 17(68%) were female. The age of the patients ranged from 13 to 62 years (Mean±SD: 39±14 years). Twelve (48%) tumors were on the right side and 13(52%) were on the left side. Table 1 presents the clinical features and structures involved.

Twenty-four patients had Glasgow Coma Scale (GCS) of 15/15 during admission. One patient had decreased consciousness during admission, which improved in the postoperative period. The smallest tumor was vagal schwannoma in the neck measuring 4.6×1.3 cm and the largest was trigeminal schwannoma measuring 7.8×5.3 cm. Six patients had hydrocephalus in which 3 underwent ventriculo-peritoneal shunt in the preoperative period. Table 2 presents the locations of the tumors in the CT/MRI scan. One vagal and one hypoglossal schwannoma were located in the neck. One LCN schwannoma was located in the posterior cranial fossa (PCF) extending to the neck. Two LCN schwannomas were located in PCF extending to the upper cervical canal. All the patients underwent surgery.

Table 3 presents the surgical approaches. During surgery, the authors used a cavitron ultrasonic surgical aspirator (CUSA) as a main tool to remove the tumor in the skull base region. It helped to remove the tumor without manipulation or damage to the surrounding structures. Initially, the tumor capsule was opened and internal debulking was done till the capsule becomes thin. Then capsule was reflected without damaging the surrounding structures. Removal of part of the tumor near the brainstem was the most difficult part of the surgery. In difficult cases, part of the tumor was left behind considering the benign nature of the lesion. Achieving hemostasis was challenging and enough time was spent on this at the end of the tumor removal. Haemostasis was achieved using bipolar electrocautery and absorbable hemostatic agents. Valsalva maneuver was used to check if any bleeding points were remaining. The dura was sutured watertight and pericranium was used for dural closure in some cases.

Thirteen patients (52%) underwent GTR, 7(28%) underwent near-total resection (NTR) and 5(20%) underwent subtotal resection (STR). Table 4 presents the extent of resection. All tumors were large (the smallest tumor size is 4.6×1.3 cm). Hence, GTR was not attempted in some cases to decrease the complications. The authors believe that experience in skull base neurosurgery can help in better surgical results.

All the patients improved in the postoperative period. Complications developed in the postoperative period (Table 5) were managed with conservative treatment. The authors believe that complications can be decreased by avoiding injury to the vital surrounding structures, such as the brainstem, by achieving proper hemostasis at the end of the surgery, watertight dural closure, and by waxing air sinuses. The duration of stay in the hospital after surgery was 7 to 46 days (Mean±SD: 16±10 days).

Illustrative case
A 42-year-old male presented with headache, intermittent difficulty in speech, and left-sided submandibular neck swelling of 8 months duration. MRI of the neck revealed a well-defined strongly enhancing lesion measuring 4.6×1.3 cm in the left carotid space at the C2 level causing compression of the cervical internal carotid artery (ICA) with proximity to the jugular foramen and compressing the internal jugular vein (Figure 1).

The patient underwent surgery with a submandibular transcervical approach. A vascular surgeon’s help was sought due to its proximity to carotid and jugular vessels. Intraoperatively digastric muscle was divided. Hypoglossal nerve, carotid bifurcation and internal jugular vein were identified and preserved. The carotid sheath was opened and a solid cystic encapsulated greyish-white lesion arising from the left vagus nerve was defined (Figure 2). The vagus nerve was not separable from the tumor. Excision of the tumor was done. Histopathology confirmed it as vagal nerve schwannoma. Postoperatively patient developed a change in voice and uvula deviation to the right side which partially improved in the follow-up period.

Experience in upper cervical neck dissection is important for operating such cases. Neurosurgeons can expose vagal nerve schwannoma with the help of a vascular surgeon given its proximity to carotid and jugular vessels. Anesthetists should be aware of the cardiac effect of the vagus nerve during surgery. Care should be taken to avoid injury to the hypoglossal nerve, carotid bifurcation, carotid vessels, and internal jugular vein. If possible, the vagus nerve should be preserved. But in the author’s case, the tumor was large and the vagus nerve was not separable and hence divided.
4. Discussion
Schwannoma is World Health Organization (WHO) grade I tumor. It occurs due to the loss of function of the Merlin gene [5]. The schwannomas form a well-demarcated, eccentric mass and deflect the nerve [1]. NVCNS are relatively rare tumors [3]. Chowdhury et al. presented their experience in 30 cases of NVCNS. The most common tumor was trigeminal schwannoma followed by glossopharyngeal+/ vagus nerve schwannoma. They achieved total resection of the tumor in 80% of cases. Postoperatively 25 cases improved, 3 cases developed new deficits and 2 patients died. They concluded that the surgical approach to these tumors is associated with significant morbidity. But correct decision-making and surgical planning can result in good outcomes [6]. In the author’s study, the most common tumors are trigeminal and LCN schwannomas. They achieved total resection in only 52% of cases, but all the patients improved in the postoperative period without any mortality. Since these are benign lesions, the authors believe that postoperative clinical improvement along with decreased complications should be the goal of the surgery.
Safavi-Abbasi et al. noted that larger tumors were treated with microsurgery or combined microsurgery and radiosurgery. Tumors with a mean tumor volume of 5.38±3.23 cc received microsurgical treatment alone. Postoperative complications include CSF leak, hydrocephalus, and meningitis. New CN deficits were identified in the 14.3% microsurgical group. Other complications include new hearing deficits, new facial numbness, transient hoarseness, and mild dysphagia [3]. Facial nerve palsy and chest infection were the most common complications in the author’s cases.
Sarma et al. reported their experience in 26 trigeminal nerve schwannomas. The mean tumor equivalent diameter (TED) was 2.52 cm. Trigeminal nerve dysfunction was the most common presenting symptom. The other CNs involved in trigeminal schwannomas are VI, II, III, IV, VII, and VIII. Twenty of these patients underwent surgery via an anterolateral craniotomy with extradural or intradural approach. The remaining six patients underwent surgery via a lateral intradural approach (partial labyrinthectomy and petrous apicectomy) or other transpetrosal approaches [4]. Our author reported 10 cases of trigeminal schwannomas. The smallest tumor measured 3.9 x 4.1 cm and the largest measured 7.8 x 5.3 cm. Five patients underwent surgery by temporal craniotomy and another 5 by retrosigmoid suboccipital approach.
Sarma et al. reported that the main presenting complaint in facial nerve schwannoma was facial nerve weakness. Other CNs involved are VIII, IX, and X. The mean tumor equivalent diameter (TED) was 0.99 cm. The tumors located at the geniculate segment of the facial nerve underwent a middle fossa and transmastoid approach. The tumors in the cerebellopontine angle underwent a retrosigmoid approach. Lower cranial nerve schwannomas occupy the jugular foramen area. Glossopharyngeal nerve schwannomas presented with CNs IX, VIII, VI, and V impairment or cerebellar impairment. Cisternal tumors underwent a retrosigmoid approach. Tumors extending into the jugular foramen underwent a retrosigmoid and transjugular approach. The vagal nerve schwannoma underwent retrosigmoid approach and the accessory nerve schwannoma underwent an extreme lateral approach. Hypoglossal nerve schwannomas presented with weakness of CNs IX, X, and XII. Complete tumor excision was achieved in all cases. Cisternal tumors underwent an extreme lateral and partial transcondylar approach [4]. In the author’s study, 2 patients underwent temporal craniotomy and 1 patient underwent retrosigmoid suboccipital approach in facial nerve schwannoma. The retrosigmoid, suboccipital approach was used in 8 cases, and the far lateral approach in 1 case of LCN schwannomas. Vagal schwannoma underwent a submandibular transcervical approach. The retrosigmoid suboccipital approach and submandibular transcervical approach were used in each case of hypoglossal schwannoma. The authors believe that the selection of surgical approaches depends on the location of the tumor and the surgeon’s preference.
Cervical vagal schwannoma is a rare, benign pathology with non-specific symptoms. It is 1.5 times more common in women with a median age of 44 years. The surgical decision is taken by balancing risks and benefits. For benign lesions, intracapsular enucleation gives better results in terms of preserving nerve function [7].
Hypoglossal schwannomas occur rarely and account for 5% of non-vestibular schwannomas. Due to its negative effects on delicate surrounding structures, treatment consists of the surgical excision of the lesion. An intracranial lesion with extracranial extension is the most common type of lesion. Isolated extracranial lesions are rare [8].
In the author’s study, NVCNS are more common in women. Findings of cerebellar involvement, VIIIth CN involvement, and headache are the most common clinical features. All the tumors are large in the author’s study. Thirteen (52%) patients underwent GTR of tumors. The retrosigmoid suboccipital approach is the most commonly used surgical approach for trigeminal and LCN schwannoma. Postoperative complications were managed conservatively with good results. All the patients recovered with good surgical outcomes.
5. Conclusion
NVCNS are more common in women. Trigeminal and lower cranial nerve schwannomas are the most common NVCNS. The retrosigmoid suboccipital approach is the most commonly used surgical approach for tumors located in the posterior cranial fossa. Facial nerve palsy is the most common cranial nerve palsy occurring in the postoperative period. Since NVCNS are benign lesions, postoperative clinical improvement along with decreasing the complications should be the goal of the surgery. Hence, though gross total resection is the most commonly achieved extent of resection, near-total or subtotal resection can be done wherever GTR is not possible.
Ethical Considerations
Compliance with ethical guidelines
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Institutional ethical committee (institutional review board) permission was obtained for the study from Government Medical College, Kozhikode, India (GMCKKD/RP 2022/IEC/29). This is a retrospective study. Individual patient consent was not obtained from all the patients. Consent was obtained from the patient whose clinical details are described in the illustrative case.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conception or design of the work: Rajeev Mandaka Parambil, Binoy Damodar Thavara; Data collection: Binoy Damodar Thavara, Vishal Mangla; Data analysis and interpretation: Pavithran Vadakkam Muriyil, Byjo Valiyaveetil Jose; Drafting the article: All authors; Critical revision of the article: All authors; Approving the final version of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The study was supported by the Department of Neurosurgery, Government Medical College, Kozhikode, India.
References
- Alemdar M. Expert commentary on rare (Nonvestibular, Nontrigenimal) cranial nerve schwannomas. Journal of Neurosciences in Rural Practice. 2018; 9(2):175-6. [DOI:10.4103/jnrp.jnrp_546_17] [PMID] [PMCID]
- Suárez C, López F, Mendenhall WM, Andreasen S, Mikkelsen LH, Langendijk JA, et al. Trends in the management of non-vestibular skull base and intracranial schwannomas. Cancer Management and Research. 2021; 13:463-78. [DOI:10.2147/CMAR.S287410] [PMID] [PMCID]
- Safavi-Abbasi S, Bambakidis NC, Zabramski JM, Workman R, Verma K, Senoglu M, et al. Nonvestibular schwannomas: An evaluation of functional outcome after radiosurgical and microsurgical management. Acta Neurochirurgica (Wien). 2010; 152(1):35-46. [DOI:10.1007/s00701-009-0403-5] [PMID]
- Sarma S, Sekhar LN, Schessel DA. Nonvestibular schwannomas of the brain: A 7-year experience. Neurosurgery. 2002; 50(3):437-48; discussion 438-9. [DOI:10.1097/00006123-200203000-00002] [PMID]
- Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathology (Zurich, Switzerland). 2014; 24(3):205-20. [DOI:10.1111/bpa.12125] [PMID] [PMCID]
- Chowdhury FH, Haque MR, Kawsar KA, Sarker MH, Hasan M, Goel AH. Intracranial nonvestibular neurinomas: Young neurosurgeons’ experience. Journal of Neurosciences in Rural Practice. 2014; 5(3):231-43. [DOI:10.4103/0976-3147.133566] [PMID] [PMCID]
- Cavallaro G, Pattaro G, Iorio O, Avallone M, Silecchia G. A literature review on surgery for cervical vagal schwannomas. World Journal of Surgical Oncology. 2015; 13:130. [DOI:10.1186/s12957-015-0541-6] [PMID] [PMCID]
- Fornaro R, Salerno A, Filip DC, Caratto E, Caratto M, Casaccia M. Schwannoma of the hypoglossal nerve: Review of the literature based on an illustrative case. Molecular and Clinical Oncology. 2017; 7(2):288-94. [PMID]
Type of Study: Research |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



