Wed, Jul 16, 2025
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 202-209 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kumar Gupta S, Faheem M. Analysis of Cases of Giant Epidural Hemorrhage Evacuated in a Rural Hospital. Iran J Neurosurg 2023; 9 : 25
URL: http://irjns.org/article-1-363-en.html
URL: http://irjns.org/article-1-363-en.html
1- Department of Neurosurgery, Uttar Pradesh University of Medical Sciences, Saifai, India , Sajag.gupta@yahoo.com
2- Department of Neurosurgery, Uttar Pradesh University of Medical Sciences, Saifai, India
2- Department of Neurosurgery, Uttar Pradesh University of Medical Sciences, Saifai, India
Full Text [PDF 1181 kb]
(453 Downloads)
| Abstract (HTML) (1836 Views)
Full Text: (599 Views)
1. Introduction
Epidural hemorrhage is a hematoma accumulation between the dura and bone. It is commonly caused by a rupture of the middle meningeal artery, but it can also be caused by a rupture of the dural venous sinuses, veins in the meningeal layer, diploic vein, and fracture line.
Trauma is almost always the etiology of epidural hemorrhage and it is generally linked to a skull fracture [1]. Epidural hemorrhages are responsible for 5%-15% of dangerous brain traumas [2]. The head computed tomography (CT) scan is the preferred examination for detecting brain damage following trauma [3]. Head CT scan also detects other factors that influence the surgical outcome, such as midline shift (MLS), traumatic subarachnoid hemorrhage, obliterated basal cisterns, hematoma size, the volume of the clot, contusion, and skull bone fracture.
Local ischemia from massive trauma, direct head injury, or venous return obstruction is most responsible for the associated brain disorders. Ischemic brain injury is a significant prognostic indicator for the pathophysiology of epidural hemorrhage and may be caused by the hematoma’s mass effects and elevated intracerebral pressures (ICP), resulting in impaired cerebral perfusion pressures (CPP). Apart from epidural hemorrhage, the underlying brain damage is responsible for impaired neurological function following trauma. The outcome of epidural hemorrhage is mostly determined by the Glasgow coma scale (GCS) score on admission and neurological state [4]. A massive epidural hemorrhage (Figure 1) is an epidural collection large enough to produce an abrupt MLS, brain herniation, and pressure over the brainstem, resulting in patient death. It is an emergency that necessitates the immediate removal of epidural hemorrhage and stabilization of the patient. Many comorbidities, concurrent brain impairment, ischemic brain, antiplatelet, anticoagulant medication, and old age influence the surgical prognosis of epidural hemorrhage. It is critical to correctly identify outcome-predicting markers in order to provide appropriate surgical treatment. We assessed the effect of massive or huge epidural hemorrhage, which is significant acute deterioration of neurological status and negative prognosis. We also examined people with huge epidural hemorrhage who underwent surgery from a therapeutic and prognosis standpoint.
2. Methods and Materials/Patients
This retrospective study was done on 112 people who underwent surgery for an epidural hemorrhage in an Emergency Neurosurgical Department of the Uttar Pradesh University of Medical Sciences (UPUMS), Saifai, Etawah, India, a rural hospital between January 1, 2022, and 31 March 2023. They were evaluated for giant epidural hemorrhage target amount and we considered >80 cc giant epidural haemorrhage. Accordingly, 15 people with giant epidural hemorrhage with hematoma >80 cc were evaluated clinically, surgically, and for outcome according to age group, gender, mode of trauma, motor response, the severity of the injury, location of hemorrhage, presentation, CT findings, amount of clot evacuated, source of bleeding, and prognosis at discharge/death. All cases admitted to the Neurosurgical Department at UPUMS, met the criteria of inclusion. Patients with epidural hemorrhage >80 CC who received surgery within the first two hours after arriving at the trauma center were included in the research. The exclusion criteria were insufficient data for the research variables, conservatively treated epidural hemorrhage patients, and giving no consent to surgery. Craniotomy with epidural hemorrhage evacuation was the surgical procedure employed. Craniectomy was performed for temporal epidural hemorrhage or comminuted skull fracture. Fresh frozen plasma was utilized to correct the international normalized ratio (INR) in anticoagulation medication patients, while pools of platelets were used in antiplatelet medication patients based on the neurosurgeon’s choice. ICU care was offered following surgery. All performed procedures were informed after receiving permission from victims or their relatives (in cases where the patient was not able to consent, namely, those with a low GCS score).
Statistical tests were done using GraphPad Prism software, version 8.3. A P<0.05 was considered significant. The null hypothesis of no significant difference in the mean and the alternative hypothesis of a significant difference between the means were considered. Outcome analysis of giant epidural hemorrhage was done using Pearson’s correlation coefficient, chi-square test, and one-way ANOVA. The Peterson and Espersen equation was used to compute the amount of the epidural hemorrhage [5] (Equation 1):
1. Epidural hemorrhage volume=XYZ/0.5.
Where X, Y, and Z denoted the hematoma’s sizes in the sagittal, axial, and coronal planes, respectively.
3. Results
In this research, most patients who underwent evacuation for giant epidural hemorrhage were in the age group of 20–40 years (n=8, 53.33%) followed by the age group of 40–60 years (n=3, 20%) (Table 1).

In this research, most patients who underwent surgery for giant epidural hemorrhage were male (n=14, 93.33%). The male-to-female (F:M) ratio was 14:1 (Table 1). Road traffic accidents (RTAs) (n=10, 66.66%) followed by assault (n=4, 26.66%) and fall from height (n=1, 6.66%) were the common modes of injury. M status was mostly M2 (n=9, 60 %) followed by M3 (n=4, 26.66 %) and M4 (n=2, 13.33%). Those with giant epidural hemorrhage had moderate (GCS score: 9–13; n=2, 13.33%) and severe head injury (GCS score: ≤8, n=13, 86.66 %). No patient had a mild head injury (GCS score: 14–15). Pupil alteration (bilateral) in eight patients (53.33%), pupil alteration (unilateral) in five patients (33.33%), and bradycardia in seven patients (46.66%) were reported. In this research, the most common site of giant epidural hemorrhage was frontotemporoparietal (n=10, 66.66%) followed by frontoparietal (n=4, 26.66%) and parietal (n=1, 6.66%) (Table 2).

The right-sided giant epidural haemorrhage was present in seven victims (46.66%), left-sided was found in six victims (40%), and bilateral giant epidural hemorrhage was seen in two victims (13.33%).
The source of bleeding was dural venous sinus bleeding in 12 patients (80%), followed by middle meningeal arterial bleeding in three patients (20%) (Table 3).
The amount of hematoma drained during the operation was >140 cc in seven patients (46.66%), 120–140 cc in five patients (33.33%), 100–120 cc in two patients (13.33%), and 80–90 cc in one patient (6.66%). In all patients, MLS and brain herniation were present. The Glasgow outcome scale (GOS) score of one was observed in eight patients (53.33%), the score of two was seen in three patients (20%), the score of three was seen in two patients (13.33%), the score of four was seen in one patient (6.66%), and the GOS score of five was seen in one patient (6.66%). In this study, the GCS score on admission, pupil alteration, and motor response were significantly associated with the outcome following surgery for a giant epidural hemorrhage. In contrast, age, gender, and severity of injury were not associated with outcomes following surgery (Table 4).

4. Discussion
Hemorrhage is commonly found in the region of a skull fracture. Epidural hemorrhage is usually rapid within a few hours, although it may have a delayed period that is recognized several days later [6]. A giant epidural hemorrhage, also known as a massive epidural hemorrhage, is an epidural collection large enough to induce sudden midline displacement, brain herniation, and pressure over the brainstem, ultimately resulting in the victim’s death. This is an emergency that necessitates the immediate evacuation of the epidural hemorrhage and stabilization of the patient. The age of victims who had massive epidural hemorrhage surgery ranges from 6 to 75 years. The 20-40 year age group (n=8, 53.33%) had the largest number of casualties, followed by the 40 to 60-year age group (n=3, 20%). There is a paucity of data sets in scientific evidence on the age distribution patterns of giant epidural hemorrhage. However, in the published studies that included all epidural hemorrhages, most of the epidural hemorrhages were in the second decade of patients’ lives, and the mean age of victims with epidural hemorrhage was between 20 to 30 years [7, 8]. Patients suffering giant epidural hemorrhage have a similar trend of age distribution. The male/female ratio of 14:1 in the current research reflects our social milieu, where most women were not exposed to outdoor careers. In individuals with giant epidural hemorrhage, we discovered male predominance (n=14, 93.33%) over female victims (n=1, 6.66%). Research is scarce on sex predominance in patients with giant epidural hemorrhage. Male predominance over females is identified in the documented series of all epidural hemorrhage cases [9]. We found a similar type of male predominance in our giant epidural hemorrhage patients.
RTAs were the most prevalent source of trauma in this research (n=10, 66.66%), followed by assault (n=4, 26.66%), resulting in the development of giant epidural hemorrhage. It is comparable to several other published datasets [8, 9] that cover all epidural hemorrhage patients. Compared to other places, epidural hemorrhage is more commonly seen in the temporoparietal and temporal regions in diverse reported series [10, 11]. There is a scarcity of material about the location of the giant epidural hemorrhage. According to our data, giant epidural hemorrhage was commonly found in the front temporoparietal area (n=10, 66.66%), followed by the frontoparietal (n=4, 26.66%) and parietal (n=1, 6.66%) regions. In the temporal area, epidural hemorrhage has been linked to a high rate of death [11]. In this investigation, the M2 motor response was reported in nine patients (60%) followed by M3 (n=4, 26.66%) and M4 (n=2, 13.33%). Poor outcomes were seen in operated patients with low motor response status. In epidural hemorrhage patients, the best motor response on admission was a crucial determinant affecting the outcome [12, 13]. The majority of the head injury patients leading to giant epidural hemorrhage in our investigation were severe (n=13, 86.66%), followed by moderate (n=2, 13.33%) head injuries. Bilateral pupil alterations (n=8, 53.33%) along with unilateral pupil alteration (n=5, 33.33%) in victims of giant epidural hemorrhage suggest brain herniation as well as pressure over the brainstem caused by the giant epidural hemorrhage. Bradycardias were found in seven victims (46.66%) suggesting brainstem compressions. In our research, the most prevalent source of bleeding resulting from a large epidural hemorrhage was dural venous sinus bleeding (n=12, 80%), followed by middle meningeal artery bleeding (n=3, 20%). In recent research of 102 pediatric victims along with 387 adults with epidural hemorrhage, arterial bleeding was found as the cause of epidural hemorrhage in 36% of adults and 18% of children [11]. The low GCS score, concomitant cerebral lesions, pupil alteration, and elevated ICP are independent indicators of poor outcomes [3, 14]. The GCS score prior to surgery is a significant predictor of operative outcome [3, 14]. Age, hematoma size, and epidural hemorrhage location were all important variables in other studies. It was discovered that pupil alteration and a lower GCS score (9) were connected to bad prognosis [15]. In this research, the important clinical factors linked to poor outcomes were GCS on admission, motor response, and pupil state. Age, sex, and the severity of damage were not substantially linked to treatment outcomes of giant epidural hemorrhage surgery.
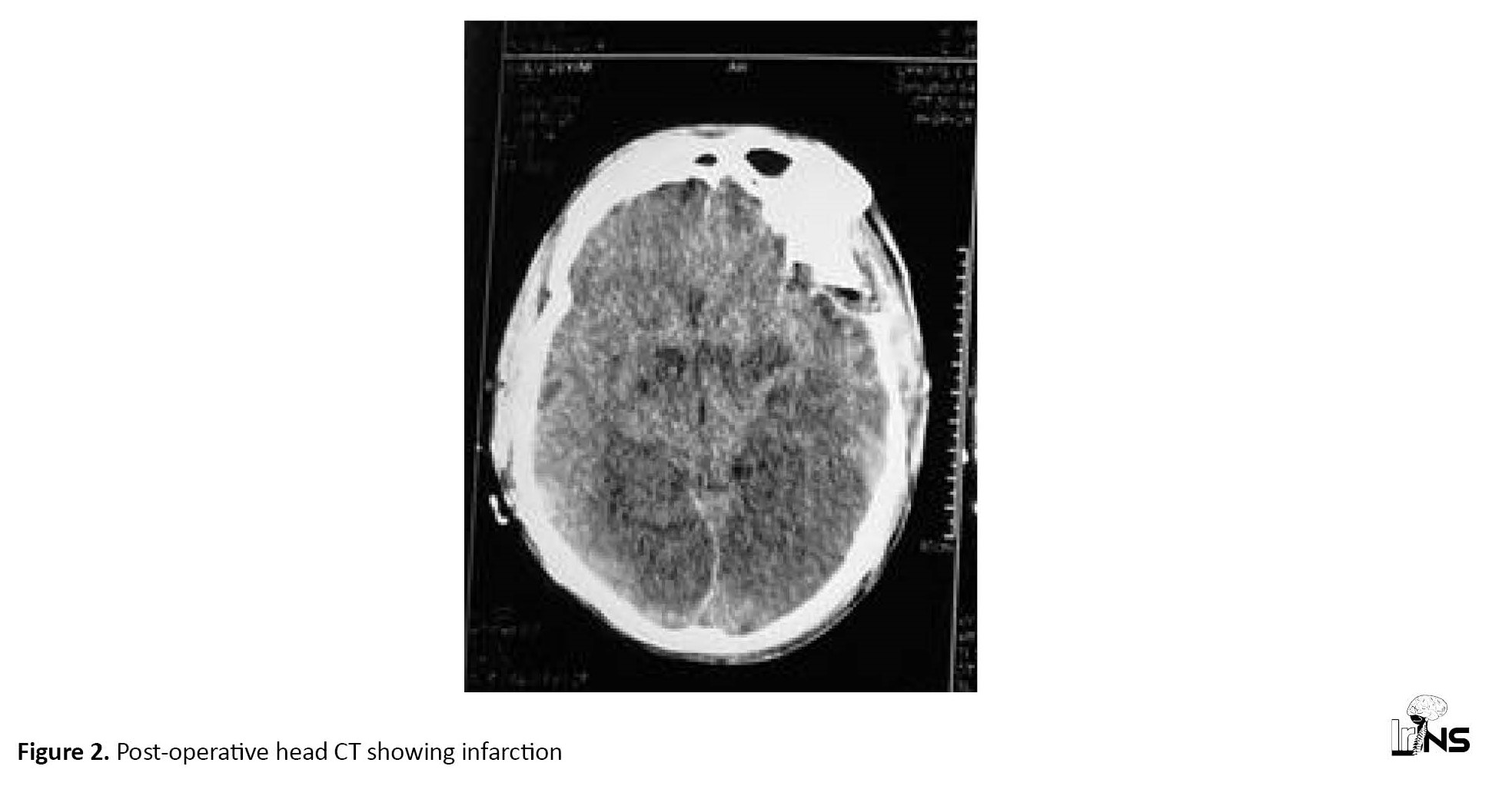
The most important predictor of prognosis in patients with epidural hemorrhage receiving surgery is the GCS score on admission or the GCS score prior to surgery [16]. There were eight deaths (53.33%) in this trial, with a GCS score of one. These findings point to bad outcomes and increased death in victims with large epidural hemorrhage (>80 cc). Research is scarce on the expiration of post-operative giant epidural bleeding. One of the patients had a heart attack after surgery and died (Figure 2).
5. Conclusion
A giant epidural hemorrhage >80 cc causes instantaneous MLS, brain herniation, and pressure over the brainstem, resulting in a higher proportion of death. Early removal of a giant epidural hemorrhage is associated with a good result. In victims with giant epidural hemorrhage, poor GCS score on admission, poor motor response, and pupil alterations are related to bad outcomes. Some notable findings in the study include the predominance of men with an M:F ratio of 14:1 and the active age group of 20 to 40 years.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Institutional Ethical Committee (Code: EC 23-008). Written informed consent was obtained from the patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All stages of the study were performed by both authors.
Conflict of interest
The author declared no conflicts of interests.
References
Epidural hemorrhage is a hematoma accumulation between the dura and bone. It is commonly caused by a rupture of the middle meningeal artery, but it can also be caused by a rupture of the dural venous sinuses, veins in the meningeal layer, diploic vein, and fracture line.
Trauma is almost always the etiology of epidural hemorrhage and it is generally linked to a skull fracture [1]. Epidural hemorrhages are responsible for 5%-15% of dangerous brain traumas [2]. The head computed tomography (CT) scan is the preferred examination for detecting brain damage following trauma [3]. Head CT scan also detects other factors that influence the surgical outcome, such as midline shift (MLS), traumatic subarachnoid hemorrhage, obliterated basal cisterns, hematoma size, the volume of the clot, contusion, and skull bone fracture.
Local ischemia from massive trauma, direct head injury, or venous return obstruction is most responsible for the associated brain disorders. Ischemic brain injury is a significant prognostic indicator for the pathophysiology of epidural hemorrhage and may be caused by the hematoma’s mass effects and elevated intracerebral pressures (ICP), resulting in impaired cerebral perfusion pressures (CPP). Apart from epidural hemorrhage, the underlying brain damage is responsible for impaired neurological function following trauma. The outcome of epidural hemorrhage is mostly determined by the Glasgow coma scale (GCS) score on admission and neurological state [4]. A massive epidural hemorrhage (Figure 1) is an epidural collection large enough to produce an abrupt MLS, brain herniation, and pressure over the brainstem, resulting in patient death. It is an emergency that necessitates the immediate removal of epidural hemorrhage and stabilization of the patient. Many comorbidities, concurrent brain impairment, ischemic brain, antiplatelet, anticoagulant medication, and old age influence the surgical prognosis of epidural hemorrhage. It is critical to correctly identify outcome-predicting markers in order to provide appropriate surgical treatment. We assessed the effect of massive or huge epidural hemorrhage, which is significant acute deterioration of neurological status and negative prognosis. We also examined people with huge epidural hemorrhage who underwent surgery from a therapeutic and prognosis standpoint.
2. Methods and Materials/Patients
This retrospective study was done on 112 people who underwent surgery for an epidural hemorrhage in an Emergency Neurosurgical Department of the Uttar Pradesh University of Medical Sciences (UPUMS), Saifai, Etawah, India, a rural hospital between January 1, 2022, and 31 March 2023. They were evaluated for giant epidural hemorrhage target amount and we considered >80 cc giant epidural haemorrhage. Accordingly, 15 people with giant epidural hemorrhage with hematoma >80 cc were evaluated clinically, surgically, and for outcome according to age group, gender, mode of trauma, motor response, the severity of the injury, location of hemorrhage, presentation, CT findings, amount of clot evacuated, source of bleeding, and prognosis at discharge/death. All cases admitted to the Neurosurgical Department at UPUMS, met the criteria of inclusion. Patients with epidural hemorrhage >80 CC who received surgery within the first two hours after arriving at the trauma center were included in the research. The exclusion criteria were insufficient data for the research variables, conservatively treated epidural hemorrhage patients, and giving no consent to surgery. Craniotomy with epidural hemorrhage evacuation was the surgical procedure employed. Craniectomy was performed for temporal epidural hemorrhage or comminuted skull fracture. Fresh frozen plasma was utilized to correct the international normalized ratio (INR) in anticoagulation medication patients, while pools of platelets were used in antiplatelet medication patients based on the neurosurgeon’s choice. ICU care was offered following surgery. All performed procedures were informed after receiving permission from victims or their relatives (in cases where the patient was not able to consent, namely, those with a low GCS score).
Statistical tests were done using GraphPad Prism software, version 8.3. A P<0.05 was considered significant. The null hypothesis of no significant difference in the mean and the alternative hypothesis of a significant difference between the means were considered. Outcome analysis of giant epidural hemorrhage was done using Pearson’s correlation coefficient, chi-square test, and one-way ANOVA. The Peterson and Espersen equation was used to compute the amount of the epidural hemorrhage [5] (Equation 1):
1. Epidural hemorrhage volume=XYZ/0.5.
Where X, Y, and Z denoted the hematoma’s sizes in the sagittal, axial, and coronal planes, respectively.
3. Results
In this research, most patients who underwent evacuation for giant epidural hemorrhage were in the age group of 20–40 years (n=8, 53.33%) followed by the age group of 40–60 years (n=3, 20%) (Table 1).

In this research, most patients who underwent surgery for giant epidural hemorrhage were male (n=14, 93.33%). The male-to-female (F:M) ratio was 14:1 (Table 1). Road traffic accidents (RTAs) (n=10, 66.66%) followed by assault (n=4, 26.66%) and fall from height (n=1, 6.66%) were the common modes of injury. M status was mostly M2 (n=9, 60 %) followed by M3 (n=4, 26.66 %) and M4 (n=2, 13.33%). Those with giant epidural hemorrhage had moderate (GCS score: 9–13; n=2, 13.33%) and severe head injury (GCS score: ≤8, n=13, 86.66 %). No patient had a mild head injury (GCS score: 14–15). Pupil alteration (bilateral) in eight patients (53.33%), pupil alteration (unilateral) in five patients (33.33%), and bradycardia in seven patients (46.66%) were reported. In this research, the most common site of giant epidural hemorrhage was frontotemporoparietal (n=10, 66.66%) followed by frontoparietal (n=4, 26.66%) and parietal (n=1, 6.66%) (Table 2).

The right-sided giant epidural haemorrhage was present in seven victims (46.66%), left-sided was found in six victims (40%), and bilateral giant epidural hemorrhage was seen in two victims (13.33%).
The source of bleeding was dural venous sinus bleeding in 12 patients (80%), followed by middle meningeal arterial bleeding in three patients (20%) (Table 3).

The amount of hematoma drained during the operation was >140 cc in seven patients (46.66%), 120–140 cc in five patients (33.33%), 100–120 cc in two patients (13.33%), and 80–90 cc in one patient (6.66%). In all patients, MLS and brain herniation were present. The Glasgow outcome scale (GOS) score of one was observed in eight patients (53.33%), the score of two was seen in three patients (20%), the score of three was seen in two patients (13.33%), the score of four was seen in one patient (6.66%), and the GOS score of five was seen in one patient (6.66%). In this study, the GCS score on admission, pupil alteration, and motor response were significantly associated with the outcome following surgery for a giant epidural hemorrhage. In contrast, age, gender, and severity of injury were not associated with outcomes following surgery (Table 4).

4. Discussion
Hemorrhage is commonly found in the region of a skull fracture. Epidural hemorrhage is usually rapid within a few hours, although it may have a delayed period that is recognized several days later [6]. A giant epidural hemorrhage, also known as a massive epidural hemorrhage, is an epidural collection large enough to induce sudden midline displacement, brain herniation, and pressure over the brainstem, ultimately resulting in the victim’s death. This is an emergency that necessitates the immediate evacuation of the epidural hemorrhage and stabilization of the patient. The age of victims who had massive epidural hemorrhage surgery ranges from 6 to 75 years. The 20-40 year age group (n=8, 53.33%) had the largest number of casualties, followed by the 40 to 60-year age group (n=3, 20%). There is a paucity of data sets in scientific evidence on the age distribution patterns of giant epidural hemorrhage. However, in the published studies that included all epidural hemorrhages, most of the epidural hemorrhages were in the second decade of patients’ lives, and the mean age of victims with epidural hemorrhage was between 20 to 30 years [7, 8]. Patients suffering giant epidural hemorrhage have a similar trend of age distribution. The male/female ratio of 14:1 in the current research reflects our social milieu, where most women were not exposed to outdoor careers. In individuals with giant epidural hemorrhage, we discovered male predominance (n=14, 93.33%) over female victims (n=1, 6.66%). Research is scarce on sex predominance in patients with giant epidural hemorrhage. Male predominance over females is identified in the documented series of all epidural hemorrhage cases [9]. We found a similar type of male predominance in our giant epidural hemorrhage patients.
RTAs were the most prevalent source of trauma in this research (n=10, 66.66%), followed by assault (n=4, 26.66%), resulting in the development of giant epidural hemorrhage. It is comparable to several other published datasets [8, 9] that cover all epidural hemorrhage patients. Compared to other places, epidural hemorrhage is more commonly seen in the temporoparietal and temporal regions in diverse reported series [10, 11]. There is a scarcity of material about the location of the giant epidural hemorrhage. According to our data, giant epidural hemorrhage was commonly found in the front temporoparietal area (n=10, 66.66%), followed by the frontoparietal (n=4, 26.66%) and parietal (n=1, 6.66%) regions. In the temporal area, epidural hemorrhage has been linked to a high rate of death [11]. In this investigation, the M2 motor response was reported in nine patients (60%) followed by M3 (n=4, 26.66%) and M4 (n=2, 13.33%). Poor outcomes were seen in operated patients with low motor response status. In epidural hemorrhage patients, the best motor response on admission was a crucial determinant affecting the outcome [12, 13]. The majority of the head injury patients leading to giant epidural hemorrhage in our investigation were severe (n=13, 86.66%), followed by moderate (n=2, 13.33%) head injuries. Bilateral pupil alterations (n=8, 53.33%) along with unilateral pupil alteration (n=5, 33.33%) in victims of giant epidural hemorrhage suggest brain herniation as well as pressure over the brainstem caused by the giant epidural hemorrhage. Bradycardias were found in seven victims (46.66%) suggesting brainstem compressions. In our research, the most prevalent source of bleeding resulting from a large epidural hemorrhage was dural venous sinus bleeding (n=12, 80%), followed by middle meningeal artery bleeding (n=3, 20%). In recent research of 102 pediatric victims along with 387 adults with epidural hemorrhage, arterial bleeding was found as the cause of epidural hemorrhage in 36% of adults and 18% of children [11]. The low GCS score, concomitant cerebral lesions, pupil alteration, and elevated ICP are independent indicators of poor outcomes [3, 14]. The GCS score prior to surgery is a significant predictor of operative outcome [3, 14]. Age, hematoma size, and epidural hemorrhage location were all important variables in other studies. It was discovered that pupil alteration and a lower GCS score (9) were connected to bad prognosis [15]. In this research, the important clinical factors linked to poor outcomes were GCS on admission, motor response, and pupil state. Age, sex, and the severity of damage were not substantially linked to treatment outcomes of giant epidural hemorrhage surgery.
The most important predictor of prognosis in patients with epidural hemorrhage receiving surgery is the GCS score on admission or the GCS score prior to surgery [16]. There were eight deaths (53.33%) in this trial, with a GCS score of one. These findings point to bad outcomes and increased death in victims with large epidural hemorrhage (>80 cc). Research is scarce on the expiration of post-operative giant epidural bleeding. One of the patients had a heart attack after surgery and died (Figure 2).
5. Conclusion
A giant epidural hemorrhage >80 cc causes instantaneous MLS, brain herniation, and pressure over the brainstem, resulting in a higher proportion of death. Early removal of a giant epidural hemorrhage is associated with a good result. In victims with giant epidural hemorrhage, poor GCS score on admission, poor motor response, and pupil alterations are related to bad outcomes. Some notable findings in the study include the predominance of men with an M:F ratio of 14:1 and the active age group of 20 to 40 years.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Institutional Ethical Committee (Code: EC 23-008). Written informed consent was obtained from the patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All stages of the study were performed by both authors.
Conflict of interest
The author declared no conflicts of interests.
References
- Rengachary SS, Ellenbogen RG. Principles in neurosurgery. New York: Elsevier Mosby; 2004.
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport: The 3rd international conference on concussion in sport held in Zurich, November 2008. British Journal of Sports Medicine. 2009; 43(Suppl 1):i76-90. [DOI:10.1136/bjsm.2009.058248] [PMID]
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute epidural haematomas. Neurosurgery. 2006; 58:52-7. [DOI:10.1227/01.NEU.0000210364.29290.C9]
- Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochirurgica. 1988; 90(3-4):111-6. [DOI:10.1007/BF01560563] [PMID]
- Petersen OF, Espersen JO. Extradural hematomas: Measurement of size by volume summation on CT scanning. Neuroradiology. 1984; 26(5):363-7. [DOI:10.1007/BF00327488] [PMID]
- Carlos UP, Joas DB, Carneiro L, Antonio R, Egmond ASS, Joas TSM. Epidural haematoma: Analysis of 30 cases. The Internet Journal of Emergency Medicine. 2005; 2:44-7.
- Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochirurgica. 1988; 90(3-4):111-6. [DOI:10.1007/BF01560563] [PMID]
- Jones NR, Molloy CJ, Kloeden CN, North JB, Simpson DA. Extradural haematoma: Trends in outcome over 35 years. British Journal of Neurosurgery. 1993; 7(5):465-71. [DOI:10.3109/02688699308995068] [PMID]
- Jamjoom AB. The difference in the outcome of surgery for traumatic extradural hematoma between patients who are admitted directly to the neurosurgical unit and those referred from another hospital. Neurosurgical Review. 1997;20(4):227-30.[DOI:10.1007/BF01105892] [PMID]
- Chowdhury NK, Raihan MZ, Chowdhury FH, Ashadullah AT, Sarkar MH, Hossain SS. Surgical management of traumatic extradural haematoma: Experiences with 610 patients and prospective analysis. Indian Journal of Neurotrauma. 2008; 5(2):75-9. [DOI:10.1016/S0973-0508(08)80004-4]
- Mohanty A, Kolluri VR, Subbakrishna DK, Satish S, Mouli BA, Das BS. Prognosis of extradural haematomas in children. Pediatric Neurosurgery. 1995; 23(2):57-63. [DOI:10.1159/000120936] [PMID]
- Erşahin Y, Mutluer S, Güzelbag E. Extradural hematoma: analysis of 146 cases. Child's Nervous System. 1993; 9(2):96-9.[DOI:10.1007/BF00305316] [PMID]
- Faheem M, Jaiswal M, Ojha BK, Chandra A, Singh SK, Srivastava C. Traumatic pediatric extradural hematoma: An institutional study of 228 patients in tertiary care center. Pediatric Neurosurgery. 2019; 54(4):237-44. [DOI:10.1159/000501043] [PMID]
- Lee EJ, Hung YC, Wang LC, Chung KC, Chen HH. Factors influencing the functional outcome of patients with acute epidural hematomas: Analysis of 200 patients undergoing surgery. The Journal of Trauma. 1998; 45(5):946-52. [DOI:10.1097/00005373-199811000-00017] [PMID]
- Dubey A, Pillai SV, Kolluri SV. Does volume of extradural hematoma influence management strategy and outcome? Neurology India. 2004; 52(4):443-5. [PMID]
- Özkan U, Kemaloğlu S, Özateş M, Güzel A, ve Tatlı M. Analyzing extradural haematomas: A retrospective clinical investigation. Dicle Tip Dergisi. 2007; 34(1):14-9. [Link]
Type of Study: Research |
Subject:
Neurotrauma
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |