Fri, May 3, 2024
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghosh T, K Singh B, Mukherjee A. Recurrence and Functional Outcome following Resection of Craniopharyngioma. Iran J Neurosurg 2023; 9 : 23
URL: http://irjns.org/article-1-372-en.html
URL: http://irjns.org/article-1-372-en.html
1- Department of Neurosurgery, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, India. , tamajyoti@gmail.com
2- Department of Neurosurgery, All India Institute of Medical Sciences, Raipur, India.
3- Department of Neurosurgery, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, India.
2- Department of Neurosurgery, All India Institute of Medical Sciences, Raipur, India.
3- Department of Neurosurgery, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, India.
Full Text [PDF 858 kb]
(117 Downloads)
| Abstract (HTML) (839 Views)
Full Text: (36 Views)
1. Introduction
Craniopharyngioma (CP) is a benign tumor of the sellar suprasellar region that is critical due to its intricate close relationship with nearby vital structures and poses a significant threat to visual, endocrine, and hypothalamus dysfunction. Although the primary goal of treatment is to eliminate the compressive effect of the tumor on nearby structures,it is still unclear whether a gross total resection (GTR) or subtotal resection (STR) with adjuvant therapy confers a better prognosis. The recurrence rate following GTR is 20.2%, which is comparable to the recurrence rate of 22.1% seen in STR followed by adjuvant therapy [1]. However, postoperative morbidity in the STR group was significantly better in the form of endocrine disturbances, visual dysfunction, and neurological deficit [1]. Different factors responsible for a higher recurrence rate of CP surgery and thereby contributing to increased morbidity in patients include male gender [2], older age (>30 years) [3], neuroimaging showing large tumor [3], neurovascular encasement [4], tumor calcification, histopathological examination (HPE) showing adamantiomatous variety [5], high Ki67 index of tumor [6]. However, preoperative hydrocephalus [3] or presentation with seizure has not been found to have poorer outcomes in such patients.
2. Materials and Methods
This study was conducted to study the morbidity, mortality, and 12-month recurrence rate of CP at our institute. In the course, we also want to determine the extent of safe resection (STR/GTR) in CP surgery with acceptable morbidity and short-term recurrence rate.
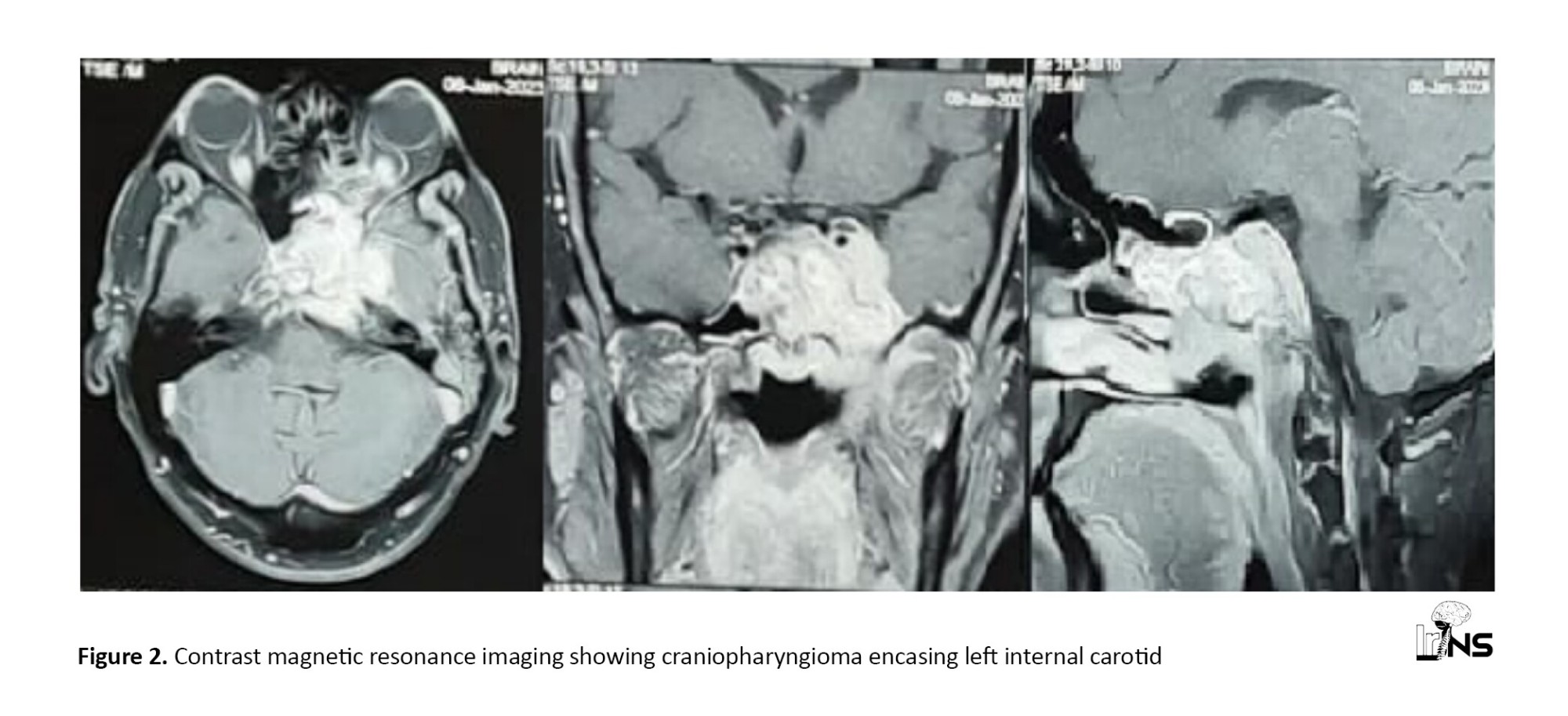
All patients presenting with CP at North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS) who met the inclusion criteria were included, and informed consent was obtained from all prospectively followed participants. Information about clinic -radiological presentation, hormonal status, visual charting, functional status, and extent of surgical resection is also obtained from hospital records/ departmental database/occupational therapy (OT) register and analyzed retrospectively. Treatment outcomesand recurrence rate will be assessed at 0, 1-month, 6-month, and 12-month intervals using the Karnofsky performance scale (KPS). Radiological outcomes will be studied based on the comparison of preoperative and follow-up scans. Statistical analysis will be performed to determine any variables (age, sex, hypothalamus invasion, extent of resection, histopathological variant) related to better outcomes (Figure 1 and Figure 2).

Inclusion criteria
The inclusion criteria included all CP-operated patients, an age group of 1-75 years, and willingness to provide informed consent.
Exclusion criteria
The exclusion criteria included patients operated elsewhere, patients <1 year and >75 years, and patients lost in follow-up.
3. Results
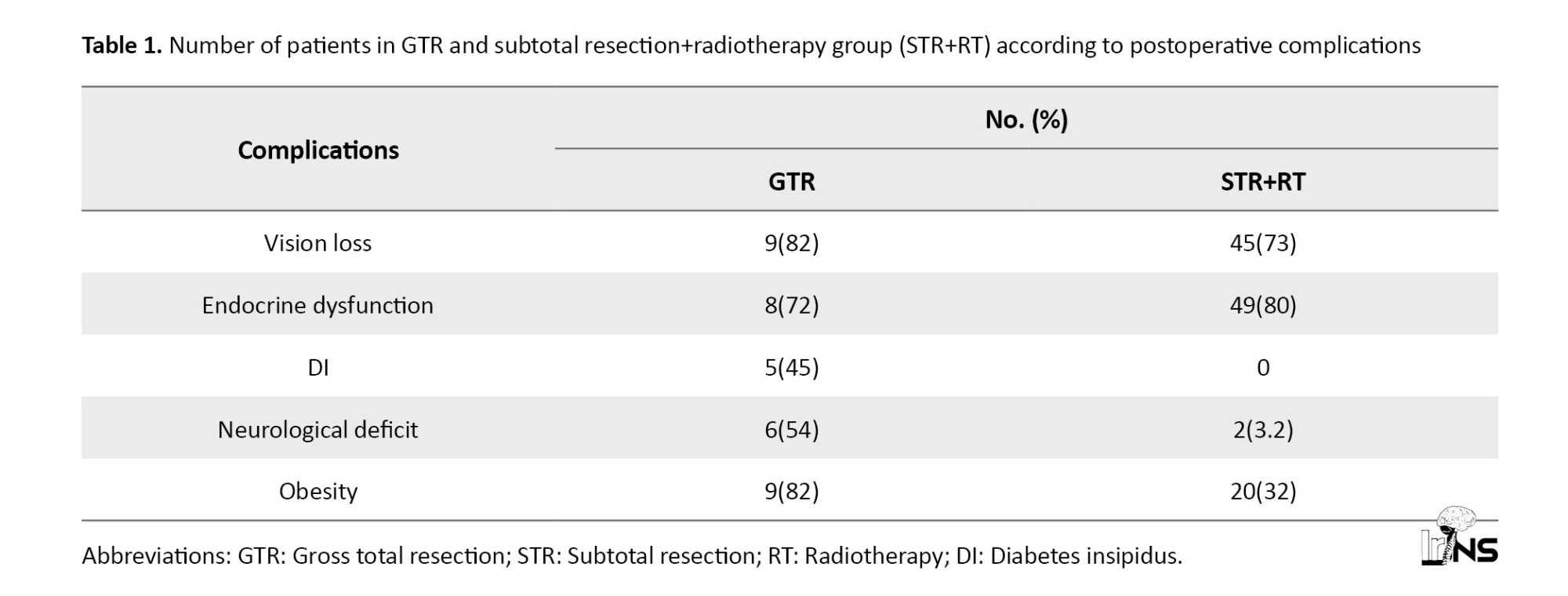
Seventy-two patients were operated at the institute between 2016 and 2021 and followed up till 2022. A total of 33 men and 39 women were in the age group of 4-45 years (mean 17.34 years). Vision loss was observed in 54 patients. Fifty-seven patients had endocrine abnormalities. Five cases of diabetes insipidus (DI) were present. Eight cases of postoperative neurological deficit were observed. Obesity was observed in 29 patients. Three cases presented with seizures (Table 1).
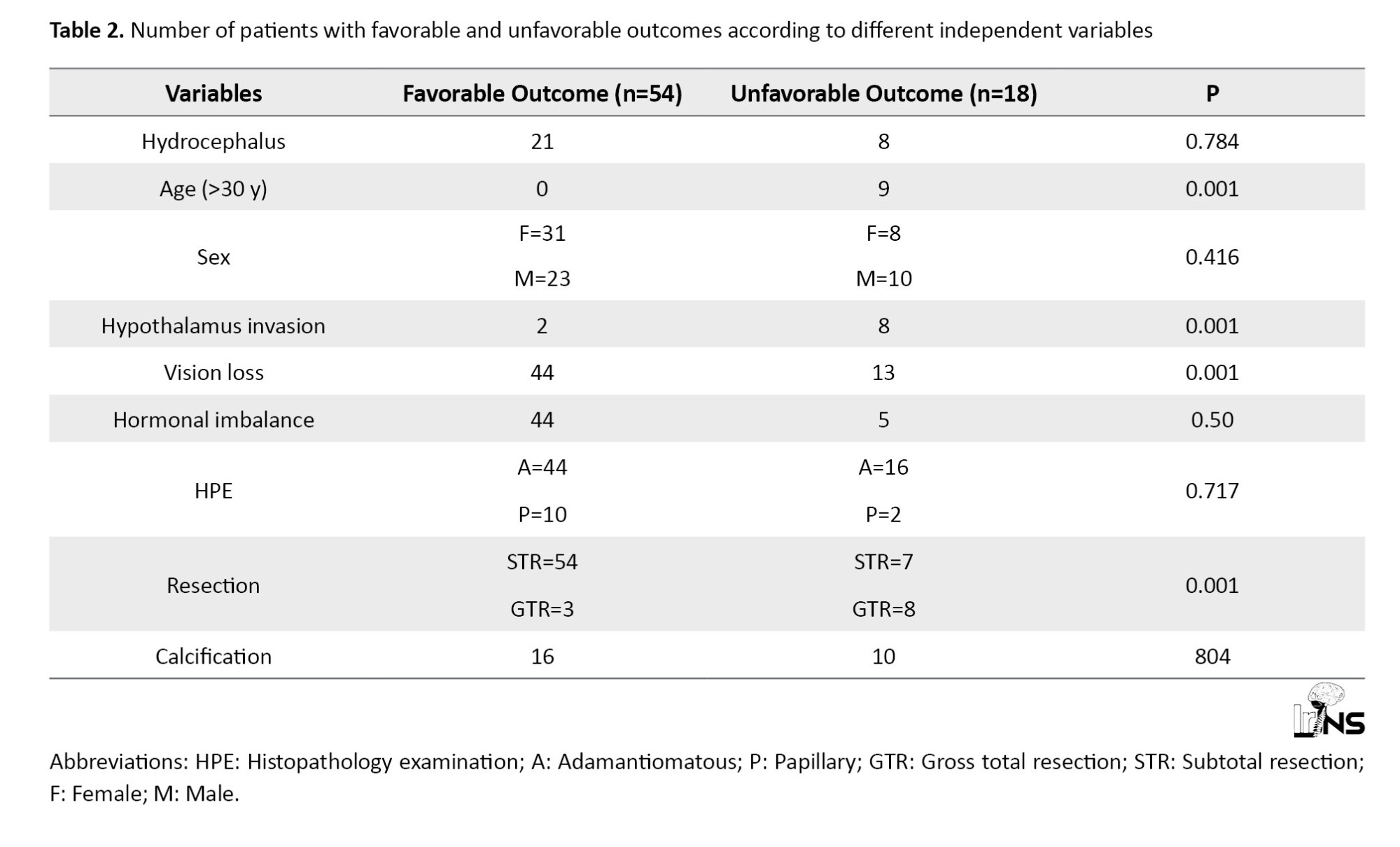
Based on HPE adamantiomatous type was observed in 60 patients and papillary type in the rest of 12 cases. A total of 11 cases underwent GTR, while the rest 61 cases underwent STR. Eighteen cases had a Karnofsky performance scale (KPS) score of less than 80 at 1-year follow-up and 5 of them had expired. The recurrence rate following GTR is 1% (n=1), while CP recurred in 24% (n=15) in patients undergoing STR and adjuvant RT (Table 2).
4. Discussion
GTR was associated with a significantly low recurrence rate (1%) compared to STR+RT (24%). Recent studies have found that the CP recurrence rate following GTR is as low as 0%-1% [7, 8]. If we compare the recurrence rate following STR+RT, our study shows acomparatively lower recurrence rate compared to 50%-100% in different studies [4, 9], which may be attributed to the short 1-year follow-up. However, as described in another study, a low recurrence rate following GTR comes at the cost of raising postoperative morbidity and mortality compared to STR+RT. The mortality rate in our study was 6.94, which is comparable to Western studies with a mortality rate between 2.88 and 9.28 [10]. In our study, most postoperative morbidity is due to endocrine abnormalities, such as panhypopituitarism and vision loss, which is similar to other studies, such as Clark et al and Park et al [11, 12]. Compared to other studies [13, 14], an increased rate of postoperative visual loss and endocrinopathies in the STR+RT group was shown. This may be attributed to the added complications following radiation therapy. A significant relationship was observed between older age, hypothalamus invasion, and GTR with unfavourable outcomes following surgery, which is consistent with other available studies in the literature [15, 16]. It was also found that preoperative vision loss is significantly associated with poor postoperative outcomes; although no direct association is reported between preoperative vision status and outcome of surgery. Several studies suggest that large tumor size >3 cm is associated with poor outcomes [16, 17].
5. Conclusion
Due to the lack of clarity regarding the extent of surgical resection of CP, significant morbidity is observed in the form of hypothalamic injury, hormonal imbalance requiring lifelong hormonal supplementation, visual deficit, infarction, and even death. Thus patient-specific tailored resection of CP is an urgent need for the patient to reduce morbidity and mortality due to tumor resection, but at the same time provides an acceptable recurrence rate.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Institutes Ethical Committee (IEC) of NEIGRIHMS Hospital (Code: NEIGR/IEC/M6/F11/2022 dated 13th, August 2022). Written informed consent was obtained from all patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, study design and data collection: Tamajyoti Ghosh and Binoy Singh; Data analysis, data interpretation, writing original draft: Tamajyoti Ghosh; Critically revising the article: Aishik Mukherjee and Binoy Singh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Craniopharyngioma (CP) is a benign tumor of the sellar suprasellar region that is critical due to its intricate close relationship with nearby vital structures and poses a significant threat to visual, endocrine, and hypothalamus dysfunction. Although the primary goal of treatment is to eliminate the compressive effect of the tumor on nearby structures,it is still unclear whether a gross total resection (GTR) or subtotal resection (STR) with adjuvant therapy confers a better prognosis. The recurrence rate following GTR is 20.2%, which is comparable to the recurrence rate of 22.1% seen in STR followed by adjuvant therapy [1]. However, postoperative morbidity in the STR group was significantly better in the form of endocrine disturbances, visual dysfunction, and neurological deficit [1]. Different factors responsible for a higher recurrence rate of CP surgery and thereby contributing to increased morbidity in patients include male gender [2], older age (>30 years) [3], neuroimaging showing large tumor [3], neurovascular encasement [4], tumor calcification, histopathological examination (HPE) showing adamantiomatous variety [5], high Ki67 index of tumor [6]. However, preoperative hydrocephalus [3] or presentation with seizure has not been found to have poorer outcomes in such patients.
2. Materials and Methods
This study was conducted to study the morbidity, mortality, and 12-month recurrence rate of CP at our institute. In the course, we also want to determine the extent of safe resection (STR/GTR) in CP surgery with acceptable morbidity and short-term recurrence rate.
All patients presenting with CP at North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS) who met the inclusion criteria were included, and informed consent was obtained from all prospectively followed participants. Information about clinic -radiological presentation, hormonal status, visual charting, functional status, and extent of surgical resection is also obtained from hospital records/ departmental database/occupational therapy (OT) register and analyzed retrospectively. Treatment outcomesand recurrence rate will be assessed at 0, 1-month, 6-month, and 12-month intervals using the Karnofsky performance scale (KPS). Radiological outcomes will be studied based on the comparison of preoperative and follow-up scans. Statistical analysis will be performed to determine any variables (age, sex, hypothalamus invasion, extent of resection, histopathological variant) related to better outcomes (Figure 1 and Figure 2).


Inclusion criteria
The inclusion criteria included all CP-operated patients, an age group of 1-75 years, and willingness to provide informed consent.
Exclusion criteria
The exclusion criteria included patients operated elsewhere, patients <1 year and >75 years, and patients lost in follow-up.
3. Results
Seventy-two patients were operated at the institute between 2016 and 2021 and followed up till 2022. A total of 33 men and 39 women were in the age group of 4-45 years (mean 17.34 years). Vision loss was observed in 54 patients. Fifty-seven patients had endocrine abnormalities. Five cases of diabetes insipidus (DI) were present. Eight cases of postoperative neurological deficit were observed. Obesity was observed in 29 patients. Three cases presented with seizures (Table 1).

Based on HPE adamantiomatous type was observed in 60 patients and papillary type in the rest of 12 cases. A total of 11 cases underwent GTR, while the rest 61 cases underwent STR. Eighteen cases had a Karnofsky performance scale (KPS) score of less than 80 at 1-year follow-up and 5 of them had expired. The recurrence rate following GTR is 1% (n=1), while CP recurred in 24% (n=15) in patients undergoing STR and adjuvant RT (Table 2).

4. Discussion
GTR was associated with a significantly low recurrence rate (1%) compared to STR+RT (24%). Recent studies have found that the CP recurrence rate following GTR is as low as 0%-1% [7, 8]. If we compare the recurrence rate following STR+RT, our study shows acomparatively lower recurrence rate compared to 50%-100% in different studies [4, 9], which may be attributed to the short 1-year follow-up. However, as described in another study, a low recurrence rate following GTR comes at the cost of raising postoperative morbidity and mortality compared to STR+RT. The mortality rate in our study was 6.94, which is comparable to Western studies with a mortality rate between 2.88 and 9.28 [10]. In our study, most postoperative morbidity is due to endocrine abnormalities, such as panhypopituitarism and vision loss, which is similar to other studies, such as Clark et al and Park et al [11, 12]. Compared to other studies [13, 14], an increased rate of postoperative visual loss and endocrinopathies in the STR+RT group was shown. This may be attributed to the added complications following radiation therapy. A significant relationship was observed between older age, hypothalamus invasion, and GTR with unfavourable outcomes following surgery, which is consistent with other available studies in the literature [15, 16]. It was also found that preoperative vision loss is significantly associated with poor postoperative outcomes; although no direct association is reported between preoperative vision status and outcome of surgery. Several studies suggest that large tumor size >3 cm is associated with poor outcomes [16, 17].
5. Conclusion
Due to the lack of clarity regarding the extent of surgical resection of CP, significant morbidity is observed in the form of hypothalamic injury, hormonal imbalance requiring lifelong hormonal supplementation, visual deficit, infarction, and even death. Thus patient-specific tailored resection of CP is an urgent need for the patient to reduce morbidity and mortality due to tumor resection, but at the same time provides an acceptable recurrence rate.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Institutes Ethical Committee (IEC) of NEIGRIHMS Hospital (Code: NEIGR/IEC/M6/F11/2022 dated 13th, August 2022). Written informed consent was obtained from all patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, study design and data collection: Tamajyoti Ghosh and Binoy Singh; Data analysis, data interpretation, writing original draft: Tamajyoti Ghosh; Critically revising the article: Aishik Mukherjee and Binoy Singh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Grewal MR, Spielman DB, Safi C, Overdevest JB, Otten M, Bruce J, et al. Gross total versus subtotal surgical resection in the management of craniopharyngiomas. Allergy & Rhinology (Providence, R.I.). 11:2152656720964158. [DOI:10.1177/2152656720964158] [PMID]
- Minamida Y, Mikami T, Hashi K, Houkin K. Surgical management of the recurrence and regrowth of craniopharyngiomas. Journal of Neurosurgery. 2005; 103(2):224-32. [DOI:10.3171/jns.2005.103.2.0224] [PMID]
- De Vile CJ, Grant DB, Kendall DB, Neville BG, Stanhope R, Watkins KE, et al. Management of childhood craniopharyngioma: Can the morbidity of radical surgery be predicted? Journal of Neurosurgery. 1996; 85(1):73–81. [DOI:10.3171/jns.1996.85.1.0073] [PMID]
- Duff J, Meyer FB, Ilstrup DM, Laws ER Jr, Schleck CD, Scheithauer BW. Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery. 2000; 46(2):291-305. [DOI:10.1097/00006123-200002000-00007] [PMID]
- Szeifert GT, Sipos L, Horvath M, Sarker MH, Major O, Salomvary B, et al. Pathological characteristics of surgically removed craniopharyngiomas: Analysis of 131 cases. Acta Neurochirurgica. 1993; 124(2-4):139-143. [DOI:10.1007/BF01401137] [PMID]
- Izumoto S, Suzuki T, Kinoshita M, Hahiba T, Kagawa N, Wada K, et al. Immunohistochemical detection of female sex hormone receptors in craniopharyngiomas: Correlation with clinical and histologic features. Surgical Neurology. 2005; 63(6):520-5. [DOI:10.1016/j.surneu.2004.08.094] [PMID]
- Patel VS, Thamboo A, Quon J, Nayak JV, Hwang PH, Edwards M, et al. Outcomes after endoscopic endonasal resection of craniopharyngiomas in the pediatric population. World Neurosurg. 2017; 108:6-14. [DOI:10.1016/j.wneu.2017.08.058] [PMID]
- Schelini JC, Cavalheiro S, Dastoli PA, Hirai ÉR, Atallah C, Costa M, et al. Endoscopic endonasal transsphenoidal approach for pediatric craniopharyngiomas: A case series. International Journal of Pediatric Otorhinolaryngology. 2020; 130:109786. [DOI:10.1016/j.ijporl.2019.109786] [PMID]
- Tomita T, Bowman RM. Craniopharyngiomas in children: Surgical experience at Children's Memorial Hospital. Child's Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 2005; 21(8-9):729-46. [DOI:10.1007/s00381-005-1202-9] [PMID]
- Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopharyngioma. Pituitary. 2013; 16(1):46-55. [DOI:10.1007/s11102-012-0428-2] [PMID]
- Park HR, Kshettry VR, Farrell CJ, Lee JM, Kim YH, Won TB, et al. Clinical outcome after extended endoscopic endonasal resection of craniopharyngiomas: Two-institution experience. World Neurosurgery. 2017; 103:465-74. [DOI:10.1016/j.wneu.2017.04.047] [PMID]
- Clark AJ, Cage TA, Aranda D, Parsa AT, Auguste KI, Gupta N. Treatment-related morbidity and the management of pediatric craniopharyngioma: A systematic review. Journal of Neurosurgery: Pediatrics. 2012; 10(4):293-301. [DOI:10.3171/2012.7.PEDS11436]
- Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, et al. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. Journal of Neuro-Oncology. 2012; 108(1):133-9. [DOI:10.1007/s11060-012-0806-7] [PMID]
- Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, et al. Longitudinal analysis of visual outcomes after surgical treatment of adult craniopharyngiomas. Neurosurgery. 2012; 71(3):715-21. [DOI:10.1227/NEU.0b013e318262146b] [PMID]
- Elwatidy SM, Jamjoom ZA, Jamjoom AB, Yakoub AO. Craniopharyngioma: Analysis of factors that affect the outcome. Neurosciences (Riyadh, Saudi Arabia). 2002; 7(1):27-31. [PMID]
- Teng H, Liu Z, Yan O, He W, Jie D, Qie Y, et al. Nomograms for predicting overall survival among patients with craniopharyngiomas at initial diagnosis: A SEER population-based analysis. International Journal of General Medicine. 2021; 14:3517-27. [DOI:10.2147/IJGM.S320643] [PMID]
- Lubuulwa J, Miao Z, Liu SW, Chen J, Wang S, Jiang, et al. Clinical, pathological and surgical risk factors associated with craniopharyngioma recurrence: A literature review. Open Journal of Modern Neurosurgery. 2019; 9(1):61-77. [DOI:10.4236/ojmn.2019.91008]
Type of Study: Research |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






