Wed, Feb 4, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 87-97 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Varshney R, Singh B K, Choudhary A. Investigating the Surgical Management of Large Basal Ganglia Hemorrhage: A Bi-institutional Experience. Iran J Neurosurg 2024; 10 : 10
URL: http://irjns.org/article-1-373-en.html
URL: http://irjns.org/article-1-373-en.html
1- Department of Neurosurgery, PGIMER & Dr. RML Hospital, New Delhi, India. , rahul_varshney1@rediffmail.com
2- Department of Neurosurgery, North Eastern Indra Gandhi Regional Institute of Health and Medical Sciences, Shillong, India.
3- Department of Neurosurgery, PGIMER & Dr. RML Hospital, New Delhi, India.
2- Department of Neurosurgery, North Eastern Indra Gandhi Regional Institute of Health and Medical Sciences, Shillong, India.
3- Department of Neurosurgery, PGIMER & Dr. RML Hospital, New Delhi, India.
Full Text [PDF 1047 kb]
(2635 Downloads)
| Abstract (HTML) (3185 Views)
Full Text: (1819 Views)
1. Introduction
Intracerebral hemorrhage (ICH) is associated with higher mortality and more severe disability compared to ischemic stroke [1]. The in-hospital mortality rate with ICH varies from 30-50% [2, 3]. In hypertensive hemorrhages, 60% occur in the basal ganglia, with a mortality rate of almost 50%. Even though many studies have been published concerning the management of ICH, no class [4]evidence has yet been published. Numerous studies [4, 5] and meta-analyses [6, 7] that assert no difference in outcome regardless of whether medical or surgical treatment is used are entirely rejected by others [8, 9].
Kaya et al. performed a study comparing conservative medical treatment to open craniotomy for putaminal hematomas in patients with a volume greater than 30 ml. Accordingly, mortality was 34% among patients treated surgically, compared to 63.1% in conservatively managed patients [8].
In contrast, surgical trials in traumatic intracerebral hemorrhage (STITCH) trials I and II have shown that early surgical evacuation of hematoma does not improve outcomes over initial best medical management. However, patients at risk of herniation or impaired level of consciousness were excluded from the study and almost 26% of patients initially treated conservatively underwent surgical decompression. Gregson et al. reanalyzed the data from STITCH trials and found that large basal ganglia bleeds will likely benefit from early surgery [9]. With advancements in postoperative critical care facilities, the outcome has improved after the surgery. Surgery has the advantage of removing the blood clot, thus limiting edema development in perihematomal white and grey matter and reducing the mass effect [10]. Surgical treatment of basal ganglia hemorrhages ranges from decompressive craniectomies to stereotactic needle aspiration of hematoma [11]. However, craniotomy remains the most common surgical procedure as it provides an added advantage of intraoperative homeostasis. Moreover, the superiority of any one of these methods over the others still needs to be completely established.
Due to differences in the incidence of hypertensive bleeding in the basal ganglia region compared to other parts of the brain, along with various factors, such as intraventricular hemorrhage (IVH), associated hydrocephalus and the impact of surrounding edema basal ganglia hemorrhage warrants distinct attention from cortical or posterior fossa bleeds. This study investigates the prognostic factors specifically associated with poor outcomes in basal ganglia hemorrhage. This study also provides an overview of our experience in managing basal ganglia hemorrhage surgical cases. The objective was to evaluate the functional outcomes using the Glasgow outcome score (GOS) three months after surgery.
2. Materials and Methods
This study involved 271 patients treated surgically for basal ganglia bleeding from December 2018 to November 2020 across two institutions. Institutional Review Board approval was obtained from both centers. Upon admission to the emergency room, all patients underwent a computed tomography scan to confirm the diagnosis of basal ganglia bleeding. All patients aged 18 years and above with ictus of less than three days who underwent surgical intervention were identified by reviewing electronic medical records. Follow-up scans were done in all patients based on their neurological conditions.
Patients with secondary causes of basal ganglia bleeding, subjects with the extension of bleeding into the brain stem, and individuals with a rebleed and those requiring a second surgery were excluded from the study. Additionally, patients with a Glasgow coma scale (GCS) 3 and fixed dilated pupils on presentation were excluded from the study. Study physicians recorded the demographics and clinical information of the patients. Every patient underwent an assessment utilizing the first available computed tomography (CT) or magnetic resonance imaging. The decision for operative intervention was made considering relevant clinical and radiological parameters. Surgery was indicated when signs of neurological deterioration or the risk of life-threatening herniation became apparent.
The volume of hematoma was calculated on CT scan using the ABC/2 method [12], which is based on a simplification of the ellipsoid volume equation in which A represents the maximum length (in cm) on the largest hemorrhage slice, B represents the diameter perpendicular to A and C represents the approximate number of axial slices multiplied by the slice thickness. Additionally, midline shift was calculated using the Foramen of Monro as a base point, and any extension of the hematoma into the ventricles as well as associated hydrocephalus was also noted.
According to the ABC protocol, all patients were admitted to and managed in a dedicated neuroscience intensive care unit or stroke unit. Antihypertensive medications, such as labetalol infusion, were used to maintain systolic blood pressure below 140 mmHg. Osmotic diuretics (such as mannitol) were administered to manage increased intracranial pressure. Anticoagulants and antiplatelets were discontinued for at least five days whenever feasible. The patients were operated on in spine position with a 30-degree elevation of the head and slight rotation toward the opposite side of the hemorrhage. Using the operating microscope and considering clot extension and surgeon preference, the clot was approached either through the prefrontal or superior temporal gyrus cortex. The hematoma was evacuated with the help of control suction and gentle irrigation. Any active bleeding point was coagulated and hemostasis was achieved. The dura was primarily closed whenever feasible and patched if necessary.
A decompressive craniectomy with lax duraplasty was performed in patients whose brains remained tensed and non-pulsatile even after hematoma evacuation when replacing the bone was not feasible. In such cases, wide decompressive craniectomy was done with a bone flap placed in the abdomen. Patients with IVH underwent extra ventricular drain insertion or venticuloperitoneal shunt placement before or after the operation, depending on the development of hydrocephalus. Subsequently, patients were monitored in the neurosurgical intensive care unit postoperatively, and any neurological or ventilator-associated complications were managed accordingly. Postoperative CT scans were done in all patients as needed based on their neurological status.
Follow-up procedure
The follow-up duration was at least three months from surgery, during which the outcome was assessed using the GOS. Patients with GOS 4 and 5 were classified as having excellent outcomes, while those with GOS scores of 3, 2 and 1 were considered to have poor outcomes [13].
Data analysis
Statistical significance was set to P<0.05 to determine the accuracy of the analyses conducted by Stata software, version 5.0.
All the variables used for prognostication were abstracted from electronic medical records at the time of admission. Study variables included age, sex, GCS, hematoma volume, IVH, midline shift, bleeding side and addictions (smoking and drinking). Continuous variables among factors related to outcome were dichotomized into different categories, which along with dichotomous variables, were compared using the chi-square tests. Multivariate regression analyses were performed subsequently, considering the effect of all the variables on the GOS.
3. Results
Of all 271 patients, 203 were male, and 68 were female (Table 1).
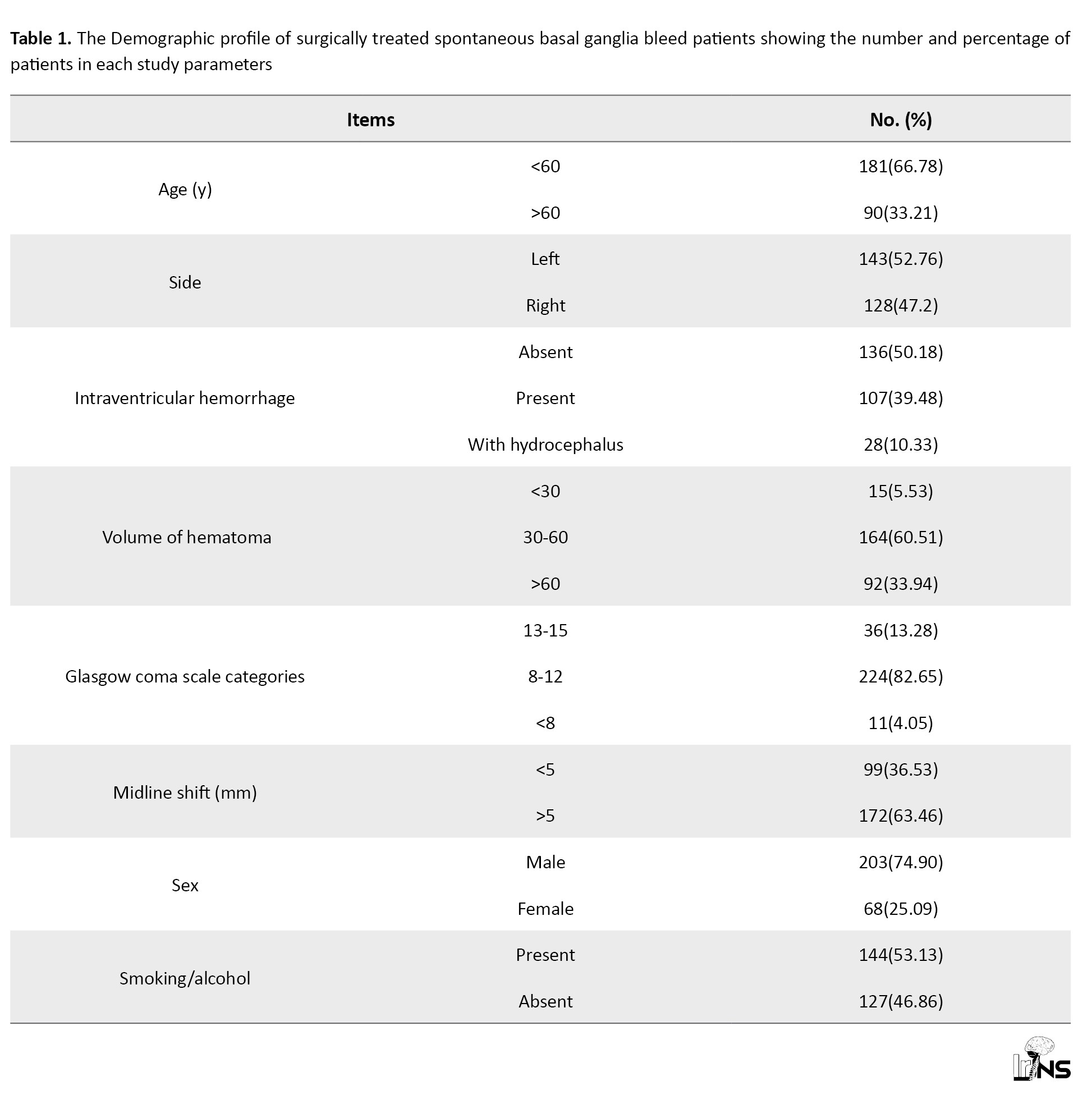
Their age ranged from 20 to 85 years (mean, 50.8±13.57 years; Table 2).

A statistically significant difference was found between the excellent and unfavorable outcomes groups, with people below 60 years having poor outcomes (GOS=1, 2, 3) rate of 38.67%, and 53.33% of patients above 60 years having poor outcomes (P<0.02; Table 2). Among patients younger than 30 years, 10 out of 13(76.92%) had a favorable outcome, compared to 3(23.08%) who had poor outcome. The age-related outcomes highlight that younger patients with basal ganglia bleed are more likely to achieve an excellent outcome following treatment.
Top of form
The 60-year cut-off was chosen because basal ganglia bleeding tends to occur in a relatively younger population in the Indian subcontinent. Thus, the patients were divided into two groups based on age. Among the patients, 35.10% (n=119) had right-sided bleeding, while 51.66% (n=131) had a hematoma on the left side (Table 2).
Patients were classified into three groups based on their GCS, following the same classification as that for head injury patients with GCS scores ranging from 4 to 15. In the GCS group <8, three patients (27.27%) out of 11 patients had a favorable outcome (GOS=4, 5), while 72.73% had a poor outcome. In patients with GCS>8, a total of 12 patients (55.35%) out of 224 patients had GOS 4, 5 (Table 2). Among the patients with a GCS 13-15, a total of 72.22% (n=26) out of 36 patients had favorable outcomes, while 27.77% had a poor outcome (Table 2). Meanwhile, a significant difference in terms of outcome was observed (P<0.0001) between the three studied groups based on the GCS score on admission (Table 2).
Hematoma volumes ranged from 30 to 120 cm3, with a mean of 54.42±23.25 cm3. Based on volume, 5.53% (n=15) patients had small hematomas (30 cm3), 60.51% (n=164) had medium-sized hematomas (30-60 cm3), and 33.94% (n=92) had large (>60 cm3) hematomas. Among patients with hematoma volumes <30 mL, the majority (n=12), 80% had favorable outcomes, while three patients (20%) had poor outcomes. In the group with hematoma volumes between 30-60 mL, 72.56% (n=119) of patients had good outcomes. However, among patients with ICH volumes >60 mL, 76.08% (n=22) had poor outcomes (Table 2). Additionally, 49.85% (n=135) of patients had hemorrhage extending into the ventricles, and 10.32% (n=28) developed associated hydrocephalus (Table 1). Among these 28 patients, an external ventricular drain was inserted in 20(71.4%) either preoperatively or postoperatively and eight patients (28.57%) underwent concurrent external ventricular drain insertion with hematoma evacuation.
Meanwhile, 72.79% of patients had excellent outcomes with no associated IVH. However, when IVH was present, only 47.66% had a GOS of 4 or 5. In cases when IVH was associated with hydrocephalus, an excellent outcome was present in only 10.7% of patients (Table 2). Thus, a hematoma volume of more than 60 mL, IVH, and hydrocephalus had a statistically significant impact on good functional outcomes (P<0.0001; Table 2). A midline shift of more than 5 mm led to a significantly worse outcome for 52.32% of patients (n=91) compared to a midline shift of <5 mm for 28.28% (n=28; P<0.0001; Table 2).
Only 71(49.65%) of the 143 patients in our study had favorable outcomes when their hematoma was located in the dominant hemisphere (left side). Out of 128 patients with hematoma in the non-dominant hemisphere, 64.06% (n=82) had a good outcome (Table 2). This difference was statistically significant in terms of good functional outcomes (P<0.016; Table 2). This distinction may be attributed to the dominant hemisphere’s involvement in more complex functions, potentially resulting in greater neurological damage when affected by a hematoma.
A total of 144 patients had a history of either smoking or drinking. Of these, 61.11% (n=88) were smokers, 24.30% (n=35) were drinkers, and 14.58% (n=21) were both smokers and drinkers. Both study groups did not show statistically significant differences in terms of outcomes (P=0.57; Table 2).
Similarly, no significant difference was observed regarding gender in terms of outcomes (P=0.53; Table 2).
Among the 34 patients who underwent decompressive craniectomy 28 patients (82.35%) had hematoma volume greater than 60 mL, and 25 patients (73.52%) had midline shift >5 mm.
Regarding GOS, out of 271 patients, 146 patients (53.87%) showed favorable outcomes (GOS 4, 5), 26 (9.59%) had poor outcomes (GOS 2, 3) and 99 patients died resulting in a mortality rate of 36.53% (GOS 1).
Univariate analysis showed that older age (P<0.021), bleeding on the left side of the brain (P<0.016), presence of IVH (P<0.0001), higher ICH volume (P<0.0001), GCS under 8 (P<0.0001), and midline shift >5mm (P<0.0001) were all associated with a poorer outcome (Table 2). The likelihood of poor outcomes was not different between males and females (P=0.53) or addicted people(P=0.57; Table 2).Once the confounders were adjusted, a multivariate analysis was performed, which demonstrated that individuals over the age of 60 years, patients with bleeding on the left side of the brain, cases in which bleeding was associated with IVH and hydrocephalus, when the hematoma volume was >60 mL and patients whose GCS was <8 had poor outcomes. The P<0.05, as shown in Table 3.

4. Discussion
Despite advances in medical science in stroke management, basal ganglia hemorrhage remains one of the most incapacitating and disabling forms of stroke. While many trials and met analyses published earlier for spontaneous ICH include both lobar and deep-seated hemorrhages, the factors deciding management, either surgical or conservative, and prognostication for the two, cannot be generalized. IVH, more commonly associated with basal ganglia hemorrhage than lobar hemorrhage, significantly impacts survival. Various studies in the past have advocated IVH as an independent predictor of mortality. Similarly, post-IVH hydrocephalus is more likely in basal ganglia hemorrhage than in cortical bleeding, leading to poor outcomes. The importance of surrounding edema and its impact on nearby structures cannot be ruled out. Basal ganglia bleed faces the major brunt due to critical structures in the vicinity. In this study, we investigated surgical outcomes and identified prognostic factors specifically related to basal ganglia hemorrhages in patients who underwent surgical evacuation with or without extraventricular drainage or ventriculoperotoneal shunt. The management of spontaneous ICH has been the subject of several trials and meta-analyses in the past. In the STITCH trial [4], the most notable stroke trial, the superiority of surgical management over first-line conservative best medical treatment was also not confirmed. However, the trial’s findings could not be generalized because there was a significant cross-over from the conservatively managed group to the surgically treated group, resulting in reduced unfavorable outcomes observed in conservatively managed patients. Furthermore, the STITCH II trial [14] was biased in patient selection as it excluded patients with low GCS, having an IVH, or those at risk of herniation (>3300).
Top of form
In 1990, Batjer et al. [15] performed a randomized clinical trial in 21 patients with hypertensive putaminal hemorrhage. They concluded that despite the best available treatment, the outcome was worse. However, with advancements in intraoperative techniques and post-operative critical care, the survival rate in such patients has gradually increased over some time.
A conservative approach has been advocated in most studies when a patient presents with a hematoma volume of up to 10 cm3, minimal neurological deficit, severe coagulopathy, or underlying medical conditions, or when the patient with advanced age (over 80), makes surgery intolerable. However, in cases of a large, life-threatening ICH, evacuation is necessary. Minimally invasive endoscopic approaches have become increasingly prevalent over the past few years for spontaneous ICH, while others have advocated decompressive craniectomies for large hematomas with midline shift, GCS score <8 and raised ICP not responding to medical management. In 2012, Zhou et al. [16] performed a meta-analysis and found that minimally invasive procedures were superior to craniotomies. However, methodological flaws in the research have been raised [17], warranting further discussion to determine the efficacy of these procedures. Decompressive craniectomies were performed in only a few patients in our study, most undergoing craniotomies and hematoma evacuation. Ensuring a clear and unobstructed view is crucial for complete hematoma evacuation. Craniotomy also facilitates easy hemostasis, leading to improved outcomes and reduced mortality rates. Among the 271 patients in our study, 237(87.45%) underwent craniotomies, while 34(12.54%) underwent decompressive craniectomies with hematoma evacuation.
In this study,the mortality within the first three months was 36.53%. However, in a study by Lahti et al. the mortality for deep-seated bleed was 16.3% [18]. This difference could be because they included hemorrhages of all sizes. In our study, we only included those patients requiring surgery for whom hematoma volume and GCS score were major deciding factors, with relatively larger volume hematoma and poor GCS requiring surgery. Yilmaz et al. reported a mortality rate of 56% for deep-seated hemorrages [19]. The improvement in surgical outcomes observed in our study may be attributed to advancements in preoperative and postoperative critical care, improved surgical techniques, and neuroanesthesia, all of which have led to better results. González-Pérez et al. reported an increase in mortality rates with an increase in age. According to them, case fatality increased with age, reaching 29.7% for individuals aged 20-49 years and 54.6% for those aged 80-89 years [20]. According to the study by Kwon et al. univariate analysis revealed that age exhibited no significant effect, despite being strongly associated with clinical outcomes [21]. Possibly explained considering that the incidence of spontaneous ICH in the western population is higher in older populations, the atrophic brain in such patients counteract the effects of raised ICP due to the hematoma. However, in the Indian subcontinent, cortical atrophy does not significantly affect the outcome due to the relatively increased incidence of basal ganglia bleeds in the younger population. The mean age among deceased patients was significantly higher, with no differences in mortality rate based on sex. Thus mortality at three months was strongly correlated with age, with a mortality rate of 53.3% in patients >60 years.
Top of form
Our research confirms previous findings that IVH with or without subsequent hydrocephalus is associated with adverse outcomes. Due to the vicinity of lateral ventricles to basal ganglia thus, with an increased incidence of IVH in basal ganglia bleeds than cortical bleed, early rupture of hemorrhage into ventricles is common, compromising the normal pressure regulation of the cranial vault. It has been demonstrated in animal studies [22] that the likelihood of animal death increases proportionately with the amount of blood injected into the ventricles. Blood in the ventricular system at the tissue level causes inflammation, fibrosis and subsequent hydrocephalus [22], further increasing mortality and morbidity rates.
The preoperative hematoma volume is the primary deciding factor for surgical evacuation. Many studies have advocated that preoperative hematoma volume on CT scans less than <20 mL can be managed conservatively since it causes little mass effect. Broderick et al. [23] in 1991 reported 93%, 64% and 23% mortality rates for hematoma volumes >60 mL, 30-60 mL and 30 mL, respectively. Similar mortality and morbidity rates have been reported by multiple cohorts, describing a solid correlation between hematoma volume and survival or functional outcome in spontaneous ICH patients [24, 25]. The pressure effect of the hematoma on the surrounding brain tissue causes initial damage to the brain. Subsequently, the lysis of erythrocytes liberates various inflammatory mediators which cause secondary damage to the brain [26, 27, 28]. All these pathological effects increase proportionately with the size of the hematoma. Thus early surgery has the theoretical advantage of preventing both types of damage.
Hematoma volumes less than 30 mL were associated with good postoperative functional outcomes [29]. Conversely, patients with larger hematoma volumes (90 mL) and the highest GCS score of 6 were linked to poorer outcomes (P<0.039) [30].
Our outcome rates were similar to Broderick’s in the < 30 mL group and the >60 mL group; however, we had a better outcome in the middle group (30-60 mL) regarding GOS. This can be attributed to many patients in the second group lying close to the minimum range value, thus having relatively less hematoma volume.
Similar to head injuries, GCS strongly predicts clinical outcomes in spontaneous ICH patients [31]. Apart from prognostication; it also helps decide the management line in patients with deep-seated hemorrhages. In contrast, a GCS Score of 13 or higher favors conservative management; surgery is recommended for patients having a GCS score below 12 [32]. Thus, GCS scores are an essential part of the treatment decision-making process, and lower scores indicate that aggressive treatment options such as surgery should be considered.
In our study majority of patients (82.65%) had GCS scores between 8-12. Patients with GCS score <8 experienced a worse outcome, with a mortality rate of 54.54%.This finding is consistent with a meta-analysis published by Gregson et al. which included 1541 randomized patients with spontaneous ICH. The meta-analysis suggested that surgery may be most beneficial for those with an intermediate GCS between 9-12, while its risks may outweigh the benefits for those with a higher or lower GCS [9]. Therefore, surgery should be reserved for patients with a GCS between 9 and 12, as this group stands to gain the most from an intervention. This finding underscores the importance of weighing surgery risks against the potential benefits for each patient with a higher or lower GCS.
While foramen of Monro displacement was not nearly as indicative of stroke mortality, displacement at the septum pellucidum and coma level were strong indicators of mortality [33].
Top of form
Missori et al. concluded that the amount of brain shift at the level of the corpus callosum was related to survival in surgically treated patients with ICH. However, brain shift at Monro’s foramen was not associated with positive outcomes [34]. However, due to the limited sample size, they also advocated the need for further investigation with a larger sample size, considering the location and volume of hematoma and associated co-morbidities. We included all the factors mentioned above in our study. We found that midline shift at the level of the foramen of Monro were significantly associated with excellent or poor surgical outcomes, with 52.32 % of patients having midline shifts greater than 5mm at the level of foramen Monro having a poor outcome. Thus, choosing an appropriate surgical approach and route with the least cortical damage may improve functional outcomes. Like any other mass lesion that causes a rise in intracranial pressure, thereby increasing mortality or morbidity, basal ganglia bleeding also carries the same potential. Therefore, large hypertensive basal ganglia bleed requires early surgical evacuation, minimizing the harmful effect of raised ICP and improving the local circulation.
5. Conclusion
Hematoma drainage through craniotomies remains a life-saving procedure in critical situations. Surgical evacuation is an effective treatment option for large hypertensive basal ganglia hemorrhages. Surgical outcomes for these hemorrhages are influenced by factors such as hematoma volume, preoperative GCS score, presence of IVH, and the location of the bleeding (left side). Predictors of a favorable surgical outcome include a hematoma volume of less than 60 mL, absence of IVH, a GCS score of 13-15, age <60 years and a bleed on the right side.
Limitations
This study faced some limitations. The two institutions’ postoperative critical care differences may have influenced the outcomes. Other factors, such as comorbidities or the use of oral anticoagulants, may also affect surgical outcomes, but they were not explicitly addressed in our study. Additionally, the relatively short follow-up period increases the possibility of patients transitioning from a poor outcome group to a favorable outcome group over time.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of PGIMER, Dr. RML Hospital (Code: ECR/78/Inst/DL/2013/RR-19). Informed consent was taken for the case series as well as for medical management and surgical procedures. Any portion of the contents of the paper was not presented previously. Routine treatment was done as per literature, and in this case series, no animal was used.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Rahul Varshney; Data collection: Binoy Kumar Singh; Drafting the article: Rahul Varshney; Critically revising: Binoy Kumar Singh; Data analysis, interpretation, review and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We would like to express my sincere appreciation to ABVIMS & Dr. RML hospital and individuals whose contributions and support have greatly enhanced the quality and rigor of this research. Also, thanks are due to Prof. Pankaj Kumar for his unwavering guidance and insights throughout the research period.
References
Intracerebral hemorrhage (ICH) is associated with higher mortality and more severe disability compared to ischemic stroke [1]. The in-hospital mortality rate with ICH varies from 30-50% [2, 3]. In hypertensive hemorrhages, 60% occur in the basal ganglia, with a mortality rate of almost 50%. Even though many studies have been published concerning the management of ICH, no class [4]evidence has yet been published. Numerous studies [4, 5] and meta-analyses [6, 7] that assert no difference in outcome regardless of whether medical or surgical treatment is used are entirely rejected by others [8, 9].
Kaya et al. performed a study comparing conservative medical treatment to open craniotomy for putaminal hematomas in patients with a volume greater than 30 ml. Accordingly, mortality was 34% among patients treated surgically, compared to 63.1% in conservatively managed patients [8].
In contrast, surgical trials in traumatic intracerebral hemorrhage (STITCH) trials I and II have shown that early surgical evacuation of hematoma does not improve outcomes over initial best medical management. However, patients at risk of herniation or impaired level of consciousness were excluded from the study and almost 26% of patients initially treated conservatively underwent surgical decompression. Gregson et al. reanalyzed the data from STITCH trials and found that large basal ganglia bleeds will likely benefit from early surgery [9]. With advancements in postoperative critical care facilities, the outcome has improved after the surgery. Surgery has the advantage of removing the blood clot, thus limiting edema development in perihematomal white and grey matter and reducing the mass effect [10]. Surgical treatment of basal ganglia hemorrhages ranges from decompressive craniectomies to stereotactic needle aspiration of hematoma [11]. However, craniotomy remains the most common surgical procedure as it provides an added advantage of intraoperative homeostasis. Moreover, the superiority of any one of these methods over the others still needs to be completely established.
Due to differences in the incidence of hypertensive bleeding in the basal ganglia region compared to other parts of the brain, along with various factors, such as intraventricular hemorrhage (IVH), associated hydrocephalus and the impact of surrounding edema basal ganglia hemorrhage warrants distinct attention from cortical or posterior fossa bleeds. This study investigates the prognostic factors specifically associated with poor outcomes in basal ganglia hemorrhage. This study also provides an overview of our experience in managing basal ganglia hemorrhage surgical cases. The objective was to evaluate the functional outcomes using the Glasgow outcome score (GOS) three months after surgery.
2. Materials and Methods
This study involved 271 patients treated surgically for basal ganglia bleeding from December 2018 to November 2020 across two institutions. Institutional Review Board approval was obtained from both centers. Upon admission to the emergency room, all patients underwent a computed tomography scan to confirm the diagnosis of basal ganglia bleeding. All patients aged 18 years and above with ictus of less than three days who underwent surgical intervention were identified by reviewing electronic medical records. Follow-up scans were done in all patients based on their neurological conditions.
Patients with secondary causes of basal ganglia bleeding, subjects with the extension of bleeding into the brain stem, and individuals with a rebleed and those requiring a second surgery were excluded from the study. Additionally, patients with a Glasgow coma scale (GCS) 3 and fixed dilated pupils on presentation were excluded from the study. Study physicians recorded the demographics and clinical information of the patients. Every patient underwent an assessment utilizing the first available computed tomography (CT) or magnetic resonance imaging. The decision for operative intervention was made considering relevant clinical and radiological parameters. Surgery was indicated when signs of neurological deterioration or the risk of life-threatening herniation became apparent.
The volume of hematoma was calculated on CT scan using the ABC/2 method [12], which is based on a simplification of the ellipsoid volume equation in which A represents the maximum length (in cm) on the largest hemorrhage slice, B represents the diameter perpendicular to A and C represents the approximate number of axial slices multiplied by the slice thickness. Additionally, midline shift was calculated using the Foramen of Monro as a base point, and any extension of the hematoma into the ventricles as well as associated hydrocephalus was also noted.
According to the ABC protocol, all patients were admitted to and managed in a dedicated neuroscience intensive care unit or stroke unit. Antihypertensive medications, such as labetalol infusion, were used to maintain systolic blood pressure below 140 mmHg. Osmotic diuretics (such as mannitol) were administered to manage increased intracranial pressure. Anticoagulants and antiplatelets were discontinued for at least five days whenever feasible. The patients were operated on in spine position with a 30-degree elevation of the head and slight rotation toward the opposite side of the hemorrhage. Using the operating microscope and considering clot extension and surgeon preference, the clot was approached either through the prefrontal or superior temporal gyrus cortex. The hematoma was evacuated with the help of control suction and gentle irrigation. Any active bleeding point was coagulated and hemostasis was achieved. The dura was primarily closed whenever feasible and patched if necessary.
A decompressive craniectomy with lax duraplasty was performed in patients whose brains remained tensed and non-pulsatile even after hematoma evacuation when replacing the bone was not feasible. In such cases, wide decompressive craniectomy was done with a bone flap placed in the abdomen. Patients with IVH underwent extra ventricular drain insertion or venticuloperitoneal shunt placement before or after the operation, depending on the development of hydrocephalus. Subsequently, patients were monitored in the neurosurgical intensive care unit postoperatively, and any neurological or ventilator-associated complications were managed accordingly. Postoperative CT scans were done in all patients as needed based on their neurological status.
Follow-up procedure
The follow-up duration was at least three months from surgery, during which the outcome was assessed using the GOS. Patients with GOS 4 and 5 were classified as having excellent outcomes, while those with GOS scores of 3, 2 and 1 were considered to have poor outcomes [13].
Data analysis
Statistical significance was set to P<0.05 to determine the accuracy of the analyses conducted by Stata software, version 5.0.
All the variables used for prognostication were abstracted from electronic medical records at the time of admission. Study variables included age, sex, GCS, hematoma volume, IVH, midline shift, bleeding side and addictions (smoking and drinking). Continuous variables among factors related to outcome were dichotomized into different categories, which along with dichotomous variables, were compared using the chi-square tests. Multivariate regression analyses were performed subsequently, considering the effect of all the variables on the GOS.
3. Results
Of all 271 patients, 203 were male, and 68 were female (Table 1).

Their age ranged from 20 to 85 years (mean, 50.8±13.57 years; Table 2).

A statistically significant difference was found between the excellent and unfavorable outcomes groups, with people below 60 years having poor outcomes (GOS=1, 2, 3) rate of 38.67%, and 53.33% of patients above 60 years having poor outcomes (P<0.02; Table 2). Among patients younger than 30 years, 10 out of 13(76.92%) had a favorable outcome, compared to 3(23.08%) who had poor outcome. The age-related outcomes highlight that younger patients with basal ganglia bleed are more likely to achieve an excellent outcome following treatment.
Top of form
The 60-year cut-off was chosen because basal ganglia bleeding tends to occur in a relatively younger population in the Indian subcontinent. Thus, the patients were divided into two groups based on age. Among the patients, 35.10% (n=119) had right-sided bleeding, while 51.66% (n=131) had a hematoma on the left side (Table 2).
Patients were classified into three groups based on their GCS, following the same classification as that for head injury patients with GCS scores ranging from 4 to 15. In the GCS group <8, three patients (27.27%) out of 11 patients had a favorable outcome (GOS=4, 5), while 72.73% had a poor outcome. In patients with GCS>8, a total of 12 patients (55.35%) out of 224 patients had GOS 4, 5 (Table 2). Among the patients with a GCS 13-15, a total of 72.22% (n=26) out of 36 patients had favorable outcomes, while 27.77% had a poor outcome (Table 2). Meanwhile, a significant difference in terms of outcome was observed (P<0.0001) between the three studied groups based on the GCS score on admission (Table 2).
Hematoma volumes ranged from 30 to 120 cm3, with a mean of 54.42±23.25 cm3. Based on volume, 5.53% (n=15) patients had small hematomas (30 cm3), 60.51% (n=164) had medium-sized hematomas (30-60 cm3), and 33.94% (n=92) had large (>60 cm3) hematomas. Among patients with hematoma volumes <30 mL, the majority (n=12), 80% had favorable outcomes, while three patients (20%) had poor outcomes. In the group with hematoma volumes between 30-60 mL, 72.56% (n=119) of patients had good outcomes. However, among patients with ICH volumes >60 mL, 76.08% (n=22) had poor outcomes (Table 2). Additionally, 49.85% (n=135) of patients had hemorrhage extending into the ventricles, and 10.32% (n=28) developed associated hydrocephalus (Table 1). Among these 28 patients, an external ventricular drain was inserted in 20(71.4%) either preoperatively or postoperatively and eight patients (28.57%) underwent concurrent external ventricular drain insertion with hematoma evacuation.
Meanwhile, 72.79% of patients had excellent outcomes with no associated IVH. However, when IVH was present, only 47.66% had a GOS of 4 or 5. In cases when IVH was associated with hydrocephalus, an excellent outcome was present in only 10.7% of patients (Table 2). Thus, a hematoma volume of more than 60 mL, IVH, and hydrocephalus had a statistically significant impact on good functional outcomes (P<0.0001; Table 2). A midline shift of more than 5 mm led to a significantly worse outcome for 52.32% of patients (n=91) compared to a midline shift of <5 mm for 28.28% (n=28; P<0.0001; Table 2).
Only 71(49.65%) of the 143 patients in our study had favorable outcomes when their hematoma was located in the dominant hemisphere (left side). Out of 128 patients with hematoma in the non-dominant hemisphere, 64.06% (n=82) had a good outcome (Table 2). This difference was statistically significant in terms of good functional outcomes (P<0.016; Table 2). This distinction may be attributed to the dominant hemisphere’s involvement in more complex functions, potentially resulting in greater neurological damage when affected by a hematoma.
A total of 144 patients had a history of either smoking or drinking. Of these, 61.11% (n=88) were smokers, 24.30% (n=35) were drinkers, and 14.58% (n=21) were both smokers and drinkers. Both study groups did not show statistically significant differences in terms of outcomes (P=0.57; Table 2).
Similarly, no significant difference was observed regarding gender in terms of outcomes (P=0.53; Table 2).
Among the 34 patients who underwent decompressive craniectomy 28 patients (82.35%) had hematoma volume greater than 60 mL, and 25 patients (73.52%) had midline shift >5 mm.
Regarding GOS, out of 271 patients, 146 patients (53.87%) showed favorable outcomes (GOS 4, 5), 26 (9.59%) had poor outcomes (GOS 2, 3) and 99 patients died resulting in a mortality rate of 36.53% (GOS 1).
Univariate analysis showed that older age (P<0.021), bleeding on the left side of the brain (P<0.016), presence of IVH (P<0.0001), higher ICH volume (P<0.0001), GCS under 8 (P<0.0001), and midline shift >5mm (P<0.0001) were all associated with a poorer outcome (Table 2). The likelihood of poor outcomes was not different between males and females (P=0.53) or addicted people(P=0.57; Table 2).Once the confounders were adjusted, a multivariate analysis was performed, which demonstrated that individuals over the age of 60 years, patients with bleeding on the left side of the brain, cases in which bleeding was associated with IVH and hydrocephalus, when the hematoma volume was >60 mL and patients whose GCS was <8 had poor outcomes. The P<0.05, as shown in Table 3.

4. Discussion
Despite advances in medical science in stroke management, basal ganglia hemorrhage remains one of the most incapacitating and disabling forms of stroke. While many trials and met analyses published earlier for spontaneous ICH include both lobar and deep-seated hemorrhages, the factors deciding management, either surgical or conservative, and prognostication for the two, cannot be generalized. IVH, more commonly associated with basal ganglia hemorrhage than lobar hemorrhage, significantly impacts survival. Various studies in the past have advocated IVH as an independent predictor of mortality. Similarly, post-IVH hydrocephalus is more likely in basal ganglia hemorrhage than in cortical bleeding, leading to poor outcomes. The importance of surrounding edema and its impact on nearby structures cannot be ruled out. Basal ganglia bleed faces the major brunt due to critical structures in the vicinity. In this study, we investigated surgical outcomes and identified prognostic factors specifically related to basal ganglia hemorrhages in patients who underwent surgical evacuation with or without extraventricular drainage or ventriculoperotoneal shunt. The management of spontaneous ICH has been the subject of several trials and meta-analyses in the past. In the STITCH trial [4], the most notable stroke trial, the superiority of surgical management over first-line conservative best medical treatment was also not confirmed. However, the trial’s findings could not be generalized because there was a significant cross-over from the conservatively managed group to the surgically treated group, resulting in reduced unfavorable outcomes observed in conservatively managed patients. Furthermore, the STITCH II trial [14] was biased in patient selection as it excluded patients with low GCS, having an IVH, or those at risk of herniation (>3300).
Top of form
In 1990, Batjer et al. [15] performed a randomized clinical trial in 21 patients with hypertensive putaminal hemorrhage. They concluded that despite the best available treatment, the outcome was worse. However, with advancements in intraoperative techniques and post-operative critical care, the survival rate in such patients has gradually increased over some time.
A conservative approach has been advocated in most studies when a patient presents with a hematoma volume of up to 10 cm3, minimal neurological deficit, severe coagulopathy, or underlying medical conditions, or when the patient with advanced age (over 80), makes surgery intolerable. However, in cases of a large, life-threatening ICH, evacuation is necessary. Minimally invasive endoscopic approaches have become increasingly prevalent over the past few years for spontaneous ICH, while others have advocated decompressive craniectomies for large hematomas with midline shift, GCS score <8 and raised ICP not responding to medical management. In 2012, Zhou et al. [16] performed a meta-analysis and found that minimally invasive procedures were superior to craniotomies. However, methodological flaws in the research have been raised [17], warranting further discussion to determine the efficacy of these procedures. Decompressive craniectomies were performed in only a few patients in our study, most undergoing craniotomies and hematoma evacuation. Ensuring a clear and unobstructed view is crucial for complete hematoma evacuation. Craniotomy also facilitates easy hemostasis, leading to improved outcomes and reduced mortality rates. Among the 271 patients in our study, 237(87.45%) underwent craniotomies, while 34(12.54%) underwent decompressive craniectomies with hematoma evacuation.
In this study,the mortality within the first three months was 36.53%. However, in a study by Lahti et al. the mortality for deep-seated bleed was 16.3% [18]. This difference could be because they included hemorrhages of all sizes. In our study, we only included those patients requiring surgery for whom hematoma volume and GCS score were major deciding factors, with relatively larger volume hematoma and poor GCS requiring surgery. Yilmaz et al. reported a mortality rate of 56% for deep-seated hemorrages [19]. The improvement in surgical outcomes observed in our study may be attributed to advancements in preoperative and postoperative critical care, improved surgical techniques, and neuroanesthesia, all of which have led to better results. González-Pérez et al. reported an increase in mortality rates with an increase in age. According to them, case fatality increased with age, reaching 29.7% for individuals aged 20-49 years and 54.6% for those aged 80-89 years [20]. According to the study by Kwon et al. univariate analysis revealed that age exhibited no significant effect, despite being strongly associated with clinical outcomes [21]. Possibly explained considering that the incidence of spontaneous ICH in the western population is higher in older populations, the atrophic brain in such patients counteract the effects of raised ICP due to the hematoma. However, in the Indian subcontinent, cortical atrophy does not significantly affect the outcome due to the relatively increased incidence of basal ganglia bleeds in the younger population. The mean age among deceased patients was significantly higher, with no differences in mortality rate based on sex. Thus mortality at three months was strongly correlated with age, with a mortality rate of 53.3% in patients >60 years.
Top of form
Our research confirms previous findings that IVH with or without subsequent hydrocephalus is associated with adverse outcomes. Due to the vicinity of lateral ventricles to basal ganglia thus, with an increased incidence of IVH in basal ganglia bleeds than cortical bleed, early rupture of hemorrhage into ventricles is common, compromising the normal pressure regulation of the cranial vault. It has been demonstrated in animal studies [22] that the likelihood of animal death increases proportionately with the amount of blood injected into the ventricles. Blood in the ventricular system at the tissue level causes inflammation, fibrosis and subsequent hydrocephalus [22], further increasing mortality and morbidity rates.
The preoperative hematoma volume is the primary deciding factor for surgical evacuation. Many studies have advocated that preoperative hematoma volume on CT scans less than <20 mL can be managed conservatively since it causes little mass effect. Broderick et al. [23] in 1991 reported 93%, 64% and 23% mortality rates for hematoma volumes >60 mL, 30-60 mL and 30 mL, respectively. Similar mortality and morbidity rates have been reported by multiple cohorts, describing a solid correlation between hematoma volume and survival or functional outcome in spontaneous ICH patients [24, 25]. The pressure effect of the hematoma on the surrounding brain tissue causes initial damage to the brain. Subsequently, the lysis of erythrocytes liberates various inflammatory mediators which cause secondary damage to the brain [26, 27, 28]. All these pathological effects increase proportionately with the size of the hematoma. Thus early surgery has the theoretical advantage of preventing both types of damage.
Hematoma volumes less than 30 mL were associated with good postoperative functional outcomes [29]. Conversely, patients with larger hematoma volumes (90 mL) and the highest GCS score of 6 were linked to poorer outcomes (P<0.039) [30].
Our outcome rates were similar to Broderick’s in the < 30 mL group and the >60 mL group; however, we had a better outcome in the middle group (30-60 mL) regarding GOS. This can be attributed to many patients in the second group lying close to the minimum range value, thus having relatively less hematoma volume.
Similar to head injuries, GCS strongly predicts clinical outcomes in spontaneous ICH patients [31]. Apart from prognostication; it also helps decide the management line in patients with deep-seated hemorrhages. In contrast, a GCS Score of 13 or higher favors conservative management; surgery is recommended for patients having a GCS score below 12 [32]. Thus, GCS scores are an essential part of the treatment decision-making process, and lower scores indicate that aggressive treatment options such as surgery should be considered.
In our study majority of patients (82.65%) had GCS scores between 8-12. Patients with GCS score <8 experienced a worse outcome, with a mortality rate of 54.54%.This finding is consistent with a meta-analysis published by Gregson et al. which included 1541 randomized patients with spontaneous ICH. The meta-analysis suggested that surgery may be most beneficial for those with an intermediate GCS between 9-12, while its risks may outweigh the benefits for those with a higher or lower GCS [9]. Therefore, surgery should be reserved for patients with a GCS between 9 and 12, as this group stands to gain the most from an intervention. This finding underscores the importance of weighing surgery risks against the potential benefits for each patient with a higher or lower GCS.
While foramen of Monro displacement was not nearly as indicative of stroke mortality, displacement at the septum pellucidum and coma level were strong indicators of mortality [33].
Top of form
Missori et al. concluded that the amount of brain shift at the level of the corpus callosum was related to survival in surgically treated patients with ICH. However, brain shift at Monro’s foramen was not associated with positive outcomes [34]. However, due to the limited sample size, they also advocated the need for further investigation with a larger sample size, considering the location and volume of hematoma and associated co-morbidities. We included all the factors mentioned above in our study. We found that midline shift at the level of the foramen of Monro were significantly associated with excellent or poor surgical outcomes, with 52.32 % of patients having midline shifts greater than 5mm at the level of foramen Monro having a poor outcome. Thus, choosing an appropriate surgical approach and route with the least cortical damage may improve functional outcomes. Like any other mass lesion that causes a rise in intracranial pressure, thereby increasing mortality or morbidity, basal ganglia bleeding also carries the same potential. Therefore, large hypertensive basal ganglia bleed requires early surgical evacuation, minimizing the harmful effect of raised ICP and improving the local circulation.
5. Conclusion
Hematoma drainage through craniotomies remains a life-saving procedure in critical situations. Surgical evacuation is an effective treatment option for large hypertensive basal ganglia hemorrhages. Surgical outcomes for these hemorrhages are influenced by factors such as hematoma volume, preoperative GCS score, presence of IVH, and the location of the bleeding (left side). Predictors of a favorable surgical outcome include a hematoma volume of less than 60 mL, absence of IVH, a GCS score of 13-15, age <60 years and a bleed on the right side.
Limitations
This study faced some limitations. The two institutions’ postoperative critical care differences may have influenced the outcomes. Other factors, such as comorbidities or the use of oral anticoagulants, may also affect surgical outcomes, but they were not explicitly addressed in our study. Additionally, the relatively short follow-up period increases the possibility of patients transitioning from a poor outcome group to a favorable outcome group over time.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of PGIMER, Dr. RML Hospital (Code: ECR/78/Inst/DL/2013/RR-19). Informed consent was taken for the case series as well as for medical management and surgical procedures. Any portion of the contents of the paper was not presented previously. Routine treatment was done as per literature, and in this case series, no animal was used.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Rahul Varshney; Data collection: Binoy Kumar Singh; Drafting the article: Rahul Varshney; Critically revising: Binoy Kumar Singh; Data analysis, interpretation, review and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We would like to express my sincere appreciation to ABVIMS & Dr. RML hospital and individuals whose contributions and support have greatly enhanced the quality and rigor of this research. Also, thanks are due to Prof. Pankaj Kumar for his unwavering guidance and insights throughout the research period.
References
- Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004; 363(9425):1925-33. [DOI:10.1016/S0140-6736(04)16405-2] [PMID]
- Bhatia R, Singh H, Singh S, Padma MV, Prasad K, Tripathi M, et al. A prospective study of in-hospital mortality and discharge outcome in spontaneous intracerebral hemorrhage. Neurology India. 2013; 61(3):244-8. [DOI:10.4103/0028-3886.115062] [PMID]
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: Systematic review and meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry. 2014; 85(6):660-7. [DOI:10.1136/jnnp-2013-306476] [PMID]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet. 2005; 365(9457):387-97. [DOI:10.1016/S0140-6736(05)17826-X] [PMID]
- Tan SH, Ng PY, Yeo TT, Wong SH, Ong PL, Venketasubramanian N. Hypertensive basal ganglia hemorrhage: A prospective study comparing surgical and nonsurgical management. Surgical Neurology. 2001; 56(5):287-92. [DOI:10.1016/S0090-3019(01)00561-4] [PMID]
- Fernandes HM, Gregson B, Siddique S, Mendelow AD. Surgery in intracerebral hemorrhage. The uncertainty continues. Stroke. 2000; 31(10):2511-6. [DOI:10.1161/01.STR.31.10.2511] [PMID]
- Prasad K, Browman G, Srivastava A, Menon G. Surgery in primary supratentorial intracerebral hematoma: A meta-analysis of randomized trials. Acta Neurologica Scandinavica. 1997; 95(2):103-10. [DOI:10.1111/j.1600-0404.1997.tb00078.x] [PMID]
- Kaya RA, Türkmenoğlu O, Ziyal IM, Dalkiliç T, Sahin Y, Aydin Y. The effects on prognosis of surgical treatment of hypertensive putaminal hematomas through transsylvian transinsular approach. Surgical Neurology. 2003; 59(3):176-83. [DOI:10.1016/S0090-3019(02)01043-1] [PMID]
- Gregson BA, Mitchell P, Mendelow AD. Surgical decision making in brain hemorrhage. Stroke. 2019; 50(5):1108-15. [DOI:10.1161/STROKEAHA.118.022694] [PMID] [PMCID]
- Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998; 29(12):2580-6. [DOI:10.1161/01.STR.29.12.2580] [PMID]
- Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage:– A randomized study. Journal of Neurosurgery. 2004; 101(3):417-20. [DOI:10.3171/jns.2004.101.3.0417] [PMID]
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27(8):1304-5. [DOI:10.1161/01.STR.27.8.1304] [PMID]
- Houseman CM, Belverud SA, Narayan RK. Closed head injury. In: Ellenbogen, RG, Abdulrauf SI, Sekhar LN, editors. Principles of neurological surgery. Amsterdam: Sciencedirect; 2012. [DOI:10.1016/B978-1-4377-0701-4.00020-8]
- Mendelow AD, Gregson BA, Mitchell PM, Murray GD, Rowan EN, Gholkar AR, et al. Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials. 2011; 12:124. [DOI:10.1186/1745-6215-12-124] [PMID] [PMCID]
- Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Archives of Neurology. 1990; 47(10):1103-6. [DOI:10.1001/archneur.1990.00530100071015] [PMID]
- Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials. Stroke. 2012; 43(11):2923-30. [DOI:10.1161/STROKEAHA.112.667535] [PMID]
- Gregson BA, Rowan EN, Mendelow AD. Letter to the editor by Gregson et al regarding article, "minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials". Stroke. 2013; 44(5):e45. [DOI:10.1161/STROKEAHA.111.000296] [PMID]
- Lahti AM, Nätynki M, Huhtakangas J, Bode M, Juvela S, Ohtonen P, et al. Long-term survival after primary intracerebral hemorrhage: A population-based case-control study spanning a quarter of a century. Eur J Neurol. 2021; 28(11):3663-9. [DOI:10.1111/ene.14988] [PMID]
- Yilmaz C, Kabatas S, Gulsen S, Cansever T, Gurkanlar D, Caner H, et al. Spontaneous supratentorial intracerebral hemorrhage: Does surgery benefit comatose patients? Annals of Indian Academy of Neurology. 2010; 13(3):184-7. [DOI:10.4103/0972-2327.70881] [PMID] [PMCID]
- González-Pérez A, Gaist D, Wallander MA, McFeat G, García-Rodríguez LA. Mortality after hemorrhagic stroke: data from general practice (the health improvement network). Neurology. 2013; 81(6):559-65. [DOI:10.1212/WNL.0b013e31829e6eff] [PMID]
- Kwon SM, Choi KS, Yi HJ, Ko Y, Kim YS, Bak KH, et al. Impact of brain atrophy on 90-day functional outcome after moderate-volume basal ganglia hemorrhage. Scientific Reports. 2018; 8(1):4819. [DOI:10.1038/s41598-018-22916-3] [PMID] [PMCID]
- Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1. Canine intraventricular blood cast model. Neurosurgery. 1986; 19(4):540-6. [DOI:10.1227/00006123-198610000-00008] [PMID]
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993; 24(7):987-93. [DOI:10.1161/01.STR.24.7.987] [PMID]
- Hallevy C, Ifergane G, Kordysh E, Herishanu Y. Spontaneous supratentorial intracerebral hemorrhage. Criteria for short-term functional outcome prediction. Journal of Neurology. 2002; 249(12):1704-9. [DOI:10.1007/s00415-002-0911-1] [PMID]
- Salihović D, Smajlović D, Ibrahimagić OĆ. Does the volume and localization of intracerebral hematoma affect short-term prognosis of patients with intracerebral hemorrhage? ISRN Neuroscience. 2013; 2013:327968. [DOI:10.1155/2013/327968] [PMID] [PMCID]
- Duan X, Wen Z, Shen H, Shen M, Chen G. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxidative Medicine and Cellular Longevity. 2016; 2016:1203285. [DOI:10.1155/2016/1203285] [PMID] [PMCID]
- Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia activation and polarization after intracerebral hemorrhage in mice: The role of protease-activated receptor-1. Translational Stroke Research. 2016; 7(6):478-87. [DOI:10.1007/s12975-016-0472-8] [PMID] [PMCID]
- Zhang Z, Zhang ZE, Lu H, Yang Q, Wu H, Wang J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Molecular Neurobiology. 2017; 54:1874-86. [DOI:10.1007/s12035-016-9785-6]
- Rathor MY, Rani MF, Jamalludin AR, Amran M, Shahrin TC, Shah A. Prediction of functional outcome in patients with primary intracerebral hemorrhage by clinical-computed tomographic correlations. Journal of Research in Medical Sciences. 2012; 17(11):1056-62. [PMID] [PMCID]
- Zhang HT, Xue S, Li PJ, Fu YB, Xu RX. Treatment of huge hypertensive putaminal hemorrhage by surgery and cerebrospinal fluid drainage. Clinical Neurology and Neurosurgery. 2013; 115(9):1602-8. [DOI:10.1016/j.clineuro.2013.02.005] [PMID]
- Wang CW, Liu YJ, Lee YH, Hueng DY, Fan HC, Yang FC, et al. Hematoma shape, hematoma size, Glasgow coma scale score and ICH score: Which predicts the 30-day mortality better for intracerebral hematoma? Plos One. 2014; 9(7):e102326. [DOI:10.1371/journal.pone.0102326] [PMID] [PMCID]
- Cho DY, Chen CC, Lee HC, Lee WY, Lin HL. Glasgow Coma Scale and hematoma volume as criteria for treatment of putaminal and thalamic intracerebral hemorrhage. Surgical Neurology. 2008; 70(6):628-33. [DOI:10.1016/j.surneu.2007.08.006] [PMID]
- Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW. Mass effect and death from severe acute stroke. Neurology. 1997; 49(4):1090-5. [DOI:10.1212/WNL.49.4.1090] [PMID]
- Missori P, La Torre G, Lazzari S, Paolini S, Peschillo S, Martini S, et al. Preoperative brain shift is a prognostic factor for survival in certain neurosurgical diseases other than severe head injury: A case series and literature review. Neurosurgical Review. 2022; 45(2):1445-50. [DOI:10.1007/s10143-021-01659-2] [PMID] [PMCID]
Type of Study: Research |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






