Sat, Dec 27, 2025
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 56-63 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Parambil R M, Thavara B D, Jose B V, Sasi P, Maniyan R, Cholakkal S et al . Clinical Profile and Surgical Management of Intramedullary
Spinal Cord Tumors. Iran J Neurosurg 2024; 10 : 6
URL: http://irjns.org/article-1-379-en.html
URL: http://irjns.org/article-1-379-en.html
Rajeev Mandaka Parambil1 

 , Binoy Damodar Thavara *2
, Binoy Damodar Thavara *2 

 , Byjo Valiyaveetil Jose1
, Byjo Valiyaveetil Jose1 

 , Premkumar Sasi1
, Premkumar Sasi1 

 , Radhakrishnan Maniyan1
, Radhakrishnan Maniyan1 

 , Shanavas Cholakkal1
, Shanavas Cholakkal1 

 , Ebby Sebastian1
, Ebby Sebastian1 




 , Binoy Damodar Thavara *2
, Binoy Damodar Thavara *2 

 , Byjo Valiyaveetil Jose1
, Byjo Valiyaveetil Jose1 

 , Premkumar Sasi1
, Premkumar Sasi1 

 , Radhakrishnan Maniyan1
, Radhakrishnan Maniyan1 

 , Shanavas Cholakkal1
, Shanavas Cholakkal1 

 , Ebby Sebastian1
, Ebby Sebastian1 


1- Department of Neurosurgery, Government Medical College, Kozhikode, India.
2- Department of Neurosurgery, Government Medical College, Kozhikode, India. ,drbinoytd@gmail.com
2- Department of Neurosurgery, Government Medical College, Kozhikode, India. ,
Full Text [PDF 2604 kb]
(1029 Downloads)
| Abstract (HTML) (3384 Views)
References
Full Text: (1303 Views)
1. Introduction
Spine lesions are classified as either intramedullary, intradural extramedullary, or extradural in origin [1]. Intramedullary spinal cord tumors (IMSCTs) are the rarest of all spine tumors [2]. Males are affected more than females [3]. The cervical region is the most common location [4]. Magnetic resonance imaging (MRI) scan is the investigation of choice for IMSCT [5]. The most common symptoms are weakness followed by pain and urinary incontinence [3]. Ependymomas and low-grade gliomas are the most common tumor types. Astrocytoma is infiltrative and lateral in location, ependymoma displaces the white matter tract and is central in location, and hemangioblastoma is postero-laterally located [6]. Microsurgical resection is the treatment of choice. Hemangioblastomas and ependymomas are amenable to gross total resection (GTR) as compared to astrocytomas. Bansal et al. noted that during follow-up, 16.4% had improved, 74.6% had not changed, and 9% had worsened. They concluded that postoperative outcomes were dependent on preoperative neurological status [3]. Authors have studied the clinical profile and surgical management of IMSCTs so that patients with this rare disease are benefited.
2. Methods and Materials/Patients
This is a retrospective study to review the IMSCTs operated between January 2007 to December 2021 in the Department of Neurosurgery. Institutional Ethics Committee clearance has been obtained. The aim is to analyze the clinical profile and surgical management of IMSCTs. All the patients underwent an MRI scan of the spine before the surgery. All the operated cases of IMSCTs were analyzed in the study. Myxopapillary ependymomas were excluded from the study. The authors analyzed the patient’s age, sex, clinical features, diagnosis, duration of symptoms, MRI findings, level of the lesion, surgical procedure, extent of resection, histopathology, postoperative neurological status, complications, and duration of stay in hospital after surgery. The results were analyzed using Mean±SD and median using Epi info software, version 7.
3. Results
There were twenty-seven operated cases of IMSCTs. The age ranges from 1-75 years (Mean±SD 39±22 years). Fifteen cases (55.5%) were male and 12(44.5%) were female patients. There were 6(22%) cervical, 7(26%) cervicodorsal, 9(33%) dorsal, and 5(19%) dorsolumbar IMSCTs. Duration of symptoms was 3 days to 60 months (Mean±SD 10±14 months). Ten (37%) patients had spinal cord syrinx. Clinical features include pain in 24(89%) patients, sensory symptoms in 20(74%) patients, motor weakness in 26(96%) patients, and sphincter involvement in 18(67%) patients. All the patients underwent laminectomy and dura was opened at the lesion site. The dura and arachnoid were longitudinally opened. Midline myelotomy was done in nonsurfacing lesions for tumor excision. Intraoperative GTR was achieved in 12(44.4%) patients, near-total resection (NT) in 4(14.8%) patients, subtotal resection (ST) in 2(7.4%) patients, decompression in 7(26%) patients and biopsy in 2(7.4%) patients (Table 1).

Postoperative neurological status was analyzed and the results showed that eighteen (66.6%) patients had the neurological status same as preoperative status. Four (14.8%) patients (3 GTR, 1 debulking) had improvement in neurological status and 5(18.5%) patients (2 GTR, 2 NT, 1 decompression) had deterioration in the neurological status. The histopathological diagnosis of operated IMSCTs is given in Table 2.
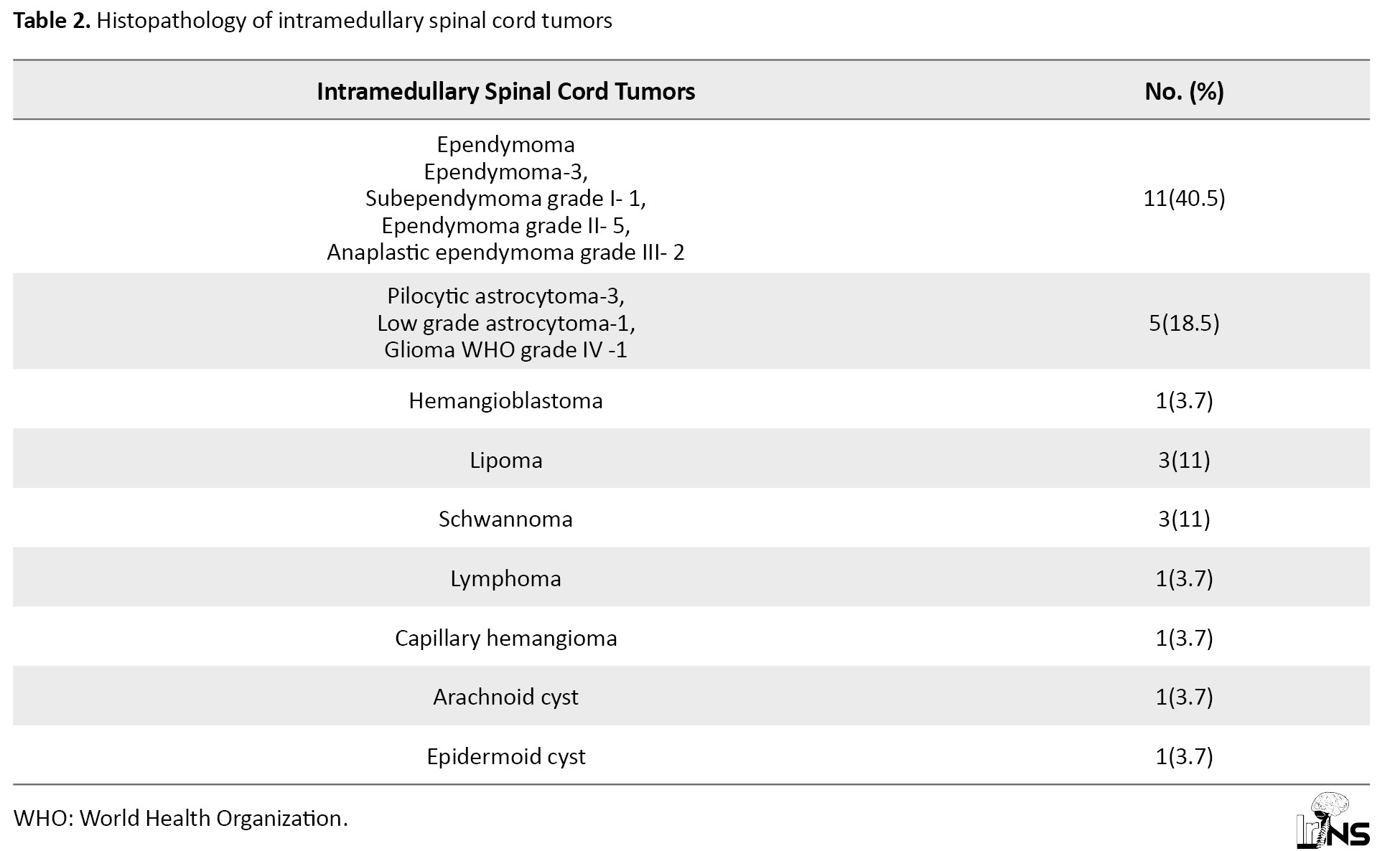
Ependymomas had a central location in the spinal cord. It is the most common histological type of IMSCT (40.5%). Other lesions are glioma, multiple hemangioblastoma (cervical), lipoma (2 dorsal and 1 lumbar), schwannoma (1 cervical, 1 dorsal, and 1 dorsolumbar), lymphoma (cervical), capillary hemangioma (dorsal), arachnoid cyst (dorsal), and epidermoid cyst with spinal dysraphism (lumbar). Only symptomatic lesion at the C5-6 level was operated in the case of multiple hemangioblastoma. Ependymomas had well-defined margins compared to other gliomas. Hence a greater number of ependymomas achieved GTR when compared to other gliomas. GTR was not attempted in cases with spinal cord infiltration to reduce the morbidity. Duration of stay in the hospital was 9±3.5 days.
Illustrative case 1
A 63-year-old male patient presented with a history of weakness in both lower limbs for 5 months, weakness in both upper limbs for 3 months, and urinary incontinence for 3 months. Examination revealed medical research council (MRC) grade 4 power of all limbs, exaggerated deep tendon reflexes in both lower limbs, and decreased sensation in all limbs. MRI scan of the spine revealed a relatively well-defined contrast-enhancing intramedullary lesion (12×10×51 mm) extending from C7 to D2 level. Polar cysts and syrinx were noted cranial and caudal to the lesion (Figure 1).
Spine lesions are classified as either intramedullary, intradural extramedullary, or extradural in origin [1]. Intramedullary spinal cord tumors (IMSCTs) are the rarest of all spine tumors [2]. Males are affected more than females [3]. The cervical region is the most common location [4]. Magnetic resonance imaging (MRI) scan is the investigation of choice for IMSCT [5]. The most common symptoms are weakness followed by pain and urinary incontinence [3]. Ependymomas and low-grade gliomas are the most common tumor types. Astrocytoma is infiltrative and lateral in location, ependymoma displaces the white matter tract and is central in location, and hemangioblastoma is postero-laterally located [6]. Microsurgical resection is the treatment of choice. Hemangioblastomas and ependymomas are amenable to gross total resection (GTR) as compared to astrocytomas. Bansal et al. noted that during follow-up, 16.4% had improved, 74.6% had not changed, and 9% had worsened. They concluded that postoperative outcomes were dependent on preoperative neurological status [3]. Authors have studied the clinical profile and surgical management of IMSCTs so that patients with this rare disease are benefited.
2. Methods and Materials/Patients
This is a retrospective study to review the IMSCTs operated between January 2007 to December 2021 in the Department of Neurosurgery. Institutional Ethics Committee clearance has been obtained. The aim is to analyze the clinical profile and surgical management of IMSCTs. All the patients underwent an MRI scan of the spine before the surgery. All the operated cases of IMSCTs were analyzed in the study. Myxopapillary ependymomas were excluded from the study. The authors analyzed the patient’s age, sex, clinical features, diagnosis, duration of symptoms, MRI findings, level of the lesion, surgical procedure, extent of resection, histopathology, postoperative neurological status, complications, and duration of stay in hospital after surgery. The results were analyzed using Mean±SD and median using Epi info software, version 7.
3. Results
There were twenty-seven operated cases of IMSCTs. The age ranges from 1-75 years (Mean±SD 39±22 years). Fifteen cases (55.5%) were male and 12(44.5%) were female patients. There were 6(22%) cervical, 7(26%) cervicodorsal, 9(33%) dorsal, and 5(19%) dorsolumbar IMSCTs. Duration of symptoms was 3 days to 60 months (Mean±SD 10±14 months). Ten (37%) patients had spinal cord syrinx. Clinical features include pain in 24(89%) patients, sensory symptoms in 20(74%) patients, motor weakness in 26(96%) patients, and sphincter involvement in 18(67%) patients. All the patients underwent laminectomy and dura was opened at the lesion site. The dura and arachnoid were longitudinally opened. Midline myelotomy was done in nonsurfacing lesions for tumor excision. Intraoperative GTR was achieved in 12(44.4%) patients, near-total resection (NT) in 4(14.8%) patients, subtotal resection (ST) in 2(7.4%) patients, decompression in 7(26%) patients and biopsy in 2(7.4%) patients (Table 1).

Postoperative neurological status was analyzed and the results showed that eighteen (66.6%) patients had the neurological status same as preoperative status. Four (14.8%) patients (3 GTR, 1 debulking) had improvement in neurological status and 5(18.5%) patients (2 GTR, 2 NT, 1 decompression) had deterioration in the neurological status. The histopathological diagnosis of operated IMSCTs is given in Table 2.

Ependymomas had a central location in the spinal cord. It is the most common histological type of IMSCT (40.5%). Other lesions are glioma, multiple hemangioblastoma (cervical), lipoma (2 dorsal and 1 lumbar), schwannoma (1 cervical, 1 dorsal, and 1 dorsolumbar), lymphoma (cervical), capillary hemangioma (dorsal), arachnoid cyst (dorsal), and epidermoid cyst with spinal dysraphism (lumbar). Only symptomatic lesion at the C5-6 level was operated in the case of multiple hemangioblastoma. Ependymomas had well-defined margins compared to other gliomas. Hence a greater number of ependymomas achieved GTR when compared to other gliomas. GTR was not attempted in cases with spinal cord infiltration to reduce the morbidity. Duration of stay in the hospital was 9±3.5 days.
Illustrative case 1
A 63-year-old male patient presented with a history of weakness in both lower limbs for 5 months, weakness in both upper limbs for 3 months, and urinary incontinence for 3 months. Examination revealed medical research council (MRC) grade 4 power of all limbs, exaggerated deep tendon reflexes in both lower limbs, and decreased sensation in all limbs. MRI scan of the spine revealed a relatively well-defined contrast-enhancing intramedullary lesion (12×10×51 mm) extending from C7 to D2 level. Polar cysts and syrinx were noted cranial and caudal to the lesion (Figure 1).
MRI features were suggestive of ependymoma. The patient underwent C7-D2 laminectomy and the dura was opened. A thin cord with a central greyish-red moderately vascular suckable tumor was noted (Figure 2).
Near total excision was done and the dura closed with continuous sutures. The wound closed in layers. Postoperatively patients had grade 4 power of upper limbs and grade 3 power of lower limbs. Histopathological examination was consistent with Grade II ependymoma.
Illustrative case 2
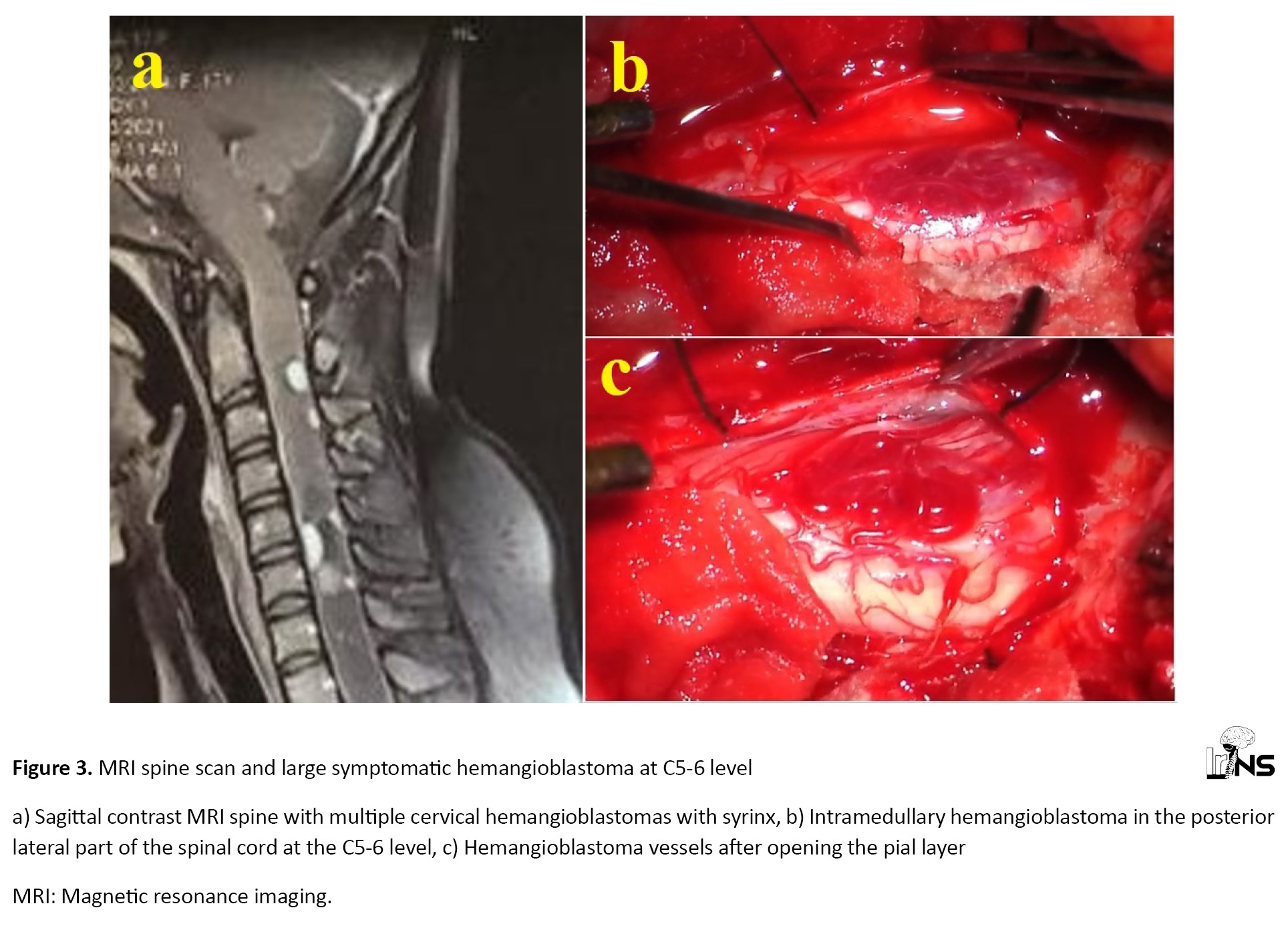
A 17-year-old female patient with Von Hippel Lindau disease (VHL) presented with a history of neck pain and weakness of upper limbs for 3 months. Examination revealed bilateral upper limbs power of MRC grade 4 and lower limbs power of MRC grade 5. MRI spine scan revealed multiple cervical hemangioblastomas with syrinx (Figure 3a).
Illustrative case 2
A 17-year-old female patient with Von Hippel Lindau disease (VHL) presented with a history of neck pain and weakness of upper limbs for 3 months. Examination revealed bilateral upper limbs power of MRC grade 4 and lower limbs power of MRC grade 5. MRI spine scan revealed multiple cervical hemangioblastomas with syrinx (Figure 3a).
Large symptomatic hemangioblastoma at C5-6 level was operated. The patient underwent C4 C5 C6 laminectomy and excision of C5-6 level hemangioblastoma. Reddish fleshy intramedullary surfacing lesion of blood vessels with pial attachments noted at C5-6 level (Figure 3b and 3c). GTR was done and syrinx fluid was drained. The patient’s upper limb power improved to MRC grade 4+. Histopathological examination confirmed the diagnosis of hemangioblastoma.
4. Discussion
The classification of the primary IMSCTs follows that of central nervous system (CNS) tumors [7]. Ependymomas and astrocytomas are the most common types. Other tumors include hemangioblastoma, metastasis, cavernomas, dermoidal cysts, and enterogenetic cysts. The most frequent site is the cervical and cervicothoracic region [8]. IMSCTs can present as either complete cord transactions or incomplete cord transactions [9].
Spinal ependymomas are classified as ependymoma, ependymoma MCYN-amplified, subependymoma, and myxopapillary ependymoma [10]. Ependymomas are more common in the cervical region. They are solitary tumors and arise from the central canal. They are associated with syrinx in half of the cases. They present with pain and central cord syndrome. During surgery, the tumor is separable from the cord because of the presence of the plane of cleavage [9]. Syrinx was seen in 4 (36%) cases of ependymoma in the author’s study. They noted that the majority of the ependymomas were excised through a clear plane between the tumor and the cord.
Astrocytomas account for about 30% to 35% of all IMSCTs. They are the most common IMSCTs in the pediatric age group. They often present with back pain and progressive weakness. They are peripherally located due to their affinity to white matter tracts and hence present with asymmetric symptoms. The average duration of symptoms is shorter for malignant astrocymoma (6 months) as compared to low-grade astrocytoma (3.5 years). They can present either with polar cysts or with intratumoral cysts. Intratumoral cysts are lined by abnormal glial cells [9].
Hemangioblastoma is a World Health Organization (WHO) grade I tumor and the most common nonglial IMSCT [11]. They are highly vascular tumors [12]. Von Hippel Lindau disease is associated with 72.5% of patients. The cervical spine is the most common site and pain is the most common symptom [13]. Small hemangioblastomas are most frequently located along the posterior aspect of the spinal cord [14]. GTR can be achieved in 93% to 99% of tumors [11]. Non-dysraphic intramedullary lipoma is very rare. Balachandar et al. presented a case of intramedullary lipoma extending from T1-T9 with distal syrinx. The surgical goal should be decompression only, to avoid neurological deterioration [15]. The authors operated 3 cases of intramedullary lipomas.
Schwannomas are benign but clinically progressive tumors. They are quite rare in the intramedullary region. Shahab et al. reported a case of D11 intramedullary schwannoma presented with a history of gait instability and numbness in bilateral lower limbs. Near total resection of the lesion was done. They noted that preoperative radiologic diagnosis is difficult and a high degree of suspicion should be present, keeping in mind one differential of intramedullary schwannoma [16]. The primary intramedullary spinal cord lymphomas present with multifocal contrast-enhancing lesions and involvement of conus medullaris or cauda equina. A biopsy of the CNS confirms the diagnosis [17]. The authors had operated 3 cases of schwannomas and one lymphoma.
Intramedullary capillary hemangiomas, arachnoid cysts, and epidermoid cysts are exceedingly rare. Total, subtotal, or even partial resection is advised for capillary hemangiomas [18]. Novegno et al., reported a case of T11-T12 dorsal intramedullary arachnoid cyst presenting with progressive paraparesis. MRI revealed a T11-T12 dorsal intramedullary arachnoid cyst. The patient was relieved of symptoms following cyst fenestration [19]. Musali et al., reported a case of D9-D12 dorsal epidermoid cyst in a 6-year-old patient. Surgical excision of the lesion was done [20]. Ependymomas can be separated from normal parenchyma rendering a GTR. Most astrocytomas are simply biopsied or decompressed. In hemangioblastoma, the pial interface surrounding the tumor is sharply incised and bipolar cautery is used to shrink the tumor bulk. Once completely detached ventrally, the tumor can be removed from the bloc [11].
In this study, the dorsal spine is the most common location of IMSCT. Syrinx formation is one of the main reasons for the patient’s symptoms. Motor weakness and pain are the most common symptoms. The extent of resection depends on the pathology of the lesion and the intraoperative decision of the surgeon. Tumors with a clear interface with the spinal cord underwent GTR. GTR was not attempted in tumors with surrounding spinal cord infiltration to decrease the morbidity. Ependymomas had well-defined resection margins when compared to other types of gliomas. Intraoperative GTR was achieved in 44.4% of patients, NTR in 14.8% of patients, STR in 7.4% of patients, decompression in 26% of patients, and biopsy in 7.4% of patients. A peculiar finding in our study is the occurrence of a greater number of rare tumors like lipoma, lymphoma, schwannoma, epidermoid cyst, and arachnoid cyst. These constituted 9 out of 27 cases. The reason may be that the authors’ work in this tertiary care referral center treats only referred cases from other centers. Common intramedullary tumors may be operated more in the peripheral centers.
5. Conclusion
The dorsal spine is the most common location of IMSCTs and ependymoma is the most common histological type. The extent of resection depends on the surgeons’ intraoperative decision based on the spinal cord infiltration and tumor type. Ependymomas have well-defined margins compared to other gliomas. GTR can be achieved in the majority of ependymomas and some other tumors like schwannoma, arachnoid cyst, and epidermoid cyst. Decreasing the morbidity and improving the quality of life should be considered during surgery on IMSCTs.
Limitations
This is a retrospective study and includes cases operated over a long period. A greater number of cases are required to obtain a better conclusion of these rare IMSCTs.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by Government Medical College (Code: GMCKKD/RP 2022/IEC/80, dated 19/09/2022). Appropriate consent was obtained from the patients whose clinical details are described in the illustrative cases.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Concept and study design: Binoy Damodar Thavara and Rajeev Mandaka Parambil; Data collection: Rajeev Mandaka Parambil, Binoy Damodar Thavara, Byjo Valiyaveetil Jose, Prem kumar Sasi, Ebby Sebastian; Data analysis and interpretation: Binoy Damodar Thavara, Byjo Valiyaveetil Jose, Prem kumar Sasi and Radhakrishnan Maniyan; Drafting the article: Prem kumar Sasi, Radhakrishnan Maniyan and Shanavas Cholakkal; Review and editing: Binoy Damodar Thavara and Rajeev Mandaka Parambil; Final approval: All authors; Supervision: Binoy Damodar Thavara.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the support of the Department of Neurosurgery, Government Medical College, Kozhikode, India.
4. Discussion
The classification of the primary IMSCTs follows that of central nervous system (CNS) tumors [7]. Ependymomas and astrocytomas are the most common types. Other tumors include hemangioblastoma, metastasis, cavernomas, dermoidal cysts, and enterogenetic cysts. The most frequent site is the cervical and cervicothoracic region [8]. IMSCTs can present as either complete cord transactions or incomplete cord transactions [9].
Spinal ependymomas are classified as ependymoma, ependymoma MCYN-amplified, subependymoma, and myxopapillary ependymoma [10]. Ependymomas are more common in the cervical region. They are solitary tumors and arise from the central canal. They are associated with syrinx in half of the cases. They present with pain and central cord syndrome. During surgery, the tumor is separable from the cord because of the presence of the plane of cleavage [9]. Syrinx was seen in 4 (36%) cases of ependymoma in the author’s study. They noted that the majority of the ependymomas were excised through a clear plane between the tumor and the cord.
Astrocytomas account for about 30% to 35% of all IMSCTs. They are the most common IMSCTs in the pediatric age group. They often present with back pain and progressive weakness. They are peripherally located due to their affinity to white matter tracts and hence present with asymmetric symptoms. The average duration of symptoms is shorter for malignant astrocymoma (6 months) as compared to low-grade astrocytoma (3.5 years). They can present either with polar cysts or with intratumoral cysts. Intratumoral cysts are lined by abnormal glial cells [9].
Hemangioblastoma is a World Health Organization (WHO) grade I tumor and the most common nonglial IMSCT [11]. They are highly vascular tumors [12]. Von Hippel Lindau disease is associated with 72.5% of patients. The cervical spine is the most common site and pain is the most common symptom [13]. Small hemangioblastomas are most frequently located along the posterior aspect of the spinal cord [14]. GTR can be achieved in 93% to 99% of tumors [11]. Non-dysraphic intramedullary lipoma is very rare. Balachandar et al. presented a case of intramedullary lipoma extending from T1-T9 with distal syrinx. The surgical goal should be decompression only, to avoid neurological deterioration [15]. The authors operated 3 cases of intramedullary lipomas.
Schwannomas are benign but clinically progressive tumors. They are quite rare in the intramedullary region. Shahab et al. reported a case of D11 intramedullary schwannoma presented with a history of gait instability and numbness in bilateral lower limbs. Near total resection of the lesion was done. They noted that preoperative radiologic diagnosis is difficult and a high degree of suspicion should be present, keeping in mind one differential of intramedullary schwannoma [16]. The primary intramedullary spinal cord lymphomas present with multifocal contrast-enhancing lesions and involvement of conus medullaris or cauda equina. A biopsy of the CNS confirms the diagnosis [17]. The authors had operated 3 cases of schwannomas and one lymphoma.
Intramedullary capillary hemangiomas, arachnoid cysts, and epidermoid cysts are exceedingly rare. Total, subtotal, or even partial resection is advised for capillary hemangiomas [18]. Novegno et al., reported a case of T11-T12 dorsal intramedullary arachnoid cyst presenting with progressive paraparesis. MRI revealed a T11-T12 dorsal intramedullary arachnoid cyst. The patient was relieved of symptoms following cyst fenestration [19]. Musali et al., reported a case of D9-D12 dorsal epidermoid cyst in a 6-year-old patient. Surgical excision of the lesion was done [20]. Ependymomas can be separated from normal parenchyma rendering a GTR. Most astrocytomas are simply biopsied or decompressed. In hemangioblastoma, the pial interface surrounding the tumor is sharply incised and bipolar cautery is used to shrink the tumor bulk. Once completely detached ventrally, the tumor can be removed from the bloc [11].
In this study, the dorsal spine is the most common location of IMSCT. Syrinx formation is one of the main reasons for the patient’s symptoms. Motor weakness and pain are the most common symptoms. The extent of resection depends on the pathology of the lesion and the intraoperative decision of the surgeon. Tumors with a clear interface with the spinal cord underwent GTR. GTR was not attempted in tumors with surrounding spinal cord infiltration to decrease the morbidity. Ependymomas had well-defined resection margins when compared to other types of gliomas. Intraoperative GTR was achieved in 44.4% of patients, NTR in 14.8% of patients, STR in 7.4% of patients, decompression in 26% of patients, and biopsy in 7.4% of patients. A peculiar finding in our study is the occurrence of a greater number of rare tumors like lipoma, lymphoma, schwannoma, epidermoid cyst, and arachnoid cyst. These constituted 9 out of 27 cases. The reason may be that the authors’ work in this tertiary care referral center treats only referred cases from other centers. Common intramedullary tumors may be operated more in the peripheral centers.
5. Conclusion
The dorsal spine is the most common location of IMSCTs and ependymoma is the most common histological type. The extent of resection depends on the surgeons’ intraoperative decision based on the spinal cord infiltration and tumor type. Ependymomas have well-defined margins compared to other gliomas. GTR can be achieved in the majority of ependymomas and some other tumors like schwannoma, arachnoid cyst, and epidermoid cyst. Decreasing the morbidity and improving the quality of life should be considered during surgery on IMSCTs.
Limitations
This is a retrospective study and includes cases operated over a long period. A greater number of cases are required to obtain a better conclusion of these rare IMSCTs.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by Government Medical College (Code: GMCKKD/RP 2022/IEC/80, dated 19/09/2022). Appropriate consent was obtained from the patients whose clinical details are described in the illustrative cases.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Concept and study design: Binoy Damodar Thavara and Rajeev Mandaka Parambil; Data collection: Rajeev Mandaka Parambil, Binoy Damodar Thavara, Byjo Valiyaveetil Jose, Prem kumar Sasi, Ebby Sebastian; Data analysis and interpretation: Binoy Damodar Thavara, Byjo Valiyaveetil Jose, Prem kumar Sasi and Radhakrishnan Maniyan; Drafting the article: Prem kumar Sasi, Radhakrishnan Maniyan and Shanavas Cholakkal; Review and editing: Binoy Damodar Thavara and Rajeev Mandaka Parambil; Final approval: All authors; Supervision: Binoy Damodar Thavara.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the support of the Department of Neurosurgery, Government Medical College, Kozhikode, India.
References
- Kandemirli SG, Reddy A, Hitchon P, Saini J, Bathla G. Intramedullary tumors and tumor mimics. Clinical Radiology. 2020; 75(11):876.e17-32. [DOI:10.1016/j.crad.2020.05.010] [PMID]
- Waguia R, Wang TY, Mehta VA, Ramirez L, McCray E, Pennington Z, et al. Risk factors for prolonged length of stay in patients undergoing surgery for intramedullary spinal cord tumors. Journal of Clinical Neuroscience. 2021; 91:396-401. [DOI:10.1016/j.jocn.2021.06.046] [PMID]
- Bansal S, Ailawadhi P, Suri A, Kale SS, Sarat Chandra P, Singh M, et al. Ten years’ experience in the management of spinal intramedullary tumors in a single institution. Journal of Clinical Neuroscience. 2013; 20(2):292-8. [DOI:10.1016/j.jocn.2012.01.056] [PMID]
- Wu J, Wu Y, Xu WL, Li GY.The surgical treatment of intramedullary spinal cord tumors: A retrospective analysis of 76 patients. CNS Neuroscience & Therapeutics. 2018; 24(6):575-8. [DOI:10.1111/cns.12819] [PMID]
- Tomura N. [Imaging of tumors of the spine and spinal cord (Japanese)]. Nippon Acta Radiologica. 2000; 60(6):302-11. [PMID]
- Cosnard G, Duprez T, Grandin C, Hernalsteen D. Moelle épinière tumorale et pseudo-tumorale [Intramedullary tumours and pseudotumours (French)]]. Journal De Radiologie. 2010; 91(9 Pt 2):988-97. [DOI:10.1016/S0221-0363(10)70144-X] [PMID]
- Park SH, Won JK, Kim CH, Phi JH, Kim SK, Choi SH, et al. Pathological classification of the intramedullary spinal cord tumors according to 2021 World Health Organization Classification of Central Nervous System Tumors, a Single-Institute Experience. Neurospine. 2022; 19(3):780-91. [DOI:10.14245/ns.2244196.098] [PMID]
- Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: Experience with 78 patients. Spinal Cord. 2005; 43(1):34-41. [DOI:10.1038/sj.sc.3101668] [PMID]
- Mechtler LL, Nandigam K. Spinal cord tumors: New views and future directions. Neurologic Clinics. 2013; 31(1):241-68. [DOI:10.1016/j.ncl.2012.09.011] [PMID]
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021; 23(8):1231-51. [DOI:10.1093/neuonc/noab106] [PMID]
- Hussain I, Parker WE, Barzilai O, Bilsky MH. Surgical management of intramedullary spinal cord tumors. Neurosurgery Clinics of North America. 2020; 31(2):237-49. [DOI:10.1016/j.nec.2019.12.004] [PMID]
- Mandigo CE, Ogden AT, Angevine PD, McCormick PC. Operative management of spinal hemangioblastoma. Neurosurgery. 2009; 65(6):1166-77. [DOI:10.1227/01.NEU.0000359306.74674.C4] [PMID]
- David P, Messerer M, Aghakhani N, Benoudiba F, Adam C, Richard S, et al. [Intramedullary hemangioblastomas (French)]. Neurochirurgie. 2017; 63(5):366-71. [DOI:10.1016/j.neuchi.2015.11.001] [PMID]
- Chu BC, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in spinal hemangioblastoma: Correlation with symptoms and with angiographic and surgical findings. AJNR. American Journal of Neuroradiology. 2001; 22(1):206-17. [PMID]
- Balachandar D, Naidu PB, Sangeetha, Selvakumar K. Non Dysraphic intramedullary spinal cord lipoma. Neurology India. 2022; 70(Supplement):S302-5. [DOI:10.4103/0028-3886.360941] [PMID]
- Shahab FB, Khan SA. Intramedullary schwannoma - A case report. Surgical Neurology International. 2022; 13:535. [DOI:10.25259/SNI_907_2022] [PMID]
- Flanagan EP, O’Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology. 2011; 77(8):784-91. [DOI:10.1212/WNL.0b013e31822b00b9] [PMID]
- Wu L, Deng X, Yang C, Xu Y. Intramedullary spinal capillary hemangiomas: Clinical features and surgical outcomes: Clinical article. Journal of Neurosurgery. Spine. 2013; 19(4):477-84. [DOI:10.3171/2013.7.SPINE1369] [PMID]
- Novegno F, Umana G, Di Muro L, Fraioli B, Fraioli MF. Spinal intramedullary arachnoid cyst: Case report and literature review. The Spine Journal. 2014; 14(6):e9-15. [DOI:10.1016/j.spinee.2013.10.051] [PMID]
- Musali SR, Mohammed I, Gollapudi PR, Maley SK. Dorsal spinal intradural intramedullary epidermoid cyst: A rare case report and review of literature. Journal of Neurosciences in Rural Practice. 2019; 10(2):352-4. [DOI:10.4103/jnrp.jnrp_304_18] [PMID]
Type of Study: Research |
Subject:
Neuroscience
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |