Wed, Jan 28, 2026
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Barde N, VV R C, Kulkarni D, Chawada M. A Comparative Study on Therapeutic Modalities of
Spontaneous Supratentorial Intracerebral Hemorrhage
at a Teaching Institute. Iran J Neurosurg 2025; 11 : 2
URL: http://irjns.org/article-1-383-en.html
URL: http://irjns.org/article-1-383-en.html
1- Department of Neurosurgery, Vilasrao Deshmukh Government Medical College, Latur Superspeciality Hospital, Latur, India.
2- Department of Neurosurgery, Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati, India.
3- Department of Neurosurgery, Vilasrao Deshmukh Government Medical College, Latur Superspeciality Hospital, Latur, India. ,devdk.kulkarni@gmail.com
2- Department of Neurosurgery, Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati, India.
3- Department of Neurosurgery, Vilasrao Deshmukh Government Medical College, Latur Superspeciality Hospital, Latur, India. ,
Keywords: Supratentorial, Intracerebral
hemorrhage, Glasgow coma
scale, Glasgow outcome
scale (GOS), Decompressive
craniectomy (DC)
Full Text [PDF 2002 kb]
(676 Downloads)
| Abstract (HTML) (2795 Views)
Full Text: (367 Views)
1. Introduction
Spontaneous supratentorial intracerebral hemorrhage (SICH) accounts for 10%-15% of all strokes [1]. Intracerebral hemorrhage (ICH) appears to be more common in Eastern countries, accounting for up to 30% of strokes, with an overall mortality rate of 40% to 50% [2, 3]. The treatment of patients with ICH is complex in many ways. Apart from standard medical treatments, no novel therapies have been introduced to improve outcomes. Despite preventing hematoma enlargement, recombinant activated factor VII treatment showed no beneficial effect on outcome in a randomized placebo-controlled clinical trial [4]. Surgery has the potential to reduce the volume of intracerebral hematomas. Clinical and experimental evidence suggests that mass removal may reduce damage to nervous tissue. There are also wide variations in surgical practices among countries. Trials in specific populations of ICH patients have focused on hematoma evacuation alone, such as the International Surgical Trial in ICH (STICH) [5]. This landmark trial demonstrated that emergent surgical hematoma evacuation of superficial lobar hemorrhage s within 72 h of onset failed to improve outcomes compared with standard medical management. The results of the International Surgical Trial in ICH (STICH)-II demonstrated that early surgery did not improve the rate of death or disability at 6 months but suggested a slight survival advantage for patients with ICH who did not have intraventricular hemorrhage (IVH) or hydrocephalus [6].
We aimed to study prognostic factors and immediate outcomes in surgical and medical treatment groups of patients with SICH because these health data are vital for individualized stroke management protocols, as no universal recommendation exists. In lower middle-income countries (LMIC), there is wide variation in healthcare infrastructure and availability of timely healthcare for emergencies, such as brain stroke. Hence, identifying definite prognostic factors, designing and implementing policies of stroke management, making prompt and accurate diagnoses, stratifying patients according to outcome predictors, and thus ensuring prompt referral of deserving critical patients to tertiary centers for intensive management may be the need of the hour as we wait to embrace the newer advances into our management protocol. This study aimed to compare the various prognostic factors in bothmedical treatment and surgical treatment groups. The immediate outcomes of the best medical management and surgery treatment groups were also estimated.
2. Methods and Materials/Patients
The study was conducted after approval by the Institutional Scientific Committee and Institutional Ethical Committee (AS/11/IEC/SVIMS/2017 vide IEC No 762). Written informed consent was obtained from all patients before the study. All patients diagnosed with spontaneous SICH who presented to the Department of Neurology and Neurosurgery at Tertiary Medical College and met the inclusion criteria between May 2018 and November 2019 were included in the study.
Our study is a prospective observational study, and the study population is mentioned below.
Inclusion criteria
1. Diagnosed spontaneous SICH on plain computed tomography (CT) brain scans
2. Age range of 18 to 80 years
3. Supratentorial ICH
Exclusion criteria
1. Age <18 and >80 years of age
2. ICH secondary to trauma, tumor, arterial venous malformation, aneurysm rupture, and cerebral sinuous venous thrombosis
3. Cerebellar hematoma
4. Unwillingness to participate in the study
Data collection
Detailed clinical history was recorded, including demographic data, presenting complaints, history of present illness, personal history, and drug history. All participants underwent detailed physical and neurological examinations. Glasgow coma scale (GCS) score, ICH score were also recorded.
The selected patients performed all necessary blood examinations, and the functional outcome of surgical and medical treatment was measured using the Glasgow outcome scale (GOS) at 3 months.
Medical treatment
The appropriate feasible medical treatment was administered according to American Heart Association/American Stroke Association guidelines. In conscious patients, a systolic blood pressure of 160 mm Hg was used. Patients with a GCS ≤8 are ventilated and sedated. Management of increased intracranial pressure included cerebrospinal fluid drainage using an external ventricular drain (EVD), neuromuscular blockade, and sedation. Anticoagulant treatment was stopped and reversed with clotting factors, vitamin K, and protamine. Intermittent pneumatic compression was used to prevent venous thrombosis, and after 36 hours, low-dose fractionated heparin was used.
Surgical treatment
The surgical approach was individualized based on the site and size of the ICH due to the lack of standardized guidelines for the allocation of operative treatment. The allowed techniques included open decompressive craniectomy (DC) and hematoma evacuation. Surgical treatment intends to remove the clot completely. Surgery was performed in patients with impending cerebral herniation, as indicated by abnormal pupil response, abnormal posture, or CT findings of absence of ambient cistern or severe midline shifting (>6 mm). Patients with IVH with hydrocephalus underwent EVD insertion.
Outcomes
All prognostic factors were measured in both treatment groups. Their immediate outcomes at 3 months were measured using GOS.
Statistical analysis
The data were recorded on a predesigned performance, managed using Microsoft Excel 2007 (Microsoft Corp, Redmond, WA), and analyzed using SPSS software, version 20. Descriptive statistics, such as percentages and frequencies, and inferential statistics, such as the chi-square test, were used during the analysis. The primary analysis was categorical frequency comparison using the chi-square test for prognosis based on favorable and unfavorable outcomes.
3. Results
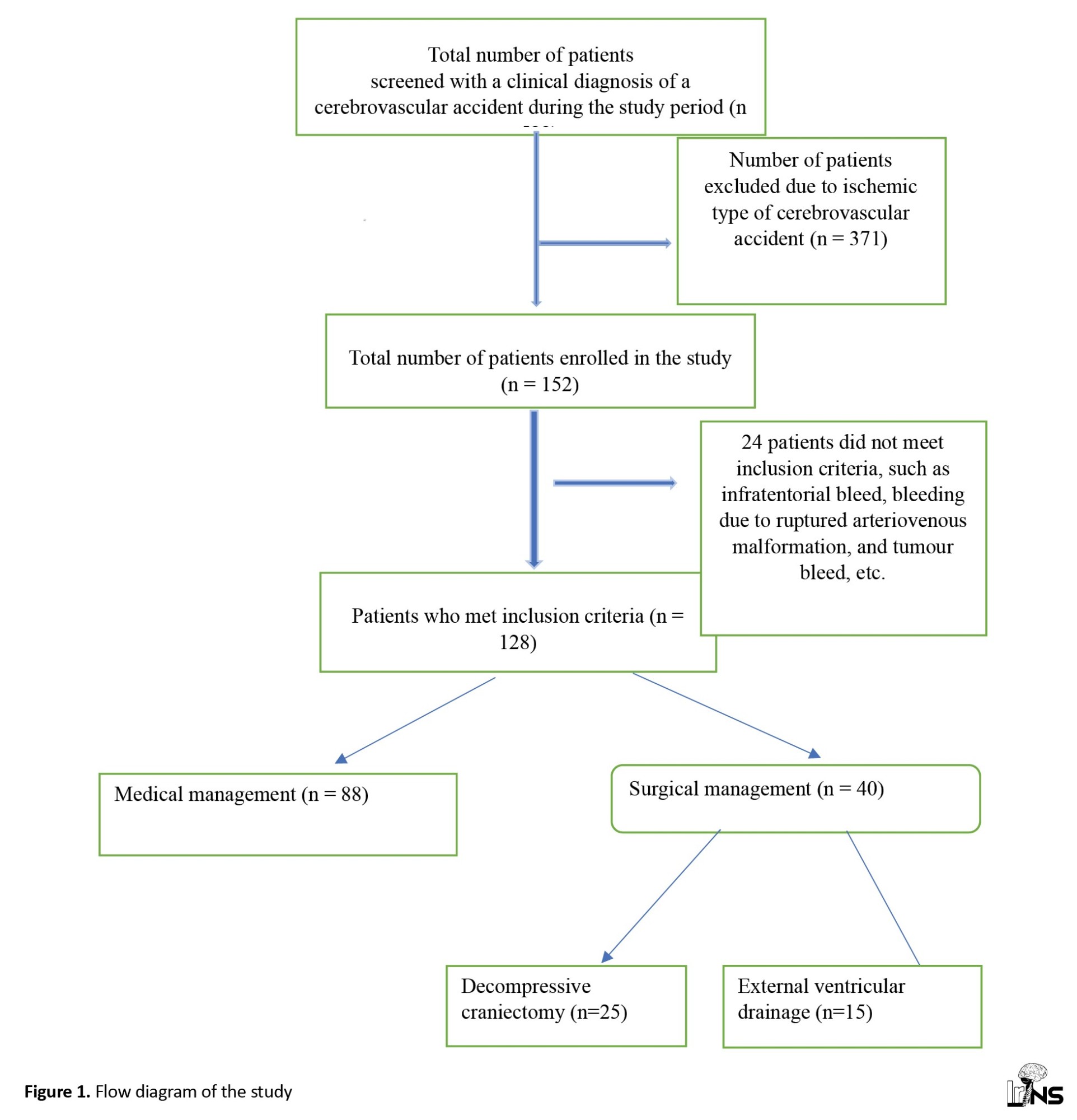
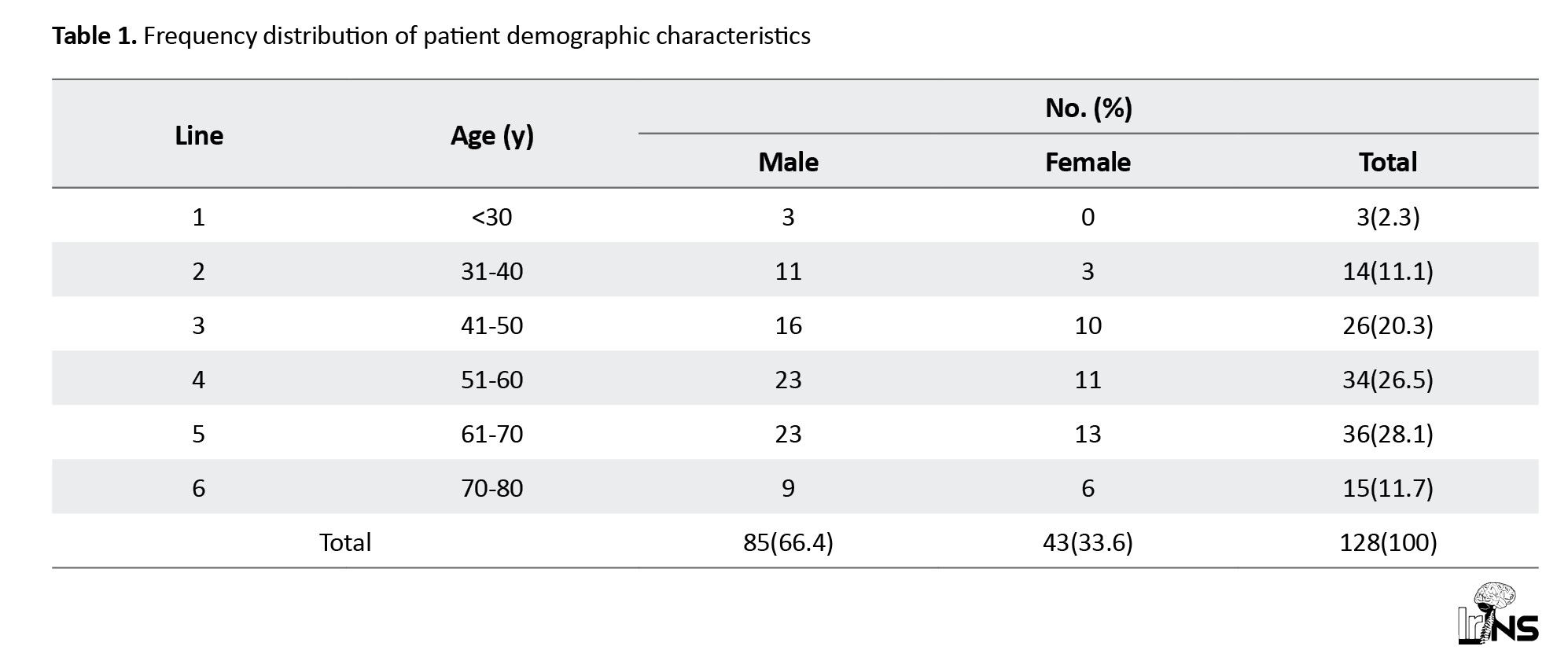
In this prospective observational study, 523 patients with features suggestive of cerebrovascular accidents were screened and subjected to brain CT. A total of 152 patients with spontaneous SICH were identified, of whom 24 were excluded because they did not meet the inclusion criteria. We studied 125 patients with spontaneous SICH, and the data were stratified into two groups based on treatment to evaluate the outcome. Among the 128 patients, 88 underwent medical treatment, 40 underwent surgical management, and among surgery, 25 underwent DC, and 15 underwent (EVD) (Figure 1). The mean age of the medical group was 57±13 years, while the mean age of the surgical group was 53.7±12 years. Our study included 85 male (66.4%) and 43 female patients (33.6%) (Table 1).
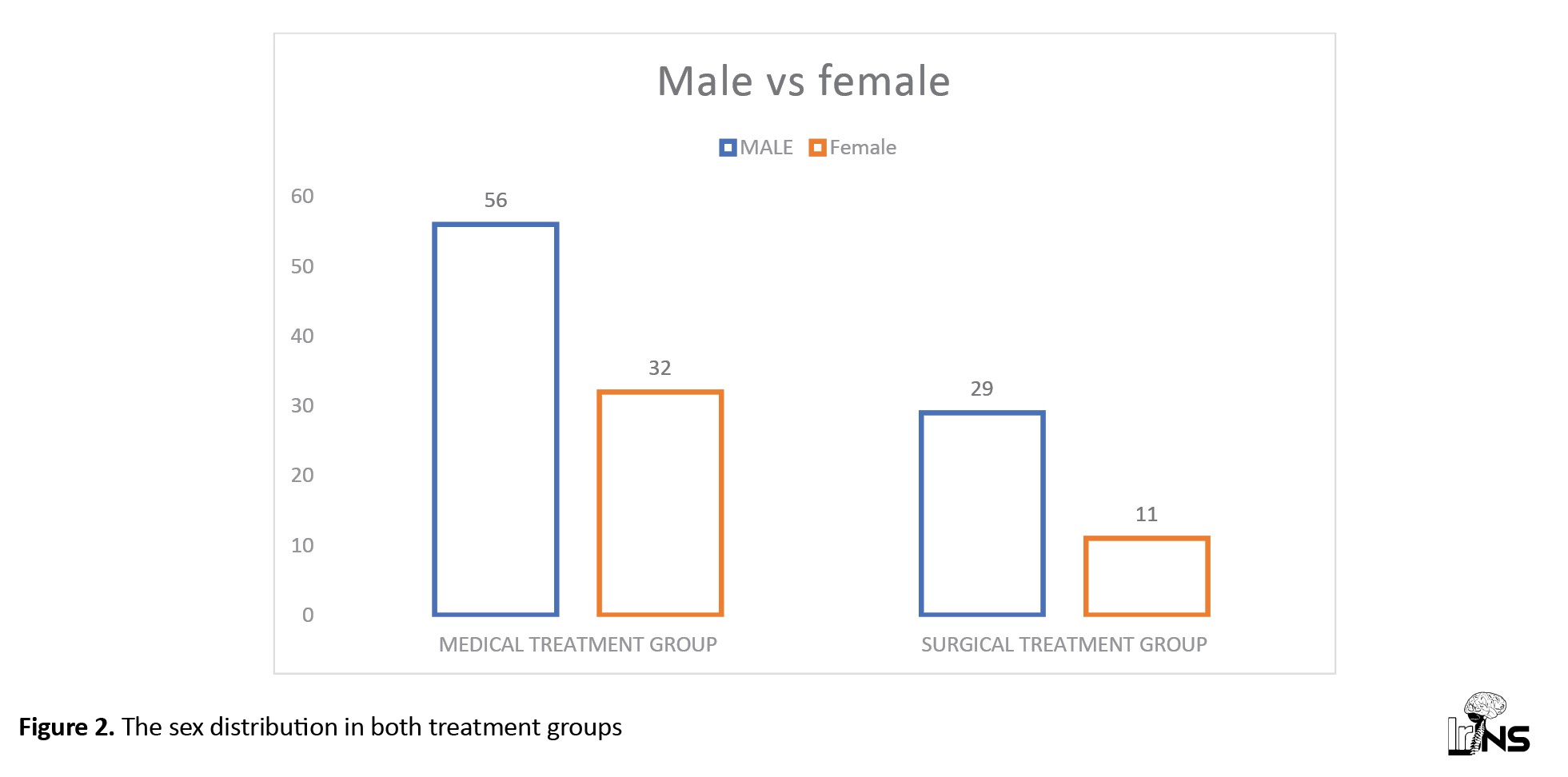
Male/female ratio (M:F) in medical group was 1.6:1; in surgical group, it was 2.6:1 and in total, M:F ratio was 1.9:1 (Figure 2).
The laterality and location of ICH
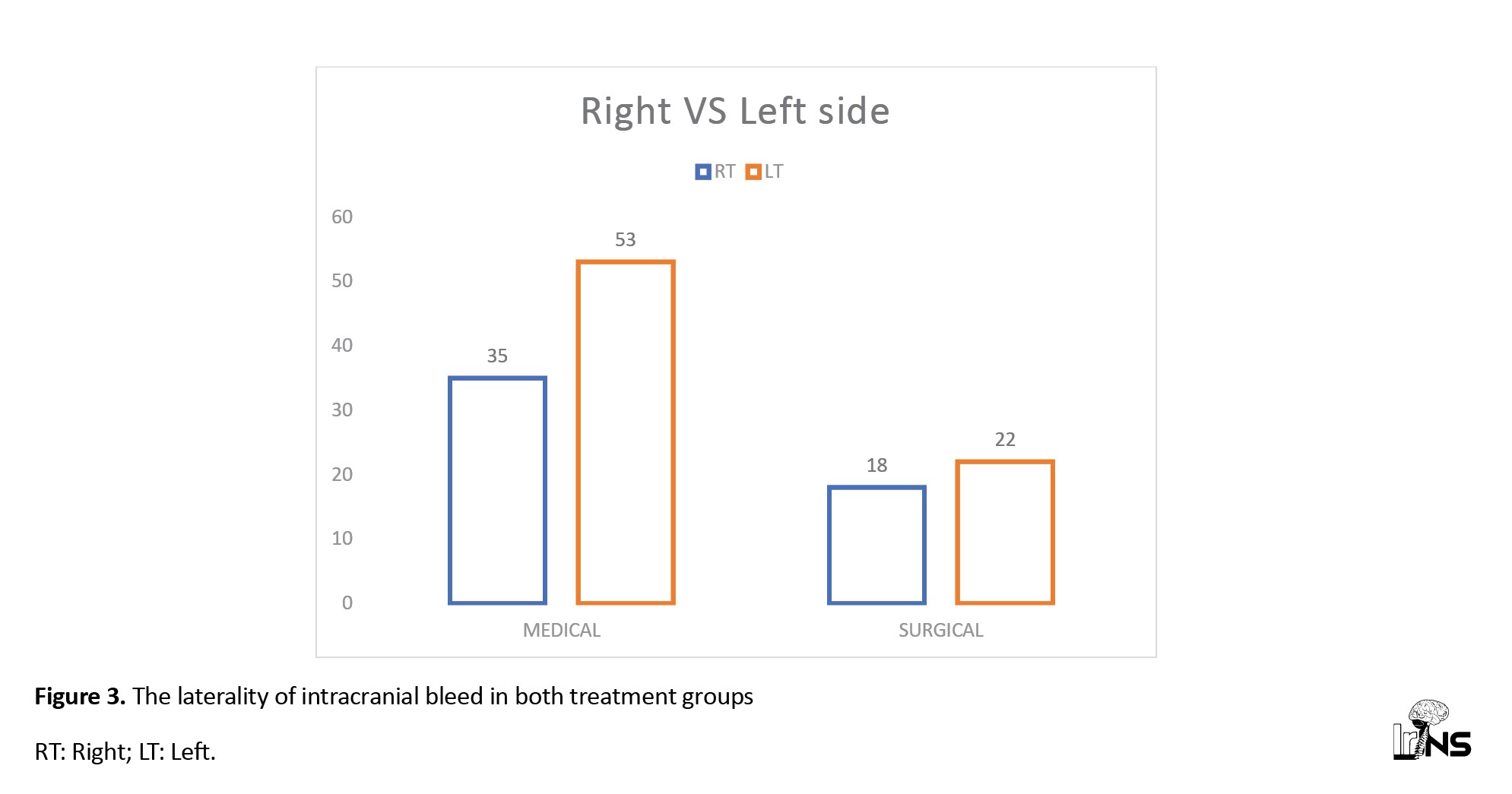
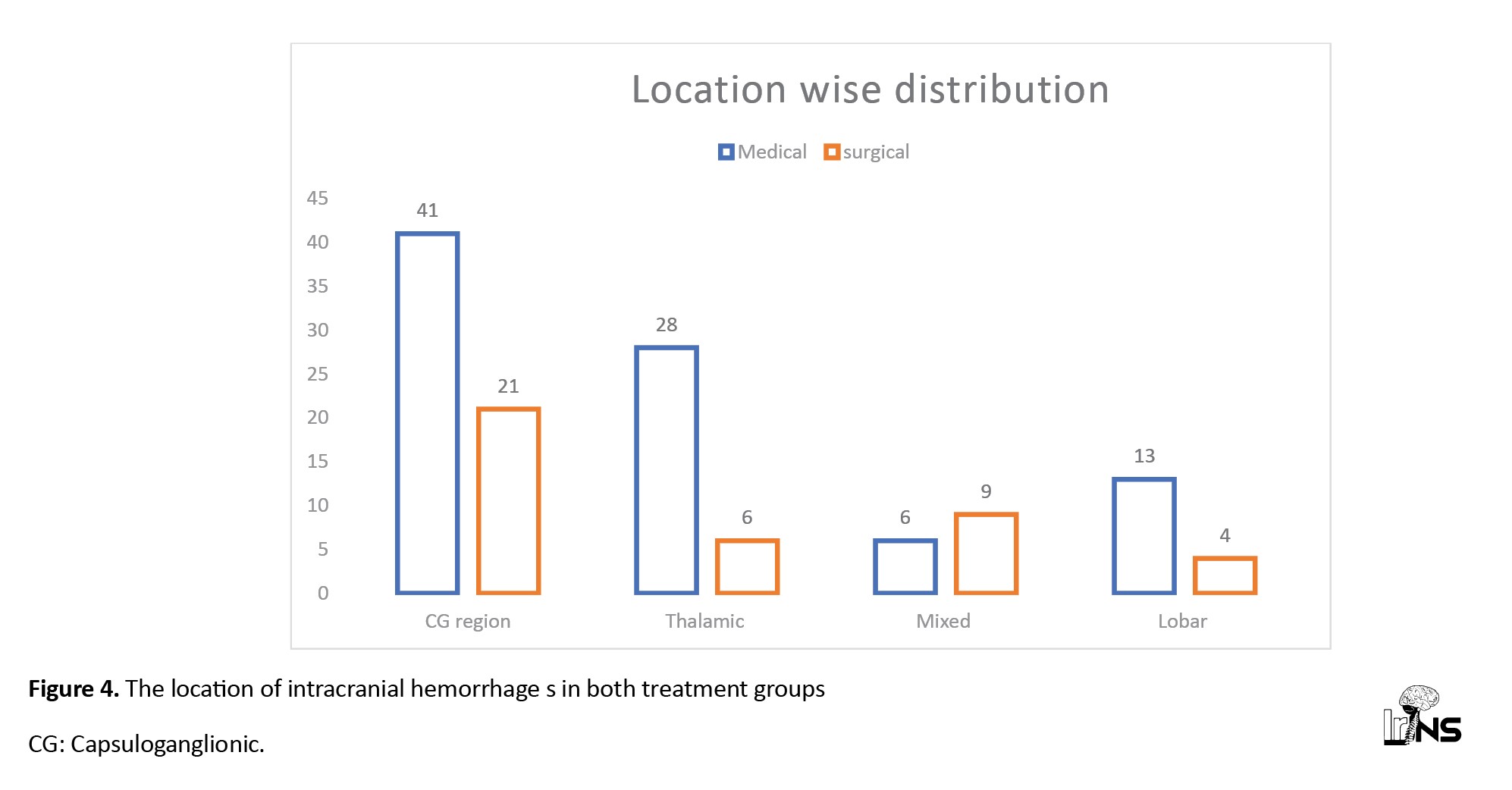
In the medical treatment group, 53 patients (60%) had left-sided bleeding, and the rest, 35 patients (40%), had right-sided bleeding, while in the surgical group, 22 patients had left-sided bleeding (55%), and 18 patient (45%) had right-sided bleeding. In both treatment groups, the bleeding locations were almost similar; capsuloganglionic region (CG) bleeding was 50% in each group, followed by 30% to 35 % thalamic region bleeding. The lobar location of bleeding was 15% and 10% in the medical group and surgical group, respectively. In the surgical treatment group, 23% of patients had bleeding in the mixed parts of the cerebrum, whereas mixed bleeding cases were less common in the medical treatment group (7%) (Figures 3 and 4).
Clinical profile
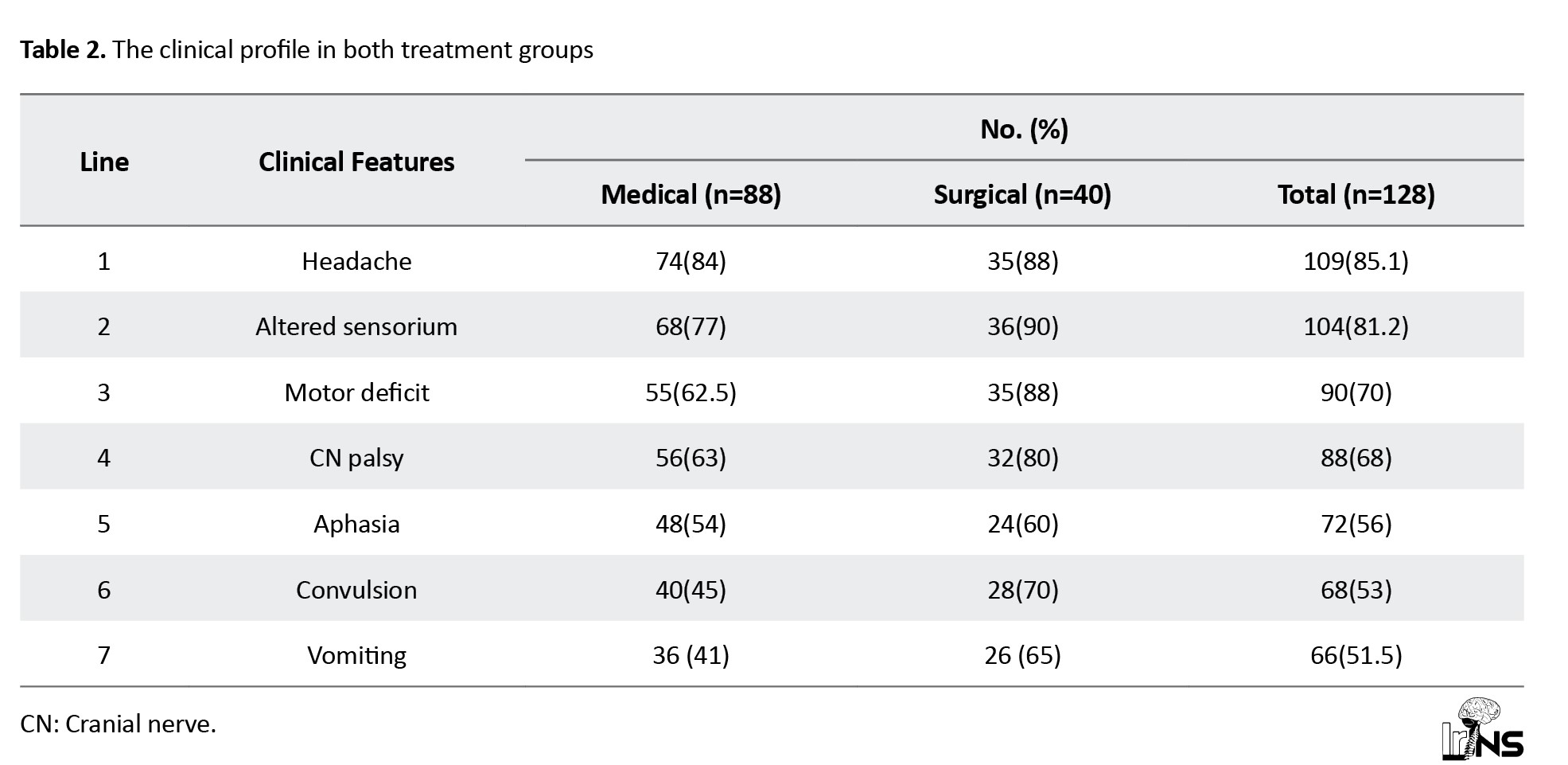
The range of the clinical spectrum was similar between groups. In our study of 128 patients, 109(85.1%) had headache, 104(81.2%) had altered sensorium, 88(68%) had cranial nerve palsy, 90(70%) had motor deficit, 72(56%) had aphasia, 68(53%) had convulsions, and 66(51.5%) had vomiting (Table 2).
The risk factors with their immediate outcomes
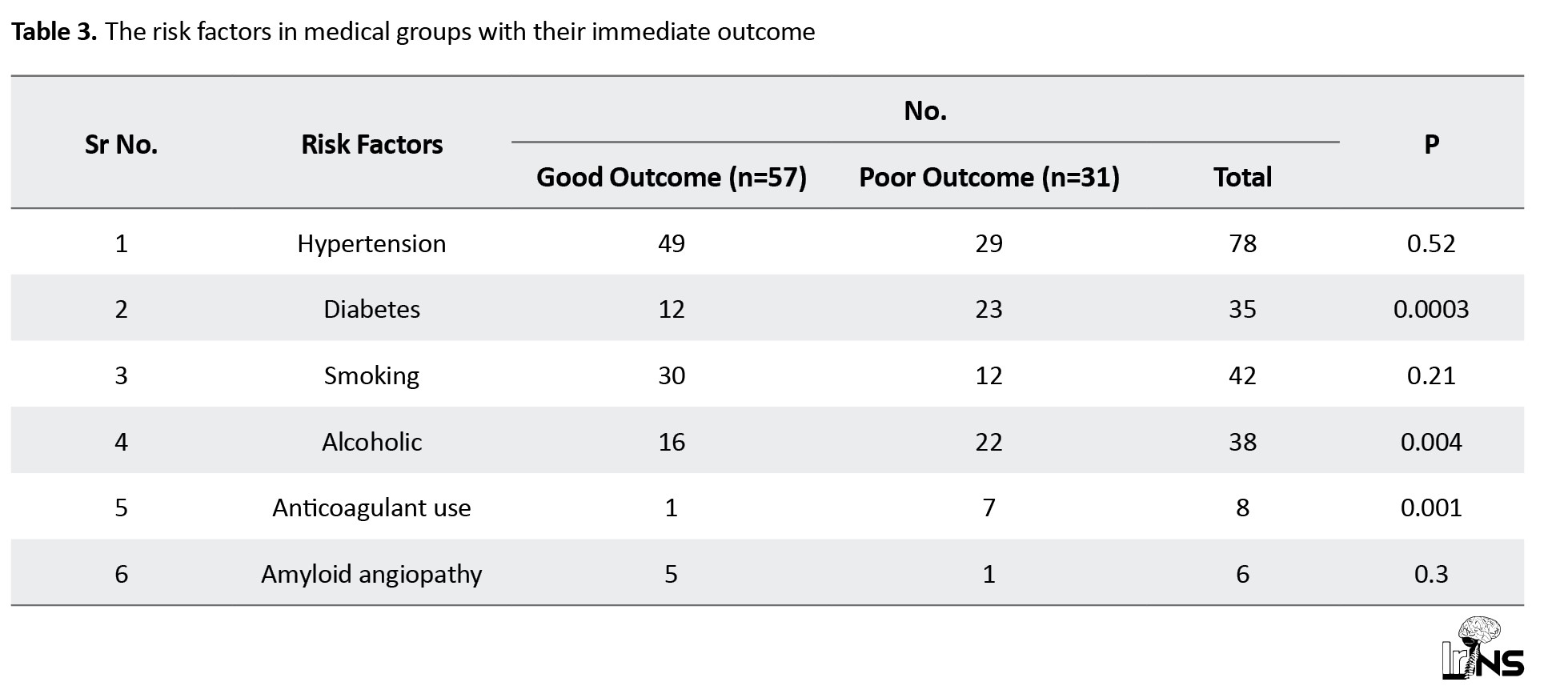
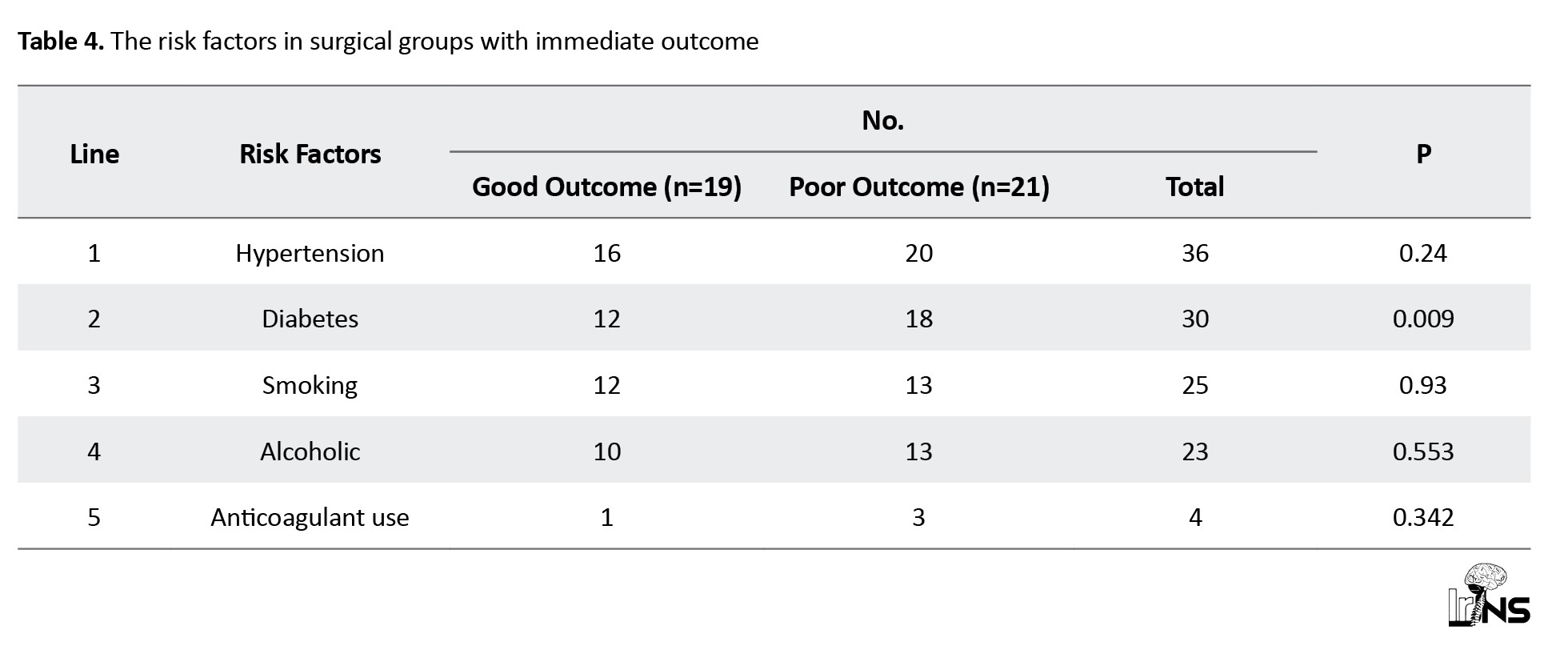
In the medical group, the most common risk factor was hypertension in 78 patients (88%), diabetes in 35 patients (40%), smoking in 42 patients (47%), and alcohol in 38 patients (43%). In the surgical group, hypertension was the most common risk factor observed in 36 patients (90%), diabetes in 30 patients (75%), and alcohol and smoking in 25 patients (62%) and 23 patients (57%), respectively (Tables 3 and 4).
GCS range
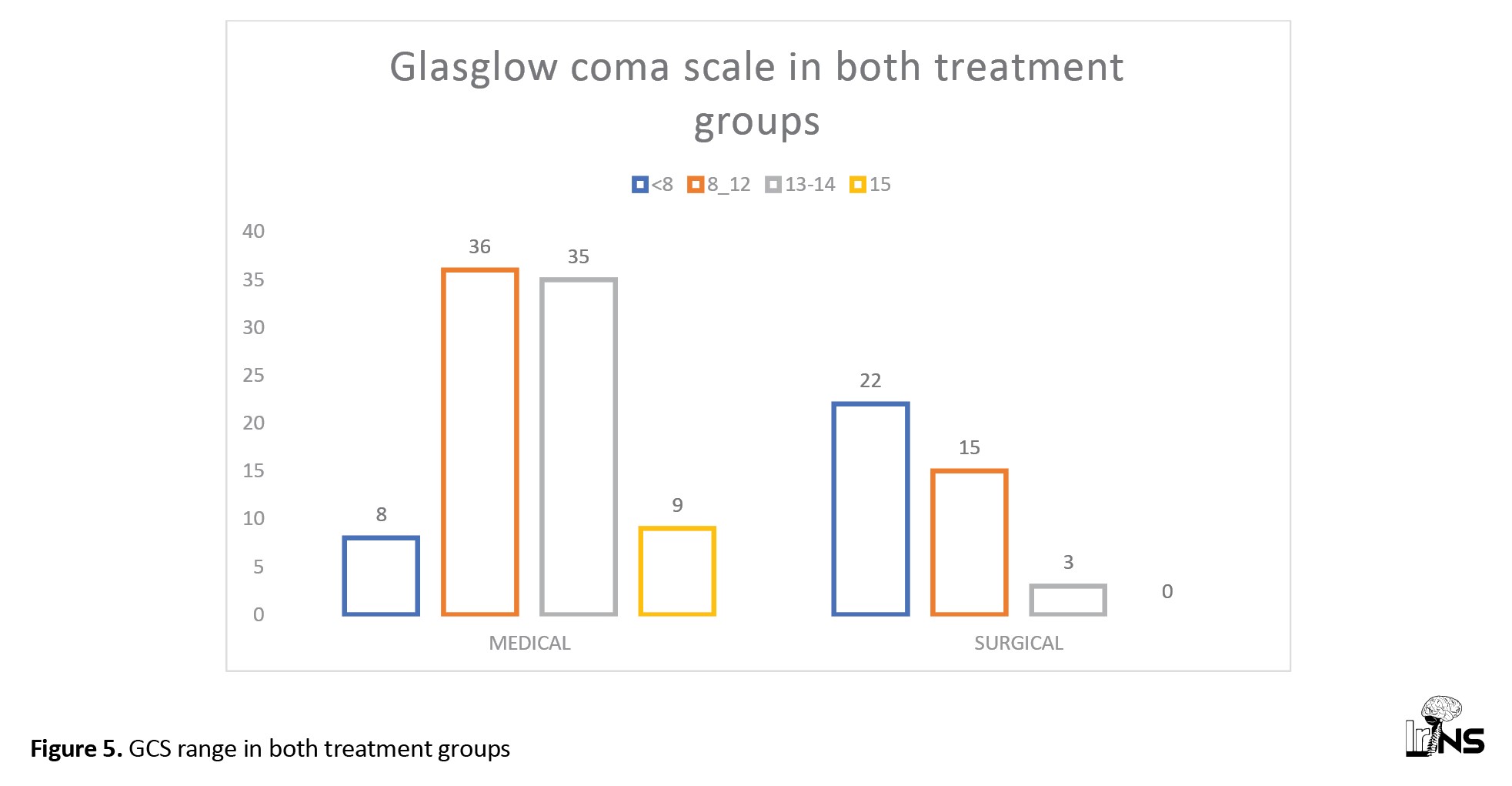
In the medical group, GCS scores were normal or mild derangement in 50% (44 patients) of SICH cases, moderate derangement in 40% (36 patients), and severe derangement (<8) in 9% of cases. In the surgical group, 37 patients (92.5%) had moderate to severe derangement in scores, among which 55% (22 cases) had GCS <8 and 38% (15 cases) had GCS 8-12. In the surgical group, the GCS score was normal or mildly deranged in only three patients (7.5%) (Figure 5).
The outcomes
In a study of 128 patients, 76 patients (59.3%) had good outcomes, and 52 patients (40.7%) had poor outcomes, 57 patients (64.7%) had good outcomes, and 31 patients (35.3%) had poor outcomes. We observed mortality rates of 25% (22 deaths) in the medical group and 30% (12 deaths) in the surgical group until 3 months.
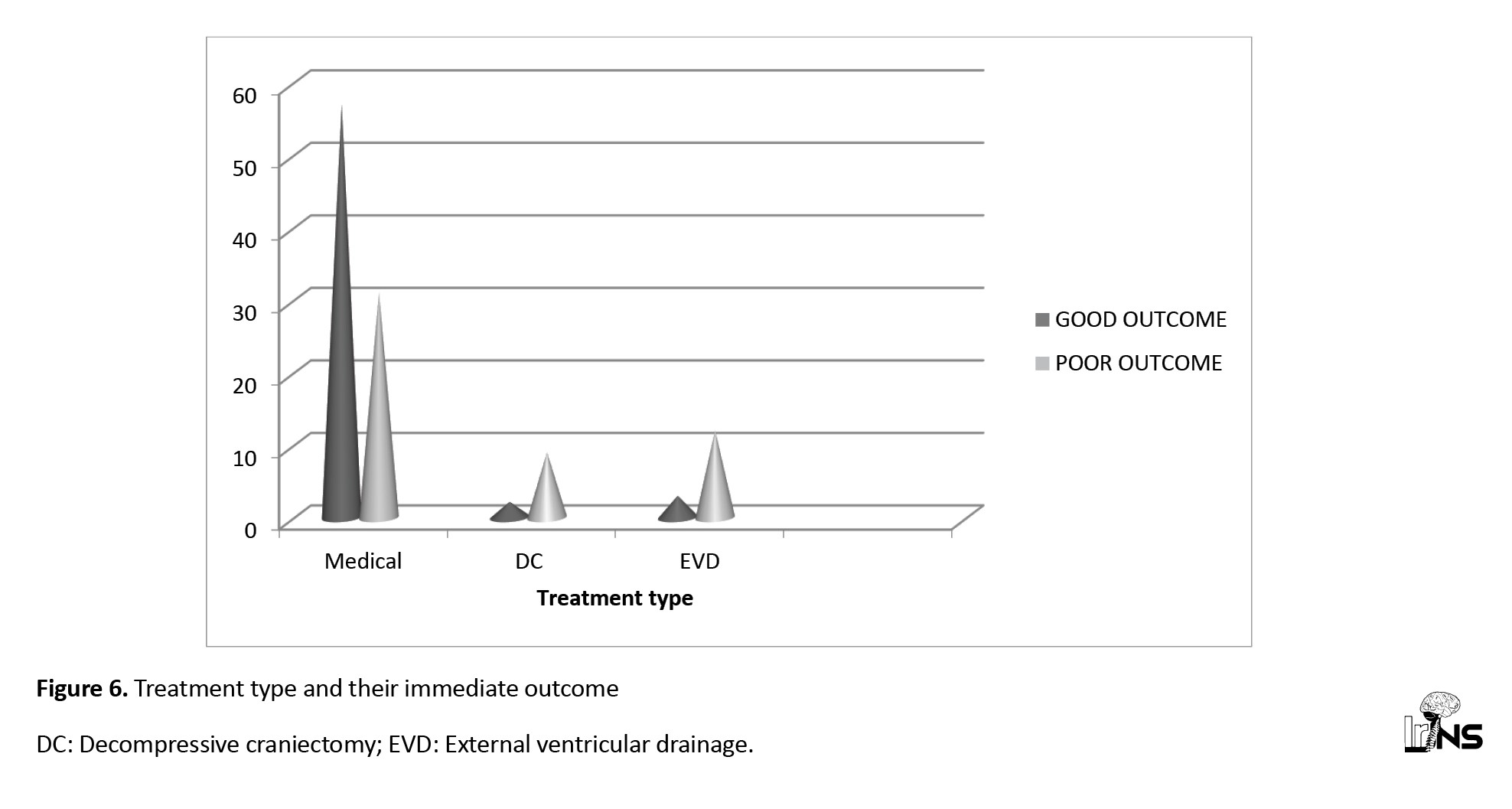
In the surgical group of 40 ICH cases, 19 patients (47.5%) had good outcomes, and 21 patients (52.5%) had poor outcomes; among the surgical group, 25 patients underwent DC, of whom 16(64%) had good outcomes, and 9 patients (36%) had poor outcomes; 15 patients underwent EVD, of whom only three patients had good outcomes, and 12 patients had poor outcomes (Figure 6).
The comparison of prognostic factors
Midline shift >6 mm
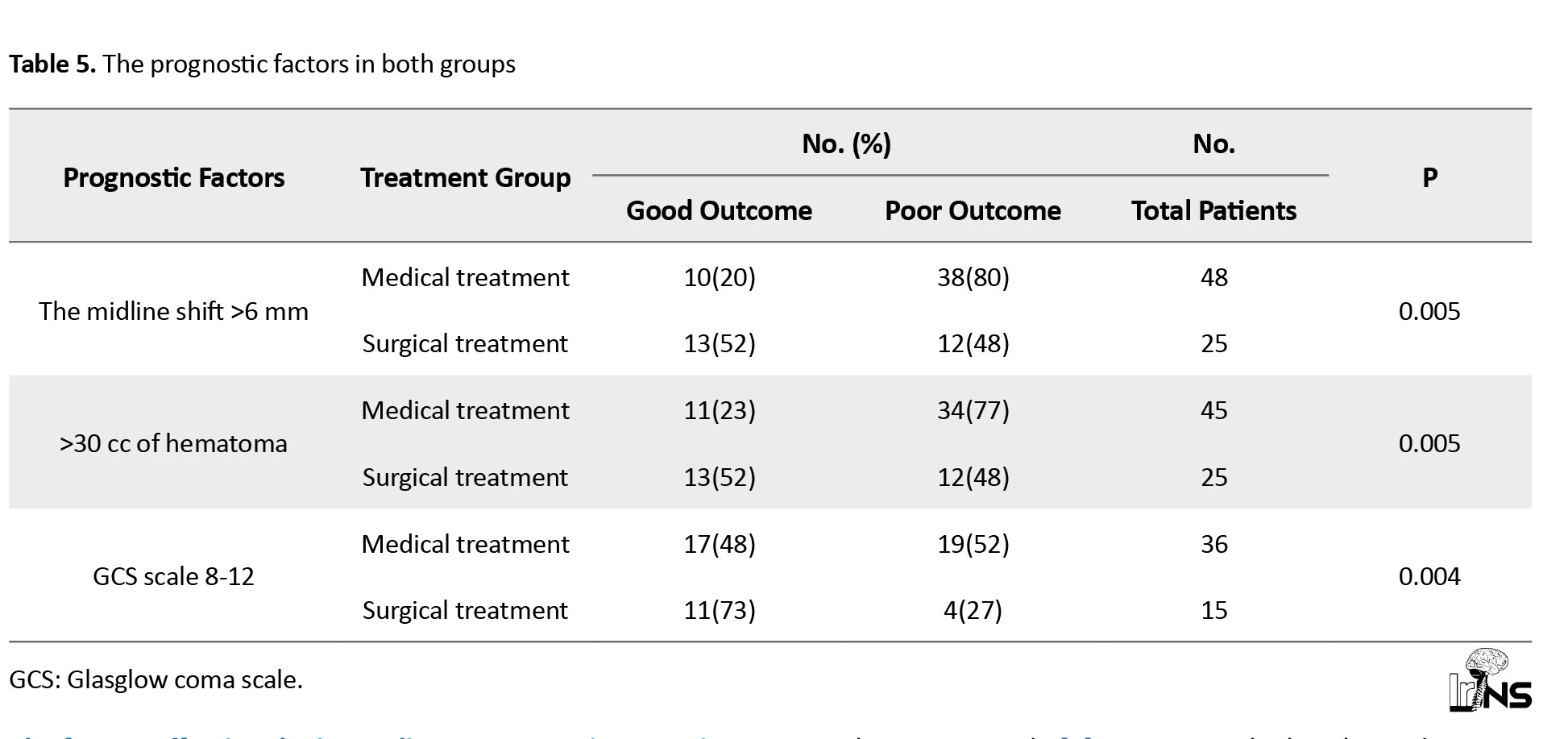
In our study, 48 patients with midline shifts >6 mm were not eligible for surgical interventions; therefore, among 48 patients, 80% had poor, and 20% had good outcomes. In the surgical group, 52% of patients had good outcomes, and 48% had poor outcomes (P<0.005) (Table 5).
>30 cc size of hematoma
In our study, 45 patients with >30 cc hematoma were not included in the surgical intervention. Among 45 patients, 77% had poor and 23% had good outcomes. In the surgical group, 52% of patients had good outcomes, and 48% had poor outcomes (P<0.005) (Table 5).
GCS 8-12
A total of 51 patients had GCS 8-12; 36 patients did not present for surgical interventions; among 36 patients, 52% had poor outcomes, and 48% had good outcomes. A total of 15 patients underwent surgical intervention; among them, 11 patients (73%) had good outcomes, and 4(27%) had poor outcomes. Among the surgical group, 73% of patients had a good outcome, and 27% had a poor outcome (P<0.005) (Table 5).
The effect of the prognostic scores and sign-in each treatment group on immediate outcome
ICH score
a) Medical group: ICH score was used to predict the outcome of ICH. In our study, in the medical group, the ICH score was 1 in 25 patients, of whom 23 had a good outcome and 2 had a poor outcome. An ICH score of 2 was observed in 45 patients, of whom 28 had a good outcome, and 17 had a poor outcome. In 18 patients, the ICH score was three among 18 patients; 12 patients had a poor outcome, and six patients had a good outcome.
b) Surgical group: In the surgical group, 18 of 18 patients had an ICH score of 2. Twelve patients had a good outcome, and 6 had a poor outcome. In 22 patients, the ICH score was 3. Among the 22 patients, 15 had poor outcomes, and only 7 had good outcomes.
GCS score
The GCS scores of patients on admission GCS was calculated, and patients were grouped into four groups: Patients with GCS <8, 8-12, 13-14 and 15.
a) Medical group: In our study, among 88 medically treated patients, eight had GCS <8, 36 had GCS 8-12, 35 had GCS 13-14, and 9 had GCS 15. Eight patients had a GCS <8, among which seven had poor outcomes and one had a good outcome. Among 36 patients in groups 8-12, 17 had good outcomes and 19 had poor outcomes. In the GCS group 13-14, 35 patients were included, among which 32 had good outcomes and only three had poor outcomes.
b) Surgical group: In our study, among 40 patients in the surgical group, 22 patients had GCS <8, 15 patients in the 8-12 groups, and three patients had GCS 13-14.
Among patients with GCS <8, 16 had poor outcomes, and 6 had good outcomes. In the 8-12 groups, 11 patients had good outcomes, and 4 patients had poor outcomes.
SICH with IVH
a) Medical group: A total of 28 cases were found to be associated with IVH, of whom 71% had poor outcomes and the remaining 29% had good outcomes. The medical group without IVH had good outcomes in 82% of cases.
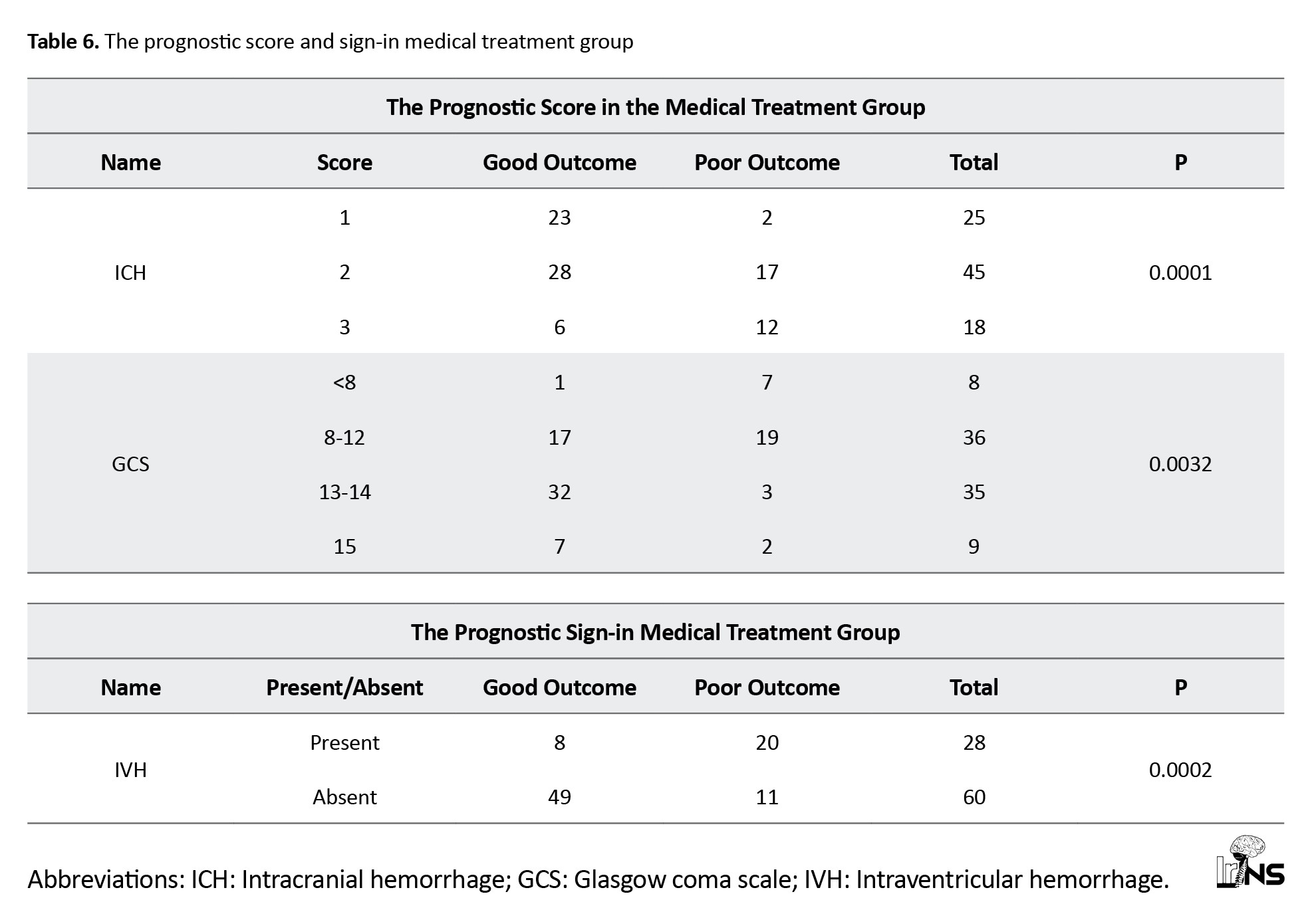
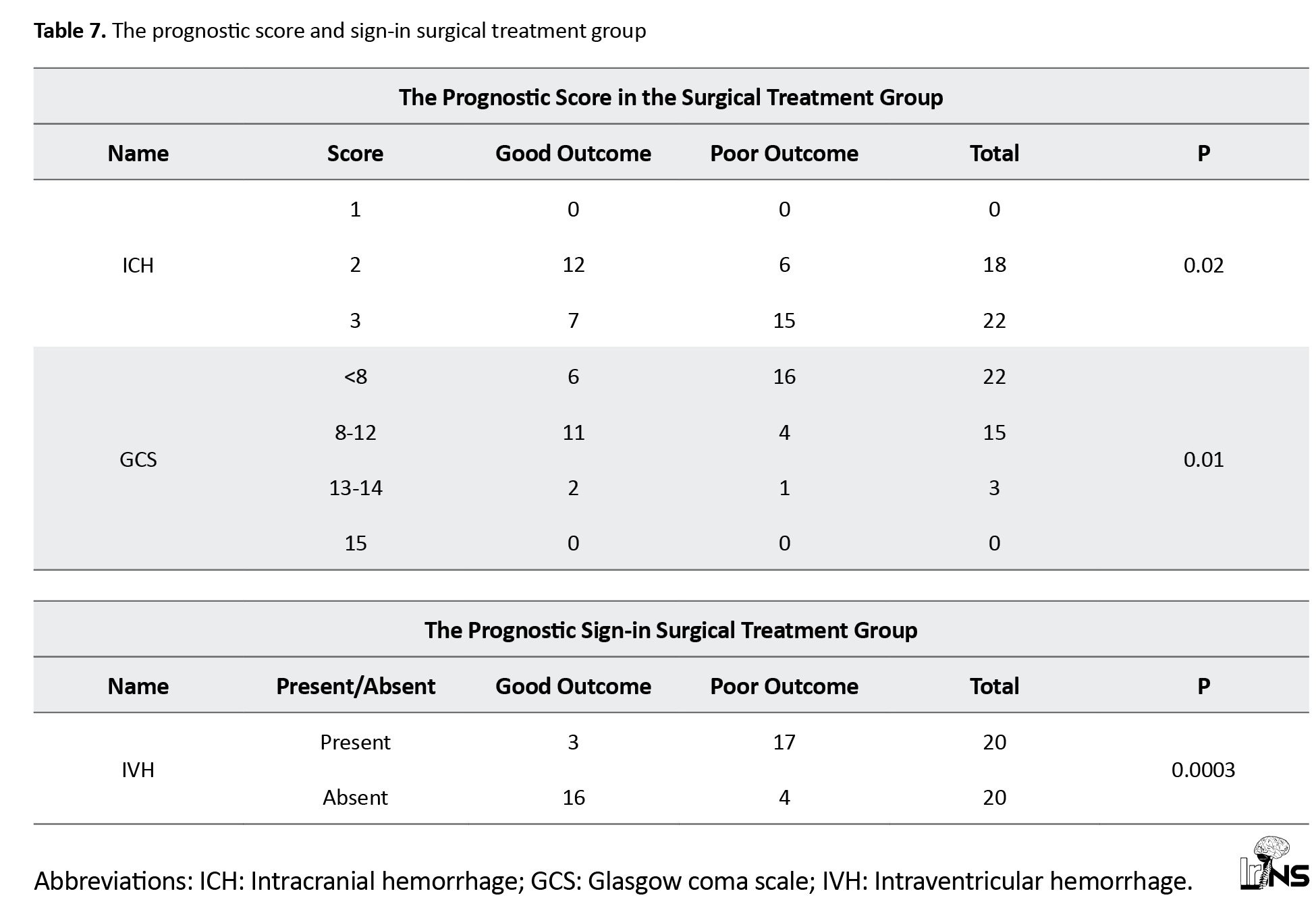
b) Surgical group: In the surgical group, 20 cases had IVH. The surgical group with IVH had 85% poor outcomes and 15% good outcomes, while the surgical group without IVH had good outcomes in 80% of cases (Tables 6 and 7).
The comparison of the health complications
a) Medical group: The common complications in the surgical group were respiratory infection (39%), hyponatremia (39%), urinary tract infection (UTI): 34%, ventilator-associated pneumonia (VAP): 20%, and 2% cases observed to have rebleeding.
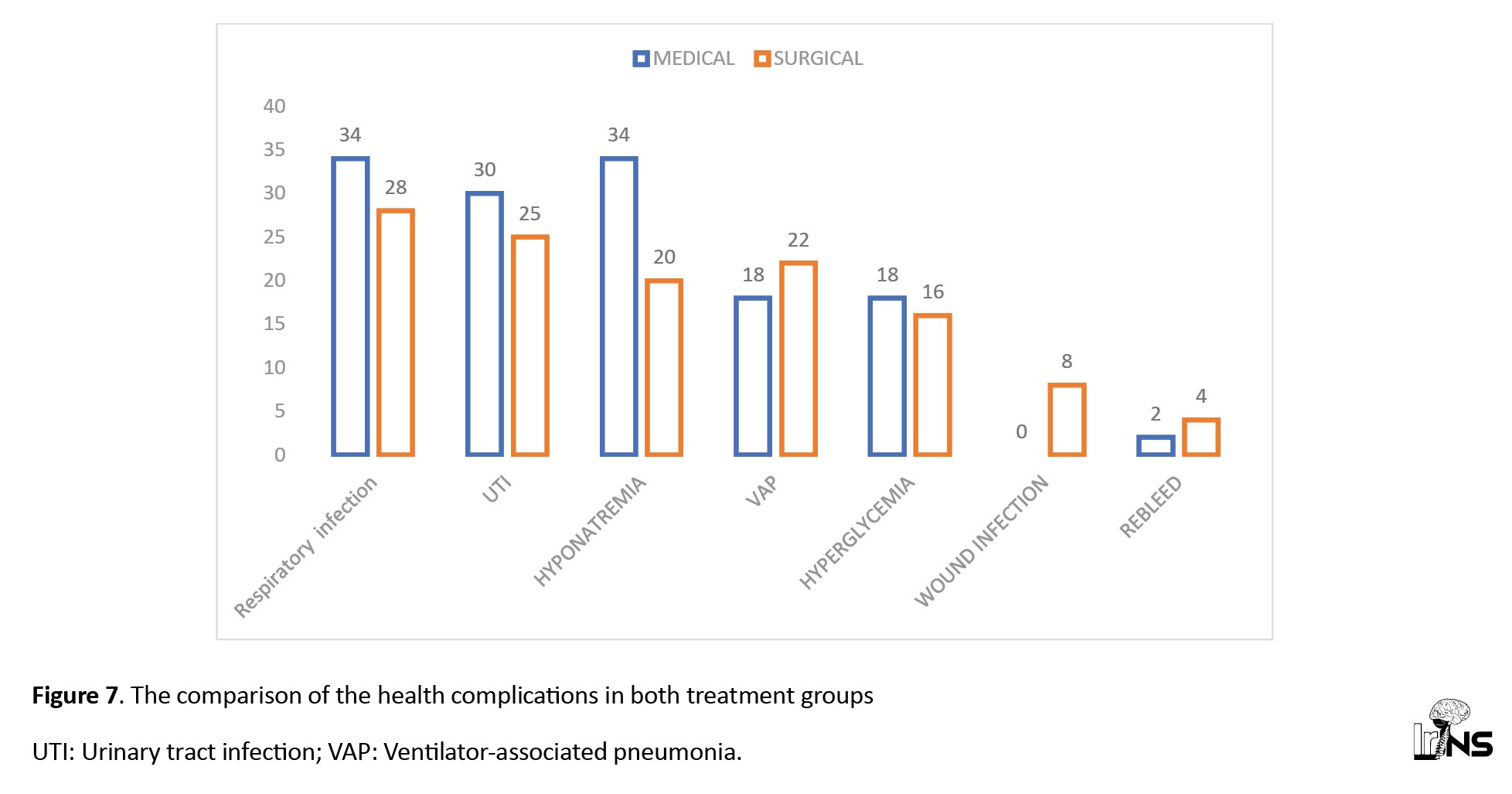
b) Surgical group: Hyponatremia was observed in maximum patient (85%). Respiratory tract infections were also observed in most cases (70%), followed by UTI (63%), VAP (55%), and hyperglycaemia (40%). The number of rebleeding cases was higher than in the medical group (10%) (Figure 7).
The factors affecting the immediate outcomes irrespective of treatment received
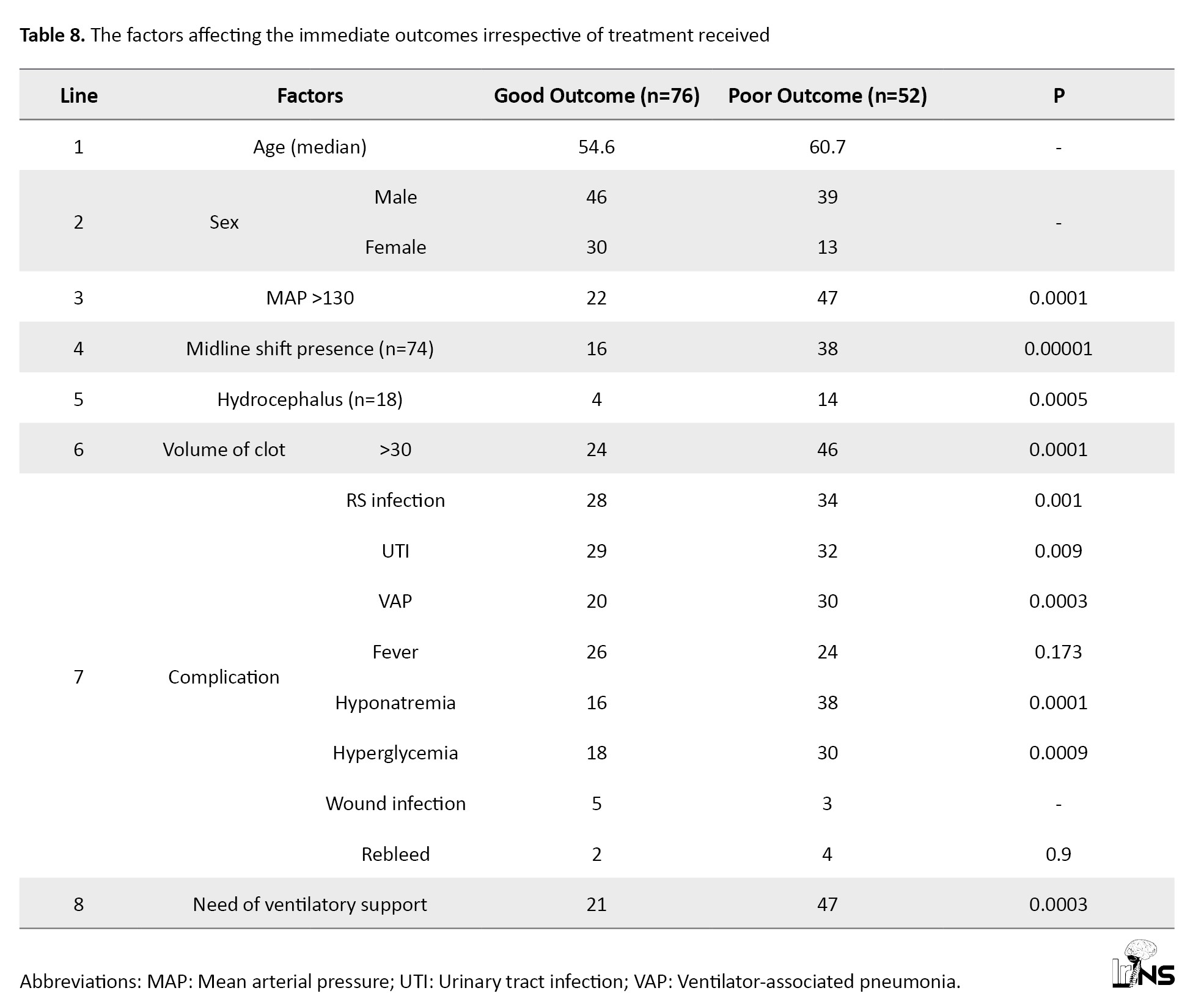
When all ICH cases had poor outcomes compared with good outcomes regardless of the treatment group, hypertension (mean arterial pressure >130), midline shift (>30 cc), and hydrocephalus were significant risk factors. We have observed that any hospital-acquired infection or ventilator support requirement also affected the outcome irrespective of the treatment group (Table 8).
4. Discussion
The maximum number of patients was in the age group 51-60 years and 61-70 years (54%) in our study. The mean age of patients in the medical group was 57±13 years, while the mean age in the surgical group was 53.7±12 years. In the study by Hu et al., the mean age was 57.9 years, similar to our study [7]. Wasay et al. also showed a mean age of 55 in their study [8]. The age of presentation also plays a pivotal role in spontaneous stroke, with a higher age indicating a higher risk of poor prognosis. However, findings of most studies from lower-middle-income countries reported that the younger patients with stroke suggested modification in age for risk calculation [9]. In our study, 85 patients (66.4%) were men and 43(33.6%) were women. Our M:F ratio was 1.9:1. In a study by Bhatia et al., male preponderance (65.4%) was observed [10]. Sang Joon An et al., in this review, concluded that the male sex is a nonmodifiable risk factor for ICH [11].
Location and laterality of spontaneous SICH
The timely management of stroke patients and prognostication require vital information about the site of ICH and, to some extent, the laterality of intracranial bleeding. The infratentorial location is a proven poor prognostic factor for ICH, but the supratentorial location still varies in type and prognosis. The most common areas of spontaneous SICH were the capsuloganglionic region (48%), thalamic region (26%), and lobar region (13%) in our study. Bhatia et al. found that the most common sites of hematoma were capsuloganglionic (70.6%), thalamic (16.8%), and lobar (4.2%), similar to ours [10]. In a study by Moussa and Khedr, the most common site for SICH was the basal ganglia (55%), and lobar bleeding was observed in 10% of cases [12]. An international multicenter randomized control trial (RCT) titled “ intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT)” by Anderson et al. showed that the posterior limb of the internal capsule and thalamus carries a high risk of mortality and significant follow-up disability [13, 14]. The INTERACT showed higher mortality in right-sided ICH. We observed a similar profile of location and laterality in both treatment groups, except for the predominance of mixed-type bleeding in the surgical group, even though the number of patients was less in a single-center study.
Clinical profile: Headache was the predominant symptom in both groups of patients. Patients in the surgical group had a higher percentage of encephalopathy, focal neurological deficits, and seizures compared to the medical group, but this difference was insignificant. There is a crossover of patients from the medical group to the surgical groups as per the progress of ICH and the need for surgical intervention for patients from the medical group. The selection of spontaneous ICH patients for surgery and the ideal timing of surgical intervention is still unknown; hence, the results of most ICH surgical randomized trials are inconclusive [15].
The risk factors for spontaneous SICH
Risk factors for spontaneous SICH have been extensively studied. The literature on risk factors for spontaneous SICH reported two major categories, lobar and non-lobar ICH. A systematic review and meta-analysis (SRMA) on the risk factors of ICH by Jolink et al. involving 26174 patients has shown that hypertension is a risk factor for both types of spontaneous ICH whereas various modifiable risk factors, such as diabetes mellitus, alcohol, lesser weight, and non-modifiable risk factors, such as male sex and black race for non-lobar ICH. Factors, such as smoking, hyperlipidemia, and obesity did not affect the risk of spontaneous SICH [16]. Hypertension (89%) was a significant risk factor in our study. We also observed no significant effect of smoking on either group. Al-Shahi Salman et al., in their patient data SRMA involving 5,076 on predictors and absolute risk of spontaneous SICH, showed that anticoagulant use is an independent predictor of ICH [17]. We observed a statistically significant effect of anticoagulant use in the medical group but not in the surgical group.
GCS range in spontaneous SICH
GCS has been a part of most of the prognostic scores used in managing ICH. Severe low GCS (<8) during ICH diagnosis is associated with poor outcomes, ranging from 60% to 100% in LMIC studies. With severely low GCS scores, we observed a poor outcome in 87% of cases in the medical group, with a slightly poorer outcome in 73% of the surgical group. Our results are consistent with those of previous studies in similar populations from LMIC [9, 18]. Compared to the medical group, a surgical group with a low GCS score had a poorer outcome, indicating that GCS alone may not be valid for the prognostication of surgical patients.
Treatment type and outcome at 3 months by GOS
In our surgical group, most patients underwent DC (63%), and the rest underwent EVD (37%). Despite advancements in the medical management of spontaneous SICH, the optimal surgical strategy remains unknown. Gregson et al.’s meta-analysis of 8 RCT concluded that patients with a GCS between 9 and 12 demonstrated a significantly improved outcome with surgery [19]. Prasad et al., in their meta-analysis of 10 RCT, concluded that surgery was associated with a statistically significant reduction in the odds of being dead or dependent on follow-up [20]. Takeuchi et al. concluded that DC with hematoma evacuation for large ICH may be a safe and effective procedure in patients with severely disturbed consciousness [21]. Moussa and Khedr, in their study on the outcomes of DC with clot evacuation, concluded that patients with high admission GCS, younger age, smaller hematoma volume, subcortical hematoma location, absent or minimal preoperative and postoperative midline shift as well as the absence of IVH had contributed significantly to a better outcome in the surgical group [12]. Various randomized trials comparing surgical treatment with medical management in the past have shown no clinical benefit (Table 9).
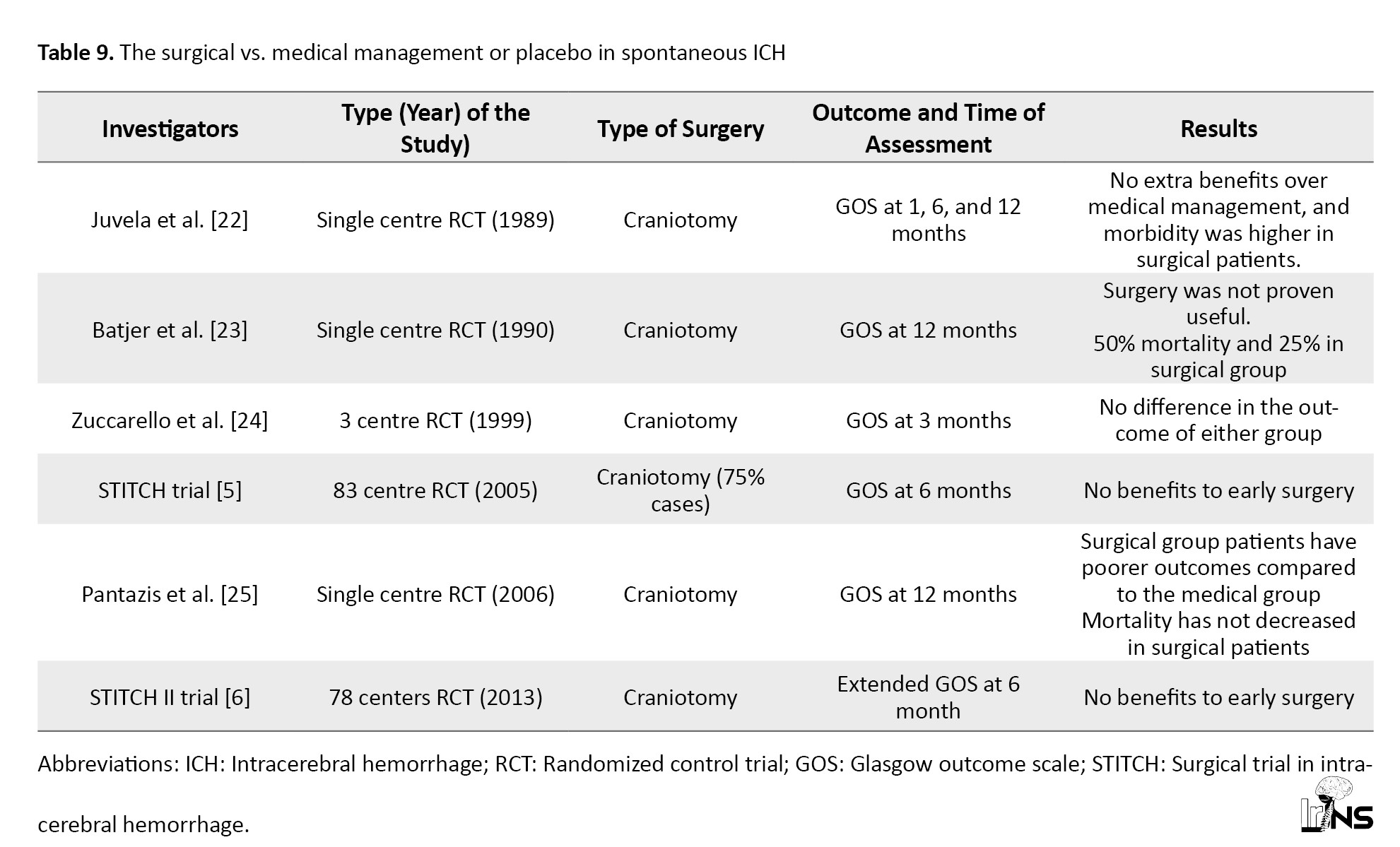
The best-designed trial, such as the surgical trial in intracerebral hemorrhage 2 (STITCH II), also showed no superiority for surgical treatment [22-25]. In STITCH II, no clinical benefit from early surgery was observed compared with medical treatment with delayed surgery if necessary [6]. The surgical trial findings may not apply to all settings because a high crossover of patients was observed. Gregson et al. performed a new analysis of all crucial surgical RCT and showed clinically relevant survival benefits in patients with GCS 9-12 (conscious patients) and large ICH [26]. In our study, the surgical group had good and poor outcomes in 48% and 52% of cases, respectively. Our patients with DC had good outcomes in 64% of cases, similar to the medical group outcomes at 3 months. In contrast, EVD surgical patients had a poorer outcome than the medical group.
The reported death rate at 3 months for spontaneous ICH cases is approximately 30% or more [27-31]. We also have a mortality rate of 25% in the medical group and 30% in the surgical group at 3 months.
The comparison of prognostic factors
The volume of spontaneous ICH is a radiological marker of a poor prognosis. Evidence suggests that mortality increases with increasing ICH [32]. Hegde et al., in their study on spontaneous SICH outcome predictors, have made two groups, <30 mL and >30 mL ICH volume, to see the effect. They reported 54% mortality and 5% rebleeding in the >30 volume group of patients with ICH [9]. We observed that the surgical treatment group with >30 cc size of ICH had better outcomes than the medical treatment group.
Kim showed that <5 mm midline shift patients had 10.9% mortality, and >5 mm midline shift had 34.4% mortality [31]. We observed an overall mortality rate of 30% in ICH cases with a midline shift >6 mm, whereas better outcomes were observed in the surgical treatment group.
Hayes et al. found that DC improves the outcome for putaminal spontaneous SICH [33]. Seeruch observed improved outcomes with DC for basal ganglia hemorrhage with midline shift [34]. In contrast, Shimamura et al. found that DC was not useful in spontaneous SICH cases [35]. The existing evidence available for the surgical outcomes may not be conclusive because the surgical treatment outcomes of spontaneous SICH vary based on the type of surgery and mostly on individual patient variables like the location of the bleed, the volume of SICH, and severity of midline shift. In spontaneous ICH with a midline shift, we observed better outcomes than the medical treatment group.
The consensus guidelines on stroke management by various associations, such as those in America and Europe, have suggested that for most SICH cases, surgical treatment benefits are not well proven, and surgery may reduce deaths in a subset of patients with comatose condition, large hematoma, or unresponsive medical treatment [36, 37]. It is a well-accepted fact that a GCS 9-12 in spontaneous ICH cases may predict good outcomes, especially with surgical treatment [38]. Gregson et al., in his meta-analysis of 8 RCT, concluded that patients with a GCS between 9 and 12 demonstrated a significantly improved outcome with surgery [19]. We also observed statistically significant improved outcomes in surgical patients with a GCS 9-12 score, suggesting that the timing of surgery may play a role in the outcomes.
The effect of the prognostic scores and sign
Various variables have been tested by estimating mortality and morbidities and are part of the different prognostic scores used in spontaneous SICH [39-41]. The ICH score is an easy clinical score used for risk assessment of ICH and a tool for choosing the treatment modality in the past at 30-day mortality [42, 43]. All our patients were below 80 years, and supratentorial location of ICH; therefore, our maximum ICH score was 3, and minimum was 0. The ICH score is estimated to have a specificity of 91.5% with a negative predictive value of 0.94 for mortality assessment. In contrast, for a good outcome, it has a sensitivity of 93.4% with a negative predictive value of 0.93 [44]. In contrast, Parry –jones et al. showed that the ICH score proposed by Hemphill is not superior to the GCS score for 30 days mortality [45]. We observed that patients with ICH scores of 1 and 2 had similar outcomes in treatment groups. Patients with ICH grade 3 in our surgical group showed higher poor outcomes (68%) and lower mortality (46%) than those in the medical group (66% poor outcome and 55% mortality), which is similar to the findings of other studies [10, 46]. In contrast, an SRMA of 21 studies with four studies of the highest quality involving spontaneous ICH by Sondag et al. concluded that surgical treatment, especially minimally invasive and early timed intervention, may be beneficial for good outcomes, and they also reported no effect of age, GCS, and volume of ICH on surgical outcomes [47].
Spontaneous SICH is frequently associated with IVH and an independent predictor of poor outcomes. Hallevi et al. have shown that patients with IVH are twice as likely to have a poor outcome compared to patients without IVH [29]. In our study, 73% of the patients had poor outcomes associated with IVH, similar to previous studies.
A SRMA done by Yuping Li et al in 2013 involving 11 trials with 680 patients showed that under EVD treatment of SICH with IVH, a newer surgical procedure such as neuroendoscopy may improve IVH evacuation, functional outcome and decrease mortality when compared to EVD with rt PA treatment but also emphasized limited data to support treatment modality directly [48, 49]. On a similar note, we had SICH with IVH in all 48 patients (37.5%). The number of patients having IVH with SICH in surgical group was more which had higher survival but showed poor outcome.
Health complications
Rebleeding events in ICH are widespread, particularly when associated hypertension is poorly controlled [50]. The estimated incidence of rebleeding is 15.4% in conventional neurosurgical cases and 10% in minimally invasive neurosurgeries [51]. Our surgical group had a higher incidence of rebleeding events than the medical group. It is well-accepted that post-neurosurgical patients have higher infection rates [52]. In their study, Lo et al. showed that DC significantly improved survival compared with medical treatment with good outcomes but had longer hospital stays and hospital-acquired infections. The hospital-acquired infections (76%) was higher in the surgical group than in the medical group (33%) [53]. Our surgical group also had higher health complications, such as pneumonia and UTI, with an almost similar percentage.
5. Conclusion
In LMIC, supratentorial SICH has predominant left-sided bleeding, with hypertension being the highest risk factor involving younger age groups with higher males. The surgical group has improved outcomes compared to the medical group, containing patients with midline shift >6 mm, >30 cc of bleed, and GCS 8-12. With a higher ICH score and IVH, the surgical group showed a poorer outcome and fewer deaths.
Ethical Considerations
Compliance with ethical guidelines
The Institutional Ethical Committee of Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati, India, approved the study (AS/11/IEC/SVIMS/2017 vide IEC No. 762). Patients or legal representatives were explained about the risks and benefits of available advised treatment options, that is, medical and surgical treatment. They were then given written informed consent before enrolment in the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and design of study: Ramesh Candra VV, Nitin Barde, Dattatray Kulkarni, Meghraj Chawada; Data collection: Nitin Barde, Ramesh Candra VV; Data analysis and interpretation: Nitin Barde, Dattatray Kulkarni, Meghraj Chawada; Writing manuscript: Dattatray Kulkarni, Ramesh Candra VV, Nitin Barde, Meghraj Chawada; Critical review of article: Ramesh Candra VV, Nitin Barde; Supervision and Materials: Nitin Barde, Dattatray Kulkarni, Meghraj Chawada; Review and approve the final version of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank BCM Prasad MCH, Professor of Neurosurgery, and B Vengamma DM, Professor of Neurology from VIMS, Tirupati, AP, India, for their continuous support and encouragement in completing and continuing this project. We also thank all healthcare staff and patients involved in this project.
References
Spontaneous supratentorial intracerebral hemorrhage (SICH) accounts for 10%-15% of all strokes [1]. Intracerebral hemorrhage (ICH) appears to be more common in Eastern countries, accounting for up to 30% of strokes, with an overall mortality rate of 40% to 50% [2, 3]. The treatment of patients with ICH is complex in many ways. Apart from standard medical treatments, no novel therapies have been introduced to improve outcomes. Despite preventing hematoma enlargement, recombinant activated factor VII treatment showed no beneficial effect on outcome in a randomized placebo-controlled clinical trial [4]. Surgery has the potential to reduce the volume of intracerebral hematomas. Clinical and experimental evidence suggests that mass removal may reduce damage to nervous tissue. There are also wide variations in surgical practices among countries. Trials in specific populations of ICH patients have focused on hematoma evacuation alone, such as the International Surgical Trial in ICH (STICH) [5]. This landmark trial demonstrated that emergent surgical hematoma evacuation of superficial lobar hemorrhage s within 72 h of onset failed to improve outcomes compared with standard medical management. The results of the International Surgical Trial in ICH (STICH)-II demonstrated that early surgery did not improve the rate of death or disability at 6 months but suggested a slight survival advantage for patients with ICH who did not have intraventricular hemorrhage (IVH) or hydrocephalus [6].
We aimed to study prognostic factors and immediate outcomes in surgical and medical treatment groups of patients with SICH because these health data are vital for individualized stroke management protocols, as no universal recommendation exists. In lower middle-income countries (LMIC), there is wide variation in healthcare infrastructure and availability of timely healthcare for emergencies, such as brain stroke. Hence, identifying definite prognostic factors, designing and implementing policies of stroke management, making prompt and accurate diagnoses, stratifying patients according to outcome predictors, and thus ensuring prompt referral of deserving critical patients to tertiary centers for intensive management may be the need of the hour as we wait to embrace the newer advances into our management protocol. This study aimed to compare the various prognostic factors in bothmedical treatment and surgical treatment groups. The immediate outcomes of the best medical management and surgery treatment groups were also estimated.
2. Methods and Materials/Patients
The study was conducted after approval by the Institutional Scientific Committee and Institutional Ethical Committee (AS/11/IEC/SVIMS/2017 vide IEC No 762). Written informed consent was obtained from all patients before the study. All patients diagnosed with spontaneous SICH who presented to the Department of Neurology and Neurosurgery at Tertiary Medical College and met the inclusion criteria between May 2018 and November 2019 were included in the study.
Our study is a prospective observational study, and the study population is mentioned below.
Inclusion criteria
1. Diagnosed spontaneous SICH on plain computed tomography (CT) brain scans
2. Age range of 18 to 80 years
3. Supratentorial ICH
Exclusion criteria
1. Age <18 and >80 years of age
2. ICH secondary to trauma, tumor, arterial venous malformation, aneurysm rupture, and cerebral sinuous venous thrombosis
3. Cerebellar hematoma
4. Unwillingness to participate in the study
Data collection
Detailed clinical history was recorded, including demographic data, presenting complaints, history of present illness, personal history, and drug history. All participants underwent detailed physical and neurological examinations. Glasgow coma scale (GCS) score, ICH score were also recorded.
The selected patients performed all necessary blood examinations, and the functional outcome of surgical and medical treatment was measured using the Glasgow outcome scale (GOS) at 3 months.
Medical treatment
The appropriate feasible medical treatment was administered according to American Heart Association/American Stroke Association guidelines. In conscious patients, a systolic blood pressure of 160 mm Hg was used. Patients with a GCS ≤8 are ventilated and sedated. Management of increased intracranial pressure included cerebrospinal fluid drainage using an external ventricular drain (EVD), neuromuscular blockade, and sedation. Anticoagulant treatment was stopped and reversed with clotting factors, vitamin K, and protamine. Intermittent pneumatic compression was used to prevent venous thrombosis, and after 36 hours, low-dose fractionated heparin was used.
Surgical treatment
The surgical approach was individualized based on the site and size of the ICH due to the lack of standardized guidelines for the allocation of operative treatment. The allowed techniques included open decompressive craniectomy (DC) and hematoma evacuation. Surgical treatment intends to remove the clot completely. Surgery was performed in patients with impending cerebral herniation, as indicated by abnormal pupil response, abnormal posture, or CT findings of absence of ambient cistern or severe midline shifting (>6 mm). Patients with IVH with hydrocephalus underwent EVD insertion.
Outcomes
All prognostic factors were measured in both treatment groups. Their immediate outcomes at 3 months were measured using GOS.
Statistical analysis
The data were recorded on a predesigned performance, managed using Microsoft Excel 2007 (Microsoft Corp, Redmond, WA), and analyzed using SPSS software, version 20. Descriptive statistics, such as percentages and frequencies, and inferential statistics, such as the chi-square test, were used during the analysis. The primary analysis was categorical frequency comparison using the chi-square test for prognosis based on favorable and unfavorable outcomes.
3. Results
In this prospective observational study, 523 patients with features suggestive of cerebrovascular accidents were screened and subjected to brain CT. A total of 152 patients with spontaneous SICH were identified, of whom 24 were excluded because they did not meet the inclusion criteria. We studied 125 patients with spontaneous SICH, and the data were stratified into two groups based on treatment to evaluate the outcome. Among the 128 patients, 88 underwent medical treatment, 40 underwent surgical management, and among surgery, 25 underwent DC, and 15 underwent (EVD) (Figure 1). The mean age of the medical group was 57±13 years, while the mean age of the surgical group was 53.7±12 years. Our study included 85 male (66.4%) and 43 female patients (33.6%) (Table 1).

Male/female ratio (M:F) in medical group was 1.6:1; in surgical group, it was 2.6:1 and in total, M:F ratio was 1.9:1 (Figure 2).
The laterality and location of ICH
In the medical treatment group, 53 patients (60%) had left-sided bleeding, and the rest, 35 patients (40%), had right-sided bleeding, while in the surgical group, 22 patients had left-sided bleeding (55%), and 18 patient (45%) had right-sided bleeding. In both treatment groups, the bleeding locations were almost similar; capsuloganglionic region (CG) bleeding was 50% in each group, followed by 30% to 35 % thalamic region bleeding. The lobar location of bleeding was 15% and 10% in the medical group and surgical group, respectively. In the surgical treatment group, 23% of patients had bleeding in the mixed parts of the cerebrum, whereas mixed bleeding cases were less common in the medical treatment group (7%) (Figures 3 and 4).
Clinical profile
The range of the clinical spectrum was similar between groups. In our study of 128 patients, 109(85.1%) had headache, 104(81.2%) had altered sensorium, 88(68%) had cranial nerve palsy, 90(70%) had motor deficit, 72(56%) had aphasia, 68(53%) had convulsions, and 66(51.5%) had vomiting (Table 2).

The risk factors with their immediate outcomes
In the medical group, the most common risk factor was hypertension in 78 patients (88%), diabetes in 35 patients (40%), smoking in 42 patients (47%), and alcohol in 38 patients (43%). In the surgical group, hypertension was the most common risk factor observed in 36 patients (90%), diabetes in 30 patients (75%), and alcohol and smoking in 25 patients (62%) and 23 patients (57%), respectively (Tables 3 and 4).


GCS range
In the medical group, GCS scores were normal or mild derangement in 50% (44 patients) of SICH cases, moderate derangement in 40% (36 patients), and severe derangement (<8) in 9% of cases. In the surgical group, 37 patients (92.5%) had moderate to severe derangement in scores, among which 55% (22 cases) had GCS <8 and 38% (15 cases) had GCS 8-12. In the surgical group, the GCS score was normal or mildly deranged in only three patients (7.5%) (Figure 5).
The outcomes
In a study of 128 patients, 76 patients (59.3%) had good outcomes, and 52 patients (40.7%) had poor outcomes, 57 patients (64.7%) had good outcomes, and 31 patients (35.3%) had poor outcomes. We observed mortality rates of 25% (22 deaths) in the medical group and 30% (12 deaths) in the surgical group until 3 months.
In the surgical group of 40 ICH cases, 19 patients (47.5%) had good outcomes, and 21 patients (52.5%) had poor outcomes; among the surgical group, 25 patients underwent DC, of whom 16(64%) had good outcomes, and 9 patients (36%) had poor outcomes; 15 patients underwent EVD, of whom only three patients had good outcomes, and 12 patients had poor outcomes (Figure 6).
The comparison of prognostic factors
Midline shift >6 mm
In our study, 48 patients with midline shifts >6 mm were not eligible for surgical interventions; therefore, among 48 patients, 80% had poor, and 20% had good outcomes. In the surgical group, 52% of patients had good outcomes, and 48% had poor outcomes (P<0.005) (Table 5).

>30 cc size of hematoma
In our study, 45 patients with >30 cc hematoma were not included in the surgical intervention. Among 45 patients, 77% had poor and 23% had good outcomes. In the surgical group, 52% of patients had good outcomes, and 48% had poor outcomes (P<0.005) (Table 5).
GCS 8-12
A total of 51 patients had GCS 8-12; 36 patients did not present for surgical interventions; among 36 patients, 52% had poor outcomes, and 48% had good outcomes. A total of 15 patients underwent surgical intervention; among them, 11 patients (73%) had good outcomes, and 4(27%) had poor outcomes. Among the surgical group, 73% of patients had a good outcome, and 27% had a poor outcome (P<0.005) (Table 5).
The effect of the prognostic scores and sign-in each treatment group on immediate outcome
ICH score
a) Medical group: ICH score was used to predict the outcome of ICH. In our study, in the medical group, the ICH score was 1 in 25 patients, of whom 23 had a good outcome and 2 had a poor outcome. An ICH score of 2 was observed in 45 patients, of whom 28 had a good outcome, and 17 had a poor outcome. In 18 patients, the ICH score was three among 18 patients; 12 patients had a poor outcome, and six patients had a good outcome.
b) Surgical group: In the surgical group, 18 of 18 patients had an ICH score of 2. Twelve patients had a good outcome, and 6 had a poor outcome. In 22 patients, the ICH score was 3. Among the 22 patients, 15 had poor outcomes, and only 7 had good outcomes.
GCS score
The GCS scores of patients on admission GCS was calculated, and patients were grouped into four groups: Patients with GCS <8, 8-12, 13-14 and 15.
a) Medical group: In our study, among 88 medically treated patients, eight had GCS <8, 36 had GCS 8-12, 35 had GCS 13-14, and 9 had GCS 15. Eight patients had a GCS <8, among which seven had poor outcomes and one had a good outcome. Among 36 patients in groups 8-12, 17 had good outcomes and 19 had poor outcomes. In the GCS group 13-14, 35 patients were included, among which 32 had good outcomes and only three had poor outcomes.
b) Surgical group: In our study, among 40 patients in the surgical group, 22 patients had GCS <8, 15 patients in the 8-12 groups, and three patients had GCS 13-14.
Among patients with GCS <8, 16 had poor outcomes, and 6 had good outcomes. In the 8-12 groups, 11 patients had good outcomes, and 4 patients had poor outcomes.
SICH with IVH
a) Medical group: A total of 28 cases were found to be associated with IVH, of whom 71% had poor outcomes and the remaining 29% had good outcomes. The medical group without IVH had good outcomes in 82% of cases.
b) Surgical group: In the surgical group, 20 cases had IVH. The surgical group with IVH had 85% poor outcomes and 15% good outcomes, while the surgical group without IVH had good outcomes in 80% of cases (Tables 6 and 7).


The comparison of the health complications
a) Medical group: The common complications in the surgical group were respiratory infection (39%), hyponatremia (39%), urinary tract infection (UTI): 34%, ventilator-associated pneumonia (VAP): 20%, and 2% cases observed to have rebleeding.
b) Surgical group: Hyponatremia was observed in maximum patient (85%). Respiratory tract infections were also observed in most cases (70%), followed by UTI (63%), VAP (55%), and hyperglycaemia (40%). The number of rebleeding cases was higher than in the medical group (10%) (Figure 7).
The factors affecting the immediate outcomes irrespective of treatment received
When all ICH cases had poor outcomes compared with good outcomes regardless of the treatment group, hypertension (mean arterial pressure >130), midline shift (>30 cc), and hydrocephalus were significant risk factors. We have observed that any hospital-acquired infection or ventilator support requirement also affected the outcome irrespective of the treatment group (Table 8).

4. Discussion
The maximum number of patients was in the age group 51-60 years and 61-70 years (54%) in our study. The mean age of patients in the medical group was 57±13 years, while the mean age in the surgical group was 53.7±12 years. In the study by Hu et al., the mean age was 57.9 years, similar to our study [7]. Wasay et al. also showed a mean age of 55 in their study [8]. The age of presentation also plays a pivotal role in spontaneous stroke, with a higher age indicating a higher risk of poor prognosis. However, findings of most studies from lower-middle-income countries reported that the younger patients with stroke suggested modification in age for risk calculation [9]. In our study, 85 patients (66.4%) were men and 43(33.6%) were women. Our M:F ratio was 1.9:1. In a study by Bhatia et al., male preponderance (65.4%) was observed [10]. Sang Joon An et al., in this review, concluded that the male sex is a nonmodifiable risk factor for ICH [11].
Location and laterality of spontaneous SICH
The timely management of stroke patients and prognostication require vital information about the site of ICH and, to some extent, the laterality of intracranial bleeding. The infratentorial location is a proven poor prognostic factor for ICH, but the supratentorial location still varies in type and prognosis. The most common areas of spontaneous SICH were the capsuloganglionic region (48%), thalamic region (26%), and lobar region (13%) in our study. Bhatia et al. found that the most common sites of hematoma were capsuloganglionic (70.6%), thalamic (16.8%), and lobar (4.2%), similar to ours [10]. In a study by Moussa and Khedr, the most common site for SICH was the basal ganglia (55%), and lobar bleeding was observed in 10% of cases [12]. An international multicenter randomized control trial (RCT) titled “ intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT)” by Anderson et al. showed that the posterior limb of the internal capsule and thalamus carries a high risk of mortality and significant follow-up disability [13, 14]. The INTERACT showed higher mortality in right-sided ICH. We observed a similar profile of location and laterality in both treatment groups, except for the predominance of mixed-type bleeding in the surgical group, even though the number of patients was less in a single-center study.
Clinical profile: Headache was the predominant symptom in both groups of patients. Patients in the surgical group had a higher percentage of encephalopathy, focal neurological deficits, and seizures compared to the medical group, but this difference was insignificant. There is a crossover of patients from the medical group to the surgical groups as per the progress of ICH and the need for surgical intervention for patients from the medical group. The selection of spontaneous ICH patients for surgery and the ideal timing of surgical intervention is still unknown; hence, the results of most ICH surgical randomized trials are inconclusive [15].
The risk factors for spontaneous SICH
Risk factors for spontaneous SICH have been extensively studied. The literature on risk factors for spontaneous SICH reported two major categories, lobar and non-lobar ICH. A systematic review and meta-analysis (SRMA) on the risk factors of ICH by Jolink et al. involving 26174 patients has shown that hypertension is a risk factor for both types of spontaneous ICH whereas various modifiable risk factors, such as diabetes mellitus, alcohol, lesser weight, and non-modifiable risk factors, such as male sex and black race for non-lobar ICH. Factors, such as smoking, hyperlipidemia, and obesity did not affect the risk of spontaneous SICH [16]. Hypertension (89%) was a significant risk factor in our study. We also observed no significant effect of smoking on either group. Al-Shahi Salman et al., in their patient data SRMA involving 5,076 on predictors and absolute risk of spontaneous SICH, showed that anticoagulant use is an independent predictor of ICH [17]. We observed a statistically significant effect of anticoagulant use in the medical group but not in the surgical group.
GCS range in spontaneous SICH
GCS has been a part of most of the prognostic scores used in managing ICH. Severe low GCS (<8) during ICH diagnosis is associated with poor outcomes, ranging from 60% to 100% in LMIC studies. With severely low GCS scores, we observed a poor outcome in 87% of cases in the medical group, with a slightly poorer outcome in 73% of the surgical group. Our results are consistent with those of previous studies in similar populations from LMIC [9, 18]. Compared to the medical group, a surgical group with a low GCS score had a poorer outcome, indicating that GCS alone may not be valid for the prognostication of surgical patients.
Treatment type and outcome at 3 months by GOS
In our surgical group, most patients underwent DC (63%), and the rest underwent EVD (37%). Despite advancements in the medical management of spontaneous SICH, the optimal surgical strategy remains unknown. Gregson et al.’s meta-analysis of 8 RCT concluded that patients with a GCS between 9 and 12 demonstrated a significantly improved outcome with surgery [19]. Prasad et al., in their meta-analysis of 10 RCT, concluded that surgery was associated with a statistically significant reduction in the odds of being dead or dependent on follow-up [20]. Takeuchi et al. concluded that DC with hematoma evacuation for large ICH may be a safe and effective procedure in patients with severely disturbed consciousness [21]. Moussa and Khedr, in their study on the outcomes of DC with clot evacuation, concluded that patients with high admission GCS, younger age, smaller hematoma volume, subcortical hematoma location, absent or minimal preoperative and postoperative midline shift as well as the absence of IVH had contributed significantly to a better outcome in the surgical group [12]. Various randomized trials comparing surgical treatment with medical management in the past have shown no clinical benefit (Table 9).

The best-designed trial, such as the surgical trial in intracerebral hemorrhage 2 (STITCH II), also showed no superiority for surgical treatment [22-25]. In STITCH II, no clinical benefit from early surgery was observed compared with medical treatment with delayed surgery if necessary [6]. The surgical trial findings may not apply to all settings because a high crossover of patients was observed. Gregson et al. performed a new analysis of all crucial surgical RCT and showed clinically relevant survival benefits in patients with GCS 9-12 (conscious patients) and large ICH [26]. In our study, the surgical group had good and poor outcomes in 48% and 52% of cases, respectively. Our patients with DC had good outcomes in 64% of cases, similar to the medical group outcomes at 3 months. In contrast, EVD surgical patients had a poorer outcome than the medical group.
The reported death rate at 3 months for spontaneous ICH cases is approximately 30% or more [27-31]. We also have a mortality rate of 25% in the medical group and 30% in the surgical group at 3 months.
The comparison of prognostic factors
The volume of spontaneous ICH is a radiological marker of a poor prognosis. Evidence suggests that mortality increases with increasing ICH [32]. Hegde et al., in their study on spontaneous SICH outcome predictors, have made two groups, <30 mL and >30 mL ICH volume, to see the effect. They reported 54% mortality and 5% rebleeding in the >30 volume group of patients with ICH [9]. We observed that the surgical treatment group with >30 cc size of ICH had better outcomes than the medical treatment group.
Kim showed that <5 mm midline shift patients had 10.9% mortality, and >5 mm midline shift had 34.4% mortality [31]. We observed an overall mortality rate of 30% in ICH cases with a midline shift >6 mm, whereas better outcomes were observed in the surgical treatment group.
Hayes et al. found that DC improves the outcome for putaminal spontaneous SICH [33]. Seeruch observed improved outcomes with DC for basal ganglia hemorrhage with midline shift [34]. In contrast, Shimamura et al. found that DC was not useful in spontaneous SICH cases [35]. The existing evidence available for the surgical outcomes may not be conclusive because the surgical treatment outcomes of spontaneous SICH vary based on the type of surgery and mostly on individual patient variables like the location of the bleed, the volume of SICH, and severity of midline shift. In spontaneous ICH with a midline shift, we observed better outcomes than the medical treatment group.
The consensus guidelines on stroke management by various associations, such as those in America and Europe, have suggested that for most SICH cases, surgical treatment benefits are not well proven, and surgery may reduce deaths in a subset of patients with comatose condition, large hematoma, or unresponsive medical treatment [36, 37]. It is a well-accepted fact that a GCS 9-12 in spontaneous ICH cases may predict good outcomes, especially with surgical treatment [38]. Gregson et al., in his meta-analysis of 8 RCT, concluded that patients with a GCS between 9 and 12 demonstrated a significantly improved outcome with surgery [19]. We also observed statistically significant improved outcomes in surgical patients with a GCS 9-12 score, suggesting that the timing of surgery may play a role in the outcomes.
The effect of the prognostic scores and sign
Various variables have been tested by estimating mortality and morbidities and are part of the different prognostic scores used in spontaneous SICH [39-41]. The ICH score is an easy clinical score used for risk assessment of ICH and a tool for choosing the treatment modality in the past at 30-day mortality [42, 43]. All our patients were below 80 years, and supratentorial location of ICH; therefore, our maximum ICH score was 3, and minimum was 0. The ICH score is estimated to have a specificity of 91.5% with a negative predictive value of 0.94 for mortality assessment. In contrast, for a good outcome, it has a sensitivity of 93.4% with a negative predictive value of 0.93 [44]. In contrast, Parry –jones et al. showed that the ICH score proposed by Hemphill is not superior to the GCS score for 30 days mortality [45]. We observed that patients with ICH scores of 1 and 2 had similar outcomes in treatment groups. Patients with ICH grade 3 in our surgical group showed higher poor outcomes (68%) and lower mortality (46%) than those in the medical group (66% poor outcome and 55% mortality), which is similar to the findings of other studies [10, 46]. In contrast, an SRMA of 21 studies with four studies of the highest quality involving spontaneous ICH by Sondag et al. concluded that surgical treatment, especially minimally invasive and early timed intervention, may be beneficial for good outcomes, and they also reported no effect of age, GCS, and volume of ICH on surgical outcomes [47].
Spontaneous SICH is frequently associated with IVH and an independent predictor of poor outcomes. Hallevi et al. have shown that patients with IVH are twice as likely to have a poor outcome compared to patients without IVH [29]. In our study, 73% of the patients had poor outcomes associated with IVH, similar to previous studies.
A SRMA done by Yuping Li et al in 2013 involving 11 trials with 680 patients showed that under EVD treatment of SICH with IVH, a newer surgical procedure such as neuroendoscopy may improve IVH evacuation, functional outcome and decrease mortality when compared to EVD with rt PA treatment but also emphasized limited data to support treatment modality directly [48, 49]. On a similar note, we had SICH with IVH in all 48 patients (37.5%). The number of patients having IVH with SICH in surgical group was more which had higher survival but showed poor outcome.
Health complications
Rebleeding events in ICH are widespread, particularly when associated hypertension is poorly controlled [50]. The estimated incidence of rebleeding is 15.4% in conventional neurosurgical cases and 10% in minimally invasive neurosurgeries [51]. Our surgical group had a higher incidence of rebleeding events than the medical group. It is well-accepted that post-neurosurgical patients have higher infection rates [52]. In their study, Lo et al. showed that DC significantly improved survival compared with medical treatment with good outcomes but had longer hospital stays and hospital-acquired infections. The hospital-acquired infections (76%) was higher in the surgical group than in the medical group (33%) [53]. Our surgical group also had higher health complications, such as pneumonia and UTI, with an almost similar percentage.
5. Conclusion
In LMIC, supratentorial SICH has predominant left-sided bleeding, with hypertension being the highest risk factor involving younger age groups with higher males. The surgical group has improved outcomes compared to the medical group, containing patients with midline shift >6 mm, >30 cc of bleed, and GCS 8-12. With a higher ICH score and IVH, the surgical group showed a poorer outcome and fewer deaths.
Ethical Considerations
Compliance with ethical guidelines
The Institutional Ethical Committee of Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati, India, approved the study (AS/11/IEC/SVIMS/2017 vide IEC No. 762). Patients or legal representatives were explained about the risks and benefits of available advised treatment options, that is, medical and surgical treatment. They were then given written informed consent before enrolment in the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and design of study: Ramesh Candra VV, Nitin Barde, Dattatray Kulkarni, Meghraj Chawada; Data collection: Nitin Barde, Ramesh Candra VV; Data analysis and interpretation: Nitin Barde, Dattatray Kulkarni, Meghraj Chawada; Writing manuscript: Dattatray Kulkarni, Ramesh Candra VV, Nitin Barde, Meghraj Chawada; Critical review of article: Ramesh Candra VV, Nitin Barde; Supervision and Materials: Nitin Barde, Dattatray Kulkarni, Meghraj Chawada; Review and approve the final version of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank BCM Prasad MCH, Professor of Neurosurgery, and B Vengamma DM, Professor of Neurology from VIMS, Tirupati, AP, India, for their continuous support and encouragement in completing and continuing this project. We also thank all healthcare staff and patients involved in this project.
References
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. The New England Journal of Medicine. 2001; 344(19):1450-60. [DOI:10.1056/NEJM200105103441907] [PMID]
- Zhang LF, Yang J, Hong Z, Yuan GG, Zhou BF, Zhao LC, et al. Proportion of different subtypes of stroke in China. Stroke. 2003; 34(9):2091-6. [DOI:10.1161/01.STR.0000087149.42294.8C] [PMID]
- Fogelholm R, Nuutila M, Vuorela AL. Primary intracerebral haemorrhage in the Jyväskylä region, central Finland, 1985-89: incidence, case fatality rate, and functional outcome. Journal of Neurology, Neurosurgery, and Psychiatry. 1992; 55(7):546-52. [DOI:10.1136/jnnp.55.7.546] [PMID]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. The New England Journal of Medicine. 2008; 358(20):2127-37. [DOI:10.1056/NEJMoa0707534] [PMID]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet. 2005; 365(9457):387-97. [DOI:10.1016/S0140-6736(05)70233-6]
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet. 2013; 382(9890):397-408. [DOI:10.1016/S0140-6736(13)60986-1] [PMID]
- Hu YZ, Wang JW, Luo BY. Epidemiological and clinical characteristics of 266 cases of intracerebral hemorrhage in Hangzhou, China. Journal of Zhejiang University. Science. B. 2013; 14(6):496-504. [DOI:10.1631/jzus.B1200332] [PMID]
- Wasay M, Yousuf A, Lal D, Awan S. Predictors of the intracerebral hemorrhage volume in hypertensive patients. Cerebrovascular Diseases Extra. 2011; 1(1):1-5. [DOI:10.1159/000323270] [PMID]
- Hegde A, Menon G, Kumar V, Lakshmi Prasad G, Kongwad LI, Nair R, et al. Clinical profile and predictors of outcome in spontaneous intracerebral hemorrhage from a tertiary care centre in South India. Stroke Research and Treatment. 2020; 2020:2192709. [DOI:10.1155/2020/2192709] [PMID]
- Bhatia R, Singh H, Singh S, Padma MV, Prasad K, Tripathi M, et al. A prospective study of in-hospital mortality and discharge outcome in spontaneous intracerebral hemorrhage. Neurology India. 2013; 61(3):244-8. [DOI:10.4103/0028-3886.115062] [PMID]
- An SJ, Kim TJ, Yoon BW. Epidemiology, Risk Factors, and clinical features of intracerebral hemorrhage: An update. Journal of Stroke. 2017; 19(1):3-10. [DOI:10.5853/jos.2016.00864] [PMID]
- Moussa WM, Khedr W. Decompressive craniectomy and expansive duraplasty with evacuation of hypertensive intracerebral hematoma, a randomized controlled trial. Neurosurgical review. 2017; 40(1):115-27. [DOI:10.1007/s10143-016-0743-6] [PMID]
- Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): A randomised pilot trial. The Lancet. Neurology. 2008; 7(5):391-9. [DOI:10.1016/S1474-4422(08)70069-3] [PMID]
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. The New England journal of medicine. 2013; 368(25):2355-65. [DOI:10.1056/NEJMoa1214609] [PMID]
- de Oliveira Manoel AL. Surgery for spontaneous intracerebral hemorrhage. Critical Care. 2020; 24(1):45. [DOI:10.1186/s13054-020-2749-2] [PMID]
- Jolink WMT, Wiegertjes K, Rinkel GJE, Algra A, de Leeuw FE, Klijn CJM. Location-specific risk factors for intracerebral hemorrhage: Systematic review and meta-analysis. Neurology. 2020; 95(13):e1807-18. [DOI:10.1212/WNL.0000000000010418] [PMID]
- Al-Shahi Salman R, Frantzias J, Lee RJ, Lyden PD, Battey TWK, Ayres AM, et al Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. The Lancet. Neurology. 2018; 17(10):885-94. [doi:10.1016/S1474-4422(18)30253-9]
- Namani G, Rampure DM, Murali M. Clinical profile and mortality in patients presenting with intracerebral hemorrhage in a tertiary care centre. Scholars Journal of Applied Medical Sciences. 2014; 2(6C):3005-10. [Link]
- Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, et al. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012; 43(6):1496-504. [DOI:10.1161/STROKEAHA.111.640284] [PMID]
- Prasad K, Mendelow AD, Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. The Cochrane Database of Systematic Reviews. 2008; (4):CD000200. [DOI:10.1002/14651858.CD000200.pub2]
- Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Decompressive hemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurgical Focus. 2013; 34(5):E5. [DOI:10.3171/2013.2.FOCUS12424] [PMID]
- Juvela S, Heiskanen O, Poranen A, Valtonen S, Kuurne T, Kaste M, et al. The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. Journal of Neurosurgery. 1989; 70(5):755-8.[DOI:10.3171/jns.1989.70.5.0755] [PMID]
- Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Archives of Neurology. 1990; 47(10):1103-6. [DOI:10.1001/archneur.1990.00530100071015] [PMID]
- Zuccarello M, Brott T, Derex L, Kothari R, Sauerbeck L, Tew J, et al. Early surgical treatment for supratentorial intracerebral hemorrhage: A randomized feasibility study. Stroke. 1999; 30(9):1833-9. [DOI:10.1161/01.STR.30.9.1833] [PMID]
- Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: A prospective randomized study. Surgical Neurology. 2006; 66(5):492-501; discussion 501-2. [DOI:10.1016/j.surneu.2006.05.054] [PMID]
- Gregson BA, Mitchell P, Mendelow AD. Surgical decision making in brain hemorrhage. Stroke. 2019; 50(5):1108-15.[DOI:10.1161/STROKEAHA.118.022694] [PMID]
- Narayan SK, Sivaprasad P, Sushma S, Sahoo RK, Dutta TK. Etiology and outcome determinants of intracerebral hemorrhage in a south Indian population, A hospital-based study. Annals of Indian Academy of Neurology. 2012; 15(4):263-6. [DOI:10.4103/0972-2327.104333] [PMID]
- Smajlović D, Salihović D, C Ibrahimagić O, Sinanović O, Vidović M. Analysis of risk factors, localization and 30-day prognosis of intracerebral hemorrhage. Bosnian Journal of Basic Medical Sciences. 2008; 8(2):121-5. [DOI:10.17305/bjbms.2008.2964] [PMID]
- Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, et al. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008; 70(11):848-52. [DOI:10.1212/01.wnl.0000304930.47751.75] [PMID]
- Togha M, Bakhtavar K. Factors associated with in-hospital mortality following intracerebral hemorrhage: A three-year study in Tehran, Iran. BMC neurology. 2004; 4:9.[DOI:10.1186/1471-2377-4-9] [PMID]
- Kim KH. Predictors of 30-day mortality and 90-day functional recovery after primary intracerebral hemorrhage: hospital based multivariate analysis in 585 patients. Journal of Korean Neurosurgical Society. 2009; 45(6):341-9. [DOI:10.3340/jkns.2009.45.6.341] [PMID]
- Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006; 66(8):1175-81. [DOI:10.1212/01.wnl.0000208408.98482.99] [PMID]
- Hayes SB, Benveniste RJ, Morcos JJ, Aziz-Sultan MA, Elhammady MS. Retrospective comparison of craniotomy and decompressive craniectomy for surgical evacuation of nontraumatic, supratentorial intracerebral hemorrhage. Neurosurgical Focus. 2013; 34(5):E3. [DOI:10.3171/2013.2.FOCUS12422] [PMID]
- Seeruch A. A comparison of treatment results between Decompressive Craniectomy with blood clot removal and Craniotomy with blood clot removal on basal Ganglion hemorrhage. The Thai Journal of Surgery. 2017; 38(1):1-6. [Link]
- Shimamura N, Munakata A, Naraoka M, Nakano T, Ohkuma H. Decompressive hemi-craniectomy is not necessary to rescue supratentorial hypertensive intracerebral hemorrhage patients: Consecutive single-center experience. Acta neurochirurgica. Supplement. 2011; 111:415-9. [DOI:10.1007/978-3-7091-0693-8_71] [PMID]
- Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46(7):2032-60. [DOI:10.1161/STR.0000000000000069] [PMID]
- Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. International Journal of Stroke. 2014; 9(7):840-55. [DOI:10.1111/ijs.12309] [PMID]
- Go GO, Park H, Lee CH, Hwang SH, Han JW, Park IS. The outcomes of spontaneous intracerebral hemorrhage in young adults - a clinical study. Journal of Cerebrovascular and Endovascular Neurosurgery. 2013; 15(3):214-20. [DOI:10.7461/jcen.2013.15.3.214] [PMID]
- Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, et al. Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Annals of neurology. 1991; 29(6):658-63. [DOI:10.1002/ana.410290614] [PMID]
- Lisk DR, Pasteur W, Rhoades H, Putnam RD, Grotta JC. Early presentation of hemispheric intracerebral hemorrhage: prediction of outcome and guidelines for treatment allocation. Neurology. 1994; 44(1):133-9. [DOI:10.1212/WNL.44.1.133] [PMID]
- Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical Care Medicine. 1999; 27(3):617-21. [DOI:10.1097/00003246-199903000-00045] [PMID]
- Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001; 32(4):891-7.[DOI:10.1161/01.STR.32.4.891] [PMID]
- Malinova V, Iliev B, Mielke D, Rohde V. Intracerebral hemorrhage-score allows a reliable prediction of mortality in patients with spontaneous intracerebral hemorrhage managed by Fibrinolytic therapy. Cerebrovascular diseases. 2019; 48(3-6):165-70. [DOI:10.1159/000504246] [PMID]
- Esmael A, El Sherif M, Saad M. Prediction of 30-days mortality of intracerebral hemorrhage by a powerful but easy to use intracerebral hemorrhage score. International Neuropsychiatric Disease Journal. 2016; 6(2):1-11. [DOI:10.9734/INDJ/2016/22414]
- Parry-Jones AR, Abid KA, Di Napoli M, Smith CJ, Vail A, Patel HC, et al. Accuracy and clinical usefulness of intracerebral hemorrhage grading scores: A direct comparison in a UK population. Stroke. 2013; 44(7):1840-5. [DOI:10.1161/STROKEAHA.113.001009] [PMID]
- Rehman WA, Anwar MS. Surgical outcome of spontaneous supra tentorial intracerebral hemorrhage. Pakistan journal of medical sciences. 2017; 33(4):804-7. [DOI:10.12669/pjms.334.12172] [PMID]
- Sondag L, Schreuder FHBM, Boogaarts HD, Rovers MM, Vandertop WP, Dammers R, et al. Neurosurgical intervention for supratentorial intracerebral hemorrhage. Annals of Neurology. 2020; 88(2):239-50. [DOI:10.1002/ana.25732] [PMID]
- Staykov D, Kuramatsu JB, Bardutzky J, Volbers B, Gerner ST, Kloska SP, et al. Efficacy and safety of combined intraventricular fibrinolysis with lumbar drainage for prevention of permanent shunt dependency after intracerebral hemorrhage with severe ventricular involvement: A randomized trial and individual patient data meta-analysis. Annals of neurology. 2017; 81(1):93-103. [DOI:10.1002/ana.24834] [PMID]
- Li Y, Zhang H, Wang X, She L, Yan Z, Zhang N, et al. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: a systematic review and meta-analysis. PLoS One. 2013; 8(11):e80599. [DOI:10.1371/journal.pone.0080599] [PMID]
- Passero S, Burgalassi L, D'Andrea P, Battistini N. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke. 1995; 26(7):1189-92. [DOI:10.1161/01.STR.26.7.1189] [PMID]
- Wu G, Shen Z, Wang L, Sun. S, Luo J, Mao Y. Post-operative re-bleeding in patients with hypertensive ICH is closely associated with the CT blend sign. BMC neurology. 2017; 17(1):131. [DOI:10.1186/s12883-017-0910-6] [PMID]
- Wong JM, Panchmatia JR, Ziewacz JE, Bader AM, Dunn IF, Laws ER, et al. Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurgical focus. 2012; 33(5):E16. [DOI:10.3171/2012.7.FOCUS12183] [PMID]
- Lo YT, See AAQ, King NKK. Decompressive craniectomy in spontaneous intracerebral hemorrhage: A case-control study. World Neurosurgery. 2017; 103:815-820.e2. [DOI: 10.1016/j.wneu.2017.04.025] [PMID].
Type of Study: Research |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |