Wed, Feb 4, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 116-125 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Balafif F, Wardhana D, Nazwar T, Mustofa M. Intraventricular Empyema Features of Surgery and Conservative’s Outcome: A Scoping Review. Iran J Neurosurg 2024; 10 : 13
URL: http://irjns.org/article-1-414-en.html
URL: http://irjns.org/article-1-414-en.html
1- Department of Surgery, Division of Neurosurgery, Facult of Medicine, Saiful Anwar Academic General Hospital, Brawijaya, Malang, Indonesia. , nsubfarhad@gmail.com
2- Department of Surgery, Division of Neurosurgery, Facult of Medicine, Saiful Anwar Academic General Hospital, Brawijaya, Malang, Indonesia.
2- Department of Surgery, Division of Neurosurgery, Facult of Medicine, Saiful Anwar Academic General Hospital, Brawijaya, Malang, Indonesia.
Full Text [PDF 1438 kb]
(715 Downloads)
| Abstract (HTML) (2500 Views)
Full Text: (1322 Views)
1. Introduction
Intraventricular empyema (IVE) is a rare and life-threatening condition characterized by the accumulation of infected pus within the brain’s ventricular system [1]. This condition poses significant challenges to healthcare providers due to its complexity, potentially devastating outcomes, and limited available data on optimal management strategies [2]. Advances in medical science and technology have led to a growing interest in understanding the best approaches to manage IVE [3]. As a result, medical professionals, researchers, and academicians from various fields, including neurosurgery, infectious diseases, and critical care, have actively pursued investigations to improve the clinical outcomes of patients suffering from this severe neurological infection [4].
Numerous microorganisms, such as bacteria, viruses, fungi, and parasites, can infect the ventricular system. Hematogenous dissemination to the subependyma of the choroid plexus, diffusion by contiguity from a brain abscess, or direct implantation as a result of trauma or surgery are all potential paths for infection of the ventricular system [5]. Hematogenous spread occurs when pathogens enter the bloodstream and are carried to the brain. The subependyma of the choroid plexus, which is responsible for producing cerebrospinal fluid (CSF), is particularly vulnerable to hematogenous spread due to its rich blood supply [6]. Once the pathogens reach the subependyma, they can infect the ventricular system and cause inflammation and damage. Direct implantation is a less common mechanism of infection and occurs when pathogens are introduced directly into the ventricular system through a traumatic injury or surgical procedure [7]. This can happen when a foreign object, such as a bullet or surgical instrument, penetrates the brain and enters the ventricular system.
Timely and effective management is essential not only to save lives but also to reduce healthcare costs and improve the overall quality of life for survivors. Investigating the most up-to-date and comprehensive information on the management and outcomes of IVE is crucial for healthcare providers, policymakers, and researchers to enhance patient care and resource allocation [3, 8].
Despite the growing interest in IVE, a substantial literature gap exists. While individual studies and case reports have contributed valuable insights, a comprehensive synthesis of available knowledge is lacking. To address this void, this scoping review will methodically summarize the current literature, pinpoint areas of agreement, and draw attention to knowledge gaps that can influence future studies. To better understand best practices and potential areas for future research, this study intends to give a comprehensive review of the existing body of information in this field by illuminating the variety of management strategies, their linked outcomes, and developing trends.
2. Materials and Methods
This review aims to consolidate existing literature on the treatment modalities, outcomes and evolving trends in IVE management. It will provide an overview of current knowledge and suggest avenues for future research and clinical practice enhancements in this medical domain. The synthesis of literature includes surgical and non-surgical interventions, their respective outcomes, complications, and practice patterns.
Research inquiry
The central inquiry of this study delves into the management options and outcomes associated with IVE. The objective is to explore the array of methods utilized, alongside their diagnostic approaches, interventions, outcomes and any emerging clinical practices. This comprehensive framework lays the groundwork for conducting a scoping review aimed at investigating the management options for IVE and evaluating the current understanding of their efficacy, outcomes and evolving trends.
Inclusion criteria
The inclusion criteria were formulated based on the population, concept and context (PCC) framework in consultation with academic librarians. The target population encompasses individuals of all ages diagnosed with IVE. Search terms were developed to encompass keywords and controlled vocabulary terms (MeSH terms) to ensure a comprehensive retrieval of relevant literature.
Concept
This review examines the gamut of management strategies employed for IVE, encompassing surgical interventions, non-surgical approaches, conservative management, minimally invasive techniques and other therapeutic strategies.
Context
The medical context under scrutiny is the treatment and management of IVE across various clinical settings, such as hospitals, medical centers, and research institutions. These settings witness the implementation of diverse management approaches to address this specific medical condition.
Type of sources
Primary research studies, whether quantitative, qualitative, or mixed methods, were considered for inclusion in this review.
Study selection
The inclusion criteria remained relatively stable throughout the review process, including primary research studies available in full text and written in English. The exclusion criteria included studies not directly addressing IVE or lacking relevance to the core of the study.
Search strategy
A literature search was conducted across PubMed, Science Direct, MEDLINE, Embase, Scopus, and Google Scholar due to their extensive collections related to IVE. Articles published in full text, involving research on the condition and written in English, were included for evaluation.
Data collection
All identified literature underwent data collection and summarization using Microsoft Excel and Zotero. Duplicate articles were eliminated during title review, followed by screening based on titles and abstracts to ensure adherence to inclusion criteria. Relevant articles were then reviewed in full text by two independent reviewers, with disagreements resolved by a third reviewer. The scoping review methodology was documented following the preferred reporting items for systematic reviews and meta-analyses scoping reviews (PRISMA-ScR) guidelines.
Data mapping
Data mapping involved extracting pertinent information from the literature relevant to the research question. Mapped data included author names, patient demographics, and symptoms, site of empyema, CSF and blood culture results, neurosurgical treatments, antibiotic regimens, and outcomes. A narrative summary was provided to elucidate key findings from each data extraction set with the research objectives.
3. Results
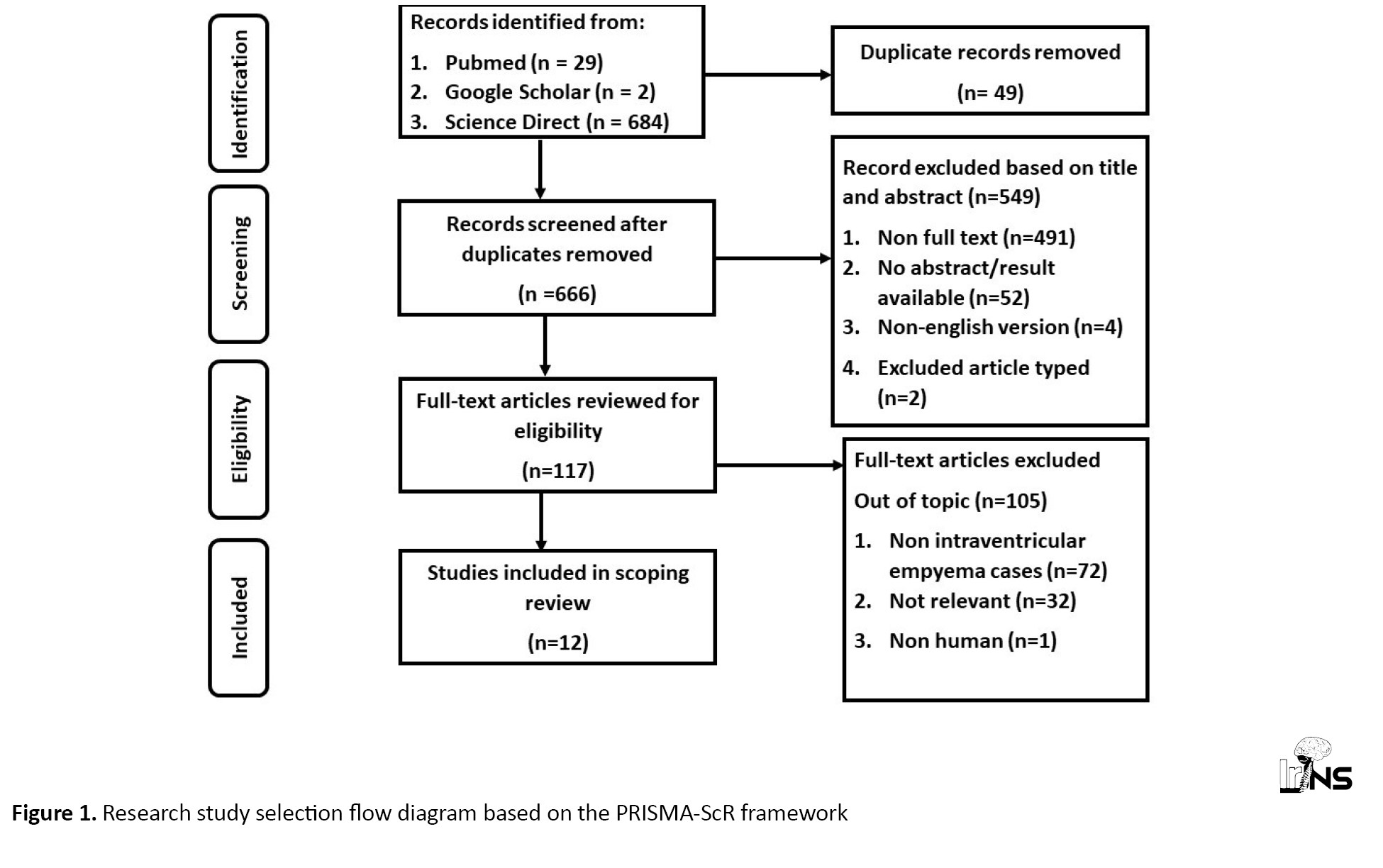
Article Search Results: The process of article search consisted of three distinct stages, identification, screening, and feasibility evaluation. The identification phase commenced by conducting a comprehensive search for pertinent publications in three databases, resulting in the retrieval of 715 papers relevant to the review’s keywords. The article titles were compiled in Microsoft Excel and organized in alphabetical order to identify duplicates. A total of 49 duplicate articles were found and subsequently excluded from the review process. Subsequently, 666 publications underwent a screening process where their titles and abstracts were evaluated to determine their relevance to the predetermined inclusion criteria. A total of 666 publications that satisfied the inclusion criteria were identified, while 549 articles failed to meet the inclusion criteria due to being review articles, lacking abstracts or results, and not being written in English. Out of the 117 papers that underwent the screening procedure, 105 articles were identified as irrelevant to the review topic and were excluded from further assessment. A total of 12 papers about this topic were discovered, leading to the review of twelve articles. Figure 1.
Article eligibility results
From the 12 kinds of literature, the incidence of IVE predominantly affects men compared to women. The age range of patients with IVE varies from 17 years to 81 years. This suggests that IVE can affect individuals in a wide age range. The condition appears to affect men and women, with a fairly balanced representation in the provided cases. Cases involved men (24, 17, 35, 69, 39, 63, 62, 66, 55 and 49 years) and women (57 and 61 years). This indicates that IVE is not limited to a particular gender.
Headache is a common clinical presentation in several cases, including patients with fever with chills, headache after a trip, intermittent occipital headache, and frontal headache. Fever is another prevalent symptom, often accompanying headache. Patients presented with fever with chills, fever, abnormal movements and acute headaches with vomiting and fever. Elevated body temperature is a common finding in IVE. Various neurological symptoms were observed, including right hemiplegia, aphasia, facial paresis and right extensor plantar response. In one case, a patient with intravenous drug abuse presented with a headache and left earache. Some patients experienced gastrointestinal symptoms, such as diarrhea, which could be associated with systemic infection or inflammation. In a few cases, psychiatric symptoms, such as anxiousness, restlessness and psychomotor retardation were noted, along with generalized weakness and unusual movements (suction, such as mouth dyskinesia). Some patients had comorbidities or other conditions, such as COVID-19.
The sites of empyema within the brain’s ventricular system listed in Table 1 indicate a diverse range of locations of pus accumulation and infection.
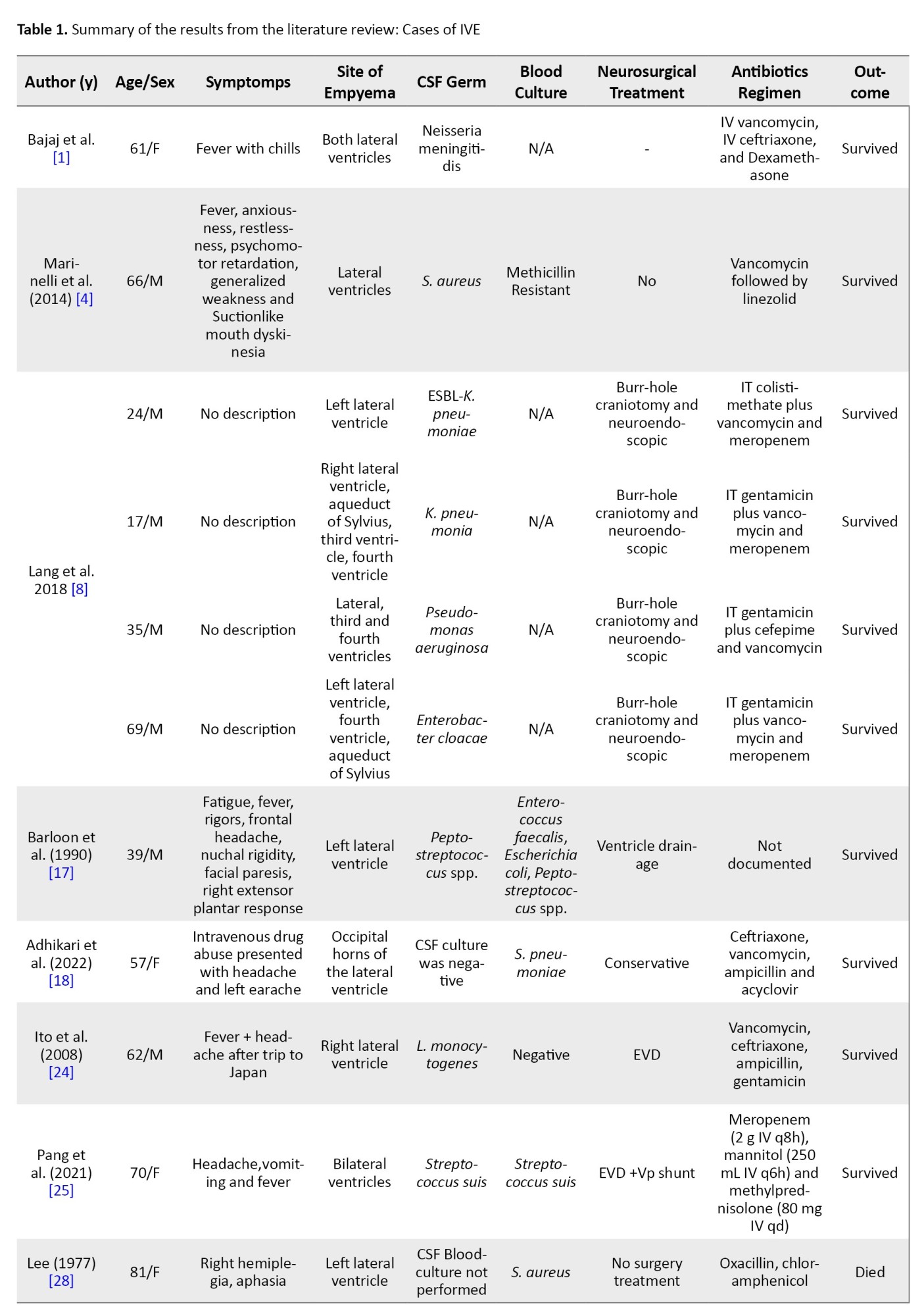
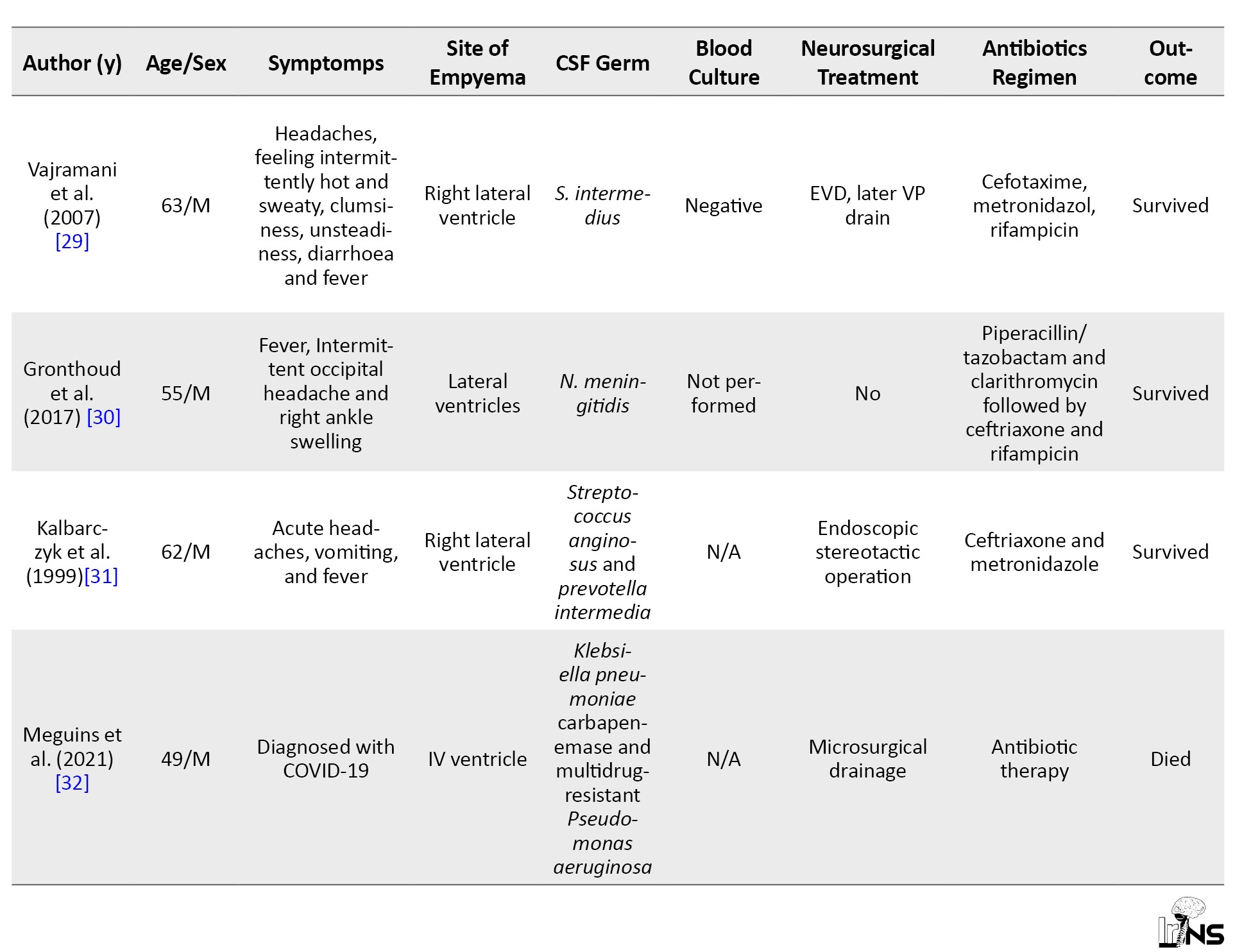
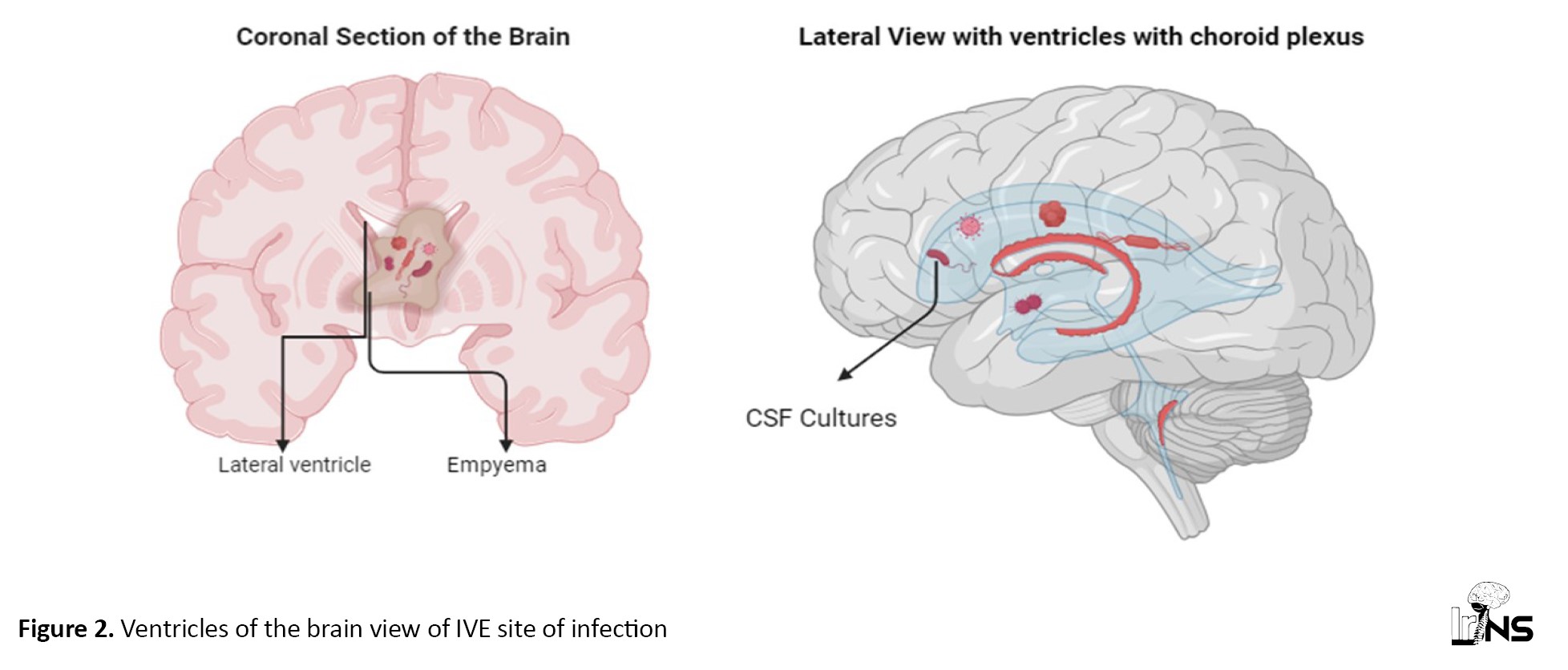
These locations encompass various parts of the ventricular system, including the lateral ventricles, third ventricle, and fourth ventricle, and specific regions within them, such as the occipital horns (Figure 2). The presence of empyema in both the left and right lateral ventricles and its involvement in regions, such as the aqueduct of Sylvius highlights the potential for extensive and complex infections. Additionally, the mention of empyema extending into the frontal and parietal lobes underscores the critical nature of diagnosing and managing this condition promptly.
Drug-resistant strains, including methicillin-resistant staphylococcus aureus and klebsiella pneumoniae carbapenemase, indicate the concerning issue of antibiotic resistance in central nervous system (CNS) infections. Several typical pathogens linked to bacterial meningitis, such as Streptococcus pneumoniae and neisseria meningitidis, are also present. Some cases yielded negative CSF culture results, underscoring the diagnostic challenges and potential need for alternative diagnostic methods. Additionally, the presence of polymicrobial infections, as seen with Streptococcus anginosus and Prevotella intermedia, highlights the complexity of CNS infections.
Conversely, patients who received conservative treatment without surgery also demonstrated favorable outcomes, further highlighting the importance of tailored antibiotic regimens. However, it’s notable that not all patients survived, as some who underwent procedures, such as bilateral frontal ventriculostomy or microsurgical drainage did not make it, underscoring the complexity and seriousness of CNS infections. The dataset emphasizes the significance of individualized treatment strategies and timely interventions to optimize patient outcomes in cases of central nervous system infections.
4. Discussion
Inflammation of the ependymal lining of the cerebral ventricular system, commonly known as IVE, ventriculitis, pyogenic ventriculitis, or intraventricular brain abscesses, is a suppurative infection of the ventricles. It typically results from meningitis, a ruptured abscess, or infections related to shunts or catheters. The accumulation of pus and other fluids in these ventricles, caused by an infection, can result in various symptoms and problems. Adults have sporadic cases of this disorder, which mostly affects newborns and infants. The neurologic examination can be a lifesaver in both routine and life-threatening situations. It aids in the diagnosis of neurologic involvement in specific diseases, which in turn allows for more targeted patient care [9].
Based on the data tabulated in this study, various symptoms can be experienced, such as a headache, left earache, fever with chills, right hemiplegia, difficulty speaking, extreme tiredness, rigors, frontal headache, nuchal rigidity, facial paresis, right extensor plantar response, random bouts of heat and sweat, dizziness, nausea, vomiting, and fever [10-13], nausea, vomiting, altered mental status, and seizures [14]. Severe headaches are a common symptom, often accompanied by increased intracranial pressure. Furthermore, depending on the extent and location of the inflammation, patients may develop neurological symptoms, such as weakness, numbness, or visual disturbances. Fever is a common symptom of many infections due to the number of events resulting in IVE. Alongside symptoms, such as anxiousness, restlessness, psychomotor retardation, generalized weakness, and suction-like mouth dyskinesia [10-13].
Additionally, intermittent occipital headaches, right ankle swelling and abnormal movements of the extremities were also observed. The diagnosis of COVID-19 suggests that some of these symptoms may have been related to the viral infection. The right hemiplegia and aphasia may have required further evaluation to rule out neurological conditions. Improving patient survival rates and making quick decisions about patient care are both made possible by accurate and timely evaluations of the extent of injuries and neurologic involvement in emergencies [15].
To better detect pyogenic ventriculitis and effect rapid therapy, it is necessary to identify computed tomography (CT) and magnetic resonance imaging (MRI) characteristics of the condition. Ventricular debris was observed. Due to the high protein concentration and, potentially, necrotic material, the shape of this substance may be uneven. The most common ventriculitis imaging sign (94%) was the presence of ventricular debris [16]. Imaging studies, such as CT scans and MRI can help diagnose IVE, but they may not always be conclusive. Among the imaging methods that have been used for these ventriculitis cases include nuclear scintigraphy, ultrasonography and CT scans [17]. The CT scan reveals an augmentation in ventricular size, while the patient exhibits an elevated count of white blood cells [8]. Brain imaging confirms the diagnosis by a CT scan, which is recently insensitive. MRI is more reliable than a CT scan in identifying pus in the cerebral ventricular system and ependymal inflammation. MRI can distinguish between blood and pus and reveals abnormal ventricular detritus [18]. Only diffusion MRI sequences showed purulent material in the ventricles for diagnosing pyogenic ventriculitis [4].
In these current case report studies, IVE mostly occurs in bilateral ventricles of the brain. Others may occur in the left or right ventricle, aqueduct of sylvius, third, fourth, and occipital horns of the lateral ventricle. In some cases, a lumbar puncture may be necessary to obtain a sample of CSF for analysis. An increase in the number of white blood cells (leucocytes) in the CSF can indicate an infection in the brain or its surrounding structures. When neutrophils predominate in a bacterial infection, lymphocytes predominate in a viral one, and vice versa. Our results corroborate the results of Adhikari and Marineli, who demonstrated the significance of patient leucocytosis screening. Following a negative neurological evaluation two weeks later, the CSF leukocyte count dropped to 64/mm3. Previously, it had been 1000/mm3, with normal values ranging from 0 to 5 [4]. CSF analysis was consistent with the bacterial infection including neutrophilic leukocytosis (2,300/mm3 of leukocytes with 83% polymorphonuclear leukocytes [PMNs]), after three days of antimicrobial therapy, the patient improved significantly and leukocytosis was back to baseline [18].
Gram-negative meningitis was the most common form of infection associated with pyogenic ventriculitis (nine [60%] of 15 cases in which an organism was established by culture or Gram stain), followed by Staphylococcus species [16]. Streptococci constitute the most cases (44.9%) of which S. pneumoniae is the primary cause of ventriculitis in patients with bacterial meningitis. Additionally, milleri group streptococci were identified as a significant source of ventriculitis associated with brain abscesses [19].
Reducing the mortality rate associated with empyema requires prompt diagnosis, treatment, and careful management of the illness. When patients experience a poor response to systemic antimicrobial therapy alone for healthcare-associated ventriculitis and meningitis, intraventricular antimicrobial therapy should be investigated [20].
Current best practices include administering antibiotics for an extended time, monitoring neurological symptoms, and conducting neuroimaging [18]. The administration of antibiotics at an earlier stage and over an extended time yields more favorable results, obviating the necessity for neurosurgical intervention [21]. How exactly antibiotics get to an intraventricular brain abscess is a mystery. Because there is less blood supply to the brain in an intraventricular abscess, antibiotic delivery may be less effective than intraparenchymal [22] It is difficult to practice intraventricular antibiotics in neonates, although they may be effective in treating ventriculitis caused by multidrug-resistant bacteria [23].
As the IVE condition is an emergency from the outset, operative treatment must be done immediately. A craniotomy is one of the operative options to address this problem. The data tabulation of this study also mentioned that the surgical procedures used included craniotomy, ventriculostomy, EVD, microsurgery and endoscopy [8, 24, 25].
Achieving CSF sterility rapidly is possible with neuroendoscopic lavage, minimally invasive treatment for purulent ventriculitis in neonates [26].
Reports indicate that the neuroendoscope has shown efficacy in treating a ruptured brain abscess within the lateral ventricles [27]. From the tabulated data, we conclude that surgery is still a necessary and immediate option following the patient's diagnosis. Thorough action will achieve optimal results to save the patient from the threat of brain infection. Hence, it cannot be refuted that performing surgical removal of the intraventricular brain abscesses at an earlier stage could have potentially averted the occurrence of isolated ventricular dilatation [22]. Clear guidelines have been established for the management of ventricular catheter-related ventriculitis. However, there is a lack of specific recommendations or expert opinions regarding the optimal treatment regimen or duration for primary bacterial ventriculitis. Further clinical competence is necessary to determine the most appropriate treatment (surgical versus conservative) for these cases.
5. Conclusion
Most patients who underwent a conservative approach combined with surgery survived their infections. This suggests that a surgical intervention, when complemented by appropriate conservative measures, can be effective in managing these conditions.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, study design, data analysis and interpretation: Farhad Balafif, Donny Wisnu Wardhana and Tommy Alfandy Nazwar; Data collection: Farhad Balafif and Mustofa Mustofa; Drafting the article: Tommy Alfandy Nazwar and Mustofa Mustofa; Critically revising, review and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We thank all SMF Bedah Saraf RSSA Malang members and staff.
References
Intraventricular empyema (IVE) is a rare and life-threatening condition characterized by the accumulation of infected pus within the brain’s ventricular system [1]. This condition poses significant challenges to healthcare providers due to its complexity, potentially devastating outcomes, and limited available data on optimal management strategies [2]. Advances in medical science and technology have led to a growing interest in understanding the best approaches to manage IVE [3]. As a result, medical professionals, researchers, and academicians from various fields, including neurosurgery, infectious diseases, and critical care, have actively pursued investigations to improve the clinical outcomes of patients suffering from this severe neurological infection [4].
Numerous microorganisms, such as bacteria, viruses, fungi, and parasites, can infect the ventricular system. Hematogenous dissemination to the subependyma of the choroid plexus, diffusion by contiguity from a brain abscess, or direct implantation as a result of trauma or surgery are all potential paths for infection of the ventricular system [5]. Hematogenous spread occurs when pathogens enter the bloodstream and are carried to the brain. The subependyma of the choroid plexus, which is responsible for producing cerebrospinal fluid (CSF), is particularly vulnerable to hematogenous spread due to its rich blood supply [6]. Once the pathogens reach the subependyma, they can infect the ventricular system and cause inflammation and damage. Direct implantation is a less common mechanism of infection and occurs when pathogens are introduced directly into the ventricular system through a traumatic injury or surgical procedure [7]. This can happen when a foreign object, such as a bullet or surgical instrument, penetrates the brain and enters the ventricular system.
Timely and effective management is essential not only to save lives but also to reduce healthcare costs and improve the overall quality of life for survivors. Investigating the most up-to-date and comprehensive information on the management and outcomes of IVE is crucial for healthcare providers, policymakers, and researchers to enhance patient care and resource allocation [3, 8].
Despite the growing interest in IVE, a substantial literature gap exists. While individual studies and case reports have contributed valuable insights, a comprehensive synthesis of available knowledge is lacking. To address this void, this scoping review will methodically summarize the current literature, pinpoint areas of agreement, and draw attention to knowledge gaps that can influence future studies. To better understand best practices and potential areas for future research, this study intends to give a comprehensive review of the existing body of information in this field by illuminating the variety of management strategies, their linked outcomes, and developing trends.
2. Materials and Methods
This review aims to consolidate existing literature on the treatment modalities, outcomes and evolving trends in IVE management. It will provide an overview of current knowledge and suggest avenues for future research and clinical practice enhancements in this medical domain. The synthesis of literature includes surgical and non-surgical interventions, their respective outcomes, complications, and practice patterns.
Research inquiry
The central inquiry of this study delves into the management options and outcomes associated with IVE. The objective is to explore the array of methods utilized, alongside their diagnostic approaches, interventions, outcomes and any emerging clinical practices. This comprehensive framework lays the groundwork for conducting a scoping review aimed at investigating the management options for IVE and evaluating the current understanding of their efficacy, outcomes and evolving trends.
Inclusion criteria
The inclusion criteria were formulated based on the population, concept and context (PCC) framework in consultation with academic librarians. The target population encompasses individuals of all ages diagnosed with IVE. Search terms were developed to encompass keywords and controlled vocabulary terms (MeSH terms) to ensure a comprehensive retrieval of relevant literature.
Concept
This review examines the gamut of management strategies employed for IVE, encompassing surgical interventions, non-surgical approaches, conservative management, minimally invasive techniques and other therapeutic strategies.
Context
The medical context under scrutiny is the treatment and management of IVE across various clinical settings, such as hospitals, medical centers, and research institutions. These settings witness the implementation of diverse management approaches to address this specific medical condition.
Type of sources
Primary research studies, whether quantitative, qualitative, or mixed methods, were considered for inclusion in this review.
Study selection
The inclusion criteria remained relatively stable throughout the review process, including primary research studies available in full text and written in English. The exclusion criteria included studies not directly addressing IVE or lacking relevance to the core of the study.
Search strategy
A literature search was conducted across PubMed, Science Direct, MEDLINE, Embase, Scopus, and Google Scholar due to their extensive collections related to IVE. Articles published in full text, involving research on the condition and written in English, were included for evaluation.
Data collection
All identified literature underwent data collection and summarization using Microsoft Excel and Zotero. Duplicate articles were eliminated during title review, followed by screening based on titles and abstracts to ensure adherence to inclusion criteria. Relevant articles were then reviewed in full text by two independent reviewers, with disagreements resolved by a third reviewer. The scoping review methodology was documented following the preferred reporting items for systematic reviews and meta-analyses scoping reviews (PRISMA-ScR) guidelines.
Data mapping
Data mapping involved extracting pertinent information from the literature relevant to the research question. Mapped data included author names, patient demographics, and symptoms, site of empyema, CSF and blood culture results, neurosurgical treatments, antibiotic regimens, and outcomes. A narrative summary was provided to elucidate key findings from each data extraction set with the research objectives.
3. Results
Article Search Results: The process of article search consisted of three distinct stages, identification, screening, and feasibility evaluation. The identification phase commenced by conducting a comprehensive search for pertinent publications in three databases, resulting in the retrieval of 715 papers relevant to the review’s keywords. The article titles were compiled in Microsoft Excel and organized in alphabetical order to identify duplicates. A total of 49 duplicate articles were found and subsequently excluded from the review process. Subsequently, 666 publications underwent a screening process where their titles and abstracts were evaluated to determine their relevance to the predetermined inclusion criteria. A total of 666 publications that satisfied the inclusion criteria were identified, while 549 articles failed to meet the inclusion criteria due to being review articles, lacking abstracts or results, and not being written in English. Out of the 117 papers that underwent the screening procedure, 105 articles were identified as irrelevant to the review topic and were excluded from further assessment. A total of 12 papers about this topic were discovered, leading to the review of twelve articles. Figure 1.
Article eligibility results
From the 12 kinds of literature, the incidence of IVE predominantly affects men compared to women. The age range of patients with IVE varies from 17 years to 81 years. This suggests that IVE can affect individuals in a wide age range. The condition appears to affect men and women, with a fairly balanced representation in the provided cases. Cases involved men (24, 17, 35, 69, 39, 63, 62, 66, 55 and 49 years) and women (57 and 61 years). This indicates that IVE is not limited to a particular gender.
Headache is a common clinical presentation in several cases, including patients with fever with chills, headache after a trip, intermittent occipital headache, and frontal headache. Fever is another prevalent symptom, often accompanying headache. Patients presented with fever with chills, fever, abnormal movements and acute headaches with vomiting and fever. Elevated body temperature is a common finding in IVE. Various neurological symptoms were observed, including right hemiplegia, aphasia, facial paresis and right extensor plantar response. In one case, a patient with intravenous drug abuse presented with a headache and left earache. Some patients experienced gastrointestinal symptoms, such as diarrhea, which could be associated with systemic infection or inflammation. In a few cases, psychiatric symptoms, such as anxiousness, restlessness and psychomotor retardation were noted, along with generalized weakness and unusual movements (suction, such as mouth dyskinesia). Some patients had comorbidities or other conditions, such as COVID-19.
The sites of empyema within the brain’s ventricular system listed in Table 1 indicate a diverse range of locations of pus accumulation and infection.


These locations encompass various parts of the ventricular system, including the lateral ventricles, third ventricle, and fourth ventricle, and specific regions within them, such as the occipital horns (Figure 2). The presence of empyema in both the left and right lateral ventricles and its involvement in regions, such as the aqueduct of Sylvius highlights the potential for extensive and complex infections. Additionally, the mention of empyema extending into the frontal and parietal lobes underscores the critical nature of diagnosing and managing this condition promptly.
Drug-resistant strains, including methicillin-resistant staphylococcus aureus and klebsiella pneumoniae carbapenemase, indicate the concerning issue of antibiotic resistance in central nervous system (CNS) infections. Several typical pathogens linked to bacterial meningitis, such as Streptococcus pneumoniae and neisseria meningitidis, are also present. Some cases yielded negative CSF culture results, underscoring the diagnostic challenges and potential need for alternative diagnostic methods. Additionally, the presence of polymicrobial infections, as seen with Streptococcus anginosus and Prevotella intermedia, highlights the complexity of CNS infections.
Conversely, patients who received conservative treatment without surgery also demonstrated favorable outcomes, further highlighting the importance of tailored antibiotic regimens. However, it’s notable that not all patients survived, as some who underwent procedures, such as bilateral frontal ventriculostomy or microsurgical drainage did not make it, underscoring the complexity and seriousness of CNS infections. The dataset emphasizes the significance of individualized treatment strategies and timely interventions to optimize patient outcomes in cases of central nervous system infections.
4. Discussion
Inflammation of the ependymal lining of the cerebral ventricular system, commonly known as IVE, ventriculitis, pyogenic ventriculitis, or intraventricular brain abscesses, is a suppurative infection of the ventricles. It typically results from meningitis, a ruptured abscess, or infections related to shunts or catheters. The accumulation of pus and other fluids in these ventricles, caused by an infection, can result in various symptoms and problems. Adults have sporadic cases of this disorder, which mostly affects newborns and infants. The neurologic examination can be a lifesaver in both routine and life-threatening situations. It aids in the diagnosis of neurologic involvement in specific diseases, which in turn allows for more targeted patient care [9].
Based on the data tabulated in this study, various symptoms can be experienced, such as a headache, left earache, fever with chills, right hemiplegia, difficulty speaking, extreme tiredness, rigors, frontal headache, nuchal rigidity, facial paresis, right extensor plantar response, random bouts of heat and sweat, dizziness, nausea, vomiting, and fever [10-13], nausea, vomiting, altered mental status, and seizures [14]. Severe headaches are a common symptom, often accompanied by increased intracranial pressure. Furthermore, depending on the extent and location of the inflammation, patients may develop neurological symptoms, such as weakness, numbness, or visual disturbances. Fever is a common symptom of many infections due to the number of events resulting in IVE. Alongside symptoms, such as anxiousness, restlessness, psychomotor retardation, generalized weakness, and suction-like mouth dyskinesia [10-13].
Additionally, intermittent occipital headaches, right ankle swelling and abnormal movements of the extremities were also observed. The diagnosis of COVID-19 suggests that some of these symptoms may have been related to the viral infection. The right hemiplegia and aphasia may have required further evaluation to rule out neurological conditions. Improving patient survival rates and making quick decisions about patient care are both made possible by accurate and timely evaluations of the extent of injuries and neurologic involvement in emergencies [15].
To better detect pyogenic ventriculitis and effect rapid therapy, it is necessary to identify computed tomography (CT) and magnetic resonance imaging (MRI) characteristics of the condition. Ventricular debris was observed. Due to the high protein concentration and, potentially, necrotic material, the shape of this substance may be uneven. The most common ventriculitis imaging sign (94%) was the presence of ventricular debris [16]. Imaging studies, such as CT scans and MRI can help diagnose IVE, but they may not always be conclusive. Among the imaging methods that have been used for these ventriculitis cases include nuclear scintigraphy, ultrasonography and CT scans [17]. The CT scan reveals an augmentation in ventricular size, while the patient exhibits an elevated count of white blood cells [8]. Brain imaging confirms the diagnosis by a CT scan, which is recently insensitive. MRI is more reliable than a CT scan in identifying pus in the cerebral ventricular system and ependymal inflammation. MRI can distinguish between blood and pus and reveals abnormal ventricular detritus [18]. Only diffusion MRI sequences showed purulent material in the ventricles for diagnosing pyogenic ventriculitis [4].
In these current case report studies, IVE mostly occurs in bilateral ventricles of the brain. Others may occur in the left or right ventricle, aqueduct of sylvius, third, fourth, and occipital horns of the lateral ventricle. In some cases, a lumbar puncture may be necessary to obtain a sample of CSF for analysis. An increase in the number of white blood cells (leucocytes) in the CSF can indicate an infection in the brain or its surrounding structures. When neutrophils predominate in a bacterial infection, lymphocytes predominate in a viral one, and vice versa. Our results corroborate the results of Adhikari and Marineli, who demonstrated the significance of patient leucocytosis screening. Following a negative neurological evaluation two weeks later, the CSF leukocyte count dropped to 64/mm3. Previously, it had been 1000/mm3, with normal values ranging from 0 to 5 [4]. CSF analysis was consistent with the bacterial infection including neutrophilic leukocytosis (2,300/mm3 of leukocytes with 83% polymorphonuclear leukocytes [PMNs]), after three days of antimicrobial therapy, the patient improved significantly and leukocytosis was back to baseline [18].
Gram-negative meningitis was the most common form of infection associated with pyogenic ventriculitis (nine [60%] of 15 cases in which an organism was established by culture or Gram stain), followed by Staphylococcus species [16]. Streptococci constitute the most cases (44.9%) of which S. pneumoniae is the primary cause of ventriculitis in patients with bacterial meningitis. Additionally, milleri group streptococci were identified as a significant source of ventriculitis associated with brain abscesses [19].
Reducing the mortality rate associated with empyema requires prompt diagnosis, treatment, and careful management of the illness. When patients experience a poor response to systemic antimicrobial therapy alone for healthcare-associated ventriculitis and meningitis, intraventricular antimicrobial therapy should be investigated [20].
Current best practices include administering antibiotics for an extended time, monitoring neurological symptoms, and conducting neuroimaging [18]. The administration of antibiotics at an earlier stage and over an extended time yields more favorable results, obviating the necessity for neurosurgical intervention [21]. How exactly antibiotics get to an intraventricular brain abscess is a mystery. Because there is less blood supply to the brain in an intraventricular abscess, antibiotic delivery may be less effective than intraparenchymal [22] It is difficult to practice intraventricular antibiotics in neonates, although they may be effective in treating ventriculitis caused by multidrug-resistant bacteria [23].
As the IVE condition is an emergency from the outset, operative treatment must be done immediately. A craniotomy is one of the operative options to address this problem. The data tabulation of this study also mentioned that the surgical procedures used included craniotomy, ventriculostomy, EVD, microsurgery and endoscopy [8, 24, 25].
Achieving CSF sterility rapidly is possible with neuroendoscopic lavage, minimally invasive treatment for purulent ventriculitis in neonates [26].
Reports indicate that the neuroendoscope has shown efficacy in treating a ruptured brain abscess within the lateral ventricles [27]. From the tabulated data, we conclude that surgery is still a necessary and immediate option following the patient's diagnosis. Thorough action will achieve optimal results to save the patient from the threat of brain infection. Hence, it cannot be refuted that performing surgical removal of the intraventricular brain abscesses at an earlier stage could have potentially averted the occurrence of isolated ventricular dilatation [22]. Clear guidelines have been established for the management of ventricular catheter-related ventriculitis. However, there is a lack of specific recommendations or expert opinions regarding the optimal treatment regimen or duration for primary bacterial ventriculitis. Further clinical competence is necessary to determine the most appropriate treatment (surgical versus conservative) for these cases.
5. Conclusion
Most patients who underwent a conservative approach combined with surgery survived their infections. This suggests that a surgical intervention, when complemented by appropriate conservative measures, can be effective in managing these conditions.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, study design, data analysis and interpretation: Farhad Balafif, Donny Wisnu Wardhana and Tommy Alfandy Nazwar; Data collection: Farhad Balafif and Mustofa Mustofa; Drafting the article: Tommy Alfandy Nazwar and Mustofa Mustofa; Critically revising, review and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We thank all SMF Bedah Saraf RSSA Malang members and staff.
References
- Bajaj D, Agrawal A, Gandhi D, Varughese R, Gupta S, Regelmann D. Intraventricular empyema caused by Neisseria meningitidis. IDCases. 2019; 15:e00503. [DOI:10.1016/j.idcr.2019.e00503] [PMID] [PMCID]
- Rashnoo F, Farsad SM, Pejhan S, Faz AA, Mirhashemi SH, Soori M, et al. A prospective study comparing treatment outcomes of empyema management techniques: Chest tube vs. video-assisted thoracoscopic surgery. Russian Open Medical Journal. 2022; 11(1):114. [DOI:10.15275/rusomj.2022.0114]
- Tandean S, Hendriansyah L, Djokomuljanto S, Saputra NA, Juliansen A, Valentina S, et al. Neuroendoscopic aspiration and lavage of intraventricular empyema following shunt infection in infants. The Pan African Medical Journal. 2018; 31:15. [DOI:10.11604/pamj.2018.31.15.16631] [PMID] [PMCID]
- Marinelli L, Trompetto C, Cocito L. Diffusion magnetic resonance imaging diagnostic relevance in pyogenic ventriculitis with an atypical presentation: A case report. BMC Research Notes. 2014; 7:149. [DOI:10.1186/1756-0500-7-149] [PMID] [PMCID]
- Schwerk C, Tenenbaum T, Schroten H. Roles of the choroid plexus in CNS infections. In: Praetorius J, Blazer-Yost B, Damkier H, editors. Role of the choroid plexus in health and disease. Berlin: Springer; 2020. [DOI:10.1007/978-1-0716-0536-3_11]
- Fields MM. How to recognize and treat neoplastic meningitis. Journal of the Advanced Practitioner in Oncology. 2013; 4(3):155. [DOI:10.6004/jadpro.2013.4.3.3]
- Diederichs A, Pawlik E, Barnbrock A, Schöning S, Konczalla J, Finger T,et al. Cerebrospinal fluid system infection in children with cancer: A retrospective analysis over 14 years in a major european pediatric cancer center. Antibiotics. 2022; 11(8):1113. [DOI:10.3390/antibiotics11081113] [PMID] [PMCID]
- Lang M, Habboub G, Moore NZ, Recinos VMR, Mohammadi AM, Nagel S, et al. Neuroendoscopic evacuation of intraventricular empyema using a side-cutting aspiration device. Journal of Clinical Neuroscience. 2018; 47:323-7. [DOI:10.1016/j.jocn.2017.09.029] [PMID]
- Kareemi H, Pratte M, English S, Hendin A. Initial diagnosis and management of acutely elevated intracranial pressure. Journal of Intensive Care Medicine. 2023; 38(7):643-50. [DOI:10.1177/08850666231156589] [PMID] [PMCID]
- Tomita Y, Shimazu Y, Kawakami M, Matsumoto H, Fujii K, Kameda M, et al. Pyogenic ventriculitis after anterior skull base surgery treated with endoscopic ventricular irrigation and reconstruction using a vascularized flap. Acta Medica Okayama. 2021; 75(2):243-8. [Link]
- Shah GS. Pyogenic ventriculitis and meningitis caused by streptococcus acidominimus in humans: A case report. The American Journal of Case Reports. 2018; 19:329-34. [DOI:10.12659/AJCR.908000] [PMID] [PMCID]
- Hatakeyama M, Kanazawa M, Ishihara A, Tanabe Y, Shimohata T, Nishizawa M. [Pathognomonic magnetic resonance imaging (MRI) finding of fluid-fluid level in pyogenic ventriculitis: two case reports (Japanese)]. Rinsho Shinkeigaku. 2014; 54(9):732-7. [DOI:10.5692/clinicalneurol.54.732] [PMID]
- Maeda K, Sanada M, Kawai H, Fujino H, Morita Y, Itoh Y. Pyogenic ventriculitis with ruptured brain abscess. Internal Medicine. 2006; 45(13):835-6. [DOI:10.2169/internalmedicine.45.1861] [PMID]
- Karvouniaris M, Brotis A, Tsiakos K, Palli E, Koulenti D. Current perspectives on the diagnosis and management of healthcare-associated ventriculitis and meningitis. Infection and Drug Resistance. 2022; 15:697-721. [DOI:10.2147/IDR.S326456] [PMID] [PMCID]
- Marklund N. The neurological wake-up test-a role in neurocritical care monitoring of traumatic brain injury patients? Frontiers in Neurology. 2017; 8:540. [DOI:10.3389/fneur.2017.00540] [PMID] [PMCID]
- Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. American Journal of Neuroradiology. 2001; 22(8):1510-6. [Link]
- Barloon TJ, Yuh WT, Knepper LE, Biller J, Ryals TJ, Sato Y. Cerebral ventriculitis: MR findings. Journal of Computer Assisted Tomography. 1990; 14(2):272-5. [DOI:10.1097/00004728-199003000-00021] [PMID]
- Adhikari P, Antala D, Pyakuryal B, Muhammed A, Pudasainee P, Friedman H, et al. Community-acquired meningitis complicated by pyogenic ventriculitis: A case report. Cureus. 2022; 14(4):e23907. [DOI:10.7759/cureus.23907] [PMID] [PMCID]
- Luque-Paz D, Revest M, Eugène F, Boukthir S, Dejoies L, Tattevin P, et al. Ventriculitis: A severe complication of central nervous system infections. Open Forum Infectious Diseases. 2021; 8(6):ofab216. [DOI:10.1093/ofid/ofab216] [PMID] [PMCID]
- Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, et al. 2017 infectious diseases society of america's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clinical Infectious Diseases. 2017; 64(6):e34-65. [DOI:10.1093/cid/ciw861] [PMID] [PMCID]
- Maheshwarappa HM, Rai AV. A rare case of primary pyogenic ventriculitis in a patient with community-acquired meningitis. Indian Journal of Critical Care Medicine. 2022; 26(7):8746. [DOI:10.5005/jp-journals-10071-24273] [PMID] [PMCID]
- Inamasu J, Moriya S, Kawazoe Y, Nagahisa S, Hasegawa M, Hirose Y. Primary intraventricular brain abscess resulting in isolated dilation of the inferior horn and unilateral hydrocephalus. Case Reports in Neurology. 2015; 7(2):156-61. [DOI:10.1159/000437255] [PMID] [PMCID]
- Hussain K, Sohail Salat M, Ambreen G, Iqbal J. Neurodevelopment outcome of neonates treated with intraventricular colistin for ventriculitis caused by multiple drug-resistant pathogens-A case series. Frontiers in Pediatrics. 2021; 8:582375. [DOI:10.3389/fped.2020.582375] [PMID] [PMCID]
- Ito H, Kobayashi S, Iino M, Kamei T, Takanashi Y. Listeria monocytogenes meningoencephalitis presenting with hydrocephalus and ventriculitis. Internal Medicine. 2008; 47(4):323-4. [DOI:10.2169/internalmedicine.47.0509] [PMID]
- Pang X, Ma L, Zhuo L, Liu L, Feng J. The first report of human primary pyogenic ventriculitis caused by Streptococcus suis: A case report. Annals of Palliative Medicine. 2021; 10(7):8448-53. [DOI:10.21037/apm-21-45] [PMID]
- Ochoa A, Argañaraz R, Mantese B. Neuroendoscopic lavage for the treatment of pyogenic ventriculitis in children: Personal series and review of the literature. Child's Nervous System. 2022; 38(3):597-604. [DOI:10.1007/s00381-021-05413-3] [PMID]
- Nishizaki T, Ikeda N, Nakano S, Sakakura T, Abiko M, Okamura T. Successful neuroendoscopic treatment of intraventricular brain abscess rupture. Clinics and Practice. 2011; 1(3):e52. [DOI:10.4081/cp.2011.e52] [PMID] [PMCID]
- Lee HK. Unilateral pyogenic ventriculitis. Journal of Nuclear Medicine. 1977; 18(4):403. [PMID]
- Vajramani GV, Akrawi H, Jones G, Sparrow OC. Primary ventriculitis caused by Streptococcus intermedius. British Journal of Neurosurgery. 2007; 21(3):293 – 96. [DOI:10.1080/02688690701246129] [PMID]
- Gronthoud F, Hassan I, Newton P. Primary pyogenic ventriculitis caused by Neisseria meningitidis: case report and review of the literature. JMM Case Reports. 2017; 4(1):e005078. [DOI:10.1099/jmmcr.0.005078] [PMID]
- Kalbarczyk A, Krauss JK, Seiler RW. Endoscopic stereotactic surgery for intraventricular loculated empyema: case report. Surgical Neurology. 1999; 52(4):412-7. [DOI:10.1016/s0090-3019(99)00109-3] [PMID]
- Meguins LC, Rocha AS, Laurenti MR, de Morais DF. Ventricular empyema associated with severe pyogenic meningitis in COVID-19 adult patient: Case report. Surgical Neurology International. 2021; 12:346. [PMID]
Type of Study: Review |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |