Wed, Feb 4, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 210-218 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ekouele Mbaki H B, Lani D R N, Thouassa G C, Elombila M, Ngackosso O B, Kinata Bambino S B et al . Acute Management of Traumatic Intracranial Hematomas in Brazzaville City, Congo: A Study of 115 Cases From 2016 to 2021. Iran J Neurosurg 2024; 10 : 24
URL: http://irjns.org/article-1-435-en.html
URL: http://irjns.org/article-1-435-en.html
Hugues Brieux Ekouele Mbaki *1 

 , Danel Rolf Nofane Lani2
, Danel Rolf Nofane Lani2 

 , Gédéon Colin Thouassa3
, Gédéon Colin Thouassa3 

 , Marie Elombila4
, Marie Elombila4 

 , Olivier Brice Ngackosso3
, Olivier Brice Ngackosso3 

 , Sinclair Brice Kinata Bambino5
, Sinclair Brice Kinata Bambino5 

 , Leon Boukassa5
, Leon Boukassa5 




 , Danel Rolf Nofane Lani2
, Danel Rolf Nofane Lani2 

 , Gédéon Colin Thouassa3
, Gédéon Colin Thouassa3 

 , Marie Elombila4
, Marie Elombila4 

 , Olivier Brice Ngackosso3
, Olivier Brice Ngackosso3 

 , Sinclair Brice Kinata Bambino5
, Sinclair Brice Kinata Bambino5 

 , Leon Boukassa5
, Leon Boukassa5 


1- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo. & Department of Multipurpose Surgery, University Hospital of Brazzaville, Brazzaville, Congo. , hugues.ekouele-mbaki@umng.cg
2- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo.
3- Department of Multipurpose Surgery, University Hospital of Brazzaville, Brazzaville, Congo.
4- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo. & Department of Intensive Care, University Hospital of Brazzaville, Brazzaville, Congo.
5- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo. & Department of Multipurpose Surgery, University Hospital of Brazzaville, Brazzaville, Congo.
2- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo.
3- Department of Multipurpose Surgery, University Hospital of Brazzaville, Brazzaville, Congo.
4- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo. & Department of Intensive Care, University Hospital of Brazzaville, Brazzaville, Congo.
5- Department of Doctoral Training, Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo. & Department of Multipurpose Surgery, University Hospital of Brazzaville, Brazzaville, Congo.
Full Text [PDF 1050 kb]
(735 Downloads)
| Abstract (HTML) (2508 Views)
Full Text: (512 Views)
1. Introduction
Traumatic brain injuries (TBI) are a significant global public health concern due to their impact on morbidity, mortality, and the economy [1, 2]. Road traffic accidents (RTA) are the main cause of these incidents and affect individuals of all ages, with a higher incidence among young adult males [3, 4]. Traumatic intracranial hematoma (TICH) is a frequent and severe outcome of TBI. Regardless of the severity of the head injury, 56% of victims experience at least one intracranial bleed. They can require surgical intervention and are associated with high morbidity and mortality [5, 6]. The management of a head injury is a crucial medical procedure, given the significant potential for adverse outcomes. The management of a head injury involves several steps. The signs and symptoms must be rapidly recognized, followed by an initial assessment and stabilization of vital functions. Imaging is then used to determine the type of brain damage. The treatment may vary from surveillance, monitoring, and management of secondary aggressions of systemic origin, to emergency surgery to evacuate hematomas or reduce intracranial pressure. The effective management of head trauma necessitates a multidisciplinary approach, involving anesthesiologists, neurosurgeons, and radiologists. The prompt and appropriate management of these patients can significantly improve their prognosis [7].
In Africa, a lack of data exists on the management of TICH in the acute phase [8]. Studies conducted in Congo on TBI have focused on epidemiological aspects and emergency treatment, but none have specifically addressed the management of TICH in the acute phase, considering the identified types of lesions [9, 10, 11]. With this in mind, we conducted a study to enhance the care of patients with TICH during the acute phase.
This study aims to describe the management of TICH during the acute phase in a neurosurgical environment at the Brazzaville University Hospital Center.
2. Methods and Materials/Patients
Study type, period, and setting
This is a descriptive cross-sectional study. The data were collected retrospectively, over six years from January 1, 2016 to December 31, 2021.
The study was conducted in the Department of Multipurpose Surgery of the Brazzaville University Hospital Center, which is a tertiary-level hospital center, with an emergency department, multipurpose resuscitation, pediatric surgery, orthopedics-traumatology, medical imaging, and functional rehabilitation.
Protocol for the management of TICH
It is standard practice for TICH cases to be admitted to the Emergency Department. Following reception and initial evaluation by the surgical unit team, the opinion of the neurosurgeon on-call duty is requested. In cases where the Glasgow coma scale (GCS) score is below 8, or in instances of multiple trauma, the opinion of the anesthesia and intensive care team is also sought.
Once the diagnosis of TICH is made, the type of lesion is identified, and the patient requires further care, he is referred to either the multipurpose surgery department or the multipurpose intensive care unit. The application of general measures is systematic. The measures employed for resuscitation (ventilatory assistance, sedation, mannitol) and medication prescriptions (analgesics and prophylaxis anti-epileptic) vary depending on the surgeon and consultation with the anesthesiologist-resuscitator. In the event of an emergency surgical indication, an operating order is issued to the family. Before the administration of anaesthesia, a pre-anaesthetic visit is conducted, during which a prescription for anaesthesia is issued. The cost of surgery is 99.75 USD.
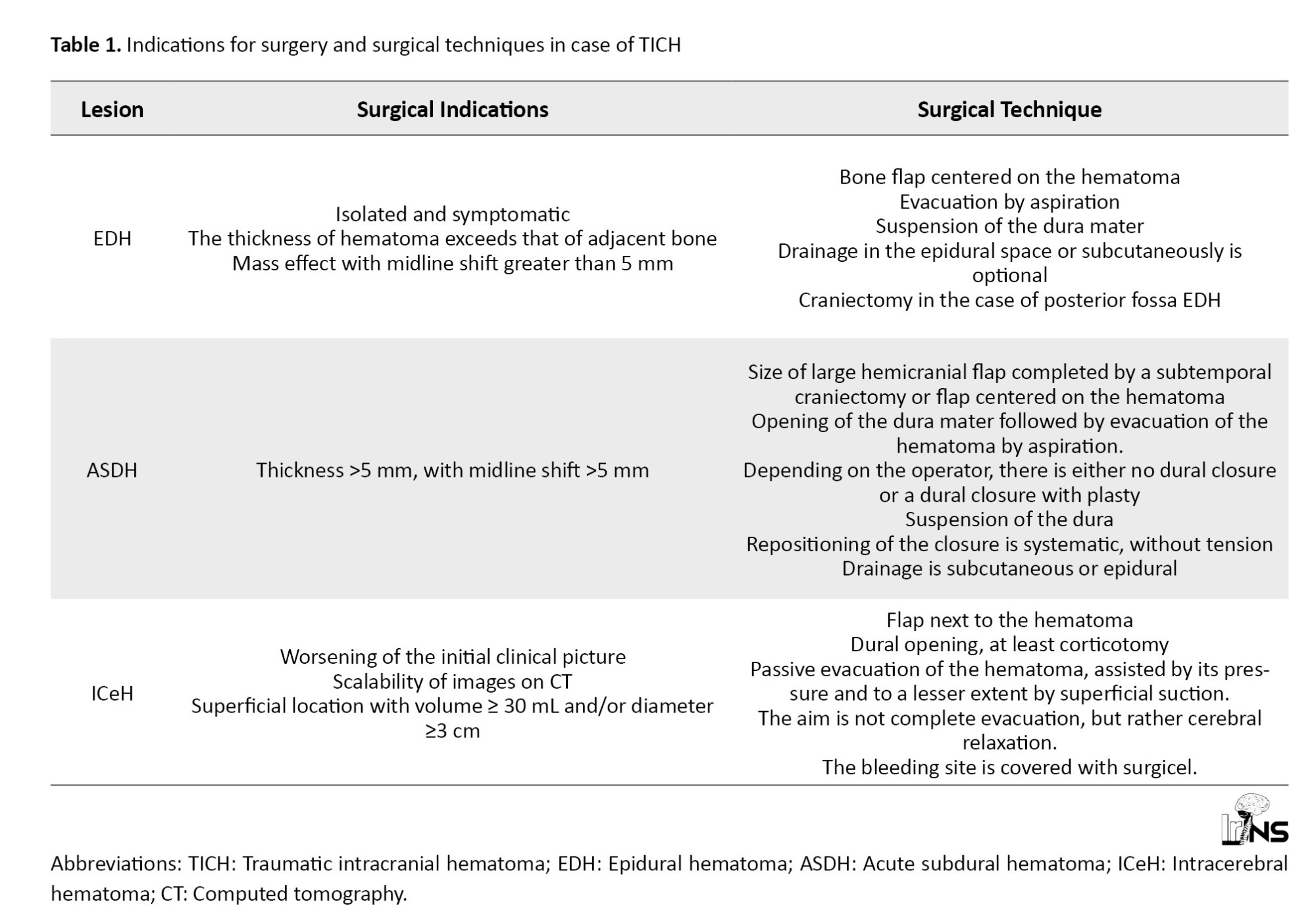
All patients underwent general anaesthesia via orotracheal intubation. Table 1 presents the surgical indications and surgical techniques agreed and used by the neurosurgical team.
Selection criteria and sampling method
The target population included all patients who were victims of TBI. The population source was all cases hospitalized. We included all patients hospitalized for TICH. We excluded all patients whose record was incomplete. We used a comprehensive sampling method.
Data collection and analysis
The data were collected from the registers of admissions and discharges and medical files, which were then collated in a survey sheet by case.
The investigated variables were socio-demographic, clinical, paraclinical, therapeutic, and evolution-related. The GCS was employed to categorize the severity of head trauma. The scale ranges from 13 to 15 for mild head trauma, 9 to 12 for moderate head trauma, and less than or equal to 8 for severe head trauma. The interval between the occurrence of the trauma and admission to the emergency room was delayed when it exceeded three hours. Similarly, the interval between the occurrence of the trauma and the performance of the brain CT was delayed by more than eight hours. Similarly, the interval between the occurrence of the trauma and the surgical intervention was delayed by more than 8 hours.
The Excel software, version 2016 was employed for the purposes of patient registration, database construction, and graph generation. The statistical analysis was conducted using Epi Info software, version 7.2.5.0. The qualitative variables were presented in numerical form and as proportions. Quantitative variables were expressed as means accompanied by their standard deviation (or median with quartiles).
3. Results
Frequency
A total of 1 045 patients were admitted for TBI. Among these patients, 130 people with TICH were identified in the acute phase, accounting for 12.4% of all TBI admissions. An additional 15 cases were excluded due to insufficient usable data. Thus, the study population included 115 cases, representing 11% of TBI admissions. Among these 115 cases, 78 cases (67.8%) had an epidural hematoma (EDH), 24 cases (20.9%) had an acute subdural hematoma (ASDH), and 13 cases (11.3%) had an intracerebral hematoma (ICeH). No relationships were found between these three types of lesions.
Socio-demographic aspects
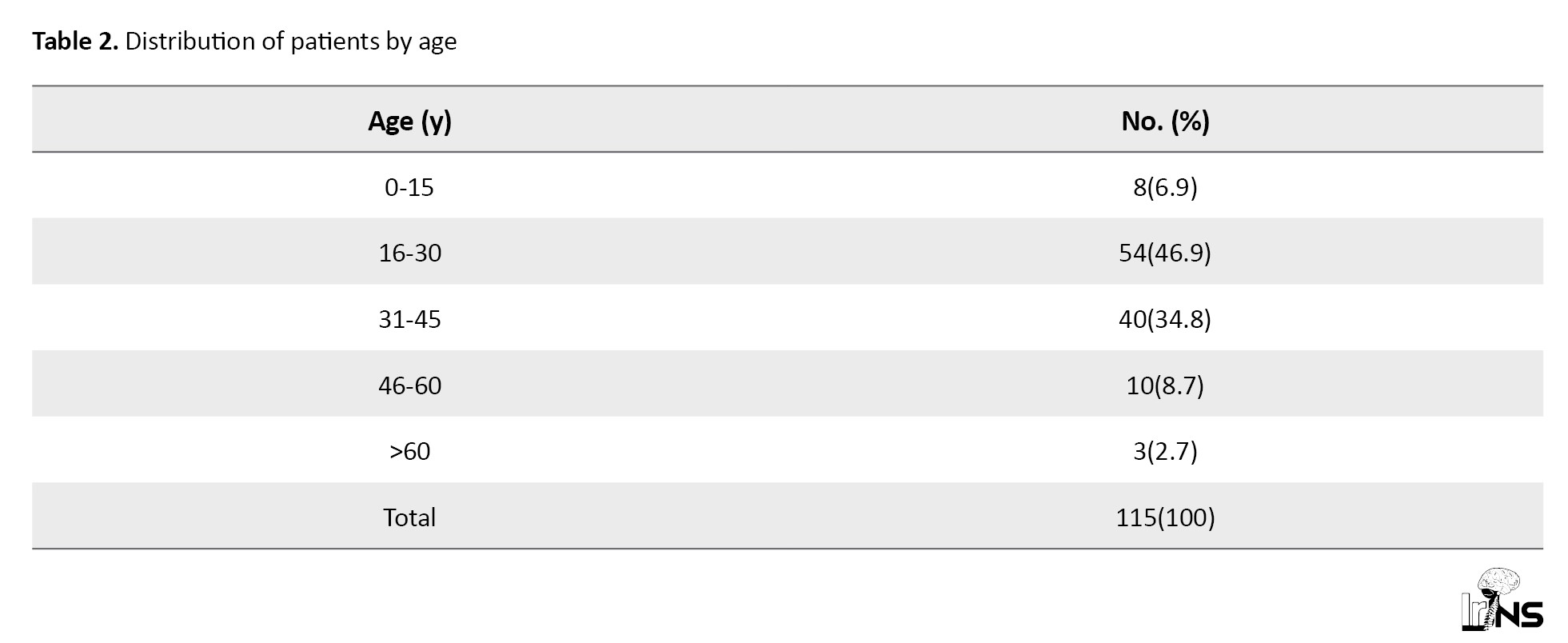
The median age for the entire series was 30 years (first quartile=24 years; third quartile=36 years), with the extremes of 3 and 64 years. Table 2 presents the distribution of all patients with TICH according to age groups.
A total of 113 male cases and two female cases were recorded, resulting in a sex ratio of 56.5.
Among the TICH cases, 111 patients (96.5%) originated from Brazzaville, while the remaining four (3.4%) were geographically distant from Brazzaville, with the furthest patients residing in Djambala (361.6 kilometers), Oyo (407 kilometers), Kindamba (140 kilometers) and Gamboma (250 kilometers).
Diagnostic
Most cases (93.9%) were attributed to head trauma resulting from a RTA. Assault was the second most common cause (5.2%), followed by falls (5.2%). Among the 115 patients included in the study, three cases (2.6%) were insured. It was not observed that any of the cases benefited from medical pick-up and transport.
The median time between the occurrence of trauma and admission of all patients with TICH was 4 hours (first quartile =3 hours; third quartile =4 hours), with the extremes of 1 and 48 hours.
The notion of initial loss of consciousness followed by a return to normal consciousness was reported in 87 cases (75.6%), while lasting disturbances of consciousness were identified in 28 cases (24.3%). Furthermore, intracranial hypertension syndrome was identified during questioning in 37 cases (32.1%).
The mean GCS score on admission was 13±1, with a range of 7 to 15. The trauma was classified as mild in 78 cases (67.8%), moderate in 34 cases (29.6%), and severe in three cases (2.6%). During the physical examination, anisocoria was reported in seven cases (6.1%), while respiratory distress was observed in three cases (2.6%). A scalp wound was reported in 75 cases (65.2%), epistaxis in 9 cases (7.8%), otorrhagia in 2 cases (1.7%), and lower limb trauma in 2 cases (1.7%).
All patients underwent a brain CT and a standard cervical spine x-ray. The median time between the occurrence of the trauma and the completion of the brain CT was 36 hours (first quartile=23 hours; third quartile=48 hours), with the extremes of 6 and 144 hours. In 76 cases (66%), this CT was performed with a delay greater than or equal to 24 hours. One percent of the lesions associated with intracranial hematoma were skull fractures, cerebral contusions, an embolism, a tibia fracture, and subarachnoid hemorrhage.
Treatment and outcome
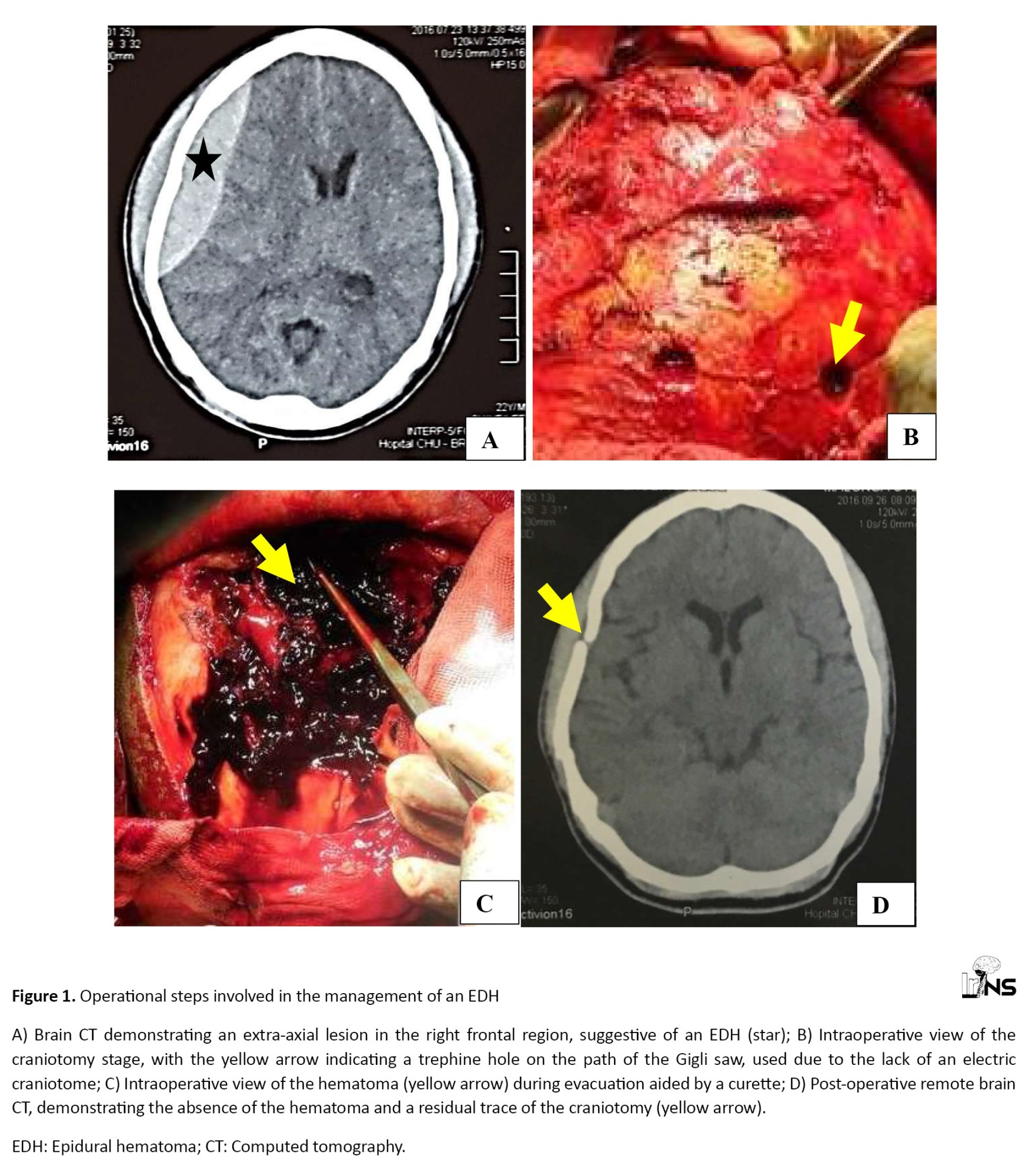
In all cases, analgesics were administered as a form of medicinal intervention, while anti-epileptic prophylaxis was also employed. Ventilatory assistance was required in the intensive care unit in three cases, representing a prevalence of 2.6%. Mannitol was administered in 7 cases (6.1%), while 31 patients (26.9%) underwent surgical intervention. Craniotomy with flap replacement was the technique employed in all patients. Among the 31 operated patients, 29 cases were operated for EDH, and the remaining two were operated for ASDH. Figure 1 depicts the operational steps involved in the management of an EDH in our context.
Traumatic brain injuries (TBI) are a significant global public health concern due to their impact on morbidity, mortality, and the economy [1, 2]. Road traffic accidents (RTA) are the main cause of these incidents and affect individuals of all ages, with a higher incidence among young adult males [3, 4]. Traumatic intracranial hematoma (TICH) is a frequent and severe outcome of TBI. Regardless of the severity of the head injury, 56% of victims experience at least one intracranial bleed. They can require surgical intervention and are associated with high morbidity and mortality [5, 6]. The management of a head injury is a crucial medical procedure, given the significant potential for adverse outcomes. The management of a head injury involves several steps. The signs and symptoms must be rapidly recognized, followed by an initial assessment and stabilization of vital functions. Imaging is then used to determine the type of brain damage. The treatment may vary from surveillance, monitoring, and management of secondary aggressions of systemic origin, to emergency surgery to evacuate hematomas or reduce intracranial pressure. The effective management of head trauma necessitates a multidisciplinary approach, involving anesthesiologists, neurosurgeons, and radiologists. The prompt and appropriate management of these patients can significantly improve their prognosis [7].
In Africa, a lack of data exists on the management of TICH in the acute phase [8]. Studies conducted in Congo on TBI have focused on epidemiological aspects and emergency treatment, but none have specifically addressed the management of TICH in the acute phase, considering the identified types of lesions [9, 10, 11]. With this in mind, we conducted a study to enhance the care of patients with TICH during the acute phase.
This study aims to describe the management of TICH during the acute phase in a neurosurgical environment at the Brazzaville University Hospital Center.
2. Methods and Materials/Patients
Study type, period, and setting
This is a descriptive cross-sectional study. The data were collected retrospectively, over six years from January 1, 2016 to December 31, 2021.
The study was conducted in the Department of Multipurpose Surgery of the Brazzaville University Hospital Center, which is a tertiary-level hospital center, with an emergency department, multipurpose resuscitation, pediatric surgery, orthopedics-traumatology, medical imaging, and functional rehabilitation.
Protocol for the management of TICH
It is standard practice for TICH cases to be admitted to the Emergency Department. Following reception and initial evaluation by the surgical unit team, the opinion of the neurosurgeon on-call duty is requested. In cases where the Glasgow coma scale (GCS) score is below 8, or in instances of multiple trauma, the opinion of the anesthesia and intensive care team is also sought.
Once the diagnosis of TICH is made, the type of lesion is identified, and the patient requires further care, he is referred to either the multipurpose surgery department or the multipurpose intensive care unit. The application of general measures is systematic. The measures employed for resuscitation (ventilatory assistance, sedation, mannitol) and medication prescriptions (analgesics and prophylaxis anti-epileptic) vary depending on the surgeon and consultation with the anesthesiologist-resuscitator. In the event of an emergency surgical indication, an operating order is issued to the family. Before the administration of anaesthesia, a pre-anaesthetic visit is conducted, during which a prescription for anaesthesia is issued. The cost of surgery is 99.75 USD.
All patients underwent general anaesthesia via orotracheal intubation. Table 1 presents the surgical indications and surgical techniques agreed and used by the neurosurgical team.

Selection criteria and sampling method
The target population included all patients who were victims of TBI. The population source was all cases hospitalized. We included all patients hospitalized for TICH. We excluded all patients whose record was incomplete. We used a comprehensive sampling method.
Data collection and analysis
The data were collected from the registers of admissions and discharges and medical files, which were then collated in a survey sheet by case.
The investigated variables were socio-demographic, clinical, paraclinical, therapeutic, and evolution-related. The GCS was employed to categorize the severity of head trauma. The scale ranges from 13 to 15 for mild head trauma, 9 to 12 for moderate head trauma, and less than or equal to 8 for severe head trauma. The interval between the occurrence of the trauma and admission to the emergency room was delayed when it exceeded three hours. Similarly, the interval between the occurrence of the trauma and the performance of the brain CT was delayed by more than eight hours. Similarly, the interval between the occurrence of the trauma and the surgical intervention was delayed by more than 8 hours.
The Excel software, version 2016 was employed for the purposes of patient registration, database construction, and graph generation. The statistical analysis was conducted using Epi Info software, version 7.2.5.0. The qualitative variables were presented in numerical form and as proportions. Quantitative variables were expressed as means accompanied by their standard deviation (or median with quartiles).
3. Results
Frequency
A total of 1 045 patients were admitted for TBI. Among these patients, 130 people with TICH were identified in the acute phase, accounting for 12.4% of all TBI admissions. An additional 15 cases were excluded due to insufficient usable data. Thus, the study population included 115 cases, representing 11% of TBI admissions. Among these 115 cases, 78 cases (67.8%) had an epidural hematoma (EDH), 24 cases (20.9%) had an acute subdural hematoma (ASDH), and 13 cases (11.3%) had an intracerebral hematoma (ICeH). No relationships were found between these three types of lesions.
Socio-demographic aspects
The median age for the entire series was 30 years (first quartile=24 years; third quartile=36 years), with the extremes of 3 and 64 years. Table 2 presents the distribution of all patients with TICH according to age groups.

A total of 113 male cases and two female cases were recorded, resulting in a sex ratio of 56.5.
Among the TICH cases, 111 patients (96.5%) originated from Brazzaville, while the remaining four (3.4%) were geographically distant from Brazzaville, with the furthest patients residing in Djambala (361.6 kilometers), Oyo (407 kilometers), Kindamba (140 kilometers) and Gamboma (250 kilometers).
Diagnostic
Most cases (93.9%) were attributed to head trauma resulting from a RTA. Assault was the second most common cause (5.2%), followed by falls (5.2%). Among the 115 patients included in the study, three cases (2.6%) were insured. It was not observed that any of the cases benefited from medical pick-up and transport.
The median time between the occurrence of trauma and admission of all patients with TICH was 4 hours (first quartile =3 hours; third quartile =4 hours), with the extremes of 1 and 48 hours.
The notion of initial loss of consciousness followed by a return to normal consciousness was reported in 87 cases (75.6%), while lasting disturbances of consciousness were identified in 28 cases (24.3%). Furthermore, intracranial hypertension syndrome was identified during questioning in 37 cases (32.1%).
The mean GCS score on admission was 13±1, with a range of 7 to 15. The trauma was classified as mild in 78 cases (67.8%), moderate in 34 cases (29.6%), and severe in three cases (2.6%). During the physical examination, anisocoria was reported in seven cases (6.1%), while respiratory distress was observed in three cases (2.6%). A scalp wound was reported in 75 cases (65.2%), epistaxis in 9 cases (7.8%), otorrhagia in 2 cases (1.7%), and lower limb trauma in 2 cases (1.7%).
All patients underwent a brain CT and a standard cervical spine x-ray. The median time between the occurrence of the trauma and the completion of the brain CT was 36 hours (first quartile=23 hours; third quartile=48 hours), with the extremes of 6 and 144 hours. In 76 cases (66%), this CT was performed with a delay greater than or equal to 24 hours. One percent of the lesions associated with intracranial hematoma were skull fractures, cerebral contusions, an embolism, a tibia fracture, and subarachnoid hemorrhage.
Treatment and outcome
In all cases, analgesics were administered as a form of medicinal intervention, while anti-epileptic prophylaxis was also employed. Ventilatory assistance was required in the intensive care unit in three cases, representing a prevalence of 2.6%. Mannitol was administered in 7 cases (6.1%), while 31 patients (26.9%) underwent surgical intervention. Craniotomy with flap replacement was the technique employed in all patients. Among the 31 operated patients, 29 cases were operated for EDH, and the remaining two were operated for ASDH. Figure 1 depicts the operational steps involved in the management of an EDH in our context.
These include a pre-operative CT scan, the illustration of the craniotomy obtained with a manual trephine and craniotomy saw, the intraoperative view of the hematoma, and the postoperative CT scan, demonstrating a satisfactory evolution with the hematoma’s disappearance and the absence of brain lesions.
The median time between the occurrence of trauma and surgery was 36 hours (first quartile =24 hours; third quartile =48 hours), with extremes of 16 and 168 hours.
In the entire series (operated as well as non-operated), the evolution during hospitalization was favorable in 110 patients (95.7%), with cognitive recovery and discharge for home care and or continued convalescence. Two patients (1.7%) experienced complications, including one case of meningitis and one case of rhabdomyolysis. Three patients (2.6%) died.
Among the 31 patients who underwent surgery, postoperative complications were recorded in two patients (6.4%), including meningitis and rhabdomyolysis. These complications only concerned patients who underwent surgery for EDH.
The two patients (6.4%) who underwent surgery for ASDH were admitted to the multi-purpose intensive care unit immediately after their operation due to impaired consciousness. One patient was admitted for five days and the other for seven days, with the death occurring in these two cases.
The mean length of hospitalization was 6.5±2.5 days (with extremes of 4 and 27 days).
4. Discussion
A hospital frequency of 12.4% was identified. A study conducted in Pakistan by Shoaib et al. [12] showed that the frequency of TICH associated with skull fracture is reported to be 16.3% over six months. In a multicenter study conducted in England, Perel et al. [5] reported a frequency of 46% over eight years. The discrepancy between these results is likely attributable to the differing inclusion criteria.
The most prevalent type of TICH was EDH, representing 67.8% of cases. This result is comparable to that of the study by Kithikii and Githinji [13], which found EDH to be the most prevalent type in 47.7% of cases. The results of this study differ from those of Shoaib et al. [12], who found ASDH to be the most common. The discrepancy with the aforementioned study is also attributable to the selection criteria because most patients in this study had suffered severe head trauma. In a study conducted in Singapore by Han et al. [14], and in a study conducted in Japan by Shibahasi et al. [15], a higher frequency of ASDH was reported compared to EDH in severe TBI.
The sex ratio in our study was 56.5, which is considerably higher than a previous study focusing on head trauma patients, with a ratio of 9.4 [9]. Male predominance has been reported by several authors [5, 12, 16], which is consistent with the results of our study. This discrepancy can be attributed to the fact that the present study exclusively focused on cases of TICH and that men are more susceptible to TBI, with a higher risk of developing TICH than women due to the circumstances and the intensity of the trauma.
The mean GCS at admission was 13±1, with extremes of 7 and 15. This result is comparable to the results of Wu et al. [17] in the USA, who found an average GCS of 12.3. However, it is higher than Fujii et al.’s study [16] which reported a GCS of 9.2, which may be attributed to the fact that the patients included in their study predominantly presented with a severe TBI. In contrast, most patients (67.8%) in our study had a mild TBI (GCS 13 to 15). This result is comparable to the results of Kiboi et al. [8] and Wu et al.’s studies [17], who demonstrated that 40.4% and 73.7% of patients, respectively, exhibited mild TBI. Consequently, a favorable GCS does not appear to be correlated with the absence of TICH.
In our series, craniotomy with flap replacement was the technique employed in all patients undergoing surgery for EDH as well as ASDH. Djientcheu et al. [18] and Gaye et al. [19] in Senegal, in their respective studies on EDH, also utilized this technique in most of their patients at respective rates of 81.1% and 57.5%. According to the literature, craniotomy with flap replacement is the optimal surgical technique for evacuating EHDs [20]. Fountain et al. [21] in England, in a study on ASDH, also employed craniotomy in all patients undergoing surgery for ASDH. Igbokwe et al. [22] in Nigeria, in a study on ASDH, used craniotomy and decompressive craniectomy (without replacing the flap). Decompressive craniectomy was indicated in cases of GCS considered low and in the presence of intraoperative edema. Karnjanasavitree et al. [23] in Thailand used decompressive craniectomy in most cases (71.7%). This can be explained by the fact that most patients in their studies presented pupillary abnormalities and had obliteration of the basal cisterns on brain CT. According to the available literature, these two techniques appear to be effective, but the superiority of each has yet to be established. The choice of surgical technique depends on the expertise of the surgeon, the neurological status of the patient, the duration of deterioration, the preoperative radiological findings, and the degree of intraoperative brain tumescence [24].
The median interval between the occurrence of trauma and surgical intervention was 36 hours, with extremes of 16 and 168 hours. In a study conducted in Kenya by Kithikii and Githinji [13], a time limit of 72 hours was identified. In contrast, Taussky et al. [25] in Switzerland reported a delay of three hours. In developing countries, the processing times are lengthy, which is attributable to the lack of comprehensive social security coverage. In the context of our study, the supply of essential surgical products was the responsibility of the patient and or their entourage. Even when the support is covered by the insurance of a vehicle in the event of a RTA, the financing procedures are lengthy.
The treatment can sometimes be medical. In the intensive care unit, treatment is aimed at preventing/limiting secondary brain damage of intracerebral (intracranial hypertension, cerebral ischemia, non-convulsive epilepsy) and systemic (hyperthermia, hyperglycemia) origin, using a standardized algorithm [26].
The evolution during hospitalization was favorable in 110 patients (95.7%). Death occurred in three patients (2.6%). This mortality rate is lower than that found by Kithikii and Githinji [13] and Perel et al. [5], who found respective frequencies of 18.4% and 22%. The difference between these results is probably related to the severity of TBI, which was not the same between these studies. The presence of a TICH represents a significant mortality factor in cases of TBI [27-29]. The postoperative mortality rate in our series was 6.4%. These deaths occurred in patients who had undergone surgery for ASDH. Tallon et al. [30] and Taussky et al. [25] reported higher postoperative mortality rates in patients who underwent surgery for ASDH, at rates of 17.1% and 41%, respectively. This can be attributed to the fact that ASDH is predominantly associated with severe TBI, which is associated with a poor prognosis.
5. Conclusion
TICH is present in 12% of TBI cases. EDH is the most common lesion. In our context, pre-hospital care is not medicalized. Most victims have TBI that is assessed as mild GCS. More than one in four patients (27%) with TICH require surgery. In most cases, the latter is performed more than 24 hours, in a context of insufficient social coverage (health insurance). The mortality of TICH is 2.6%. Postoperative complications and mortality are around 6%.
While this study provides valuable insights into the management of TICH in the acute phase, it has limitations. The retrospective nature of the study may introduce biases related to data accuracy and completeness because we relied on existing medical records. Additionally, the single-center design limits the generalizability of the results to other settings or populations. However, it is crucial to note that the study was conducted at the University Hospital Center of Brazzaville, the national referral center for neurosurgical conditions, which adds significant value to the results. Despite these limitations, the study offers a comprehensive overview of TICH cases, highlighting critical aspects of patient management and outcomes in a resource-limited context. These results can serve as a foundation for future research and improvements in clinical practice, contributing to better care for TBI patients globally.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo, and by the Health Sciences Research Ethics Committee (Code: 0029/MESRSIT/DGRST/CERSSA/22). The confidentiality of information was guaranteed, and participants were permitted to withdraw from the study at any time. Furthermore, if they wished, the research results would be made available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and design: Hugues Brieux Ekouele Mbaki and Danel Rolf Nofane Lani; Data collection: Hugues Brieux Ekouele Mbaki and Danel Rolf Nofane Lani; Analysis and interpretation of data: Hugues Brieux Ekouele Mbaki, Danel Rolf Nofane Lani, Gédéon Thouassa; Methodology, data collection, and writing: Hugues Brieux Ekouele Mbaki and Danel Rolf Nofane Lani; Data analysis, review and editing: Gedeon Colin Thouassa, Olivier Brice Ngackosso, Sinclair Brice Kinata Bambino, Marie Elombila, and Léon Boukassa; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
Thanks to Mbou Essie Darius and Bingui Outman Diogene for their contribution to the statistical processing of the data.
The median time between the occurrence of trauma and surgery was 36 hours (first quartile =24 hours; third quartile =48 hours), with extremes of 16 and 168 hours.
In the entire series (operated as well as non-operated), the evolution during hospitalization was favorable in 110 patients (95.7%), with cognitive recovery and discharge for home care and or continued convalescence. Two patients (1.7%) experienced complications, including one case of meningitis and one case of rhabdomyolysis. Three patients (2.6%) died.
Among the 31 patients who underwent surgery, postoperative complications were recorded in two patients (6.4%), including meningitis and rhabdomyolysis. These complications only concerned patients who underwent surgery for EDH.
The two patients (6.4%) who underwent surgery for ASDH were admitted to the multi-purpose intensive care unit immediately after their operation due to impaired consciousness. One patient was admitted for five days and the other for seven days, with the death occurring in these two cases.
The mean length of hospitalization was 6.5±2.5 days (with extremes of 4 and 27 days).
4. Discussion
A hospital frequency of 12.4% was identified. A study conducted in Pakistan by Shoaib et al. [12] showed that the frequency of TICH associated with skull fracture is reported to be 16.3% over six months. In a multicenter study conducted in England, Perel et al. [5] reported a frequency of 46% over eight years. The discrepancy between these results is likely attributable to the differing inclusion criteria.
The most prevalent type of TICH was EDH, representing 67.8% of cases. This result is comparable to that of the study by Kithikii and Githinji [13], which found EDH to be the most prevalent type in 47.7% of cases. The results of this study differ from those of Shoaib et al. [12], who found ASDH to be the most common. The discrepancy with the aforementioned study is also attributable to the selection criteria because most patients in this study had suffered severe head trauma. In a study conducted in Singapore by Han et al. [14], and in a study conducted in Japan by Shibahasi et al. [15], a higher frequency of ASDH was reported compared to EDH in severe TBI.
The sex ratio in our study was 56.5, which is considerably higher than a previous study focusing on head trauma patients, with a ratio of 9.4 [9]. Male predominance has been reported by several authors [5, 12, 16], which is consistent with the results of our study. This discrepancy can be attributed to the fact that the present study exclusively focused on cases of TICH and that men are more susceptible to TBI, with a higher risk of developing TICH than women due to the circumstances and the intensity of the trauma.
The mean GCS at admission was 13±1, with extremes of 7 and 15. This result is comparable to the results of Wu et al. [17] in the USA, who found an average GCS of 12.3. However, it is higher than Fujii et al.’s study [16] which reported a GCS of 9.2, which may be attributed to the fact that the patients included in their study predominantly presented with a severe TBI. In contrast, most patients (67.8%) in our study had a mild TBI (GCS 13 to 15). This result is comparable to the results of Kiboi et al. [8] and Wu et al.’s studies [17], who demonstrated that 40.4% and 73.7% of patients, respectively, exhibited mild TBI. Consequently, a favorable GCS does not appear to be correlated with the absence of TICH.
In our series, craniotomy with flap replacement was the technique employed in all patients undergoing surgery for EDH as well as ASDH. Djientcheu et al. [18] and Gaye et al. [19] in Senegal, in their respective studies on EDH, also utilized this technique in most of their patients at respective rates of 81.1% and 57.5%. According to the literature, craniotomy with flap replacement is the optimal surgical technique for evacuating EHDs [20]. Fountain et al. [21] in England, in a study on ASDH, also employed craniotomy in all patients undergoing surgery for ASDH. Igbokwe et al. [22] in Nigeria, in a study on ASDH, used craniotomy and decompressive craniectomy (without replacing the flap). Decompressive craniectomy was indicated in cases of GCS considered low and in the presence of intraoperative edema. Karnjanasavitree et al. [23] in Thailand used decompressive craniectomy in most cases (71.7%). This can be explained by the fact that most patients in their studies presented pupillary abnormalities and had obliteration of the basal cisterns on brain CT. According to the available literature, these two techniques appear to be effective, but the superiority of each has yet to be established. The choice of surgical technique depends on the expertise of the surgeon, the neurological status of the patient, the duration of deterioration, the preoperative radiological findings, and the degree of intraoperative brain tumescence [24].
The median interval between the occurrence of trauma and surgical intervention was 36 hours, with extremes of 16 and 168 hours. In a study conducted in Kenya by Kithikii and Githinji [13], a time limit of 72 hours was identified. In contrast, Taussky et al. [25] in Switzerland reported a delay of three hours. In developing countries, the processing times are lengthy, which is attributable to the lack of comprehensive social security coverage. In the context of our study, the supply of essential surgical products was the responsibility of the patient and or their entourage. Even when the support is covered by the insurance of a vehicle in the event of a RTA, the financing procedures are lengthy.
The treatment can sometimes be medical. In the intensive care unit, treatment is aimed at preventing/limiting secondary brain damage of intracerebral (intracranial hypertension, cerebral ischemia, non-convulsive epilepsy) and systemic (hyperthermia, hyperglycemia) origin, using a standardized algorithm [26].
The evolution during hospitalization was favorable in 110 patients (95.7%). Death occurred in three patients (2.6%). This mortality rate is lower than that found by Kithikii and Githinji [13] and Perel et al. [5], who found respective frequencies of 18.4% and 22%. The difference between these results is probably related to the severity of TBI, which was not the same between these studies. The presence of a TICH represents a significant mortality factor in cases of TBI [27-29]. The postoperative mortality rate in our series was 6.4%. These deaths occurred in patients who had undergone surgery for ASDH. Tallon et al. [30] and Taussky et al. [25] reported higher postoperative mortality rates in patients who underwent surgery for ASDH, at rates of 17.1% and 41%, respectively. This can be attributed to the fact that ASDH is predominantly associated with severe TBI, which is associated with a poor prognosis.
5. Conclusion
TICH is present in 12% of TBI cases. EDH is the most common lesion. In our context, pre-hospital care is not medicalized. Most victims have TBI that is assessed as mild GCS. More than one in four patients (27%) with TICH require surgery. In most cases, the latter is performed more than 24 hours, in a context of insufficient social coverage (health insurance). The mortality of TICH is 2.6%. Postoperative complications and mortality are around 6%.
While this study provides valuable insights into the management of TICH in the acute phase, it has limitations. The retrospective nature of the study may introduce biases related to data accuracy and completeness because we relied on existing medical records. Additionally, the single-center design limits the generalizability of the results to other settings or populations. However, it is crucial to note that the study was conducted at the University Hospital Center of Brazzaville, the national referral center for neurosurgical conditions, which adds significant value to the results. Despite these limitations, the study offers a comprehensive overview of TICH cases, highlighting critical aspects of patient management and outcomes in a resource-limited context. These results can serve as a foundation for future research and improvements in clinical practice, contributing to better care for TBI patients globally.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Faculty of Health Sciences, Marien Ngouabi University, Brazzaville, Congo, and by the Health Sciences Research Ethics Committee (Code: 0029/MESRSIT/DGRST/CERSSA/22). The confidentiality of information was guaranteed, and participants were permitted to withdraw from the study at any time. Furthermore, if they wished, the research results would be made available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and design: Hugues Brieux Ekouele Mbaki and Danel Rolf Nofane Lani; Data collection: Hugues Brieux Ekouele Mbaki and Danel Rolf Nofane Lani; Analysis and interpretation of data: Hugues Brieux Ekouele Mbaki, Danel Rolf Nofane Lani, Gédéon Thouassa; Methodology, data collection, and writing: Hugues Brieux Ekouele Mbaki and Danel Rolf Nofane Lani; Data analysis, review and editing: Gedeon Colin Thouassa, Olivier Brice Ngackosso, Sinclair Brice Kinata Bambino, Marie Elombila, and Léon Boukassa; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
Thanks to Mbou Essie Darius and Bingui Outman Diogene for their contribution to the statistical processing of the data.
References
- Wee JZ, Yang YR, Lee QY, Cao K, Chong CT. Demographic profile and extent of healthcare resource utilisation of patients with severe traumatic brain injury: Still a major public health problem. Singapore Medical Journal. 2016; 57(9):491-6. [DOI:10.11622/smedj.2015162] [PMID] [PMCID]
- Masson F. [Epidemiology of severe cranial injuries (French)]. Annales Francaises D'anesthesie et de Reanimation. 2000; 19(4):261-9. [DOI:10.1016/S0750-7658(99)00145-8] [PMID]
- Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochirurgica. 2015; 157(10):1683-96. [DOI:10.1007/s00701-015-2512-7] [PMID] [PMCID]
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery. 2018; 130(4):1080-97. [DOI:10.3171/2017.10.JNS17352] [PMID]
- Perel P, Roberts I, Bouamra O, Woodford M, Mooney J, Lecky F. Intracranial bleeding in patients with traumatic brain injury: A prognostic study. BMC Emergency Medicine. 2009; 9:15. [DOI:10.1186/1471-227X-9-15] [PMID] [PMCID]
- Subaiya S, Roberts I, Komolafe E, Perel P. Predicting intracranial hemorrhage after traumatic brain injury in low and middle-income countries: A prognostic model based on a large, multi-center, international cohort. BMc Emergency Medicine. 2012; 12:17. [DOI:10.1186/1471-227X-12-17] [PMID] [PMCID]
- Geeraerts T, Velly L, Abdennour L, Asehnoune K, Audibert G, Bouzat P, et al. [Management of severe traumatic brain injury (first 24 hours) (French)]. Anesthésie & Réanimation. 2016; 2(6):431-3. [DOI:10.1016/j.anrea.2016.09.007]
- Kiboi JG, Kitunguu PK, Angwenyi P, Mbuthia F, Sagina LS. Predictors of functional recovery in African patients with traumatic intracranial hematomas. World Neurosurgery. 2011; 75(5-6):586-91. [DOI:10.1016/j.wneu.2010.05.041] [PMID]
- Ekouele Mbaki HB, Otiobanda GF, Elombila M, Boukassa L, Moyikoua R, Gombet TR, et al. [Traumatismes crânio-encéphaliques de l’adulte: Aspects épidémiologiques et prise en charge au Centre Hospitalier Universitaire de Brazzaville, Congo (French)]. Revue Africaine d’Anesthésiologie et Médecine d’Urgence. 2016 ; 21(2):27-32. [Link]
- Elombila M, Ekouele Mbaki HB, Mpoy Emy Monkessa CM, Niengo Outsouta G, Bokoba Nde Ngala MA, Otiobanda GF. [Aspects épidémiologiques, cliniques et évolutifs des traumatismes crânio-encéphaliques en réanimation polyvalente du Centre Hospitalier Universitaire de Brazzaville (French)]. Health Sciences & Disease. 2022; 23(5):117-21. [Link]
- Ekouele Mbaki HB, Bingui Outman DP, Elombila M, Mbou Essie DE, Mpoy Emy Monkessa CM, Boukaka Kala RG. Socio-demographic profile of adults admitted in emergency for brain trauma injuries at the university hospital of Brazzaville. Open Journal of Modern Neurosurgery. 2019; 9(1):43-8. [DOI:10.4236/ojmn.2019.91006]
- Shoaib M, Khan S, Ui Haq N, Ullah Z, Ali S, Jamal B, et al. Frequency of intracranial hemorrhage among patients with skull fractures in blunt head trauma. American Journal of Health, Medicine and Nursing Practice. 2022; 7(5):8-17. [DOI:10.47672/ajhmn.980]
- Kithikii KP, Githinji KJ. Risk factors related to hospital mortality in kenyan patients with traumatic intracranial hematomas. East and Central African Journal of Surgery. 2011; 16(1):122-4. [Link]
- Han JX, See AAQ, Gandhi M, King NKK. Models of mortality and morbidity in severe traumatic brain injury: An analysis of a singapore neurotrauma database. World Neurosurgery. 2017; 108:885-93. [DOI:10.1016/j.wneu.2017.08.147] [PMID]
- Shibahashi K, Sugiyama K, Okura Y, Hoda H, Hamabe Y. Multicenter retrospective cohort study of "talk and die" after traumatic brain injury. World Neurosurgery. 2017; 107:82-6. [DOI:10.1016/j.wneu.2017.07.117] [PMID]
- Fujii T, Moriel G, Kramer DR, Attenello F, Zada G. Prognostic factors of early outcome and discharge status in patients undergoing surgical intervention following traumatic intracranial hemorrhage. Journal of Clinical Neuroscience. 2016; 31:152-6. [DOI:10.1016/j.jocn.2016.03.007] [PMID]
- Wu E, Marthi S, Asaad WF. Predictors of mortality in traumatic intracranial hemorrhage: A national trauma data bank Study. Frontiers in Neurology. 2020; 11:587587. [DOI:10.3389/fneur.2020.587587] [PMID] [PMCID]
- Djientcheu VP, Bisso AN, Njamnski AK, Ongolo-Zogo P, Hell-Medjo E, Sosso MA. [Les hématomes extraduraux post traumatiques: Prise en charge médicochirurgicale à Yaounde (French)]. African Journal of Neurological Sciences. 2005; 24(2):33-9. [Link]
- Gaye M, Diatta B, Ndoye N, Ba MC, Thiam AB, Diop AA et al. [Prise en charge de l’hématome extradural à Dakar à propos de 40 cas (French)]. African Journal of Neurological Sciences. 2010 ; 29(1):47-56. [Link]
- Alliez JR, Balan C, Leone M, Kaya JM, Reynier Y, Alliez B. [Hématomes intracrâniens post-traumatiques en phase aiguë (French)]. EMC-Neurologie. 2008; 5(1):1-17 [DOI:10.1016/S0246-0378(08)45269-7]
- Fountain DM, Kolias AG, Lecky FE, Bouamra O, Lawrence T, Adams H, et al. Survival trends after surgery for acute subdural hematoma in adults over a 20-year period. Annals of Surgery. 2017; 265(3):590-6. [DOI:10.1097/SLA.0000000000001682] [PMID] [PMCID]
- Igbokwe KK, Ayogu OM, Onobun DE, Essiet EA, Ugwuanyi UC. The outcomes of traumatic acute subdural hematoma in a tertiary center in Abuja, Nigeria. Cureus. 2021; 13(11):e20016. [DOI:10.7759/cureus.20016] [PMID] [PMCID]
- Karnjanasavitree W, Phuenpathom N, Tunthanathip T. The optimal operative timing of traumatic intracranial acute subdural hematoma correlated with outcome. Asian Journal of Neurosurgery. 2018; 13(4):1158-64. [DOI:10.4103/ajns.AJNS_199_18] [PMID] [PMCID]
- Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T. Surgical management of traumatic acute subdural hematoma in adults: A review. Neurologia Medico-Chirurgica. 2014; 54(11):887-94. [DOI:10.2176/nmc.cr.2014-0204] [PMID]
- Taussky P, Widmer HR, Takala J, Fandino J. Outcome after acute traumatic subdural and epidural haematoma in Switzerland: A single-centre experience. Swiss Medical Weekly. 2008; 138(19-20):281-5. [DOI:10.4414/smw.2008.12056] [PMID]
- Ben Hamouda N, Oddo M. [Prise en charge du traumatisme crânien cérébral grave (French)]. Réanimation. 2013; 22(2):479-87. [DOI:10.1007/s13546-012-0620-4]
- Kulesza B, Mazurek M, Nogalski A, Rola R. Factors with the strongest prognostic value associated with in-hospital mortality rate among patients operated for acute subdural and epidural hematoma. European Journal of Trauma and Emergency Surgery. 2021; 47(5):1517-25. [DOI:10.1007/s00068-020-01460-8] [PMID] [PMCID]
- Scotter J, Hendrickson S, Marcus HJ, Wilson MH. Prognosis of patients with bilateral fixed dilated pupils secondary to traumatic extradural or subdural haematoma who undergo surgery: A systematic review and meta-analysis. Emergency Medicine Journal. 2015; 32(8):654-9. [DOI:10.1136/emermed-2014-204260] [PMID]
- Ryan CG, Thompson RE, Temkin NR, Crane PK, Ellenbogen RG, Elmore JG. Acute traumatic subdural hematoma: Current mortality and functional outcomes in adult patients at a Level I trauma center. The Journal of Trauma and Acute Care Surgery. 2012; 73(5):1348-54. [DOI:10.1097/TA.0b013e31826fcb30] [PMID] [PMCID]
- Tallon JM, Ackroyd-Stolarz S, Karim SA, Clarke DB. The epidemiology of surgically treated acute subdural and epidural hematomas in patients with head injuries: A population-based study. Canadian Journal of Surgery. 2008; 51(5):339-45. [PMID]
Type of Study: Research |
Subject:
Neurotrauma
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |