Sat, Jan 31, 2026
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghorbani M, Hasanpoor M, Mirsardoo H, Jafari S, Ghasemigeskeminjan F, Shams Amiri R. Improving Diagnosis and Management of Nonhypertensive Intracerebral Hemorrhage With Cone Beam
CT Angiography. Iran J Neurosurg 2025; 11 : 9
URL: http://irjns.org/article-1-452-en.html
URL: http://irjns.org/article-1-452-en.html
Mohammad Ghorbani1 
 , Mohammad Hasanpoor1
, Mohammad Hasanpoor1 
 , Hojjat Mirsardoo2
, Hojjat Mirsardoo2 
 , Seifollah Jafari3
, Seifollah Jafari3 
 , Fakhrialsadat Ghasemigeskeminjan3
, Fakhrialsadat Ghasemigeskeminjan3 
 , Rouzbeh Shams Amiri *4
, Rouzbeh Shams Amiri *4 


 , Mohammad Hasanpoor1
, Mohammad Hasanpoor1 
 , Hojjat Mirsardoo2
, Hojjat Mirsardoo2 
 , Seifollah Jafari3
, Seifollah Jafari3 
 , Fakhrialsadat Ghasemigeskeminjan3
, Fakhrialsadat Ghasemigeskeminjan3 
 , Rouzbeh Shams Amiri *4
, Rouzbeh Shams Amiri *4 

1- Division of Vascular and Endovascular Neurosurgery, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Hojjat, Department of Neurosurgery, Kerman University of Medical Sciences, Kerman, Iran.
3- Department of Neurosurgery, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Neurological Surgery, Neuroscience Research Center, Golestan University of Medical Sciences. Gorgan, Iran. ,rshamsa@gmail.com
2- Hojjat, Department of Neurosurgery, Kerman University of Medical Sciences, Kerman, Iran.
3- Department of Neurosurgery, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Neurological Surgery, Neuroscience Research Center, Golestan University of Medical Sciences. Gorgan, Iran. ,
Keywords: Cone-beam computed tomography (CBCT), Intracranial arteriovenous malformations (AVMs), Endovascular procedures
Full Text [PDF 1492 kb]
(438 Downloads)
| Abstract (HTML) (1537 Views)
Full Text: (309 Views)
1. Background and Importance
Cerebral micro-arteriovenous malformations (micro-AVMs) are a rare subgroup, constituting approximately 8% of intracranial AVMs in surgical series, with a potential increase to 21% in cases presenting with hemorrhage among young adults [1]. While spontaneous hemorrhage occurs in approximately 87% of micro-AVM cases, prior studies have indicated a 100% incidence [2]. Micro-AVMs are a distinct subtype of pial AVMs characterized by a concealed nidus measuring less than 1 mm, which is typically undetectable on angiography or gross pathology but may be identified through histological analysis after hematoma evacuation [1]. Diagnosing micro-AVMs using cerebral digital subtraction angiography (DSA) is challenging due to their subtle imaging characteristics, which can closely mimic dural or pial arteriovenous fistulas. The small size of micro-AVMs and their association with hemorrhage further complicate treatment planning.
Modern angiographic equipment, leveraging digital flat panel detector computed tomography (CT) technology, enables advanced imaging modalities, such as intra-arterial cone-beam computed tomography angiography (CBCT-A) [1, 3]. This imaging technique integrates high spatial vascular resolution, enhancing the detailed visualization of blood vessels and surrounding tissues. This technique enables submillimeter reconstructions, thereby enhancing diagnostic sensitivity and mapping capabilities. Previous studies have demonstrated the efficacy of intra-arterial CBCT-A in locating intracranial and spinal dural arteriovenous fistulas [4, 5]. This case report discusses a 58-year-old woman with a spontaneous intracerebral hemorrhage (ICH) caused by a small AVM, highlighting the diagnostic and therapeutic journey that led to successful nidus occlusion using endovascular treatment (EVT).
2. Case Presentation
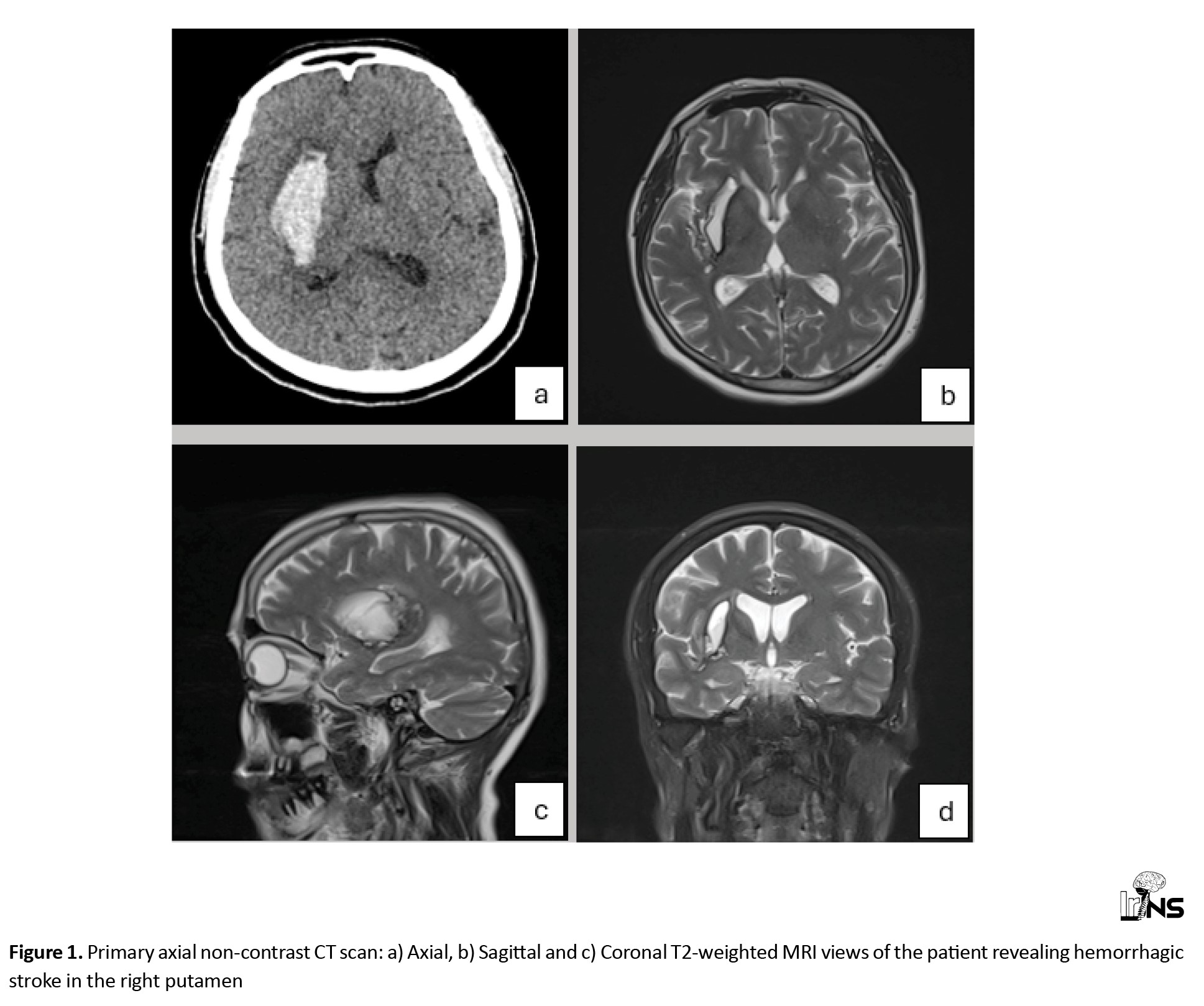
Herein, we present the case of a 58-year-old woman who was transferred to our hospital with headache, drowsiness and left hemiparesis. The patient had upper extremity force of 2/5 and lower extremity force of 3/5. The patient underwent a primary work-up using a non-contrast CT scan and magnetic resonance imaging (MRI) and was diagnosed with a suspected stroke. Imaging revealed a typical hypertension-related ICH in the right putamen (Figure 1). However, the patient did not have a history of hypertension or hyperlipidemia and did not take any medications. Medical management was planned for the patient in the intensive care unit (ICU), which helped stabilize her medical condition. Subsequently, brain Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) were performed, revealing no positive findings regarding the underlying macro-vascular lesion. The patient was treated accordingly, and her symptoms were resolved.
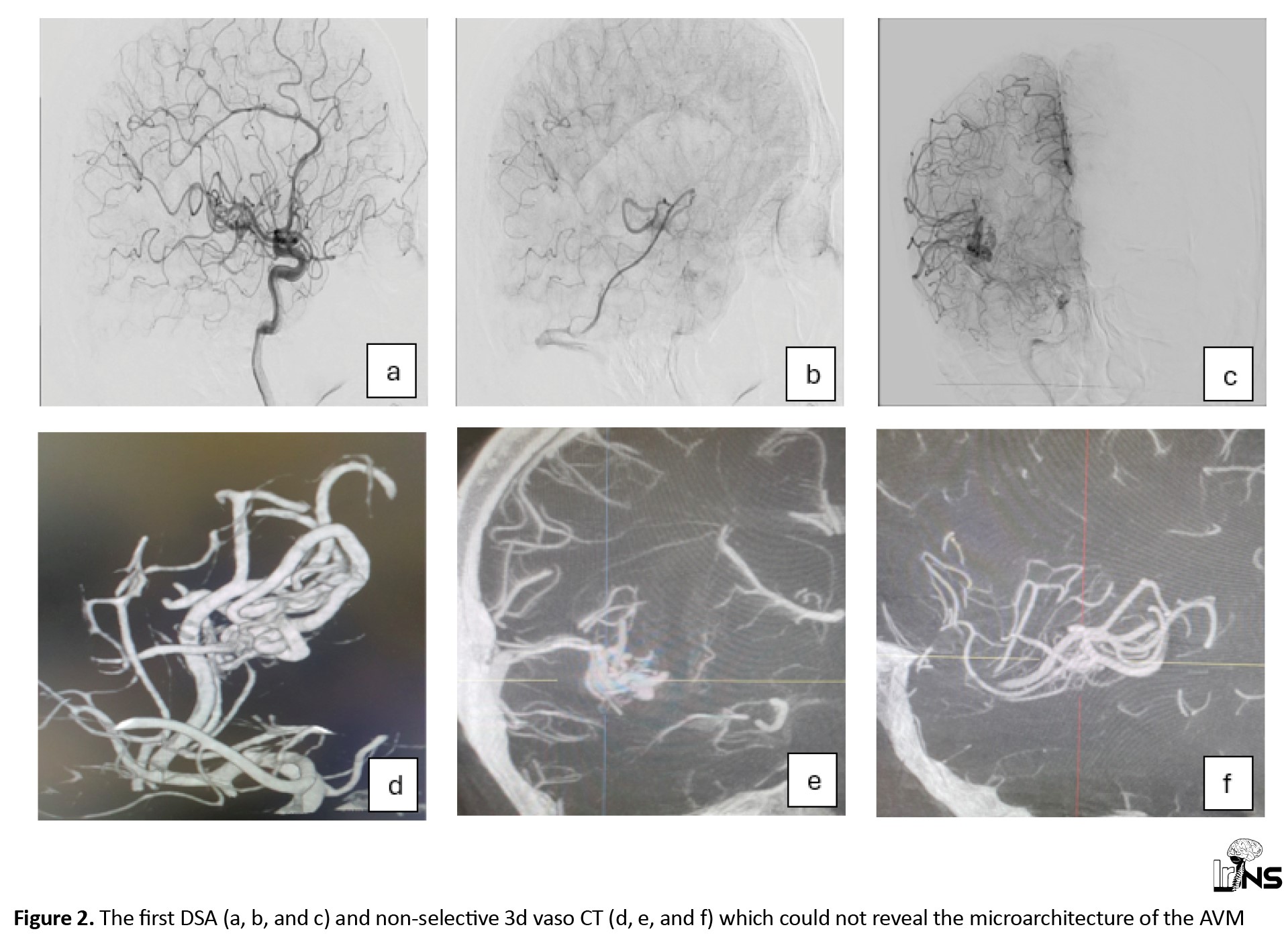
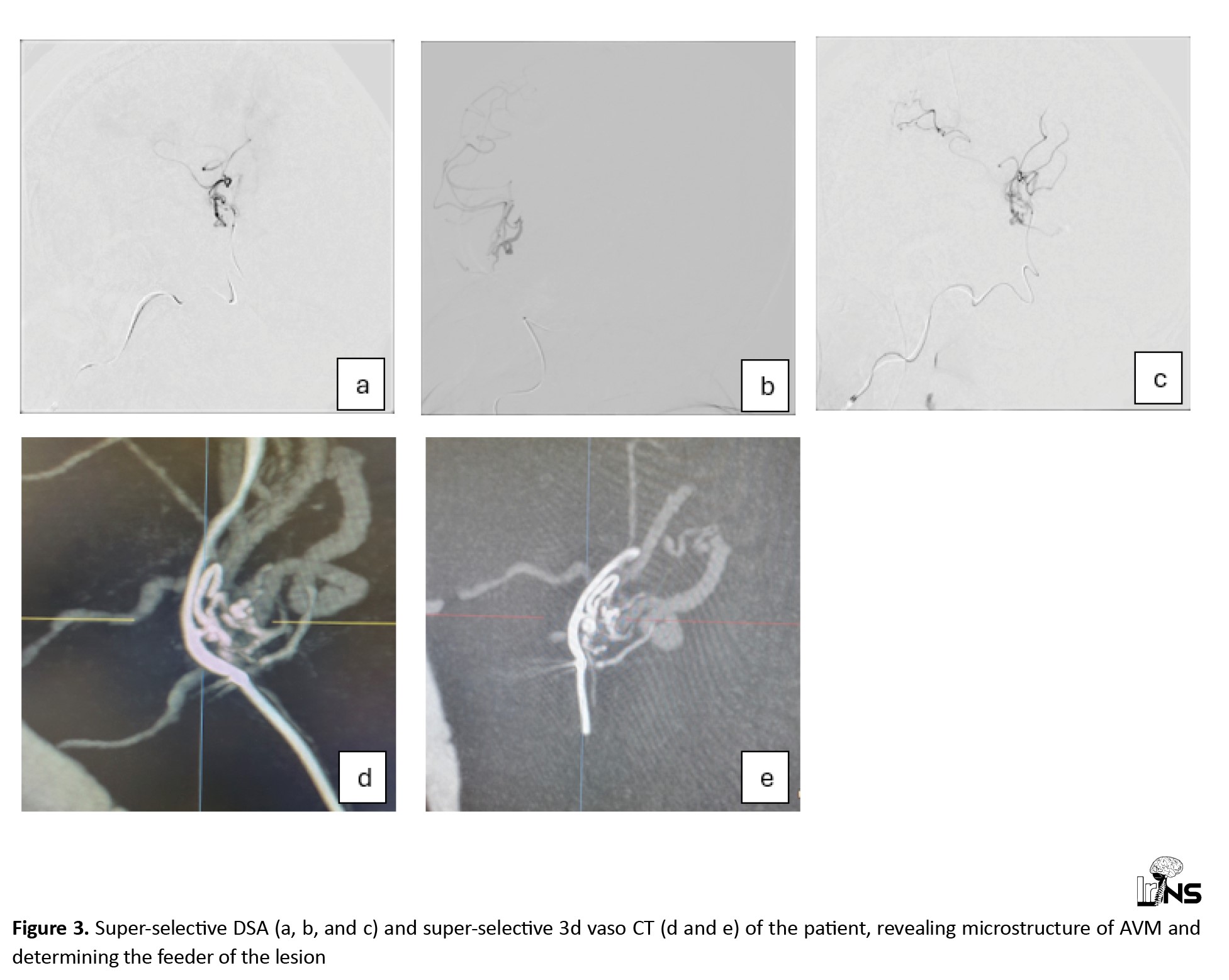
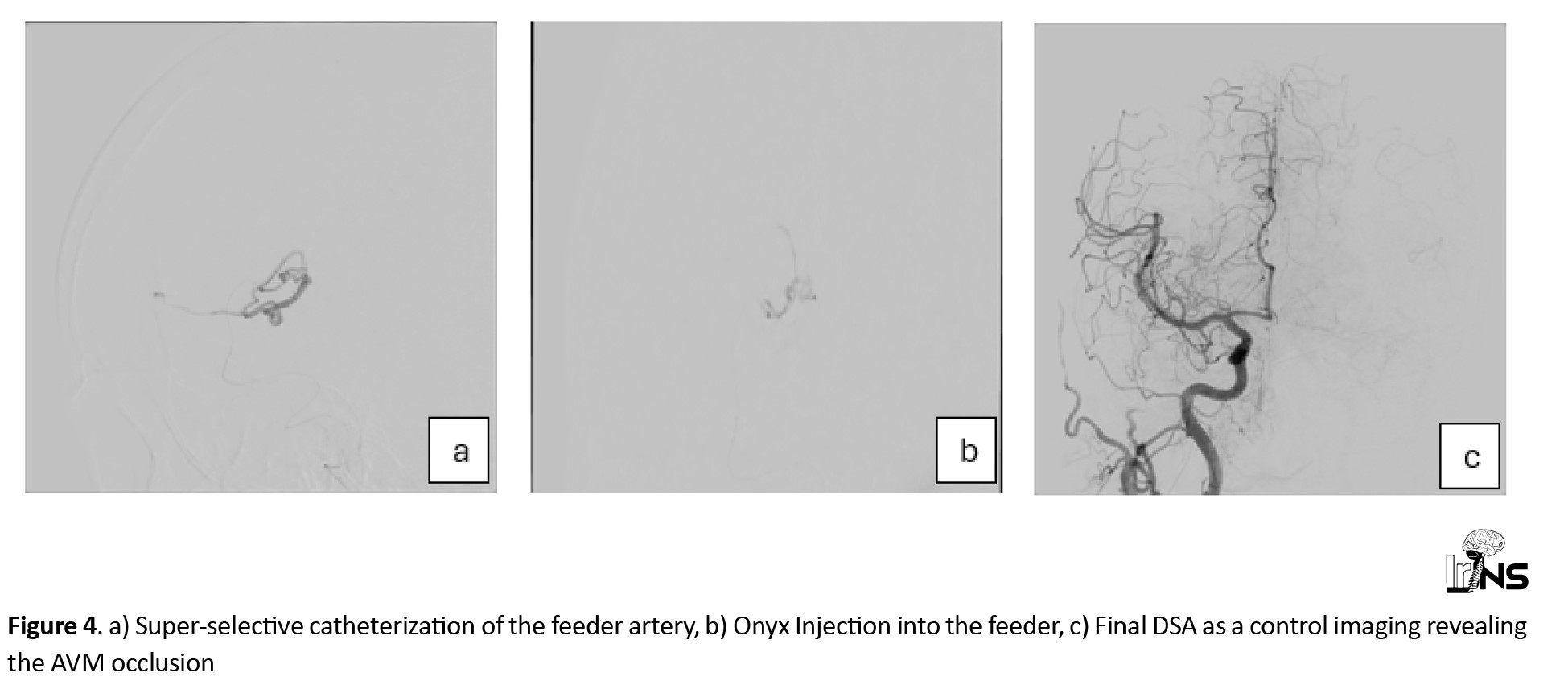
The American Heart Association (AHA)/American Stroke Association (ASA) recommends performing catheter-based intra-arterial DSA imaging in patients with spontaneous ICH, age <70 years, with negative non-invasive imaging, and without a history of hypertension to exclude a macrovascular cause [6]. Therefore, she underwent elective catheter intra-arterial DSA two months after her symptom onset (Figure 2). Imaging revealed a minimal low-flow nidus, suggesting that the AVM was the main cause of the stroke. We then attempted to perform a super-selective catheterization of the lesion’s feeder by using selective DSA and non-selective 3D Vaso CT, which yielded inconclusive outcomes. We utilized a selective CBCT-A or a selective 3D Vaso CT scan to obtain a detailed evaluation of the lesion’s angioarchitecture, which aided in precise 3D roadmap navigation during the procedure (Figure 3). The contrast medium was injected at a rate of 0.6 mL/s with a two-second pre-injection delay, and the CBCT-A acquisition was performed in 20 seconds [7, 8]. The very small feeder vessel of the nidus was successfully catheterized using a Marathon microcatheter and Mirage 0.008 microwire. Subsequently, a small amount of Onyx 18, a liquid embolic agent, was carefully injected through the microcatheter, leading to complete occlusion of the nidus. Follow-up DSA confirmed total occlusion of the lesion without any complications, ensuring the success of the intervention (Figure 4).
3. Discussion
This case illustrates the crucial role of advanced imaging and endovascular techniques in managing AVMs. In our patient, traditional non-invasive imaging modalities, including MRI and MRA, failed to identify the underlying vascular anomaly, prompting the use of catheter-based DSA, which ultimately revealed a small, low-flow nidus indicative of an AVM. The selective use of CBCT-A and 3D Vaso CT was pivotal in evaluating the lesion’s angioarchitecture, enabling precise catheterization and effective treatment.
The assessment of AVMs involves various imaging modalities, such as CT, CTA, MRI, and DSA, each with its own drawbacks, such as time consumption and limited spatial or temporal resolution [9]. Although MRI is beneficial for evaluating the brain parenchyma, it lacks detailed angioarchitecture. CTA offers quality imaging of large vessels but struggles with smaller vasculature [10, 11]. In the search for macrovascular causes of ICH, catheter intra-arterial DSA remains the established standard, demonstrating the highest diagnostic efficacy compared to CT or MRI-based vascular imaging. This holds true particularly for specific patient groups: those under 70 years with lobar ICH, those under 45 years with deep or posterior fossa ICH, those aged 45-70 years with deep or posterior fossa ICH lacking a history of hypertension or signs of small vessel disease on imaging, all ICH patients with evidence of macrovascular lesions on CT or MRI, and patients with primary intraventricular hemorrhage [6]. Combining DSA and CBCT-A maximizes modern angiography capabilities, providing crucial AVM information and overcoming the limitations of other modalities [7]. CBCT-A enables precise assessment of the AVM nidus for treatment planning, especially post-hemorrhage. It complements rather than replaces DSA, offering unique advantages, including a more accurate assessment of AVM dimensions, nidal density, feeders, and drainage [7, 9]. MRI can be combined with CBCT-A for comprehensive evaluation of AVM [9]. Cone beam CT angiography’s benefits, including real-time guidance, make it a valuable adjunct to traditional DSA, enhancing AVM management.
Recent studies have highlighted the effectiveness and safety of EVT for AVMs, especially in cases where microsurgical resection carries a high risk. Burel et al. investigated Spetzler-Martin grade III AVMs. They reported a 42.2% complete obliteration rate and a 4.3% major complication rate, indicating that EVT can serve as a viable first-line treatment for selected AVMs when performed by skilled specialists [12]. Similarly, a nationwide surveillance study reported favorable outcomes with EVT, highlighting the importance of embolization strategies tailored to the individual characteristics of AVMs [13].
The use of Onyx 18 (ethylene vinyl alcohol copolymer), a liquid embolic agent, facilitated complete occlusion of the nidus in our patient without complications, corroborating the findings of previous studies that demonstrated the agent’s effectiveness in achieving durable occlusion of AVMs [14]. Moreover, selective catheterization using microcatheters and microwires enabled the precise delivery of the embolic material, thereby minimizing the risk of non-target embolization and associated complications.
4. Conclusion
In conclusion, this case highlights the effectiveness of advanced endovascular techniques and imaging modalities, including CBCT-A, in managing AVMs, particularly in patients with atypical presentations and those without common risk factors for cerebrovascular disease. Continued advancements in imaging and embolization materials hold promise for further improving the safety and efficacy of AVM treatment.
Ethical Considerations
Compliance with ethical guidelines
Informed consent was obtained from the patient.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Mohammad Ghorbani and Rouzbeh Shams Amiri; Data collection: Rouzbeh Shams Amiri and Hojjat Mirsardoo; Writing the original draft: Rouzbeh Shams Amiri and Seifollah Jafari; Critically revising: Mohammad Ghorbani, Rouzbeh Shams Amiri and Mohammad Hasanpoor; Review and editing: Seifollah Jafari; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Cerebral micro-arteriovenous malformations (micro-AVMs) are a rare subgroup, constituting approximately 8% of intracranial AVMs in surgical series, with a potential increase to 21% in cases presenting with hemorrhage among young adults [1]. While spontaneous hemorrhage occurs in approximately 87% of micro-AVM cases, prior studies have indicated a 100% incidence [2]. Micro-AVMs are a distinct subtype of pial AVMs characterized by a concealed nidus measuring less than 1 mm, which is typically undetectable on angiography or gross pathology but may be identified through histological analysis after hematoma evacuation [1]. Diagnosing micro-AVMs using cerebral digital subtraction angiography (DSA) is challenging due to their subtle imaging characteristics, which can closely mimic dural or pial arteriovenous fistulas. The small size of micro-AVMs and their association with hemorrhage further complicate treatment planning.
Modern angiographic equipment, leveraging digital flat panel detector computed tomography (CT) technology, enables advanced imaging modalities, such as intra-arterial cone-beam computed tomography angiography (CBCT-A) [1, 3]. This imaging technique integrates high spatial vascular resolution, enhancing the detailed visualization of blood vessels and surrounding tissues. This technique enables submillimeter reconstructions, thereby enhancing diagnostic sensitivity and mapping capabilities. Previous studies have demonstrated the efficacy of intra-arterial CBCT-A in locating intracranial and spinal dural arteriovenous fistulas [4, 5]. This case report discusses a 58-year-old woman with a spontaneous intracerebral hemorrhage (ICH) caused by a small AVM, highlighting the diagnostic and therapeutic journey that led to successful nidus occlusion using endovascular treatment (EVT).
2. Case Presentation
Herein, we present the case of a 58-year-old woman who was transferred to our hospital with headache, drowsiness and left hemiparesis. The patient had upper extremity force of 2/5 and lower extremity force of 3/5. The patient underwent a primary work-up using a non-contrast CT scan and magnetic resonance imaging (MRI) and was diagnosed with a suspected stroke. Imaging revealed a typical hypertension-related ICH in the right putamen (Figure 1). However, the patient did not have a history of hypertension or hyperlipidemia and did not take any medications. Medical management was planned for the patient in the intensive care unit (ICU), which helped stabilize her medical condition. Subsequently, brain Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) were performed, revealing no positive findings regarding the underlying macro-vascular lesion. The patient was treated accordingly, and her symptoms were resolved.
The American Heart Association (AHA)/American Stroke Association (ASA) recommends performing catheter-based intra-arterial DSA imaging in patients with spontaneous ICH, age <70 years, with negative non-invasive imaging, and without a history of hypertension to exclude a macrovascular cause [6]. Therefore, she underwent elective catheter intra-arterial DSA two months after her symptom onset (Figure 2). Imaging revealed a minimal low-flow nidus, suggesting that the AVM was the main cause of the stroke. We then attempted to perform a super-selective catheterization of the lesion’s feeder by using selective DSA and non-selective 3D Vaso CT, which yielded inconclusive outcomes. We utilized a selective CBCT-A or a selective 3D Vaso CT scan to obtain a detailed evaluation of the lesion’s angioarchitecture, which aided in precise 3D roadmap navigation during the procedure (Figure 3). The contrast medium was injected at a rate of 0.6 mL/s with a two-second pre-injection delay, and the CBCT-A acquisition was performed in 20 seconds [7, 8]. The very small feeder vessel of the nidus was successfully catheterized using a Marathon microcatheter and Mirage 0.008 microwire. Subsequently, a small amount of Onyx 18, a liquid embolic agent, was carefully injected through the microcatheter, leading to complete occlusion of the nidus. Follow-up DSA confirmed total occlusion of the lesion without any complications, ensuring the success of the intervention (Figure 4).
3. Discussion
This case illustrates the crucial role of advanced imaging and endovascular techniques in managing AVMs. In our patient, traditional non-invasive imaging modalities, including MRI and MRA, failed to identify the underlying vascular anomaly, prompting the use of catheter-based DSA, which ultimately revealed a small, low-flow nidus indicative of an AVM. The selective use of CBCT-A and 3D Vaso CT was pivotal in evaluating the lesion’s angioarchitecture, enabling precise catheterization and effective treatment.
The assessment of AVMs involves various imaging modalities, such as CT, CTA, MRI, and DSA, each with its own drawbacks, such as time consumption and limited spatial or temporal resolution [9]. Although MRI is beneficial for evaluating the brain parenchyma, it lacks detailed angioarchitecture. CTA offers quality imaging of large vessels but struggles with smaller vasculature [10, 11]. In the search for macrovascular causes of ICH, catheter intra-arterial DSA remains the established standard, demonstrating the highest diagnostic efficacy compared to CT or MRI-based vascular imaging. This holds true particularly for specific patient groups: those under 70 years with lobar ICH, those under 45 years with deep or posterior fossa ICH, those aged 45-70 years with deep or posterior fossa ICH lacking a history of hypertension or signs of small vessel disease on imaging, all ICH patients with evidence of macrovascular lesions on CT or MRI, and patients with primary intraventricular hemorrhage [6]. Combining DSA and CBCT-A maximizes modern angiography capabilities, providing crucial AVM information and overcoming the limitations of other modalities [7]. CBCT-A enables precise assessment of the AVM nidus for treatment planning, especially post-hemorrhage. It complements rather than replaces DSA, offering unique advantages, including a more accurate assessment of AVM dimensions, nidal density, feeders, and drainage [7, 9]. MRI can be combined with CBCT-A for comprehensive evaluation of AVM [9]. Cone beam CT angiography’s benefits, including real-time guidance, make it a valuable adjunct to traditional DSA, enhancing AVM management.
Recent studies have highlighted the effectiveness and safety of EVT for AVMs, especially in cases where microsurgical resection carries a high risk. Burel et al. investigated Spetzler-Martin grade III AVMs. They reported a 42.2% complete obliteration rate and a 4.3% major complication rate, indicating that EVT can serve as a viable first-line treatment for selected AVMs when performed by skilled specialists [12]. Similarly, a nationwide surveillance study reported favorable outcomes with EVT, highlighting the importance of embolization strategies tailored to the individual characteristics of AVMs [13].
The use of Onyx 18 (ethylene vinyl alcohol copolymer), a liquid embolic agent, facilitated complete occlusion of the nidus in our patient without complications, corroborating the findings of previous studies that demonstrated the agent’s effectiveness in achieving durable occlusion of AVMs [14]. Moreover, selective catheterization using microcatheters and microwires enabled the precise delivery of the embolic material, thereby minimizing the risk of non-target embolization and associated complications.
4. Conclusion
In conclusion, this case highlights the effectiveness of advanced endovascular techniques and imaging modalities, including CBCT-A, in managing AVMs, particularly in patients with atypical presentations and those without common risk factors for cerebrovascular disease. Continued advancements in imaging and embolization materials hold promise for further improving the safety and efficacy of AVM treatment.
Ethical Considerations
Compliance with ethical guidelines
Informed consent was obtained from the patient.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Mohammad Ghorbani and Rouzbeh Shams Amiri; Data collection: Rouzbeh Shams Amiri and Hojjat Mirsardoo; Writing the original draft: Rouzbeh Shams Amiri and Seifollah Jafari; Critically revising: Mohammad Ghorbani, Rouzbeh Shams Amiri and Mohammad Hasanpoor; Review and editing: Seifollah Jafari; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Al-Smadi AS, Elmokadem A, Shaibani A, Hurley MC, Potts MB, & Jahromi BS, et al. Adjunctive efficacy of intra-arterial conebeam CT angiography relative to DSA in the diagnosis and surgical planning of micro-arteriovenous malformations. AJNR. American Journal of Neuroradiology. 2018; 39(9):1689-95. [DOI:10.3174/ajnr.A5745] [PMID]
- Alén JF, Lagares A, Paredes I, Campollo J, Navia P, & Ramos A, et al. Cerebral microarteriovenous malformations: A series of 28 cases. Journal of Neurosurgery. 2013; 119(3):594-602. [DOI:10.3171/2013.4.JNS121740] [PMID]
- Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC,& Soulez G, et al. Three-dimensional C-arm cone-beam CT: Applications in the interventional suite. Journal of Vascular and Interventional Radiology. 2008; 19(6):799-813. [DOI:10.1016/j.jvir.2008.02.018] [PMID]
- Aadland TD, Thielen KR, Kaufmann TJ, Morris JM, Lanzino G, & Kallmes DF,et al. 3D C-arm conebeam CT angiography as an adjunct in the precise anatomic characterization of spinal dural arteriovenous fistulas. AJNR. American Journal of Neuroradiology. 2010; 31(3):476-80. [DOI:10.3174/ajnr.A1840] [PMID]
- Honarmand AR, Gemmete JJ, Hurley MC, Shaibani A, Chaudhary N, & Pandey AS, et al. Adjunctive value of intra-arterial cone beam CT angiography relative to DSA in the evaluation of cranial and spinal arteriovenous fistulas. Journal of Neurointerventional Surgery. 2015; 7(7):517-23. [DOI:10.1136/neurintsurg-2014-011139] [PMID]
- Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke. 2022; 53(7):e282-361. [DOI:10.1161/STR.0000000000000407]
- Rahal JP, Malek AM. Benefit of cone-beam computed tomography angiography in acute management of angiographically undetectable ruptured arteriovenous malformations. Journal of Neurosurgery. 2013; 119(4):1015-20. [DOI:10.3171/2013.4.JNS1390] [PMID]
- Raz E, Nossek E, Sahlein DH, Sharashidze V, Narayan V, & Ali A, et al. Principles, techniques and applications of high resolution cone beam CT angiography in the neuroangio suite. Journal of Neurointerventional Surgery. 2023; 15(6):600-7. [DOI:10.1136/jnis-2022-018722] [PMID]
- Srinivasan VM, Schafer S, Ghali MG, Arthur A, Duckworth EA. Cone-beam CT angiography (Dyna CT) for intraoperative localization of cerebral arteriovenous malformations. Journal of Neurointerventional Surgery. 2016; 8(1):69-74. [DOI:10.1136/neurintsurg-2014-011422] [PMID]
- St George EJ, Butler P, Plowman PN. Can magnetic resonance imaging alone accurately define the arteriovenous nidus for gamma knife radiosurgery? Journal of Neurosurgery. 2002; 97(5 Suppl):464-70. [DOI:10.3171/jns.2002.97.supplement_5.0464] [PMID]
- Kumamaru KK, Hoppel BE, Mather RT, Rybicki FJ. CT angiography: Current technology and clinical use. Radiologic Clinics of North America. 2010; 48(2):213-35. [DOI:10.1016/j.rcl.2010.02.006] [PMID]
- Burel J, Papagiannaki C, Sourour N, Talbi A, Garnier M, & Hermary C, et al. Endovascular treatment as first-line therapy in Spetzler-Martin grade III brain arteriovenous malformations: A multicenter retrospective study. Journal of Neurosurgery. 2023; 139(4):1070-7. [DOI:10.3171/2023.1.JNS222745] [PMID]
- Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP, et al. Management of brain arteriovenous malformations: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017; 48(8):e200-24. [DOI:10.1161/STR.0000000000000134]
- Né R, Chevallier O, Falvo N, Facy O, Berthod PE, & Galland C,et al. Embolization with ethylene vinyl alcohol copolymer (Onyx®) for peripheral hemostatic and non-hemostatic applications: A feasibility and safety study. Quantitative Imaging in Medicine and Surgery. 2018; 8(3):280-90. [DOI:10.21037/qims.2018.04.03] [PMID]
Type of Study: Case report |
Subject:
Functional Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |