Thu, Jan 29, 2026
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Izadi G, Pourkarim arabi K, Moayedmortazavi S A, Askariardehjani N. Investigating the Correlation of Magnetic Resonance Imaging Findings and Clinical Outcome in Patients With Spondylodiscitis. Iran J Neurosurg 2025; 11 : 12
URL: http://irjns.org/article-1-453-en.html
URL: http://irjns.org/article-1-453-en.html
1- Department of Radiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Neurosurgery, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Neurosurgery, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
Full Text [PDF 1014 kb]
(478 Downloads)
| Abstract (HTML) (1054 Views)
Full Text: (247 Views)
Introduction
Pyogenic spinal infection (PSI) is a rare but serious condition that includes a wide range of conditions, including discitis, spinal osteomyelitis, and spinal epidural abscess. The annual incidence of PSI is 0.5 to 2.2 per 100000 subjects [1]. The three following ways of spread of infection have been described for PSI: Exogenous, endogenous, and contiguous. The risk factors affecting the incidence of spinal infection include male gender, old age, immunodeficiency, HIV, and history of injecting drug abuse [2]. Staphylococcus aureus is the most common microorganism known to cause PSI. Lumbar spine and then thoracic spine, neck, and to a lesser extent, sacral spine are the most common sites of involvement in spinal infection [3]. Post-operative spine infection is one of the complications of spine surgery, which is problematic both in the short term and in the long term. Its incidence is variable and has been reported up to 18% [4]. The surgical method is also involved in the incidence of infection, and surgery with a posterior approach in the cervical area, and also the use of instrumented fusion has the highest incidence rate [5]. The removal of soft tissue changes and fatty deposition in the vertebral bone marrow are reliable findings of recovery in magnetic resonance imaging (MRI), but as said, these findings are late and contradict the evidence of the patient’s clinical recovery [6]. Although the role of imaging in diagnosis has been proven, the relationship between specific imaging findings with the clinical course and prognosis of patients is unclear [7]. According to previous studies, some MRI findings are related to clinical and laboratory outcomes of patients and the need for surgical interventions [8]. Treatment of infectious spondylodiscitis includes antibiotic treatment and surgical treatment [9]. So far, the prognostic value of MRI in the clinical outcome of spondylodiscitis patients, as well as the effective diagnostic criteria in the management of patients, has not been accurately determined. Hence, this study is to investigate the relationship between MRI imaging findings and clinical outcomes in these patients. This matter is important from the point of view that the imaging findings that help in determining the adverse outcome of the disease will be noticed by clinical and surgical experts and will be effective in the management of the disease.
Methods and Materials/Patients
This is a cohort study that was conducted in 2023 on patients with infectious spondylodiscitis in previous surgery who were referred to Shohadaye Tajrish Hospital in Tehran City, Iran. The data were collected prospectively (or retrospectively, as applicable), allowing for a comprehensive assessment of patient outcomes over time.
Inclusion criteria were being over 18 years of age, MRI findings consistent with spondylodiscitis, clinical and laboratory findings consistent with infectious spondylodiscitis, and history of recent spine surgery (defined as surgery within the last 6 months). Meanwhile, the exclusion criteria were pregnancy, age under 18 years, spontaneous infectious spondylodiscitis, spondylitis (tuberculosis, brucellosis, fungal), patients who did not refer for follow-up, and patients who did not accept medication and did not continue antibiotic treatment. Spondylodiscitis was diagnosed with MRI imaging findings with contrast injection, and laboratory and clinical findings. In addition to MRI with contrast, all patients underwent imaging with plain radiography and, if necessary, computed tomography (CT) scans without contrast to evaluate the decrease in vertebral height and vertebral destruction. The findings of all patients were confirmed by radiologists and neurosurgeons. To diagnose the microbiology of the infection, the blood culture was prepared from all patients, and, if necessary, diagnostic aspiration was performed under CT scan guidance. Based on previous studies, MRI findings were divided into 7 categories as follows: Height reduction and vertebra destruction, soft tissue abscess, paravertebral soft tissue involvement, disc destruction, compressive effect on the cord or thecal sac, epidural inflammation, and enhancement pattern in injection images. The enhancement pattern in MRI images was classified into the following three categories: 1) Disc or vertebra enhancement without soft tissue and dural enhancement; 2) Diffuse enhancement in soft or dural tissue; 3) Ring enhancement in soft or dural tissue. Also, neurological deficits were diagnosed and recorded for patients at the beginning of the visit by clinical examination.
Also, suffering from concomitant diseases leading to a weak immune system, such as diabetes, steroid use, hemodialysis, and kidney failure, was included in the initial information of the patients. All patients were treated with injectable antibiotics according to the organism cultured in the secretions or blood cultures during hospitalization and were discharged with oral antibiotics for at least 3 weeks. C-reactive protein (CRP) of the patients was measured and recorded at the beginning of treatment (CRP admission) and two weeks after discharge (CRP follow-up). Patients underwent clinical evaluation for pain and disability at least 3 months after the start of treatment, and were excluded from the study if they did not cooperate. In the outcome evaluation of patients, surgical or conservative treatment, assessment of improvement of systemic symptoms and pain (absence of pain, zero points, pain that can be controlled without painkillers, one point, pain that can be controlled with non-narcotic painkillers, two points, intractable pain control of three points), and improvement of laboratory parameters of inflammation (CRP) were considered. The initial MRI findings were compared with the treatment outcome of the patients with statistical tests. A good outcome was defined based on clinical criteria, including no need for surgical intervention during hospitalization and patient-reported improvement in pain scores during follow-up. CRP level was analyzed as an independent continuous and categorical variable (≤10 vs >10) concerning clinical and imaging findings.
Mean±SD, median, and interquartile range were used to describe quantitative data, and frequency and percentage were used for qualitative variables. Mann-Whitney, chi-square test score, or the Fisher exact test were used to compare the results between the two groups, depending on the type of answer under investigation and the results of the data normality test. Analysis was performed using the SPSS software, version 26. Also, a P<0.05 was considered statistically significant.
Results
A total of 39 patients matching the diagnostic criteria of spondylodiscitis after recent surgery (post-operative spondylodiscitis) based on clinical and laboratory findings and radiological findings (MRI) during the mentioned period, were referred to the Hospital. Of these, 6 patients were excluded from the study due to non-cooperation, and 1 patient had with non-pyogenic culture of secretions (tuberculosis bacillus in the context of previous discitis). All patients had a history of spinal fixation/laminectomy surgery in the last three months secondary to degenerative intervertebral disc diseases and had been referred to this center with complaints of pain and radicular symptoms.
The site of infection was in the lumbar spine in 28 patients and the lower thoracic spine in 4 patients. The average age of the whole sample is 56.78±12.59 years. Meanwhile, 20 patients (62.5%) were male and 12 patients (37.5%) were female. In terms of age and sex, there was no statistically significant difference between the two groups.
According to Table 1, all the variables in the whole sample were examined separately and by outcome.
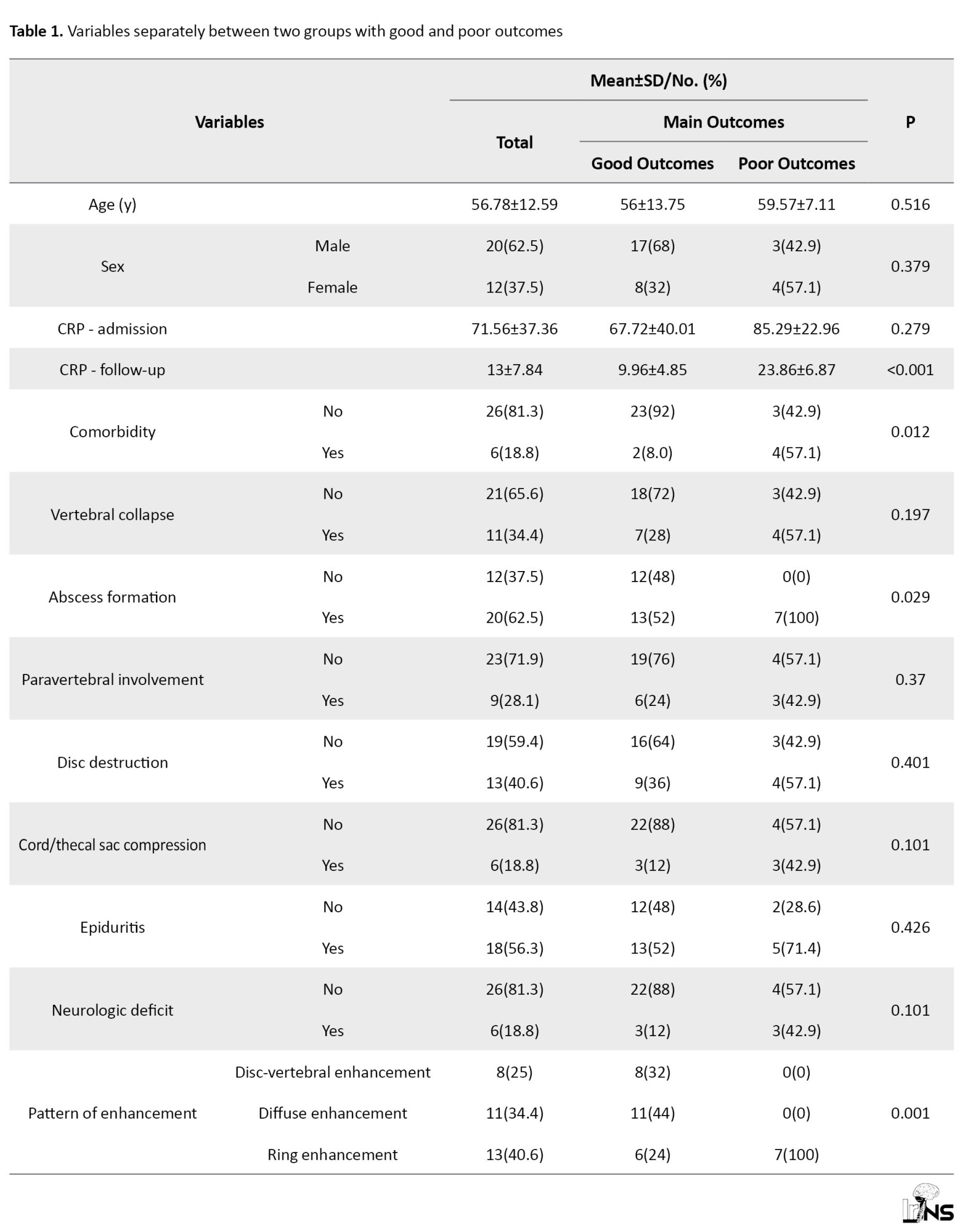
Out of the total number of 32 patients who were included in the study, 25(78.1%) patients had a good outcome, and the rest had a poor outcome.
Follow-up CRP levels were lower among patients with good clinical outcomes, given that CRP was part of the outcome definition. Therefore, no independent conclusion should be drawn from this comparison. However, we separately analyzed CRP as an outcome-related biomarker concerning initial imaging findings (Table 1).
According to Table 2, we examined and compared imaging and clinical variables with the pain level of patients after recovery and discontinuation of antibiotic treatment.
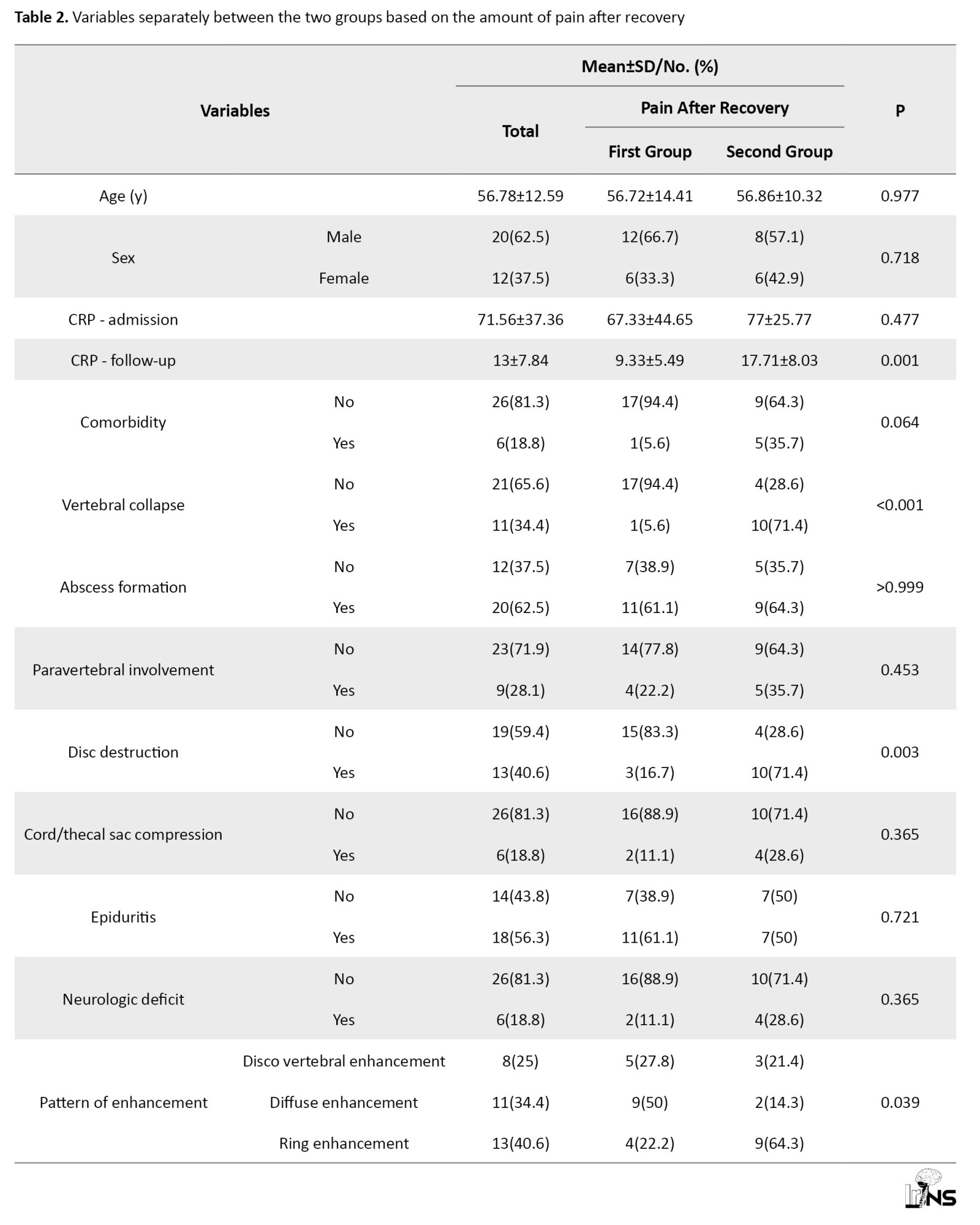
Patients who had no pain at the end of recovery or had tolerable pain without taking painkillers were placed in a group with a frequency of 18 people (56.3%). In addition, patients with controllable pain using non-narcotic painkillers or uncontrollable pain were separated into another group, with a frequency of 14 people (43.8%).
According to Table 3, the patients were divided into two groups.
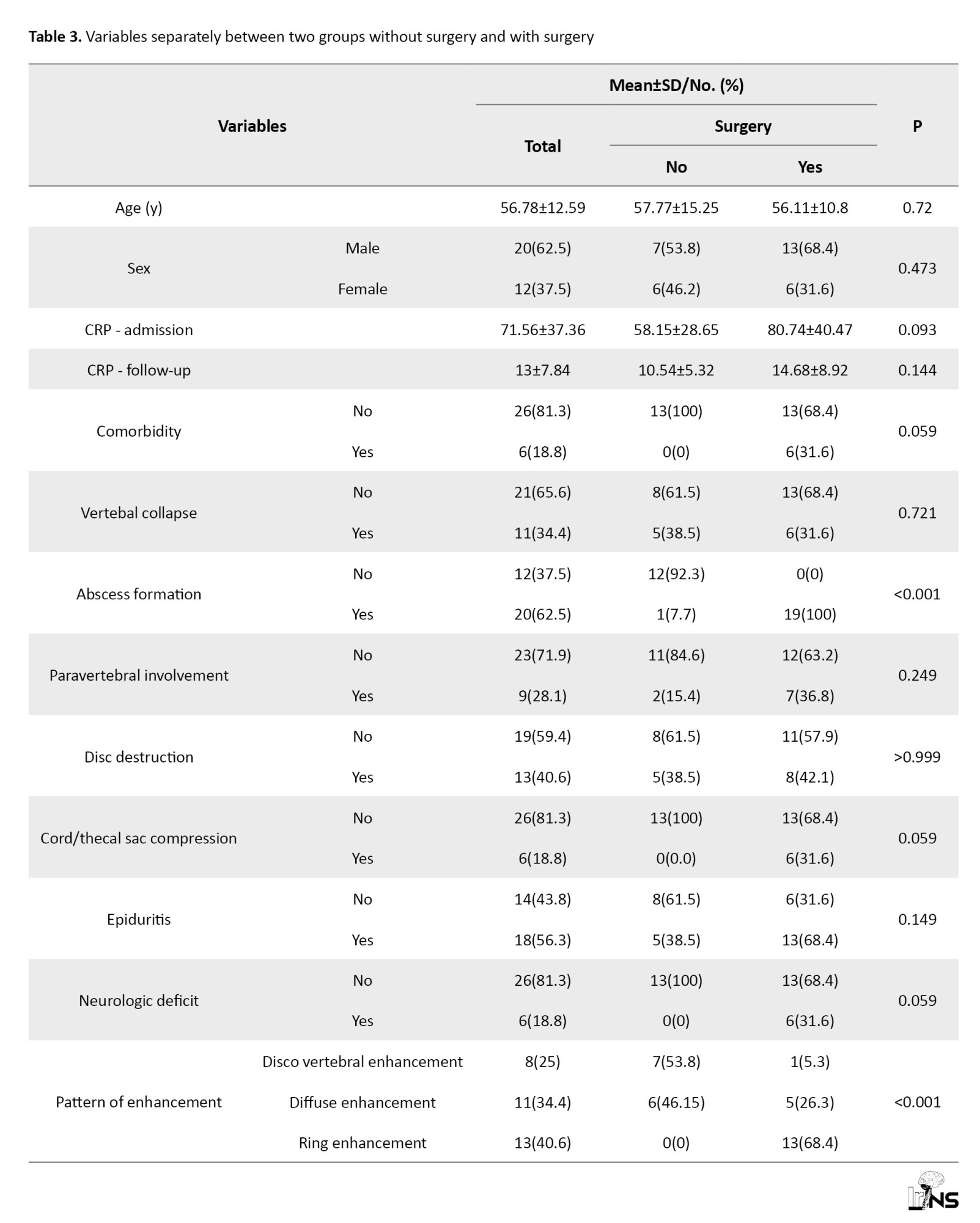
The first group, with a number of 19 people (59.4%), were patients who needed surgery during hospitalization. The second group, included 13 (40.6%) patients who recovered with non-surgical and conservative treatment. Imaging and clinical variables were compared in these two groups.
According to Table 4, patients were divided based on follow-up CRP variable in such a way that the first group is less than 10 and the second group is more than 10, and the clinical and imaging variables were compared in the two groups.

None of the imaging variables showed significant differences in the above two groups.
Discussion
MRI is the most sensitive imaging modality, whose findings in combination with increased inflammatory markers and clinical symptoms of the patient suggest infectious discitis. In the present study, all patients had findings of infectious spondylodiscitis in MRI with increased inflammatory markers (CRP) and positive cultures from blood samples, infectious collection, or aspiration. The incidence of postoperative infectious spondylodiscitis (POS) varies from 0% to 18% depending on the type of surgery. The lowest incidence is seen in decompression surgery and simple lumbar discectomy (0.6% to 3%) compared to instrumental fusion (6% to 18%) [10]. Regarding the pathogenesis of POS, there are two possibilities, firstly, during surgery, contamination is directly implanted in the site, and secondly, the patient has a previous or current infection, and secondary to that infection spreads to the surgical site. For this reason, the risk of infection increases in patients who have underlying conditions leading to weakening of the immune system or infection in other organs. Several risk factors for discitis after spine surgery are known, including diabetes mellitus, chronic steroid use and underlying malignancy, dialysis, and HIV [11]. In the current study, out of 32 patients who were eligible to enter the study, 6 patients had underlying disease (4 patients with uncontrolled diabetes mellitus and 2 patients with diabetes and end-stage renal disease). None of the patients had other major organ infections. People who had an underlying disease had a significantly worse clinical outcome, but on the other hand, there was no significant relationship with the need for surgery in these patients. This problem could be due to the fact that clinicians prefer conservative treatment in this group of patients, although the overall clinical outcome will be unfavorable. Of course, based on previous studies, diabetes mellitus and hemodialysis are known risk factors for pyogenic spondylodiscitis (and not just POS).
The mean age of the patients in the present study was 56.78±12.59, and the frequency of male patients (62.5%) was higher than female patients (37.5%), which is similar to our results reported in other studies [12]. In this study, there was no relationship between the age of patients alone and the clinical outcome, which is the result in the study of Pola et al. [13] and Appalanaidu et al. [14], is also observed. Therefore, the underlying disease (comorbidity) is known to be a more important factor for the adverse outcome of these patients. The inflammatory marker CRP currently has the highest sensitivity in the diagnosis of POS and is also a more reliable marker in response to treatment than erythrocyte sedimentation rate (ESR), because it decreases faster and with a more reliable pattern. After surgery, CRP reaches its peak level within 2-3 days and decreases within 14 days [15] and returns to a normal level. Accordingly, 82% sensitivity in previous studies [16] is one of the cases that should direct the attention of clinicians to treatment failure. In acute conditions, CRP increases faster than ESR, and vice versa, during recovery, CRP reaches a normal level earlier than ESR [17]. In the present study, the level of CRP was measured and recorded at least two weeks after discharge, and patients with CRP less than 10 were classified as having a favorable outcome. Our study showed that patients who had follow-up CRP levels more than 10 are associated with more pain (back pain) in follow-up clinical examination. On the other hand, there was no significant relationship between initial MRI findings and follow-up CRP.
In the study of Ahn et al. They examined the relationship between initial and follow-up MRI findings with CRP and ESR, and soft tissue changes were related to CRP changes, and bone changes in vertebrae were related to ESR levels [18]. Also, Kapsalaki et al. [19] showed in their study that in the follow-up MRI, soft tissue changes are more related to the patient’s clinical condition (clinical signs and symptoms) than bone changes. In the study of Foreman et al. [20], patients with CRP greater than 10.1 mg/dL showed more recurrence and required close monitoring for persistence of infection.
According to the obtained results, the patients whose CRP did not return to normal level in the follow-up examination needed antibiotic treatment, and longer treatment period, and a higher inflammatory level [21]. As a result, inflammatory cysts may be more permanent in these patients and lead to back pain.
Considering the significant relationship between disc destruction and reduction of vertebral height with patients’ pain and overall unfavorable outcome in our study, it is more reasonable to investigate the relationship between follow-up ESR and initial MRI findings and the pain level of patients after recovery.
In most of the previous studies, clinical factors (for example, duration of symptoms, age of patients, presence of comorbidities, and neurological deficits) were compared with the outcome of patients [22]. In the current study, considering the importance of MRI in the early diagnosis of POS, it was discussed which initial MRI findings are more important to identify patients at risk of adverse outcomes.
Understanding the relationship between imaging findings, elevated CRP, and persistent pain is crucial for refining treatment strategies.
Imaging techniques, such as MRI and CT, can reveal structural abnormalities, including disc herniation, nerve root compression, and inflammatory changes. These findings are critical in diagnosing conditions like spondylodiscitis, which can lead to significant pain.
CRP is an acute-phase protein produced by the liver in response to inflammation. Elevated levels of CRP indicate an ongoing inflammatory process, which may correlate with the severity of tissue damage observed on imaging. The inflammatory response, indicated by elevated CRP, can sensitize nociceptive (pain-sensing) pathways. This sensitization may lead to heightened pain perception, even in the absence of significant structural damage.
Imaging findings may reveal changes in the spinal cord or nerve roots that contribute to neurogenic inflammation. This condition can exacerbate pain through the release of pro-inflammatory mediators, which further sensitizes pain pathways.
Persistent pain can be a result of maladaptive neuroplastic changes, where the nervous system becomes increasingly sensitive to stimuli. This phenomenon can be linked to both the structural changes seen on imaging and the biochemical markers of inflammation, such as elevated CRP.
Understanding the interplay between imaging findings and inflammatory markers can lead to more targeted treatment approaches. For instance, patients with high CRP levels and specific imaging findings may benefit from anti-inflammatory medications or biologics that target the underlying inflammatory process.
By elucidating the pathophysiological mechanisms underlying the relationship between imaging findings, elevated CRP, and persistent pain, clinicians can better tailor treatment strategies to address the complex nature of pain syndromes.
In the study of Urrutia et al. [23], Patients who had evidence of abscess formation as well as neurological deficits required more surgery. In the present study, the evidence of the presence of an abscess had a significant relationship with the outcome of surgery. In the study of Widdrington et al. [24], Factors that influenced the adverse outcome of patients, including diabetes mellitus, neurological deficits at the time of presentation, and radiological evidence of cord compression, were evaluated. In our study, all patients who had evidence of compressive effect on the sac or cord and had neurological deficits at the time of referral underwent decompression surgery, but in the follow-up study, there was no significant relationship with persistent pain in these patients. Patients with spondylodiscitis who require surgery in the course of the disease usually experience a more severe form of the disease; however, it has been well demonstrated that patients who underwent surgery had a better neurological outcome [19].
In the study of Uchida et al., the pattern of epidural enhancement and soft tissue in MRI injection images was divided into two categories, the diffuse pattern, which suggests simple cellulitis or granulomatous tissue with micro abscess and indicates the phlegmon stage of infection; and the ring-like pattern, which suggests an abscess surrounded by a wall of granulation tissue [25]. In this study, it was shown that ring-like enhancement has a stronger relationship with the amount of surgery, and the current study is consistent with these results. In previous studies, it was shown that epidural linear enhancement based on epididymitis is seen in two-thirds of patients with spondylodiscitis [26], but the only clinically and surgically important factor is epidural abscess and circular enhancement pattern [27]. It is also noteworthy that in previous studies, epidural linear intensification remains in follow-up MRI images despite clinical improvement. In our study, 56% of the patients had evidence of epididymitis, and there was no significant relationship between this factor and the clinical outcome of the patients.
Conclusion
The study highlights the importance of MRI findings and CRP levels in assessing clinical outcomes in patients with infectious spondylodiscitis. Our findings indicate that lower CRP levels correlate with better outcomes, supporting the use of CRP as a valuable indicator in clinical practice.
Limitations
In terms of The relatively small number of participants can limit the generalizability of the results. A larger sample size would provide more robust data and enhance the reliability of the conclusions drawn. Meanwhile, the study included only postoperative spondylodiscitis cases, which may introduce selection bias. This limitation affects the applicability of the findings to a broader population of patients with spondylodiscitis, as those who did not undergo surgery were not represented. Also, the follow-up period for some aspects of the study was limited to only three months. This short duration may not adequately represent the long-term outcomes and complications associated with surgical interventions, potentially skewing the understanding of the treatment’s effectiveness.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP.REC.1400.149). Written informed consent was obtained from all patients or their legal guardians before inclusion in the study. The participants were assured that their information would remain confidential and would be used solely for research purposes. This study was conducted following the Declaration of Helsinki.
Funding
The present article was extracted from the PhD dissertation of Golnaz Izadi, approved by the Department of Radiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization and study design: Golnaz Izadi; Data collection: Kowsar Pourkarim Arabi and Seyed Ahmad Moayedmortazavi; Data analysis and interpretation: Golnaz Izadi, Kowsar Pourkarim Arabi, and Seyed Ahmad Moayedmortazavi; Writing the original draft: Navid Askariardehjani; Review and editing: Navid Askariardehjani; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The author would like to thank the staff of Shohadaye Tajrish Hospital, Tehran, Iran, for their support during data collection and all the patients who participated in the study. The author also appreciates the guidance and academic support provided by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
Pyogenic spinal infection (PSI) is a rare but serious condition that includes a wide range of conditions, including discitis, spinal osteomyelitis, and spinal epidural abscess. The annual incidence of PSI is 0.5 to 2.2 per 100000 subjects [1]. The three following ways of spread of infection have been described for PSI: Exogenous, endogenous, and contiguous. The risk factors affecting the incidence of spinal infection include male gender, old age, immunodeficiency, HIV, and history of injecting drug abuse [2]. Staphylococcus aureus is the most common microorganism known to cause PSI. Lumbar spine and then thoracic spine, neck, and to a lesser extent, sacral spine are the most common sites of involvement in spinal infection [3]. Post-operative spine infection is one of the complications of spine surgery, which is problematic both in the short term and in the long term. Its incidence is variable and has been reported up to 18% [4]. The surgical method is also involved in the incidence of infection, and surgery with a posterior approach in the cervical area, and also the use of instrumented fusion has the highest incidence rate [5]. The removal of soft tissue changes and fatty deposition in the vertebral bone marrow are reliable findings of recovery in magnetic resonance imaging (MRI), but as said, these findings are late and contradict the evidence of the patient’s clinical recovery [6]. Although the role of imaging in diagnosis has been proven, the relationship between specific imaging findings with the clinical course and prognosis of patients is unclear [7]. According to previous studies, some MRI findings are related to clinical and laboratory outcomes of patients and the need for surgical interventions [8]. Treatment of infectious spondylodiscitis includes antibiotic treatment and surgical treatment [9]. So far, the prognostic value of MRI in the clinical outcome of spondylodiscitis patients, as well as the effective diagnostic criteria in the management of patients, has not been accurately determined. Hence, this study is to investigate the relationship between MRI imaging findings and clinical outcomes in these patients. This matter is important from the point of view that the imaging findings that help in determining the adverse outcome of the disease will be noticed by clinical and surgical experts and will be effective in the management of the disease.
Methods and Materials/Patients
This is a cohort study that was conducted in 2023 on patients with infectious spondylodiscitis in previous surgery who were referred to Shohadaye Tajrish Hospital in Tehran City, Iran. The data were collected prospectively (or retrospectively, as applicable), allowing for a comprehensive assessment of patient outcomes over time.
Inclusion criteria were being over 18 years of age, MRI findings consistent with spondylodiscitis, clinical and laboratory findings consistent with infectious spondylodiscitis, and history of recent spine surgery (defined as surgery within the last 6 months). Meanwhile, the exclusion criteria were pregnancy, age under 18 years, spontaneous infectious spondylodiscitis, spondylitis (tuberculosis, brucellosis, fungal), patients who did not refer for follow-up, and patients who did not accept medication and did not continue antibiotic treatment. Spondylodiscitis was diagnosed with MRI imaging findings with contrast injection, and laboratory and clinical findings. In addition to MRI with contrast, all patients underwent imaging with plain radiography and, if necessary, computed tomography (CT) scans without contrast to evaluate the decrease in vertebral height and vertebral destruction. The findings of all patients were confirmed by radiologists and neurosurgeons. To diagnose the microbiology of the infection, the blood culture was prepared from all patients, and, if necessary, diagnostic aspiration was performed under CT scan guidance. Based on previous studies, MRI findings were divided into 7 categories as follows: Height reduction and vertebra destruction, soft tissue abscess, paravertebral soft tissue involvement, disc destruction, compressive effect on the cord or thecal sac, epidural inflammation, and enhancement pattern in injection images. The enhancement pattern in MRI images was classified into the following three categories: 1) Disc or vertebra enhancement without soft tissue and dural enhancement; 2) Diffuse enhancement in soft or dural tissue; 3) Ring enhancement in soft or dural tissue. Also, neurological deficits were diagnosed and recorded for patients at the beginning of the visit by clinical examination.
Also, suffering from concomitant diseases leading to a weak immune system, such as diabetes, steroid use, hemodialysis, and kidney failure, was included in the initial information of the patients. All patients were treated with injectable antibiotics according to the organism cultured in the secretions or blood cultures during hospitalization and were discharged with oral antibiotics for at least 3 weeks. C-reactive protein (CRP) of the patients was measured and recorded at the beginning of treatment (CRP admission) and two weeks after discharge (CRP follow-up). Patients underwent clinical evaluation for pain and disability at least 3 months after the start of treatment, and were excluded from the study if they did not cooperate. In the outcome evaluation of patients, surgical or conservative treatment, assessment of improvement of systemic symptoms and pain (absence of pain, zero points, pain that can be controlled without painkillers, one point, pain that can be controlled with non-narcotic painkillers, two points, intractable pain control of three points), and improvement of laboratory parameters of inflammation (CRP) were considered. The initial MRI findings were compared with the treatment outcome of the patients with statistical tests. A good outcome was defined based on clinical criteria, including no need for surgical intervention during hospitalization and patient-reported improvement in pain scores during follow-up. CRP level was analyzed as an independent continuous and categorical variable (≤10 vs >10) concerning clinical and imaging findings.
Mean±SD, median, and interquartile range were used to describe quantitative data, and frequency and percentage were used for qualitative variables. Mann-Whitney, chi-square test score, or the Fisher exact test were used to compare the results between the two groups, depending on the type of answer under investigation and the results of the data normality test. Analysis was performed using the SPSS software, version 26. Also, a P<0.05 was considered statistically significant.
Results
A total of 39 patients matching the diagnostic criteria of spondylodiscitis after recent surgery (post-operative spondylodiscitis) based on clinical and laboratory findings and radiological findings (MRI) during the mentioned period, were referred to the Hospital. Of these, 6 patients were excluded from the study due to non-cooperation, and 1 patient had with non-pyogenic culture of secretions (tuberculosis bacillus in the context of previous discitis). All patients had a history of spinal fixation/laminectomy surgery in the last three months secondary to degenerative intervertebral disc diseases and had been referred to this center with complaints of pain and radicular symptoms.
The site of infection was in the lumbar spine in 28 patients and the lower thoracic spine in 4 patients. The average age of the whole sample is 56.78±12.59 years. Meanwhile, 20 patients (62.5%) were male and 12 patients (37.5%) were female. In terms of age and sex, there was no statistically significant difference between the two groups.
According to Table 1, all the variables in the whole sample were examined separately and by outcome.

Out of the total number of 32 patients who were included in the study, 25(78.1%) patients had a good outcome, and the rest had a poor outcome.
Follow-up CRP levels were lower among patients with good clinical outcomes, given that CRP was part of the outcome definition. Therefore, no independent conclusion should be drawn from this comparison. However, we separately analyzed CRP as an outcome-related biomarker concerning initial imaging findings (Table 1).
According to Table 2, we examined and compared imaging and clinical variables with the pain level of patients after recovery and discontinuation of antibiotic treatment.

Patients who had no pain at the end of recovery or had tolerable pain without taking painkillers were placed in a group with a frequency of 18 people (56.3%). In addition, patients with controllable pain using non-narcotic painkillers or uncontrollable pain were separated into another group, with a frequency of 14 people (43.8%).
According to Table 3, the patients were divided into two groups.

The first group, with a number of 19 people (59.4%), were patients who needed surgery during hospitalization. The second group, included 13 (40.6%) patients who recovered with non-surgical and conservative treatment. Imaging and clinical variables were compared in these two groups.
According to Table 4, patients were divided based on follow-up CRP variable in such a way that the first group is less than 10 and the second group is more than 10, and the clinical and imaging variables were compared in the two groups.

None of the imaging variables showed significant differences in the above two groups.
Discussion
MRI is the most sensitive imaging modality, whose findings in combination with increased inflammatory markers and clinical symptoms of the patient suggest infectious discitis. In the present study, all patients had findings of infectious spondylodiscitis in MRI with increased inflammatory markers (CRP) and positive cultures from blood samples, infectious collection, or aspiration. The incidence of postoperative infectious spondylodiscitis (POS) varies from 0% to 18% depending on the type of surgery. The lowest incidence is seen in decompression surgery and simple lumbar discectomy (0.6% to 3%) compared to instrumental fusion (6% to 18%) [10]. Regarding the pathogenesis of POS, there are two possibilities, firstly, during surgery, contamination is directly implanted in the site, and secondly, the patient has a previous or current infection, and secondary to that infection spreads to the surgical site. For this reason, the risk of infection increases in patients who have underlying conditions leading to weakening of the immune system or infection in other organs. Several risk factors for discitis after spine surgery are known, including diabetes mellitus, chronic steroid use and underlying malignancy, dialysis, and HIV [11]. In the current study, out of 32 patients who were eligible to enter the study, 6 patients had underlying disease (4 patients with uncontrolled diabetes mellitus and 2 patients with diabetes and end-stage renal disease). None of the patients had other major organ infections. People who had an underlying disease had a significantly worse clinical outcome, but on the other hand, there was no significant relationship with the need for surgery in these patients. This problem could be due to the fact that clinicians prefer conservative treatment in this group of patients, although the overall clinical outcome will be unfavorable. Of course, based on previous studies, diabetes mellitus and hemodialysis are known risk factors for pyogenic spondylodiscitis (and not just POS).
The mean age of the patients in the present study was 56.78±12.59, and the frequency of male patients (62.5%) was higher than female patients (37.5%), which is similar to our results reported in other studies [12]. In this study, there was no relationship between the age of patients alone and the clinical outcome, which is the result in the study of Pola et al. [13] and Appalanaidu et al. [14], is also observed. Therefore, the underlying disease (comorbidity) is known to be a more important factor for the adverse outcome of these patients. The inflammatory marker CRP currently has the highest sensitivity in the diagnosis of POS and is also a more reliable marker in response to treatment than erythrocyte sedimentation rate (ESR), because it decreases faster and with a more reliable pattern. After surgery, CRP reaches its peak level within 2-3 days and decreases within 14 days [15] and returns to a normal level. Accordingly, 82% sensitivity in previous studies [16] is one of the cases that should direct the attention of clinicians to treatment failure. In acute conditions, CRP increases faster than ESR, and vice versa, during recovery, CRP reaches a normal level earlier than ESR [17]. In the present study, the level of CRP was measured and recorded at least two weeks after discharge, and patients with CRP less than 10 were classified as having a favorable outcome. Our study showed that patients who had follow-up CRP levels more than 10 are associated with more pain (back pain) in follow-up clinical examination. On the other hand, there was no significant relationship between initial MRI findings and follow-up CRP.
In the study of Ahn et al. They examined the relationship between initial and follow-up MRI findings with CRP and ESR, and soft tissue changes were related to CRP changes, and bone changes in vertebrae were related to ESR levels [18]. Also, Kapsalaki et al. [19] showed in their study that in the follow-up MRI, soft tissue changes are more related to the patient’s clinical condition (clinical signs and symptoms) than bone changes. In the study of Foreman et al. [20], patients with CRP greater than 10.1 mg/dL showed more recurrence and required close monitoring for persistence of infection.
According to the obtained results, the patients whose CRP did not return to normal level in the follow-up examination needed antibiotic treatment, and longer treatment period, and a higher inflammatory level [21]. As a result, inflammatory cysts may be more permanent in these patients and lead to back pain.
Considering the significant relationship between disc destruction and reduction of vertebral height with patients’ pain and overall unfavorable outcome in our study, it is more reasonable to investigate the relationship between follow-up ESR and initial MRI findings and the pain level of patients after recovery.
In most of the previous studies, clinical factors (for example, duration of symptoms, age of patients, presence of comorbidities, and neurological deficits) were compared with the outcome of patients [22]. In the current study, considering the importance of MRI in the early diagnosis of POS, it was discussed which initial MRI findings are more important to identify patients at risk of adverse outcomes.
Understanding the relationship between imaging findings, elevated CRP, and persistent pain is crucial for refining treatment strategies.
Imaging techniques, such as MRI and CT, can reveal structural abnormalities, including disc herniation, nerve root compression, and inflammatory changes. These findings are critical in diagnosing conditions like spondylodiscitis, which can lead to significant pain.
CRP is an acute-phase protein produced by the liver in response to inflammation. Elevated levels of CRP indicate an ongoing inflammatory process, which may correlate with the severity of tissue damage observed on imaging. The inflammatory response, indicated by elevated CRP, can sensitize nociceptive (pain-sensing) pathways. This sensitization may lead to heightened pain perception, even in the absence of significant structural damage.
Imaging findings may reveal changes in the spinal cord or nerve roots that contribute to neurogenic inflammation. This condition can exacerbate pain through the release of pro-inflammatory mediators, which further sensitizes pain pathways.
Persistent pain can be a result of maladaptive neuroplastic changes, where the nervous system becomes increasingly sensitive to stimuli. This phenomenon can be linked to both the structural changes seen on imaging and the biochemical markers of inflammation, such as elevated CRP.
Understanding the interplay between imaging findings and inflammatory markers can lead to more targeted treatment approaches. For instance, patients with high CRP levels and specific imaging findings may benefit from anti-inflammatory medications or biologics that target the underlying inflammatory process.
By elucidating the pathophysiological mechanisms underlying the relationship between imaging findings, elevated CRP, and persistent pain, clinicians can better tailor treatment strategies to address the complex nature of pain syndromes.
In the study of Urrutia et al. [23], Patients who had evidence of abscess formation as well as neurological deficits required more surgery. In the present study, the evidence of the presence of an abscess had a significant relationship with the outcome of surgery. In the study of Widdrington et al. [24], Factors that influenced the adverse outcome of patients, including diabetes mellitus, neurological deficits at the time of presentation, and radiological evidence of cord compression, were evaluated. In our study, all patients who had evidence of compressive effect on the sac or cord and had neurological deficits at the time of referral underwent decompression surgery, but in the follow-up study, there was no significant relationship with persistent pain in these patients. Patients with spondylodiscitis who require surgery in the course of the disease usually experience a more severe form of the disease; however, it has been well demonstrated that patients who underwent surgery had a better neurological outcome [19].
In the study of Uchida et al., the pattern of epidural enhancement and soft tissue in MRI injection images was divided into two categories, the diffuse pattern, which suggests simple cellulitis or granulomatous tissue with micro abscess and indicates the phlegmon stage of infection; and the ring-like pattern, which suggests an abscess surrounded by a wall of granulation tissue [25]. In this study, it was shown that ring-like enhancement has a stronger relationship with the amount of surgery, and the current study is consistent with these results. In previous studies, it was shown that epidural linear enhancement based on epididymitis is seen in two-thirds of patients with spondylodiscitis [26], but the only clinically and surgically important factor is epidural abscess and circular enhancement pattern [27]. It is also noteworthy that in previous studies, epidural linear intensification remains in follow-up MRI images despite clinical improvement. In our study, 56% of the patients had evidence of epididymitis, and there was no significant relationship between this factor and the clinical outcome of the patients.
Conclusion
The study highlights the importance of MRI findings and CRP levels in assessing clinical outcomes in patients with infectious spondylodiscitis. Our findings indicate that lower CRP levels correlate with better outcomes, supporting the use of CRP as a valuable indicator in clinical practice.
Limitations
In terms of The relatively small number of participants can limit the generalizability of the results. A larger sample size would provide more robust data and enhance the reliability of the conclusions drawn. Meanwhile, the study included only postoperative spondylodiscitis cases, which may introduce selection bias. This limitation affects the applicability of the findings to a broader population of patients with spondylodiscitis, as those who did not undergo surgery were not represented. Also, the follow-up period for some aspects of the study was limited to only three months. This short duration may not adequately represent the long-term outcomes and complications associated with surgical interventions, potentially skewing the understanding of the treatment’s effectiveness.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP.REC.1400.149). Written informed consent was obtained from all patients or their legal guardians before inclusion in the study. The participants were assured that their information would remain confidential and would be used solely for research purposes. This study was conducted following the Declaration of Helsinki.
Funding
The present article was extracted from the PhD dissertation of Golnaz Izadi, approved by the Department of Radiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization and study design: Golnaz Izadi; Data collection: Kowsar Pourkarim Arabi and Seyed Ahmad Moayedmortazavi; Data analysis and interpretation: Golnaz Izadi, Kowsar Pourkarim Arabi, and Seyed Ahmad Moayedmortazavi; Writing the original draft: Navid Askariardehjani; Review and editing: Navid Askariardehjani; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The author would like to thank the staff of Shohadaye Tajrish Hospital, Tehran, Iran, for their support during data collection and all the patients who participated in the study. The author also appreciates the guidance and academic support provided by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- Pola E, Autore G, Formica VM, Pambianco V, Colangelo D, Cauda R, et al. New classification for the treatment of pyogenic spondylodiscitis: Validation study on a population of 250 patients with a follow-up of 2 years. European Spine Journal. 2017; 26(Suppl 4):479-88. [DOI:10.1007/s00586-017-5043-5] [PMID]
- Aljawadi A, Jahangir N, Jeelani A, Ferguson Z, Niazi N, Arnall F, et al. Management of pyogenic spinal infection, review of literature. Journal of Orthopaedics. 2019; 16(6):508-12. [DOI:10.1016/j.jor.2019.08.014] [PMID] [PMCID]
- Almansour H, Pepke W, Akbar M. Pyogenic spondylodiscitis: The quest towards a clinical-radiological classification. Orthopade. 2020; 49(6):482-93. [DOI:10.1007/s00132-019-03836-0] [PMID]
- Sans N, Faruch M, Lapègue F, Ponsot A, Chiavassa H, Railhac JJ. Infections of the spinal column--spondylodiscitis. Diagnostic and Interventional Imaging. 2012; 93(6):520-9. [DOI:10.1016/j.diii.2012.04.003] [PMID]
- Basu S, Ghosh JD, Malik FH, Tikoo A. Postoperative discitis following single-level lumbar discectomy: Our experience of 17 cases. Indian Journal of Orthopaedics. 2012; 46(4):427-33. [DOI:10.4103/0019-5413.98831] [PMID] [PMCID]
- Bateman JL, Pevzner MM. Spinal osteomyelitis: A review of 10 years’ experience. Orthopedics. 1995; 18(6):561-5. [DOI:10.3928/0147-7447-19950601-10] [PMID]
- Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 Infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clinical Infectious Diseases. 2015; 61(6):e26-46. [DOI:10.1093/cid/civ482] [PMID]
- Braun A, Germann T, Wünnemann F, Weber MA, Schiltenwolf M, Akbar M, et al. Impact of MRI, CT, and clinical characteristics on microbial pathogen detection using CT-guided biopsy for suspected spondylodiscitis. Journal of Clinical Medicine. 2019; 9(1):32. [DOI:10.3390/jcm9010032] [PMID] [PMCID]
- Camino Willhuber G, Guiroy A, Zamorano J, Astur N, Valacco M. Independent reliability analysis of a new classification for pyogenic spondylodiscitis. Global Spine Journal. 2021; 11(5):669-73. [DOI:10.1177/2192568220919091] [PMID] [PMCID]
- Chaudhary SB, Vives MJ, Basra SK, Reiter MF. Postoperative spinal wound infections and postprocedural diskitis. The Journal of Spinal Cord Medicine. 2007; 30(5):441-51. [DOI:10.1080/10790268.2007.11753476] [PMID] [PMCID]
- Corona-Cedillo R, Saavedra-Navarrete MT, Espinoza-Garcia JJ, Mendoza-Aguilar AN, Ternovoy SK, Roldan-Valadez E. Imaging assessment of the postoperative spine: An updated pictorial review of selected complications. Biomed Res Int. 2021; 2021:9940001. [DOI:10.1155/2021/9940001] [PMID] [PMCID]
- Dipaola CP, Saravanja DD, Boriani L, Zhang H, Boyd MC, Kwon BK, et al. Postoperative infection treatment score for the spine (PITSS): Construction and validation of a predictive model to define need for single versus multiple irrigation and debridement for spinal surgical site infection. The Spine Journal. 2012; 12(3):218-30. [DOI:10.1016/j.spinee.2012.02.004] [PMID]
- Pola E, Taccari F, Autore G, Giovannenze F, Pambianco V, Cauda R, et al. Multidisciplinary management of pyogenic spondylodiscitis: Epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. European Spine Journal. 2018; 27(Suppl 2):229-36. [DOI:10.1007/s00586-018-5598-9] [PMID]
- Appalanaidu N, Shafafy R, Gee C, Brogan K, Karmani S, Morassi G, et al. Predicting the need for surgical intervention in patients with spondylodiscitis: The Brighton spondylodiscitis score (BSDS). European Spine Journal. 2019; 28(4):751-61. [DOI:10.1007/s00586-018-5775-x] [PMID]
- Euba G, Narváez JA, Nolla JM, Murillo O, Narváez J, Gómez-Vaquero C, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Seminars in Arthritis and Rheumatism. 2008; 38(1):28-40. [DOI:10.1016/j.semarthrit.2007.08.007] [PMID]
- Zadran S, Pedersen PH, Eiskjær S. Vertebral osteomyelitis: A mortality analysis comparing surgical and conservative management. Global Spine Journal. 2020; 10(4):456-63. [DOI:10.1177/2192568219862213] [PMID] [PMCID]
- Gillams AR, Chaddha B, Carter AP. MR appearances of the temporal evolution and resolution of infectious spondylitis. American Journal of Roentgenology. 1996; 166(4):903-7. [DOI:10.2214/ajr.166.4.8610571] [PMID]
- Ahn KS, Kang CH, Hong SJ, Kim BH, Shim E. The correlation between follow-up MRI findings and laboratory results in pyogenic spondylodiscitis. BMc Musculoskeletal Disorders. 2020; 21(1):428. [DOI:10.1186/s12891-020-03446-4] [PMID] [PMCID]
- Foreman SC, Schwaiger BJ, Meyer B, Gersing AS, Zimmer C, Gempt J, et al. Computed tomography and magnetic resonance imaging parameters associated with poor clinical outcome in spondylodiscitis. World Neurosurgery. 2017; 104:919-26.e2. [DOI:10.1016/j.wneu.2017.05.102] [PMID]
- Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: Diagnosis and treatment options. Deutsches Arzteblatt International. 2017; 114(51-52):875-82. [DOI:10.3238/arztebl.2017.0875] [PMID] [PMCID]
- Homagk L, Marmelstein D, Homagk N, Hofmann GO. SponDT (spondylodiscitis diagnosis and treatment): Spondylodiscitis scoring system. Journal of Orthopaedic Surgery and Research. 2019; 14(1):100. [DOI:10.1186/s13018-019-1134-9] [PMID] [PMCID]
- Urrutia J, Besa P, Meissner-Haecker A, Delgado B. An independent validation of the brighton spondylodiscitis score and a proposal to modify the score. The Journal of the American Academy of Orthopaedic Surgeons. 2020; 28(17):701-6. [DOI:10.5435/JAAOS-D-19-00505] [PMID]
- Widdrington JD, Emmerson I, Cullinan M, Narayanan M, Klejnow E, Watson A, et al. Pyogenic spondylodiscitis: Risk factors for adverse clinical outcome in routine clinical practice. Medical Sciences. 2018; 6(4):96. [DOI:10.3390/medsci6040096] [PMID] [PMCID]
- Uchida K, Nakajima H, Yayama T, Sato R, Kobayashi S, Chen KB, et al. Epidural abscess associated with pyogenic spondylodiscitis of the lumbar spine; evaluation of a new MRI staging classification and imaging findings as indicators of surgical management: A retrospective study of 37 patients. Archives of Orthopaedic and Trauma Surgery. 2010; 130(1):111-8. [DOI:10.1007/s00402-009-0928-3] [PMID]
- Kowalski TJ, Layton KF, Berbari EF, Steckelberg JM, Huddleston PM, Wald JT, et al. Follow-up MR imaging in patients with pyogenic spine infections: Lack of correlation with clinical features. American Journal of Neuroradiology. 2007; 28(4):693-9. [Link]
- Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clinical Infectious Diseases. 2006; 43(2):172-9. [DOI:10.1086/505118] [PMID]
- Homagk L, Homagk N, Klauss JR, Roehl K, Hofmann GO, Marmelstein D. Spondylodiscitis severity code: Scoring system for the classification and treatment of non-specific spondylodiscitis. European Spine Journal. 2016; 25(4):1012-20. [DOI:10.1007/s00586-015-3936-8] [PMID]
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






