Wed, Feb 4, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 239-248 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Karimzadeh A, Yousefzadeh-Chabok S, Hemmati H, Andalib S. Gut-brain Axis Roles in Brain Tumors: Pathophysiological Insights and Research Advances. Iran J Neurosurg 2024; 10 : 29
URL: http://irjns.org/article-1-454-en.html
URL: http://irjns.org/article-1-454-en.html
1- School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Guilan Road Trauma Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of General Surgery, Faculty of Medicine, Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran.
4- Research Unit of Neurology, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. & Department of Neurology, Odense University Hospital, Odense, Denmark. & Neuroscience Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran. ,sasan.andalib@health.sdu.dk
2- Guilan Road Trauma Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of General Surgery, Faculty of Medicine, Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran.
4- Research Unit of Neurology, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. & Department of Neurology, Odense University Hospital, Odense, Denmark. & Neuroscience Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran. ,
Full Text [PDF 1000 kb]
(693 Downloads)
| Abstract (HTML) (2431 Views)
Full Text: (920 Views)
1. Introduction
The interconnections between neurological conditions and the gut are not a newly founded topic. As Avicenna wrote in his book, The Canon, the stomach plays a major role in many neurological disturbances [1]. A millennium later, the potential therapeutic approaches to many central nervous system (CNS) disorders are meticulously being investigated within another system, the gastrointestinal (GI) tract. Although the brain is an isolated Immune-privileged site in the human body, there is a growing body of evidence indicating that the primary pathogenesis of a few chronic neurological diseases originates in the gut. This change in our perspective has occurred in the past decade with the discovery of a new organ called “gut microbiota”. Gut microbiota weighs roughly 1.5 kilograms [2] and encompasses more than 3 million genes, almost 140 times greater than that of the human genome [3]. These findings highlight the importance of these organisms’ communication with our organs through metabolites, neurotransmitters, and the mucosal immune system.
Within the human CNS, primary malignancies are still a major challenge to tackle. One of the major reasons is that the expanding tumoral tissue is in an isolated physical and physiological environment. Therefore, brain cancer research has a focus on optimized targeted therapeutic approaches. These methods try to harness brain tumors not only by early detection and debulking the mass using neurosurgical techniques but also by changing their uncontrolled behavior to minimize the damage to the surrounding tissue. A potential intervention is through modifying the tumor’s microenvironment.
A growing body of literature has deciphered molecular signals involved in the pathogenesis of brain tumors that are continuously being exchanged between the brain and gut, shedding light on new paths to modify diagnostic, prognostic, and therapeutic guidelines. These molecular signals have been classified based on their oncolytic or oncogenic effects [4]. Gut microbiota can impact the tumor microenvironment by changing the levels of immunomodulating agents, including interleukin (IL)-18, IL-17, IL-12, IL-6, tumor necrosis factor-α (TNF-α) [5], granulocyte-macrophage colony-stimulating factor signaling (GM-CSF), IL-4, IL-13, interferon-gamma (IFN-γ), and tumor necrosis factor-β (TGF-β) [4]. These chemokines and cytokines have been the targets of immunotherapy, although not enough to suppress malignant cells through apoptosis or autophagy mechanisms [6]. Gut microbiota plays a role in these pathways by modulating oxidative stress biomarkers such as reactive oxygen and nitrogen species [7]. Bacterial antigens and metabolites, namely short-chain fatty acids (SCFAs), tryptophan, arginine, and other products are also involved in manipulating tumor microenvironment [4, 8]. The gut-brain axis (GBA) is a bidirectional network linking the GI tract and CNS. Gut dysbiosis is the alteration of gut microbiota and activity. In this narrative review, we provide a brief overview of the interconnections between gut microbiota and the pathophysiology of malignancies and CNS homeostasis, aiming to highlight the potential routes of intervention through the gut in CNS malignancies.
2. Methods and Materials/Patients
A literature search was conducted for a narrative review related to the microbial components of the GBA and their potential effects on brain tumors’ formation, growth, and course of treatment with a focus on recent publications.
3. Results and Discussion
Gut microbiota and host immunity
Immediately after birth, human skin and mucous membranes are colonized with microbial flora, which is the beginning of interactions between the host and its microbiologically replete environment. Meanwhile, the host immune system is developed by consistently recruiting and training immune cells to maintain the integrity of immune barriers. The composition of the microbiota is affected by external factors and varies in health and disease. Previous studies indicated that microbiota in vaginally born infants were similar to their mother’s vaginal microbiota, but the microbiota in those who were born via c-section was mainly composed of Propionibacterium and Staphylococcus species but lacked Lactobacillus, Bifidobacterium, and Bacteroides [9, 10]. This change is also affected by antibiotics used during hospitalization and maternal diet during pregnancy [9].
The gut mucosal immune system is functionally divided into two main sites. Introductory sites, where antigens are introduced in Peyer’s patches, isolated lymphoid follicles, and mesenteric lymph nodes, and the action sites of epithelium and lamina propria where activated B and T lymphocytes are present [11]. Intestinal epithelial cells, macrophages, and dendritic cells in the introductory sites recognize the commensal or pathogen microorganisms via pattern recognition receptor systems (PRRs) [12, 13]. Microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs) are identifiable by RPRs [14]. The immune response initiated following the interactions between PAMPS and RPRs leads to an immune tolerance or triggers an inflammatory cascade and production of pro-inflammatory cytokines [15]. Commensal microbiota regulates local and systemic inflammatory responses through induction of the IgA secretion [10], and production of fermentation metabolites that limit the growth of pathogens [16]. One of the key roles of microbiota in influencing host immune responses, whether through enhancement, suppression, or modulation—originates from their metabolic byproducts. These metabolites can impact the mucosal layers or enter systemic circulation. Accordingly, the host immune system and microbiota metabolism are interconnected mechanisms.
Gut microbiota and host metabolism
With the emergence of high-throughput quantification of biological molecules and the development of techniques such as 16rRNA sequencing [17], many bacterial enzymes and consequently their metabolites have been traced. Bacterial enzymes metabolize a wide range of lipids, carbohydrates, and proteins, and are involved in the bioavailability of minerals and vitamins. Among this diverse range of metabolites, the immunoregulatory roles of SCFAs (SCFAs: Acetic acid, butyric acid, propionic acid), aryl hydrocarbon receptor (AHR) ligands, and polyamines were widely investigated [18]. Aside from providing an energy source for intestinal epithelial cells, SCFAs function as signaling molecules on polymorphonuclear leukocytes, macrophages, dendritic cells, and T regulatory cells. Subsequently, the nuclear factor-κB (NF-κB) is inactivated, which leads to a decrease in pro-inflammatory cytokines including tumor necrosis factor (TNF) [18]. Moreover, SCFAs cross through the blood-brain barrier (BBB) [19] and inactivate inflammatory microglia [20]. The essential amino acid tryptophan is metabolized by host cells to the neurotransmitter serotonin, vitamin B3 (niacin), kynurenines, and the hormone melatonin [21, 22]. It is also metabolized by a specific group of bacteria such as Lactobacilli species to produce AHR ligands, indole, indolic acid, skatole, and tryptamine which plays a crucial role in mucosal barrier integrity and immunosuppression in tumors [22, 23].
Healthy gut and tumor suppression
In the field of cancer immunotherapy, many attempts have been made to modulate the tumor microenvironment by modifying the gut microbiota. Microbiota can metabolize dietary ingredients to oncogenic metabolites such as hydrogen sulfide and secondary bile acids or tumor-suppressive agents such as SCFAs and urolithins [24]. Such an interplay between the microbiota and diet is also a subject of epigenetic modifications. Moreover, some species of commensal bacteria such as Akkermansia muciniphila, Bacteroides fragilis, Bifidobacterium species, and Faecalibacterium species enhance the effect of anti-tumor therapy when targeting T lymphocytes by blocking the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) [25-28].
Manipulation of gut microbiota using oral antibiotics was shown to potentially change the behavior of solid tumors including melanoma, pancreatic, and colon cancers in mice; however, a healthy functioning mature lymphoid cell lineage is needed for immunotherapeutic interventions through the gut [29]. In a recent study, Puerarin, which is a bioactive agent derived from Chinese herbal medicine, was shown to have the ability to change gut microbiota composition [30]. Through analysis of metabolic pathways, it was demonstrated that changes in ovarian cancer tumoral metabolism were affected by microbial metabolites, and subsequently led to the activation of apoptotic signals [30].
Gut dysbiosis and tumorigenesis
The term Oncobiome was coined since genomic research has expanded our understanding of the relationship between the gut microbiota genetic material and carcinogenesis [31]. Helicobacter pylori is the first pathogen whose strong association with GI cancers was discovered. In the context of chronic inflammation, its virulence factors including cytotoxin-associated gene A (CagA), vacuolating cytotoxin (VacA), and outer membrane proteins (OMPs) can lead to mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma [32, 33]. Unlike H. pylori, which is not a commensal microorganism in the colon, some cancer-related bacteria are considered opportunistic pathogens that are related to a specific stage of cancer [34, 35]. Dysbiosis, the alteration in composition and activity of microbiota, may result from either host-specific or environmental factors [36] and was previously observed in saliva, bile culture, and feces in pancreatic, gall bladder, and colorectal cancer patients, respectively [37].
Of the community of commensal bacteria, E. coli and Bacteroides fragilis can accelerate the progression of colorectal cancers (CRC) in genetically susceptible individuals (familial adenomatous polyposis) via damaging DNA and IL-17-induced inflammation. Additionally, Fusobacterium nucleatum is involved in the occurrence of CRC and metastasis through the up-regulation of microRNA-21 and nuclear Factor-kappaB signaling pathway [38]. The role of lymphoid cells, namely the proinflammatory T-helper 17 (TH17) and T-regulatory (T reg), are not fully understood in the tumor microenvironment [39]. Studies in germ-free mice discovered the dependence of TH17 on microbiota [39]. Naive Tregs also depend on Butyrate-mediated microbiota metabolites to mature. These findings highlight the need for further investigations to elucidate the precise mechanisms underlying immune dysregulation and chronic inflammation because of gut dysbiosis.
The gut and the brain
GBA is said to be a physiologically interconnected system composed of the CNS, the enteric nervous system (ENS), the autonomic nervous system (ANS), the hypothalamic pituitary adrenal (HPA) axis and the entero-endocrine system (EES) [40]. It functions as a pathway for the microbiome to send signals to the brain and vice versa. SCFAs are among these signals and can circulate throughout the body and maintain not only the stability of the intestinal epithelial barrier but also the BBB, by regulating the expression of Occludin, a transmembrane protein [41]. Therefore, changes in microbiota metabolome can endanger the integrity of BBB. Furthermore, a study on germ-free mice indicated that SFCAs are essential for microglial maturation [42]. Microglia, the immune regulator of the brain, constitutes 10-15 % of all CNS cells. They have two opposite phenotypes. M1 phenotype promotes inflammation and neurotoxicity, and M2 induces anti-inflammatory and neuroprotective effects [43]. The transformation of microglia to either one of these phenotypes is under the control of cytokines released from T helper lymphocytes. Overstimulation by anti-inflammatory cytokines, such as IL-4 and IL-13, turn microglia into M2 and has been shown to cause exhaustive tissue remodeling and eventually the formation of tumoral mass in the brain [44]. As a result, maintaining an immune balance, in addition to a favorable metabolic state, is vital in preventing the formation and growth of tumoral cells.
Gut microbiota and CNS malignancies in pre-clinical investigations
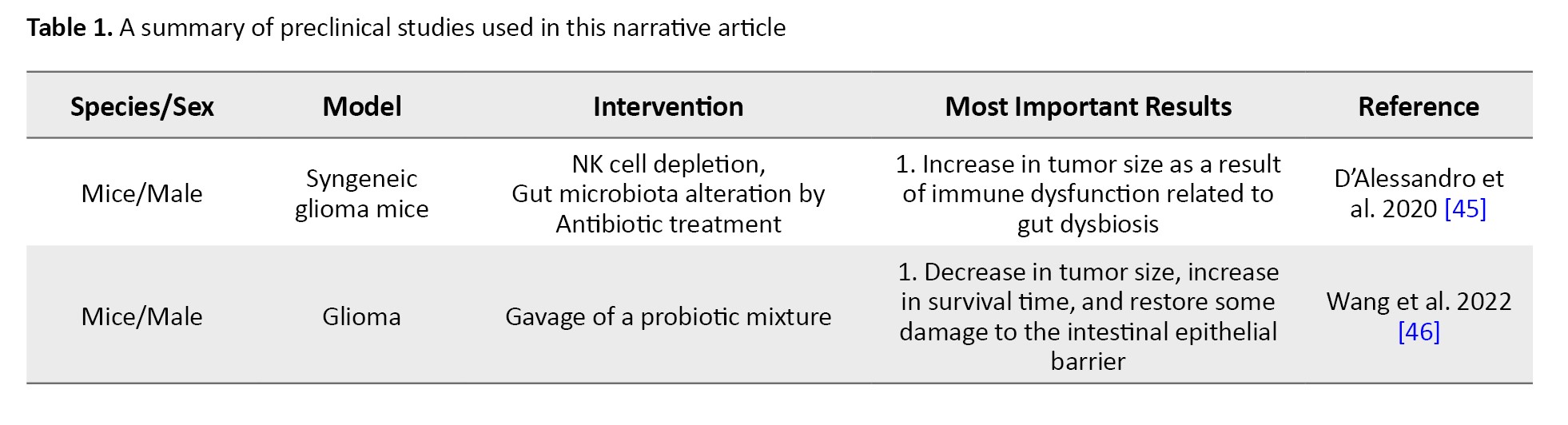
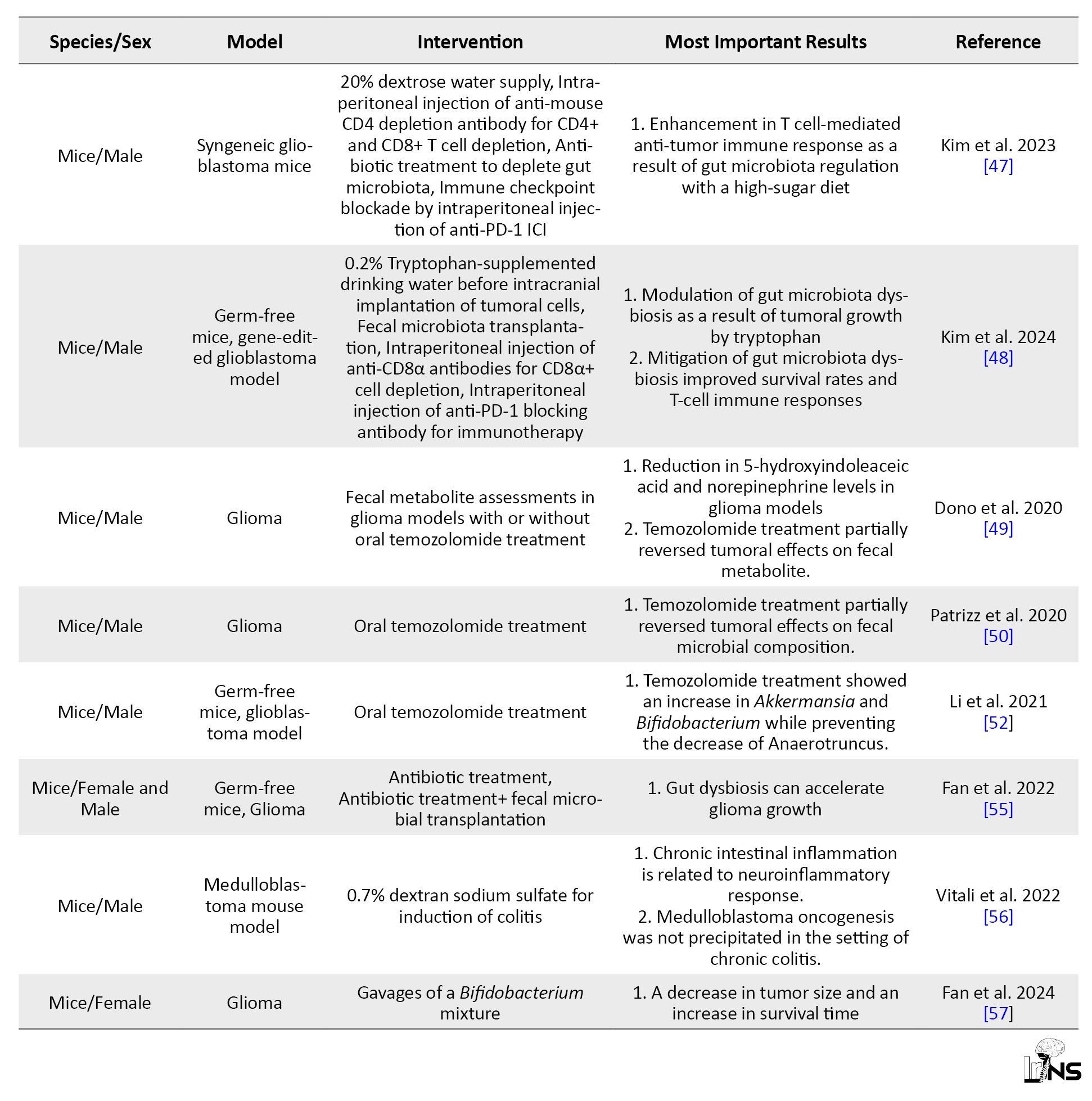
Many attempts have been made to enhance the efficacy of anti-tumor therapies in brain tumors by modifying the GBA. A study evaluated the effects of manipulated gut microbiota on glioma growth and innate immune cells including microglia, monocytes/macrophages, and infiltrating T and NK cells [45]. In this study, 5 weeks of oral non-absorbable antibiotic therapy with vancomycin and gentamicin in a glioma syngenetic mouse model, led to the growth of Burkholderiales families and a decrease in the Prevotellaceae, Rikenellacaea, and Helicobacteraceae families. The results of the flowcytometric analysis showed a reduction in the cytotoxic NK cell (CD27+/CD11b+) population, while the immature NK cell (CD27+/CD11b-) subset increased relatively in the tumoral hemisphere. An overall growth in the size of the tumor was also observed in the experimental group whose microbiota composition was altered [45].
Findings of the effects of microbiota modulation on brain glioma growth were promising. A probiotic regimen containing Bifidobacterium lactis and Lactobacillus plantarum, inhibited the PI3K/AKT pathway and decreased the expression of Ki-67 and N-cadherin, therefore slowing down the growth of glioma in mice models [46]. Another study modified the gut microbiota of GL261 syngeneic glioblastoma mice by adding high-glucose drinks to their diet for a short period, during which high-sugar metabolic complications were not observed [47]. This study demonstrated that the increase in the Desulfovibrionaceae family in the gut microbiota resulting from the modified diet was related to enhanced cytotoxic CD4+ T cell immune response and tumor growth restriction [47]. Elsewhere, the regimen of two types of glioblastoma mouse models (orthotopic implantation and genetically engineered GBM models) was supplemented with tryptophan [48]. This intervention caused a rise in CD8+ T cell numbers in circulation and boosted the antitumor response synergized with anti-PD-1 cancer immunotherapy [48].
Conversely, the effects of glioma on fecal neurotransmitters and metabolites have also been explored. In a study, a decline in the levels of SCFAs (butyrate, propionate, and acetate) and neurotransmitters, including norepinephrine were observed in fecal samples of both glioma patients and glioma-transplanted mouse models [49]. Whereas the detected levels of serotonin, 3-methyl valerate, caproate, and acetylcholine increased following tumor growth. These changes in metabolic pathways also impacted the gut microbiota composition. Results of the study showed a deterioration in Bacteroidetes and Firmicutes phyla levels and a rise in Verrucomicrobia phylum, in mice [49]. Consistent with these findings, another study reported that Firmicutes to Bacteroides ratio significantly decreased in glioma-induced models, and Verrucomicrobia phyla, specifically the Akkermansiaceae family in this phylum, demonstrated a relative growth and subsequent gut dysbiosis [50].
Gut microbiota dysbiosis can also result from the administration of chemotherapy agents within the course of treatment. Temozolomide, an oral alkylating agent used in the treatment of glioblastoma multiforme and anaplastic astrocytoma [51], can directly affect gut microbiota composition [52]. In a mouse model of brain glioma, the relative amounts of Akkermansia and Bifidobacterium increased in fecal samples of mice treated with temozolomide compared to control subjects [52]. Both bacteria are beneficial components of microbiota because of their role in metabolism and immunomodulation [53, 54].
In a recent study, it was shown that gut dysbiosis caused by oral antibiotics led to a decrease in the expression of Foxp3, a key transcriptional regulator in the function of Regulatory T lymphocytes, and fecal microbial transplantation successfully restored its expression in glioma-induced mice [55]. Within the GBA, the inflammatory pathways have been investigated in pre-clinical studies.
It was shown that acute and chronic inflammatory processes in the colon of inflammatory bowel disease models resulted in astrocyte activation in the hippocampus of mice and interfered with the process of adult hippocampal neurogenesis [56]. In this study, colitis was indicated by the raised levels of oxidative stress markers (Il-6 and iNOS) and macrophage infiltration. However, intestinal inflammation was not related to medulloblastoma tumorigenesis measured by tumor markers of Gfap and Ki67.
More recent studies are equipped with the powerful tool of multi-omics analyses to integrate biological findings related to the physiological pathways between the gut and the brain. In a multi-omics-based investigation, gut microbiota modification with oral administration of a mixture containing Bifidobacterium species in a mouse model of glioma successfully inhibited tumoral expansion and increased the survival time [57]. In this investigation, transcriptome sequencing revealed that the suppression of MEK/ERK cascade and Wnt5a mRNA levels was the underlying mechanism of growth arrest in glioma. Table 1 summarizes pre-clinical studies used in this narrative review article.
Gut microbiota and CNS malignancies in clinical investigations
Intricate molecular findings of gut microbiota and CNS malignancies have been translated into clinical research, expanding the survival rates and improving the clinical outcomes of aggressive brain malignancies, such as glioblastoma. Many attempts have been made to enhance treatment protocols in brain tumor patients.
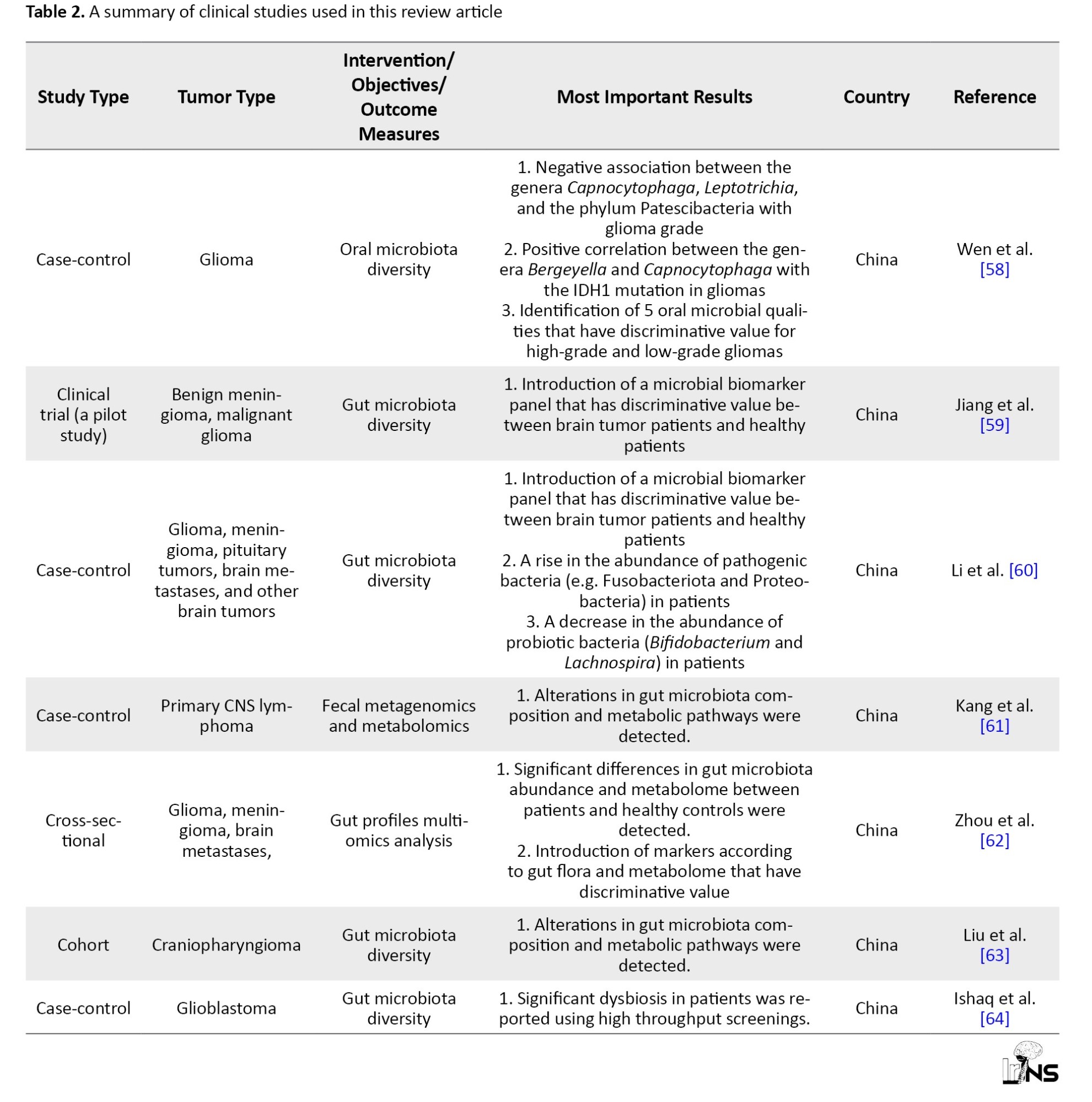
In a case-control study, the association between five oral microbiota and high-grade human brain glioma, as opposed to low-grade glioma, was investigated [58]. Additionally, the bacterial genes that are related to glioma grade were analyzed functionally. These genes play various roles in cell adhesion, focal adhesion, extracellular matrix molecule-receptor interaction, and modulation of actin cytoskeleton [58]. To detect the gut microbial biomarkers that are distinguishable in brain tumor patients compared with healthy individuals, a shortage of SCFA-producing bacteria was reported in the fecal samples of benign meningioma and malignant glioma patients [59].
An investigation of fecal microbiota composition in 101 brain tumor patients (65 benign and 36 malignant types) compared with 57 healthy controls, revealed that the levels of pathogenic bacteria, such as Fusobacteriota and Proteobacteria increased, while the amounts of probiotic bacteria, such as Bifidobacterium or Lachnospira deteriorated [60].
In a multi-omics study on fecal samples of primary CNS lymphoma (PCNSL) patients and healthy individuals, increased ratios of the Firmicutes/Bacteroides (F/B) and the proteobacteria were observed in PCNSL cases [61 ]. More to the point, amino acids, thiamine, biotin, and 2- oxocarboxylic acid metabolic pathways were diverted in PCNSL patients as compared with controls. Similarly, another study on changes in the gut microbiome and metabolome in patients with meningioma, glioma, and brain metastasis, reported a remarkable reduction of Gram-positive bacteria such as Lachnospiraceae and a considerable growth of Gram-negative bacteria such as Enterobacteriaceae [62]. A shift in metabolite composition was also detected; however, most fatty acid and amino acid metabolites increased, and bile acids (BAs) and carbohydrates decreased [62]. Multi-omics studies of gut microbiota have also been performed on different types of intracranial neoplasia, such as craniopharyngioma, and yielded consistent results [63]. A more recent study investigated gut dysbiosis in glioblastoma patients at the phylum, family, and genus levels using polymerase chain reaction-denature gradient gel electrophoreses (PCR-DGGE) analysis, and revealed major changes in microbial diversity in these patients [64]. Table 2 summarizes clinical studies used in this narrative review article.
The present article is a narrative review, and therefore there are caveats to be taken into account. Narrative reviews do not have the methodology seen commonly in systematic reviews. Narrative reviews have inherent weaknesses of non-standardized literature search, potential bias in the extracted articles’ appraisal, and findings’ interpretation; nevertheless, they provide readers with sources of quick up-to-date reference on specific areas of interest [65].
4. Conclusion
The amount and diversity of essential dietary nutrients that are synthesized by microbial enzymes is manifold. The role of microbiota in the metabolism of many ingredients that are otherwise not consumable by intestinal cells has been previously studied; however, the role of such microbial products that enter our systemic circulation could be a subject of future studies. Many of these products are neurotransmitters and signaling molecules that act as ligands on immune cell receptors, playing a pivotal role in their maturation and function. These findings highlight the significant role of healthy gut microbiota in regulating immunologic responses and inflammatory cascades within the CNS. This fact led researchers to be wary of possible interventions that can be made through gut microbiomes for brain cancer treatment; however, their effects should be further investigated. Effective interventions should not only emphasize applicable methods as adjunct treatments in the management of brain tumors but also investigate the long-term effects of microbiome modulation on brain tumor outcomes.
Ethical Considerations
Compliance with ethical guidelines
This article is a narrative review with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
The interconnections between neurological conditions and the gut are not a newly founded topic. As Avicenna wrote in his book, The Canon, the stomach plays a major role in many neurological disturbances [1]. A millennium later, the potential therapeutic approaches to many central nervous system (CNS) disorders are meticulously being investigated within another system, the gastrointestinal (GI) tract. Although the brain is an isolated Immune-privileged site in the human body, there is a growing body of evidence indicating that the primary pathogenesis of a few chronic neurological diseases originates in the gut. This change in our perspective has occurred in the past decade with the discovery of a new organ called “gut microbiota”. Gut microbiota weighs roughly 1.5 kilograms [2] and encompasses more than 3 million genes, almost 140 times greater than that of the human genome [3]. These findings highlight the importance of these organisms’ communication with our organs through metabolites, neurotransmitters, and the mucosal immune system.
Within the human CNS, primary malignancies are still a major challenge to tackle. One of the major reasons is that the expanding tumoral tissue is in an isolated physical and physiological environment. Therefore, brain cancer research has a focus on optimized targeted therapeutic approaches. These methods try to harness brain tumors not only by early detection and debulking the mass using neurosurgical techniques but also by changing their uncontrolled behavior to minimize the damage to the surrounding tissue. A potential intervention is through modifying the tumor’s microenvironment.
A growing body of literature has deciphered molecular signals involved in the pathogenesis of brain tumors that are continuously being exchanged between the brain and gut, shedding light on new paths to modify diagnostic, prognostic, and therapeutic guidelines. These molecular signals have been classified based on their oncolytic or oncogenic effects [4]. Gut microbiota can impact the tumor microenvironment by changing the levels of immunomodulating agents, including interleukin (IL)-18, IL-17, IL-12, IL-6, tumor necrosis factor-α (TNF-α) [5], granulocyte-macrophage colony-stimulating factor signaling (GM-CSF), IL-4, IL-13, interferon-gamma (IFN-γ), and tumor necrosis factor-β (TGF-β) [4]. These chemokines and cytokines have been the targets of immunotherapy, although not enough to suppress malignant cells through apoptosis or autophagy mechanisms [6]. Gut microbiota plays a role in these pathways by modulating oxidative stress biomarkers such as reactive oxygen and nitrogen species [7]. Bacterial antigens and metabolites, namely short-chain fatty acids (SCFAs), tryptophan, arginine, and other products are also involved in manipulating tumor microenvironment [4, 8]. The gut-brain axis (GBA) is a bidirectional network linking the GI tract and CNS. Gut dysbiosis is the alteration of gut microbiota and activity. In this narrative review, we provide a brief overview of the interconnections between gut microbiota and the pathophysiology of malignancies and CNS homeostasis, aiming to highlight the potential routes of intervention through the gut in CNS malignancies.
2. Methods and Materials/Patients
A literature search was conducted for a narrative review related to the microbial components of the GBA and their potential effects on brain tumors’ formation, growth, and course of treatment with a focus on recent publications.
3. Results and Discussion
Gut microbiota and host immunity
Immediately after birth, human skin and mucous membranes are colonized with microbial flora, which is the beginning of interactions between the host and its microbiologically replete environment. Meanwhile, the host immune system is developed by consistently recruiting and training immune cells to maintain the integrity of immune barriers. The composition of the microbiota is affected by external factors and varies in health and disease. Previous studies indicated that microbiota in vaginally born infants were similar to their mother’s vaginal microbiota, but the microbiota in those who were born via c-section was mainly composed of Propionibacterium and Staphylococcus species but lacked Lactobacillus, Bifidobacterium, and Bacteroides [9, 10]. This change is also affected by antibiotics used during hospitalization and maternal diet during pregnancy [9].
The gut mucosal immune system is functionally divided into two main sites. Introductory sites, where antigens are introduced in Peyer’s patches, isolated lymphoid follicles, and mesenteric lymph nodes, and the action sites of epithelium and lamina propria where activated B and T lymphocytes are present [11]. Intestinal epithelial cells, macrophages, and dendritic cells in the introductory sites recognize the commensal or pathogen microorganisms via pattern recognition receptor systems (PRRs) [12, 13]. Microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs) are identifiable by RPRs [14]. The immune response initiated following the interactions between PAMPS and RPRs leads to an immune tolerance or triggers an inflammatory cascade and production of pro-inflammatory cytokines [15]. Commensal microbiota regulates local and systemic inflammatory responses through induction of the IgA secretion [10], and production of fermentation metabolites that limit the growth of pathogens [16]. One of the key roles of microbiota in influencing host immune responses, whether through enhancement, suppression, or modulation—originates from their metabolic byproducts. These metabolites can impact the mucosal layers or enter systemic circulation. Accordingly, the host immune system and microbiota metabolism are interconnected mechanisms.
Gut microbiota and host metabolism
With the emergence of high-throughput quantification of biological molecules and the development of techniques such as 16rRNA sequencing [17], many bacterial enzymes and consequently their metabolites have been traced. Bacterial enzymes metabolize a wide range of lipids, carbohydrates, and proteins, and are involved in the bioavailability of minerals and vitamins. Among this diverse range of metabolites, the immunoregulatory roles of SCFAs (SCFAs: Acetic acid, butyric acid, propionic acid), aryl hydrocarbon receptor (AHR) ligands, and polyamines were widely investigated [18]. Aside from providing an energy source for intestinal epithelial cells, SCFAs function as signaling molecules on polymorphonuclear leukocytes, macrophages, dendritic cells, and T regulatory cells. Subsequently, the nuclear factor-κB (NF-κB) is inactivated, which leads to a decrease in pro-inflammatory cytokines including tumor necrosis factor (TNF) [18]. Moreover, SCFAs cross through the blood-brain barrier (BBB) [19] and inactivate inflammatory microglia [20]. The essential amino acid tryptophan is metabolized by host cells to the neurotransmitter serotonin, vitamin B3 (niacin), kynurenines, and the hormone melatonin [21, 22]. It is also metabolized by a specific group of bacteria such as Lactobacilli species to produce AHR ligands, indole, indolic acid, skatole, and tryptamine which plays a crucial role in mucosal barrier integrity and immunosuppression in tumors [22, 23].
Healthy gut and tumor suppression
In the field of cancer immunotherapy, many attempts have been made to modulate the tumor microenvironment by modifying the gut microbiota. Microbiota can metabolize dietary ingredients to oncogenic metabolites such as hydrogen sulfide and secondary bile acids or tumor-suppressive agents such as SCFAs and urolithins [24]. Such an interplay between the microbiota and diet is also a subject of epigenetic modifications. Moreover, some species of commensal bacteria such as Akkermansia muciniphila, Bacteroides fragilis, Bifidobacterium species, and Faecalibacterium species enhance the effect of anti-tumor therapy when targeting T lymphocytes by blocking the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) [25-28].
Manipulation of gut microbiota using oral antibiotics was shown to potentially change the behavior of solid tumors including melanoma, pancreatic, and colon cancers in mice; however, a healthy functioning mature lymphoid cell lineage is needed for immunotherapeutic interventions through the gut [29]. In a recent study, Puerarin, which is a bioactive agent derived from Chinese herbal medicine, was shown to have the ability to change gut microbiota composition [30]. Through analysis of metabolic pathways, it was demonstrated that changes in ovarian cancer tumoral metabolism were affected by microbial metabolites, and subsequently led to the activation of apoptotic signals [30].
Gut dysbiosis and tumorigenesis
The term Oncobiome was coined since genomic research has expanded our understanding of the relationship between the gut microbiota genetic material and carcinogenesis [31]. Helicobacter pylori is the first pathogen whose strong association with GI cancers was discovered. In the context of chronic inflammation, its virulence factors including cytotoxin-associated gene A (CagA), vacuolating cytotoxin (VacA), and outer membrane proteins (OMPs) can lead to mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma [32, 33]. Unlike H. pylori, which is not a commensal microorganism in the colon, some cancer-related bacteria are considered opportunistic pathogens that are related to a specific stage of cancer [34, 35]. Dysbiosis, the alteration in composition and activity of microbiota, may result from either host-specific or environmental factors [36] and was previously observed in saliva, bile culture, and feces in pancreatic, gall bladder, and colorectal cancer patients, respectively [37].
Of the community of commensal bacteria, E. coli and Bacteroides fragilis can accelerate the progression of colorectal cancers (CRC) in genetically susceptible individuals (familial adenomatous polyposis) via damaging DNA and IL-17-induced inflammation. Additionally, Fusobacterium nucleatum is involved in the occurrence of CRC and metastasis through the up-regulation of microRNA-21 and nuclear Factor-kappaB signaling pathway [38]. The role of lymphoid cells, namely the proinflammatory T-helper 17 (TH17) and T-regulatory (T reg), are not fully understood in the tumor microenvironment [39]. Studies in germ-free mice discovered the dependence of TH17 on microbiota [39]. Naive Tregs also depend on Butyrate-mediated microbiota metabolites to mature. These findings highlight the need for further investigations to elucidate the precise mechanisms underlying immune dysregulation and chronic inflammation because of gut dysbiosis.
The gut and the brain
GBA is said to be a physiologically interconnected system composed of the CNS, the enteric nervous system (ENS), the autonomic nervous system (ANS), the hypothalamic pituitary adrenal (HPA) axis and the entero-endocrine system (EES) [40]. It functions as a pathway for the microbiome to send signals to the brain and vice versa. SCFAs are among these signals and can circulate throughout the body and maintain not only the stability of the intestinal epithelial barrier but also the BBB, by regulating the expression of Occludin, a transmembrane protein [41]. Therefore, changes in microbiota metabolome can endanger the integrity of BBB. Furthermore, a study on germ-free mice indicated that SFCAs are essential for microglial maturation [42]. Microglia, the immune regulator of the brain, constitutes 10-15 % of all CNS cells. They have two opposite phenotypes. M1 phenotype promotes inflammation and neurotoxicity, and M2 induces anti-inflammatory and neuroprotective effects [43]. The transformation of microglia to either one of these phenotypes is under the control of cytokines released from T helper lymphocytes. Overstimulation by anti-inflammatory cytokines, such as IL-4 and IL-13, turn microglia into M2 and has been shown to cause exhaustive tissue remodeling and eventually the formation of tumoral mass in the brain [44]. As a result, maintaining an immune balance, in addition to a favorable metabolic state, is vital in preventing the formation and growth of tumoral cells.
Gut microbiota and CNS malignancies in pre-clinical investigations
Many attempts have been made to enhance the efficacy of anti-tumor therapies in brain tumors by modifying the GBA. A study evaluated the effects of manipulated gut microbiota on glioma growth and innate immune cells including microglia, monocytes/macrophages, and infiltrating T and NK cells [45]. In this study, 5 weeks of oral non-absorbable antibiotic therapy with vancomycin and gentamicin in a glioma syngenetic mouse model, led to the growth of Burkholderiales families and a decrease in the Prevotellaceae, Rikenellacaea, and Helicobacteraceae families. The results of the flowcytometric analysis showed a reduction in the cytotoxic NK cell (CD27+/CD11b+) population, while the immature NK cell (CD27+/CD11b-) subset increased relatively in the tumoral hemisphere. An overall growth in the size of the tumor was also observed in the experimental group whose microbiota composition was altered [45].
Findings of the effects of microbiota modulation on brain glioma growth were promising. A probiotic regimen containing Bifidobacterium lactis and Lactobacillus plantarum, inhibited the PI3K/AKT pathway and decreased the expression of Ki-67 and N-cadherin, therefore slowing down the growth of glioma in mice models [46]. Another study modified the gut microbiota of GL261 syngeneic glioblastoma mice by adding high-glucose drinks to their diet for a short period, during which high-sugar metabolic complications were not observed [47]. This study demonstrated that the increase in the Desulfovibrionaceae family in the gut microbiota resulting from the modified diet was related to enhanced cytotoxic CD4+ T cell immune response and tumor growth restriction [47]. Elsewhere, the regimen of two types of glioblastoma mouse models (orthotopic implantation and genetically engineered GBM models) was supplemented with tryptophan [48]. This intervention caused a rise in CD8+ T cell numbers in circulation and boosted the antitumor response synergized with anti-PD-1 cancer immunotherapy [48].
Conversely, the effects of glioma on fecal neurotransmitters and metabolites have also been explored. In a study, a decline in the levels of SCFAs (butyrate, propionate, and acetate) and neurotransmitters, including norepinephrine were observed in fecal samples of both glioma patients and glioma-transplanted mouse models [49]. Whereas the detected levels of serotonin, 3-methyl valerate, caproate, and acetylcholine increased following tumor growth. These changes in metabolic pathways also impacted the gut microbiota composition. Results of the study showed a deterioration in Bacteroidetes and Firmicutes phyla levels and a rise in Verrucomicrobia phylum, in mice [49]. Consistent with these findings, another study reported that Firmicutes to Bacteroides ratio significantly decreased in glioma-induced models, and Verrucomicrobia phyla, specifically the Akkermansiaceae family in this phylum, demonstrated a relative growth and subsequent gut dysbiosis [50].
Gut microbiota dysbiosis can also result from the administration of chemotherapy agents within the course of treatment. Temozolomide, an oral alkylating agent used in the treatment of glioblastoma multiforme and anaplastic astrocytoma [51], can directly affect gut microbiota composition [52]. In a mouse model of brain glioma, the relative amounts of Akkermansia and Bifidobacterium increased in fecal samples of mice treated with temozolomide compared to control subjects [52]. Both bacteria are beneficial components of microbiota because of their role in metabolism and immunomodulation [53, 54].
In a recent study, it was shown that gut dysbiosis caused by oral antibiotics led to a decrease in the expression of Foxp3, a key transcriptional regulator in the function of Regulatory T lymphocytes, and fecal microbial transplantation successfully restored its expression in glioma-induced mice [55]. Within the GBA, the inflammatory pathways have been investigated in pre-clinical studies.
It was shown that acute and chronic inflammatory processes in the colon of inflammatory bowel disease models resulted in astrocyte activation in the hippocampus of mice and interfered with the process of adult hippocampal neurogenesis [56]. In this study, colitis was indicated by the raised levels of oxidative stress markers (Il-6 and iNOS) and macrophage infiltration. However, intestinal inflammation was not related to medulloblastoma tumorigenesis measured by tumor markers of Gfap and Ki67.
More recent studies are equipped with the powerful tool of multi-omics analyses to integrate biological findings related to the physiological pathways between the gut and the brain. In a multi-omics-based investigation, gut microbiota modification with oral administration of a mixture containing Bifidobacterium species in a mouse model of glioma successfully inhibited tumoral expansion and increased the survival time [57]. In this investigation, transcriptome sequencing revealed that the suppression of MEK/ERK cascade and Wnt5a mRNA levels was the underlying mechanism of growth arrest in glioma. Table 1 summarizes pre-clinical studies used in this narrative review article.


Gut microbiota and CNS malignancies in clinical investigations
Intricate molecular findings of gut microbiota and CNS malignancies have been translated into clinical research, expanding the survival rates and improving the clinical outcomes of aggressive brain malignancies, such as glioblastoma. Many attempts have been made to enhance treatment protocols in brain tumor patients.
In a case-control study, the association between five oral microbiota and high-grade human brain glioma, as opposed to low-grade glioma, was investigated [58]. Additionally, the bacterial genes that are related to glioma grade were analyzed functionally. These genes play various roles in cell adhesion, focal adhesion, extracellular matrix molecule-receptor interaction, and modulation of actin cytoskeleton [58]. To detect the gut microbial biomarkers that are distinguishable in brain tumor patients compared with healthy individuals, a shortage of SCFA-producing bacteria was reported in the fecal samples of benign meningioma and malignant glioma patients [59].
An investigation of fecal microbiota composition in 101 brain tumor patients (65 benign and 36 malignant types) compared with 57 healthy controls, revealed that the levels of pathogenic bacteria, such as Fusobacteriota and Proteobacteria increased, while the amounts of probiotic bacteria, such as Bifidobacterium or Lachnospira deteriorated [60].
In a multi-omics study on fecal samples of primary CNS lymphoma (PCNSL) patients and healthy individuals, increased ratios of the Firmicutes/Bacteroides (F/B) and the proteobacteria were observed in PCNSL cases [61 ]. More to the point, amino acids, thiamine, biotin, and 2- oxocarboxylic acid metabolic pathways were diverted in PCNSL patients as compared with controls. Similarly, another study on changes in the gut microbiome and metabolome in patients with meningioma, glioma, and brain metastasis, reported a remarkable reduction of Gram-positive bacteria such as Lachnospiraceae and a considerable growth of Gram-negative bacteria such as Enterobacteriaceae [62]. A shift in metabolite composition was also detected; however, most fatty acid and amino acid metabolites increased, and bile acids (BAs) and carbohydrates decreased [62]. Multi-omics studies of gut microbiota have also been performed on different types of intracranial neoplasia, such as craniopharyngioma, and yielded consistent results [63]. A more recent study investigated gut dysbiosis in glioblastoma patients at the phylum, family, and genus levels using polymerase chain reaction-denature gradient gel electrophoreses (PCR-DGGE) analysis, and revealed major changes in microbial diversity in these patients [64]. Table 2 summarizes clinical studies used in this narrative review article.

The present article is a narrative review, and therefore there are caveats to be taken into account. Narrative reviews do not have the methodology seen commonly in systematic reviews. Narrative reviews have inherent weaknesses of non-standardized literature search, potential bias in the extracted articles’ appraisal, and findings’ interpretation; nevertheless, they provide readers with sources of quick up-to-date reference on specific areas of interest [65].
4. Conclusion
The amount and diversity of essential dietary nutrients that are synthesized by microbial enzymes is manifold. The role of microbiota in the metabolism of many ingredients that are otherwise not consumable by intestinal cells has been previously studied; however, the role of such microbial products that enter our systemic circulation could be a subject of future studies. Many of these products are neurotransmitters and signaling molecules that act as ligands on immune cell receptors, playing a pivotal role in their maturation and function. These findings highlight the significant role of healthy gut microbiota in regulating immunologic responses and inflammatory cascades within the CNS. This fact led researchers to be wary of possible interventions that can be made through gut microbiomes for brain cancer treatment; however, their effects should be further investigated. Effective interventions should not only emphasize applicable methods as adjunct treatments in the management of brain tumors but also investigate the long-term effects of microbiome modulation on brain tumor outcomes.
Ethical Considerations
Compliance with ethical guidelines
This article is a narrative review with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Zargaran A, Rezaeizadeh H. The role of stomach in neurological disorders: 1000 years historical background. Ann Gastroenterol. 2016; 29(1):99-100. [PMID]
- Scheperjans F. Can microbiota research change our understanding of neurodegenerative diseases? Neurodegenerative Disease Management. 2016; 6(2):81-5. [DOI:10.2217/nmt-2015-0012] [PMID]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019; 7(1):14. [DOI:10.3390/microorganisms7010014] [PMID] [PMCID]
- Dehhaghi M, Kazemi Shariat Panahi H, Heng B, Guillemin GJ. The gut microbiota, kynurenine pathway, and immune system interaction in the development of brain cancer.Frontiers in Cell and Developmental Biology. 2020; 8:562812. [DOI:10.3389/fcell.2020.562812] [PMID] [PMCID]
- Zhou H, Yuan Y, Wang H, Xiang W, Li S, Zheng H, et al. Gut microbiota: A potential target for cancer interventions. Cancer Management and Research. 2021; 13:8281-96. [DOI:10.2147/CMAR.S328249] [PMID] [PMCID]
- Yun CW, Lee SH. The roles of autophagy in cancer. International Journal of Molecular Sciences. 2018; 19(11):3466. [DOI:10.3390/ijms19113466] [PMID] [PMCID]
- Aljarrah D, Chalour N, Zorgani A, Nissan T, Pranjol MZI. Exploring the gut microbiota and its potential as a biomarker in gliomas. Biomedicine & Pharmacotherapy. 2024; 173:116420. [DOI:10.1016/j.biopha.2024.116420] [PMID]
- Zou S, Wang X, Liu P, Ke C, Xu S. Arginine metabolism and deprivation in cancer therapy. Biomedicine & Pharmacotherapy. 2019; 118:109210. [DOI:10.1016/j.biopha.2019.109210] [PMID]
- Hoang DM, Levy EI, Vandenplas Y. The impact of caesarean section on the infant gut microbiome. Acta Paediatrica. 2021; 110(1):60-67. [DOI:10.1111/apa.15501] [PMID]
- Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Frontiers in Immunology. 2018; 9:1830. [DOI:10.3389/fimmu.2018.01830] [PMID] [PMCID]
- Ahluwalia B, Magnusson MK, Öhman L. Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scandinavian Journal of Gastroenterology. 2017; 52(11):1185-93. [DOI:10.1080/00365521.2017.1349173] [PMID]
- Athman R, Philpott D. Innate immunity via Toll-like receptors and Nod proteins. Current Opinion in Microbiology. 2004; 7(1):25-32. [DOI:10.1016/j.mib.2003.12.013] [PMID]
- den Besten G, Gerding A, van Dijk TH, Ciapaite J, Bleeker A, van Eunen K, et al. Protection against the metabolic syndrome by guar gum-derived short-chain fatty acids depends on peroxisome proliferator-activated receptor γ and Glucagon-Like Peptide-1. Plos One. 2015; 10(8):e0136364. [DOI:10.1371/journal.pone.0136364] [PMID] [PMCID]
- Oviedo-Boyso J, Bravo-Patiño A, Baizabal-Aguirre VM. Collaborative action of toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediators of Inflammation. 2014; 2014:432785. [DOI:10.1155/2014/432785] [PMID] [PMCID]
- Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut microbiota and immune system interactions. Microorganisms. 2020; 8(10):1587. [DOI:10.3390/microorganisms8101587] [PMID] [PMCID]
- Martin-Gallausiaux C, Béguet-Crespel F, Marinelli L, Jamet A, Ledue F, Blottière HM, et al. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Scientific Reports. 2018; 8(1):9742. [DOI:10.1038/s41598-018-28048-y] [PMID] [PMCID]
- Franco-Duarte R, Černáková L, Kadam S, Kaushik KS, Salehi B, Bevilacqua A, et al. Advances in chemical and biological methods to identify microorganisms-from past to present. Microorganisms. 2019; 7(5):130. [DOI:10.3390/microorganisms7050130] [PMID] [PMCID]
- Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nature Reviews. Immunology. 2016; 16(6):341-52. [DOI:10.1038/nri.2016.42] [PMID] [PMCID]
- Mirzaei R, Bouzari B, Hosseini-Fard SR, Mazaheri M, Ahmadyousefi Y, Abdi M, et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed Pharmacother. 2021; 139:111661. [DOI:10.1016/j.biopha.2021.111661] [PMID]
- Caetano-Silva ME, Rund L, Hutchinson NT, Woods JA, Steelman AJ, Johnson RW. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Scientific Reports. 2023; 13(1):2819. [DOI:10.1038/s41598-022-27086-x] [PMID] [PMCID]
- Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Letters. 2002; 511(1-3):102-6. [DOI:10.1016/S0014-5793(01)03319-1] [PMID]
- Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Frontiers in Cellular and Infection Microbiology. 2018; 8:13. [DOI:10.3389/fcimb.2018.00013] [PMID] [PMCID]
- Seo SK, Kwon B. Immune regulation through tryptophan metabolism. Experimental & Molecular Medicine. 2023; 55(7):1371-9. [DOI:10.1038/s12276-023-01028-7] [PMID] [PMCID]
- Bultman SJ. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Molecular Nutrition & Food Research. 2017; 61(1):10.1002/mnfr.201500902. [DOI:10.1002/mnfr.201500902] [PMID] [PMCID]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015; 350(6264):1084-9. [DOI:10.1126/science.aac4255] [PMID] [PMCID]
- Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nature Reviews. Clinical Oncology. 2018; 15(6):382-96. [DOI:10.1038/s41571-018-0006-2] [PMID]
- Elkrief A, Derosa L, Zitvogel L, Kroemer G, Routy B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes. 2019; 10(3):424-8. [DOI:10.1080/19490976.2018.1527167] [PMID] [PMCID]
- Luu M, Schütz B, Lauth M, Visekruna A. The impact of gut microbiota-derived metabolites on the tumor immune microenvironment. Cancers. 2023; 15(5):1588. [DOI:10.3390/cancers15051588] [PMID] [PMCID]
- Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018; 155(1):33-7. [DOI:10.1053/j.gastro.2018.04.001] [PMID] [PMCID]
- Ye Y, Gao Y, Fang Y, Xu L, He F. Anticancer effect of puerarin on ovarian cancer progression contributes to the tumor suppressor gene expression and gut microbiota modulation. Journal of Immunology Research. 2022; 2022:4472509. [DOI:10.1155/2022/4472509] [PMID] [PMCID]
- Ruiz-Garcia E, Astudillo-de la Vega H. Translational research and onco-omics applications in the era of cancer personal genomics. Berlin: Springer; 2019. [DOI:10.1007/978-3-030-24100-1]
- Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, et al. Gut microbiota and cancer: From pathogenesis to therapy. Cancers. 2019; 11(1):38. [DOI:10.3390/cancers11010038] [PMID] [PMCID]
- Chattopadhyay I, Gundamaraju R, Jha NK, Gupta PK, Dey A, Mandal CC, et al. Interplay between dysbiosis of gut microbiome, lipid metabolism, and tumorigenesis: Can gut dysbiosis stand as a prognostic marker in cancer? Disease Markers. 2022; 2022:2941248. [DOI:10.1155/2022/2941248] [PMID] [PMCID]
- Khajuria N, Metgud R. Role of bacteria in oral carcinogenesis. Indian Journal of Dentistry. 2015; 6(1):37-43. [DOI:10.4103/0975-962X.151709] [PMID] [PMCID]
- Alfarouk KO, Bashir AHH, Aljarbou AN, Ramadan AM, Muddathir AK, AlHoufie STS, et al. The possible role of helicobacter pylori in gastric cancer and its management. Frontiers in Oncology. 2019; 9:75. [DOI:10.3389/fonc.2019.00075] [PMID] [PMCID]
- Hrncir T. Gut microbiota dysbiosis: Triggers, consequences, diagnostic and therapeutic options. Microorganisms. 2022; 10(3):578. [DOI:10.3390/microorganisms10030578] [PMID] [PMCID]
- Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014; 35(2):249-55. [DOI:10.1093/carcin/bgt392] [PMID] [PMCID]
- Xu JY, Liu MT, Tao T, Zhu X, Fei FQ. The role of gut microbiota in tumorigenesis and treatment. Biomedicine & Pharmacotherapy. 2021; 138:111444. [DOI:10.1016/j.biopha.2021.111444] [PMID]
- Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA: A Cancer Journal for Clinicians. 2017; 67(4):326-44. [DOI:10.3322/caac.21398] [PMID] [PMCID]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology. 2015; 28(2):203-9. [PMID]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014; 6(263):263ra158. [DOI:10.1126/scitranslmed.3009759] [PMID] [PMCID]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience. 2015; 18(7):965-77. [DOI:10.1038/nn.4030] [PMID] [PMCID]
- Darwish SF, Elbadry AMM, Elbokhomy AS, Salama GA, Salama RM. The dual face of microglia (M1/M2) as a potential target in the protective effect of nutraceuticals against neurodegenerative diseases. Frontiers in Aging. 2023; 4:1231706. [DOI:10.3389/fragi.2023.1231706] [PMID] [PMCID]
- Mehrian-Shai R, Reichardt JKV, Harris CC, Toren A. The gut-brain axis, paving the way to brain cancer. Trends in Cancer. 2019; 5(4):200-7. [DOI:10.1016/j.trecan.2019.02.008] [PMID] [PMCID]
- D'Alessandro G, Antonangeli F, Marrocco F, Porzia A, Lauro C, Santoni A, et al. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. European Journal of immunology. 2020; 50(5):705-11. [DOI:10.1002/eji.201948354] [PMID] [PMCID]
- Wang L, Li S, Fan H, Han M, Xie J, Du J, et al. Bifidobacterium lactis combined with Lactobacillus plantarum inhibit glioma growth in mice through modulating PI3K/AKT pathway and gut microbiota. Frontiers in Microbiology. 2022; 13:986837. [DOI:10.3389/fmicb.2022.986837] [PMID] [PMCID]
- Kim J, Kim Y, La J, Park WH, Kim HJ, Park SH, et al. Supplementation with a high-glucose drink stimulates anti-tumor immune responses to glioblastoma via gut microbiota modulation. Cell reports. 2023; 42(10):113220. [DOI:10.1016/j.celrep.2023.113220] [PMID]
- Kim HC, Kim HJ, La J, Park WH, Park SH, Kang BH, et al. Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy. bioRxiv. 2024; [Unpublished]. [DOI:10.1101/2024.08.18.608488]
- Dono A, Patrizz A, McCormack RM, Putluri N, Ganesh BP, Kaur B, et al. Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncology. 2020; 9(2):CNS57. [DOI:10.2217/cns-2020-0007] [PMID] [PMCID]
- Patrizz A, Dono A, Zorofchian S, Hines G, Takayasu T, Husein N, et al. Glioma and temozolomide induced alterations in gut microbiome. Scientific Reports. 2020; 10(1):21002. [DOI:10.1038/s41598-020-77919-w] [PMID] [PMCID]
- Wesolowski JR, Rajdev P, Mukherji SK. Temozolomide (Temodar). AJNR. American Journal of Neuroradiology. 2010; 31(8):1383-4. [DOI:10.3174/ajnr.A2170] [PMID] [PMCID]
- Li XC, Wu BS, Jiang Y, Li J, Wang ZF, Ma C, et al. Temozolomide-induced changes in gut microbial composition in a mouse model of brain glioma. Drug Design, Development and Therapy. 2021; 15:1641-52. [DOI:10.2147/DDDT.S298261] [PMID] [PMCID]
- Naito Y, Uchiyama K, Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. Journal of Clinical Biochemistry and Nutrition. 2018; 63(1):33-5. [DOI:10.3164/jcbn.18-57] [PMID] [PMCID]
- Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011; 3(1):118-34. [DOI:10.3390/nu3010118] [PMID] [PMCID]
- Fan Y, Su Q, Chen J, Wang Y, He S. Gut microbiome alterations affect glioma development and foxp3 expression in tumor microenvironment in mice. Frontiers in Oncology. 2022; 12:836953. [DOI:10.3389/fonc.2022.836953] [PMID] [PMCID]
- Vitali R, Prioreschi C, Lorenzo Rebenaque L, Colantoni E, Giovannini D, Frusciante S, et al. Gut-brain axis: Insights from hippocampal neurogenesis and brain tumor development in a mouse model of experimental colitis induced by dextran sodium sulfate. International Journal of Molecular Sciences. 2022; 23(19):11495. [DOI:10.3390/ijms231911495] [PMID] [PMCID]
- Fan H, Wang Y, Han M, Wang L, Li X, Kuang X, et al. Multi-omics-based investigation of Bifidobacterium's inhibitory effect on glioma: Regulation of tumor and gut microbiota, and MEK/ERK cascade. Frontiers in Microbiology. 2024; 15:1344284. [DOI:10.3389/fmicb.2024.1344284] [PMID] [PMCID]
- Wen Y, Feng L, Wang H, Zhou H, Li Q, Zhang W, et al. Association between oral microbiota and human brain glioma grade: A case-control study. Frontiers in Microbiology. 2021; 12:746568. [DOI:10.3389/fmicb.2021.746568] [PMID] [PMCID]
- Jiang H, Zeng W, Zhang X, Pei Y, Zhang H, Li Y. The role of gut microbiota in patients with benign and malignant brain tumors: A pilot study. Bioengineered. 2022; 13(3):7847-59. [DOI:10.1080/21655979.2022.2049959] [PMID] [PMCID]
- Li Y, Jiang H, Wang X, Liu X, Huang Y, Wang Z, et al. Crosstalk between the gut and brain: Importance of the fecal microbiota in patient with brain tumors. Frontiers in Cellular and Infection Microbiology. 2022; 12:881071. [DOI:10.3389/fcimb.2022.881071] [PMID] [PMCID]
- Kang Z, Zhang R, Wang C, Liu B, Li S, Huang M, et al. Integrated analysis of fecal metagenomics and metabolomics reveals the role of gut microbiota in the pathogenesis of primary central nervous system lymphoma. Research Square. 2023. [Unpublished]. [DOI:10.21203/rs.3.rs-3051515/v1]
- Zhou Y, Zhao M, Li S, Wen Y, Wang K, Wang M, et al. Multiomics analysis reveals gut profiles in patients with different brain tumors. Research Square. 2023. [Unpublished]. [DOI:10.21203/rs.3.rs-3383550/v1]
- Liu C, Liu F, Nie D, Xiao Y, Wu W, Jia Y, et al. Gut microbiota composition and metabolic characteristics in patients with Craniopharyngioma. BMC Cancer. 2024; 24(1):521. [DOI:10.1186/s12885-024-12283-w] [PMID] [PMCID]
- Ishaq HM, Yasin R, Mohammad IS, Fan Y, Li H, Shahzad M, et al. The gut-brain-axis: A positive relationship between gut microbial dysbiosis and glioblastoma brain tumour. Heliyon. 2024; 10(9):e30494. [DOI:10.1016/j.heliyon.2024.e30494] [PMID] [PMCID]
- Basheer A. The art and science of writing narrative reviews. International Journal of Advanced Medical and Health Research. 2022; 9(2):124-6. [DOI:10.4103/ijamr.ijamr_234_22]
Type of Study: Review |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






