Sat, Jan 31, 2026
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adhikesavan S, Krishnaswamy V, Swamiyappan S S, Visvesvaran V, Kannan B. Radiological and Biochemical Characteristics of Chronic Subdural Hematoma. Iran J Neurosurg 2025; 11 : 17
URL: http://irjns.org/article-1-455-en.html
URL: http://irjns.org/article-1-455-en.html
Sangeetha Adhikesavan1 
 , Visvanathan Krishnaswamy1
, Visvanathan Krishnaswamy1 

 , Sai Sriram Swamiyappan *2
, Sai Sriram Swamiyappan *2 

 , Vivek Visvesvaran1
, Vivek Visvesvaran1 
 , Balasubramanian Kannan1
, Balasubramanian Kannan1 


 , Visvanathan Krishnaswamy1
, Visvanathan Krishnaswamy1 

 , Sai Sriram Swamiyappan *2
, Sai Sriram Swamiyappan *2 

 , Vivek Visvesvaran1
, Vivek Visvesvaran1 
 , Balasubramanian Kannan1
, Balasubramanian Kannan1 

1- Sri Ramachandra Medical College and Research Institute, Chennai, India.
2- Sri Ramachandra Medical College and Research Institute, Chennai, India. ,saisriramswamiyappan@gmail.com
2- Sri Ramachandra Medical College and Research Institute, Chennai, India. ,
Full Text [PDF 1253 kb]
(380 Downloads)
| Abstract (HTML) (1430 Views)
Full Text: (280 Views)
1. Introduction
Chronic subdural hematomas (CSDH) have an incidence of 13 to 39 per 100,000 persons per year [1]. This makes them a common neurosurgical issue. It is postulated that they develop over a period due to a self-perpetuating cycle of recurrent hemorrhage, fibrinolysis, inflammation, and angiogenesis. Although the osmotic gradient theory and inflammation have been mentioned in the pathogenesis, the occurrence of a hematoma capsule and an inherent hyperfibrinolysis that leads to rebleeding is currently the most widely accepted theory [2-4].
One of the sensitive markers for fibrinolysis is D-dimer, a fibrin degradation product [5]. All patients with CSDH have a high level of D-dimer in their clots. Studies have attempted to correlate the level of D-dimer in the clot and the risk of recurrence, as well as radiological subtypes. In this study, we attempt to measure the D-dimer levels in the clots and correlate these markers with the radiological appearance and recurrence.
2. Methods and Materials/Patients
Patient selection
This was a prospective study done in a series of 80 patients who underwent evacuation of CSDH in our center between 2018 and 2020. Patients underwent imaging in the form of a computed tomography (CT) scan and were grouped based on Nomura’s classification [6], which divided CSDH into high density, iso density, low density, layered, and mixed types. We grouped the former three into the homogeneous group and the latter two into a heterogeneous group.
Inclusion criteria
All patients with a CT or a magnetic resonance imaging scan suggestive of a CSDH who presented with clinical symptoms accounted for by their scan findings-except those excluded.
Exclusion criteria
The exclusion criteria were as follows: Patients on antiplatelets, anticoagulants, anti-inflammatory agents, thrombolytic agents, and those on dialysis; patients with an active septic foci or hematological disorders, or neoplastic disorders, liver dysfunction, or coagulopathy; subjects with a ventriculo-peritoneal shunt.
The above patients were excluded as their conditions could, by themselves, cause a recurrence of the disease anticoagulants and anti-platelets added an independent bleeding risk, as do sepsis and liver diseases, sepsis and neoplasms. Ventriculo-peritoneal shunts can overdrain and precipitate CSDH; hence, they were excluded.
Patients underwent surgical evacuation through a single burr hole or two burr holes, or a mini craniotomy, depending on the clinical condition of the patient and the imaging characteristics of the CSDH.
Sample collection
During the surgery, subdural hematoma fluid was sampled after durotomy. Simultaneously, peripheral venous blood samples were drawn and collected in a tube with sodium citrate. In addition, they were centrifuged at 3000 round per min for 10 min. The supernatant was stored in sealed tubes at −70° Celsius until turbidimetric immunoassay (Sysmex CA7000) was used for quantification, and the reference value was <0.324mg/L.
Data analysis
All statistical analysis was performed using the SPSS software, version 17, for Microsoft Windows. The data were not normally distributed, and therefore, parametric/non-parametric tests were performed. Descriptive statistics were presented as numbers and percentages. The data were expressed as Mean±SD. The Chi-squared test was used for comparison between two attributes. Multiple Logistic regression method used to predict the independent variables. A two-sided P<0.05 was considered statistically significant.
3. Results
Patient demographics
We had a total of 80 patients in this study, with 82 bleeds (2 patients had bilateral bleeds). The average age of our study group was 64.35 years. There were 10 women, and the remaining 70 participants were men. There was no statistically significant correlation between either the biochemical marker or recurrence with the age or gender of the population. The average symptom duration was 13 days. A history of trauma was elicited in 67 patients, and the average time between admission and the trauma was 46 days.
Chronic subdural hematoma characteristics
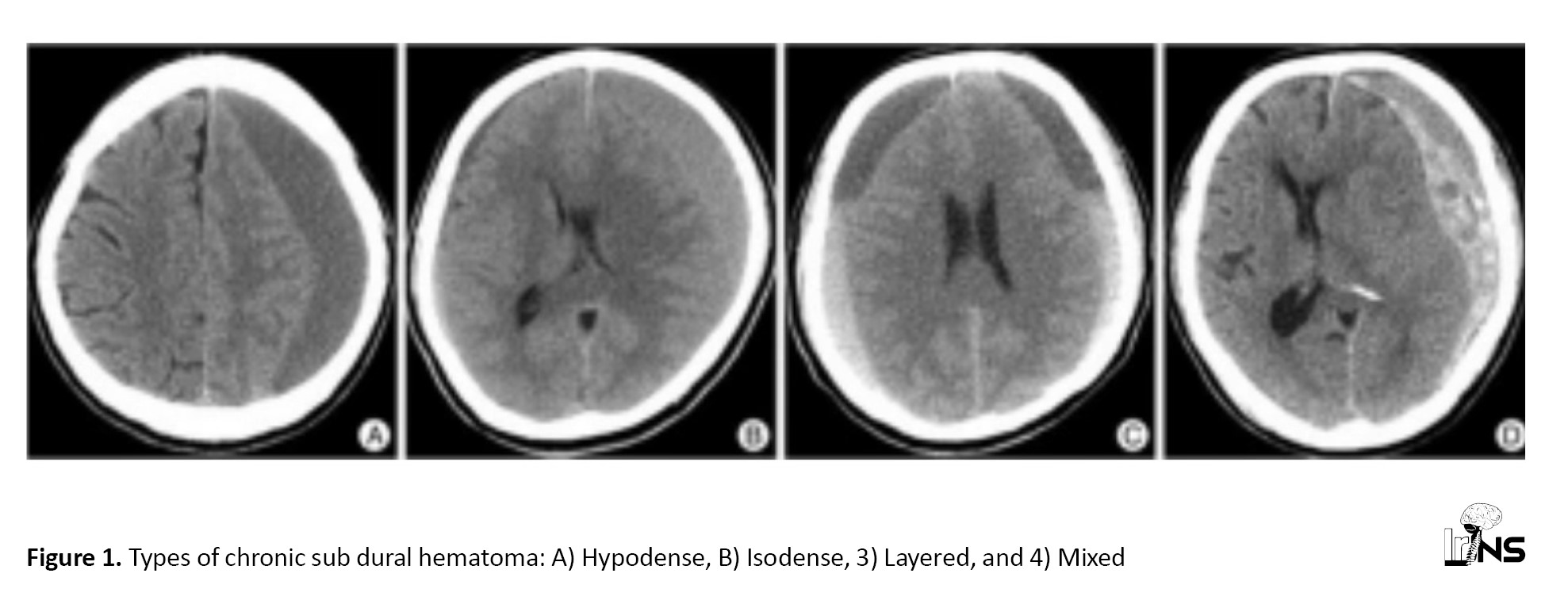
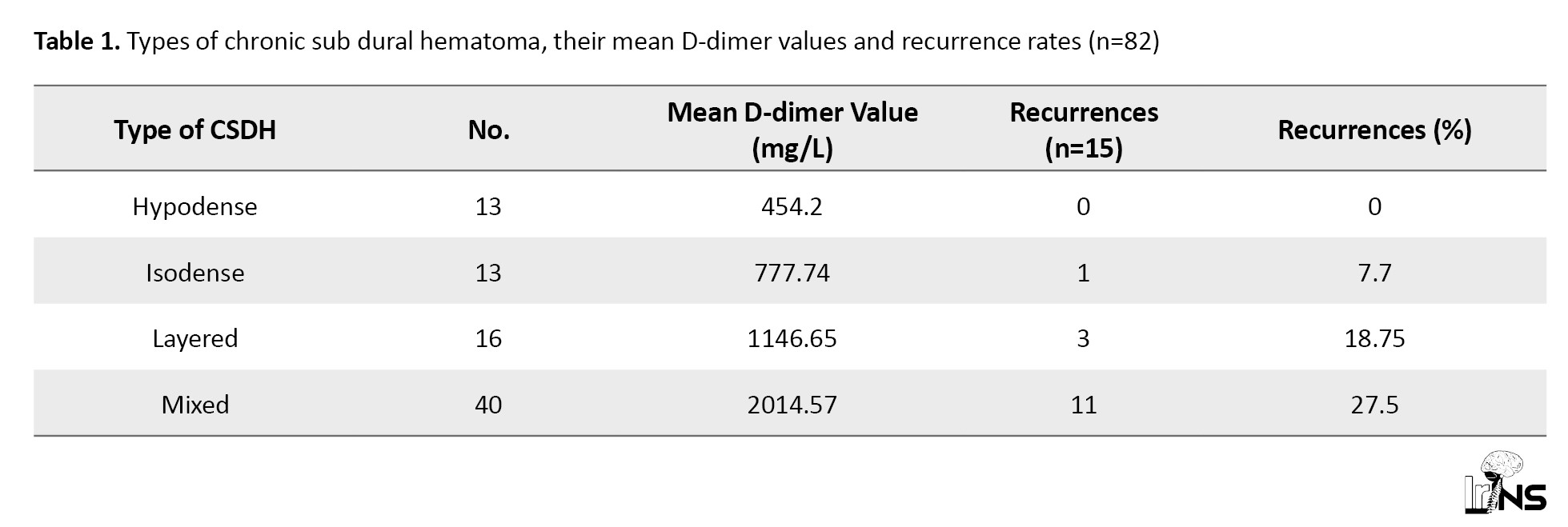
A total of 43 patients had a left-sided bleed, and 35 patients had a right-sided bleed. Meanwhile, two of them had bilateral CSDH. The average thickness of the CSDH in the present study was 19.93 mm, while the average midline shift was 9.46 mm. We had 13 bleeds, each with iso and low-density subtypes. Additionally, 40 bleeds were mixed subtype, while the remaining 16 were a layered type of CSDH. Figure 1 demonstrates the different types of CSDH (Table 1).
Management
A total of 23 patients underwent evacuation with two burr holes, while 11 patients underwent a mini-craniotomy. The rest of the clots were evacuated by a single burr hole. A total of 63 patients were placed on dexamethasone for one week postoperatively.
D-dimer analysis
All D-dimer values obtained from the CSDH were above the reference range of 0-0.325 mg/L. The mean D-dimer value in the Hypodense group was 454.2 mg/L, while the value in the isodense group was 777.74 mg/L. The highest mean value was noted in the mixed subgroup, where the value was 2370 mg/L. The layered group had a mean value of 1146.85 mg/L. The mean D-dimer value in the homogenous group was 622.48 mg/L, while the mean in the heterogeneous group was 2014.57 mg/L. There was a statistically significant correlation between the imaging subtypes and the D-dimer values (P=0.01). The serum levels of D-dimer were within normal limits in all four groups, and there was no statistically significant correlation.
Recurrences
We had 15 cases of recurrences, of whom 11 had undergone evacuation with a single burr hole, three with two burr holes, and one underwent mini craniotomy. Meanwhile, 11 of these cases were of mixed type, and a further three were layered type. There was a single case of isodense type that recurred.
14 of the 15 recurrences occurred in the heterogeneous group. The mean D-dimer value in this group was 1912.52 mg/L. There was a statistically significant correlation between recurrences and both imaging subtype (P=0.02) and the D-dimer values (P=0.01). There was no correlation between the procedure performed, although a majority had undergone a single burr hole evacuation.
Dexamethasone was administered in only 4 of the patients who had recurrence, against 59 of the 65 cases without recurrence. Given the anti-angiogenic and anti-inflammatory effects of steroids, statistical analysis was carried out, which showed a statistically significant correlation between recurrence and non-usage of steroids in the post-operative period, with a P=0.04.
A logistic regression analysis was done, and the associated significance levels are provided in Table 2.
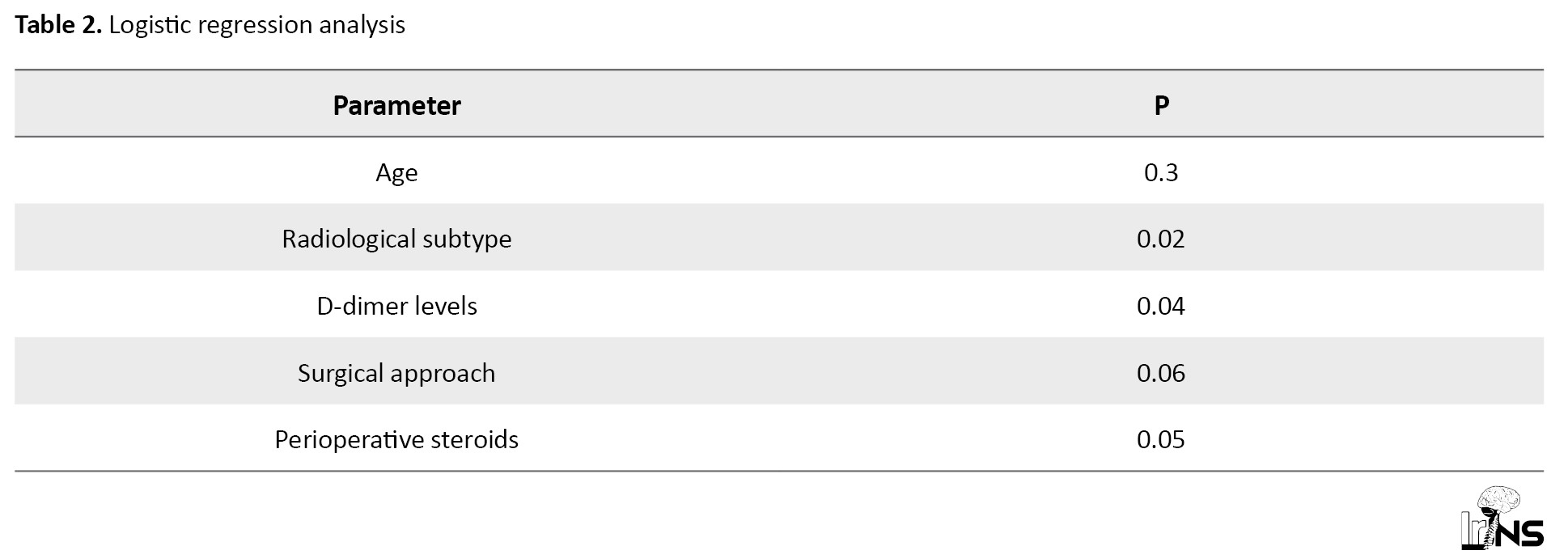
Accordingly, the radiological subtype, levels of D-dimer, and perioperative steroid use had a statistically significant correlation with recurrences, while the surgical approach neared statistical significance.
4. Discussion
This study notes that a major factor in recurrence is the radiological subtype of the bleed. There is a correlation between the subtype and the D-dimer values, and although not statistically significant, the heterogeneous types and their membranes are factors causing recurrence. Based on the D-dimer values in the CSDH fluid, it may be possible to decide on a more extensive procedure, such as a craniotomy, to address the membrane against the use of two burr holes.
Several hypotheses have been put forth to explain the pathogenesis of CSDH, with the hyperfibrinolysis and re-bleeding theory having gained the widest traction. The presence of a hematoma capsule and inherent hyperfibrinolysis that leads to rebleeding is considered central to the development and maintenance of CSDHs [1, 3, 7]. D-dimer is a by-product of the coagulation cascade and can be measured via analysis of a blood sample. It is a marker with relative sensitivity towards fibrinolysis. This cross-linked fibrin degradation product is released when a blood clot begins to break down [5]. Ito et al. stated that local hyperfibrinolysis prevents complete hemostasis and causes rebleeding into the hematoma cavity [1, 8, 9].
A study was conducted in Brazil involving 778 patients, where 60.4% had a history of trauma. The mean age of that study was 64.3 years, with men accounting for 82.6% of the study population [10]. In this study, about 66% of the patients had a history of trauma, and the average age of our study population was 64.35 years, with a male preponderance of 87.5%. The brain in the elderly weighs roughly 200 g less, allowing for an 11% increase in extra cerebral volume, which may account for the higher incidence in the elderly population [11].
Ducruet et al. stated that burr hole craniotomy is the most performed surgery, and this notion has been reiterated by studies conducted in Canada, the Netherlands, and the United Kingdom [12-14]. It seems to provide the best balance between maximal efficacy and minimal invasiveness [14].
A total of 23 patients underwent evacuation with two burr holes, while 11 patients underwent a mini-craniotomy. The rest of the clots were evacuated by a single burr hole. There were 11 recurrences in the single burr hole group, with three recurrences in the two burr holes group, and one patient who had undergone mini craniotomy had a recurrence. There was no statistically significant correlation between surgery performed and recurrence, even though a higher proportion of recurrences occurred in those who had an evacuation with a single burr hole.
Nomura et al. proposed a system [6] where CSDH was classified based on CT imaging as isodense, hypodense, mixed dense, and layering types of hematoma. They described higher fibrinogen and higher D-dimer levels in the layering and mixed density types of CSDHs compared to the high, iso, and low-density types on CT scans. This was comparable to the findings in the present study, where D-dimer levels were the highest in the mixed group, followed by the Layering type, with the hypodense and isodense groups having lower levels of D-dimer.
A meta-analysis done by Miah et al. where 22 studies were identified involving 5566 patients, mixed density was the strongest prognostic factor of recurrence. Layered type also revealed higher recurrence rates [14, 15]. Similar findings were noted in our study, where the mixed density group had the highest rates of recurrence.
Recurrence rates after surgical evacuation were placed between 4 to 26% by Mori et al. [16]. In our study recurrence rate was 18.29%. Higher recurrence was noted in the mixed and layered types, which had a high D-dimer value. Park et al. concluded that there was a difference in the concentration of D-dimer and fibrinogen between the different types of CSDH on CT, with the heterogeneous group consisting of the layering and mixed types having a higher concentration of both markers when compared to the homogenous group [17]. These are associated intimately with the origins of heterogeneous density, which needs repeated cycles of rebleeding. The findings of our study concur with their inferences. A study on recurrent CSDH concluded that the type of procedure performed doesn’t have a bearing on recurrence [18].
We noted that steroids could be of use in preventing recurrence. Sun et al. [19] suggested that administration of steroids may be associated with a decrease in the tendency of the CSDH to recur, especially in the heterogeneous population. Although statistically significant, given the small sample size, further evaluation needs to be done to ascertain the role of steroids in CSDH, although their role in hemostasis and their anti-fibrinolytic properties may break the cycle of rebleeding, coagulation, and fibrinolysis and prevent recurrence [20].
We noted that all recurrences had a D-dimer value of more than 1000 mg/L in the CSDH fluid. A proof-of-concept analysis after letting out the CSDH may help us decide if a more aggressive approach is needed to tackle the disease. This may be an area of further research. The major limitation of our study was its limited sample size and, conduction in a single center.
5. Conclusion
We noted a higher incidence of D-dimer values in patients with a heterogeneous group of CSDH, which includes the mixed and layering subtypes, compared to the homogenous group consisting of hypo- and isodense groups. Higher D-dimer values and heterogeneous appearance on scan correlated with recurrences, with maximum recurrence noted in the mixed group. Steroids may have a role in the prevention of recurrence in CSDH, although further studies are needed to elucidate their significance in that regard.
Ethical Considerations
Compliance with ethical guidelines
The study received ethical approval from the Institutional Ethics Committee of Sri Ramachandra Institute of Higher Education and Research (Code: CSP-MED/18/SEP46/145). Informed consent was obtained from the participants.
Funding
This study was a part of a Post Graduate Dissertation at Sri Ramachandra Institute of Higher Education and Research.
Authors' contributions
Resources: Visvanathan Krishnaswamy and Vivek Visvesvaran; Writing the original drafts and data analysis: Sangeetha Adhikesavan and Sai Sriram Swamiyappan; Data collection: Sangeetha Adhikesavan and Balasubramanian Kannan; Visualization: Visvanathan Krishnaswamy; Conceptualization, critical reviewand and software: Sai Sriram Swamiyappan; Supervision: Vivek Visvesvaran.
Conflict of interest
The authors declared no conflict of interest.
References
Chronic subdural hematomas (CSDH) have an incidence of 13 to 39 per 100,000 persons per year [1]. This makes them a common neurosurgical issue. It is postulated that they develop over a period due to a self-perpetuating cycle of recurrent hemorrhage, fibrinolysis, inflammation, and angiogenesis. Although the osmotic gradient theory and inflammation have been mentioned in the pathogenesis, the occurrence of a hematoma capsule and an inherent hyperfibrinolysis that leads to rebleeding is currently the most widely accepted theory [2-4].
One of the sensitive markers for fibrinolysis is D-dimer, a fibrin degradation product [5]. All patients with CSDH have a high level of D-dimer in their clots. Studies have attempted to correlate the level of D-dimer in the clot and the risk of recurrence, as well as radiological subtypes. In this study, we attempt to measure the D-dimer levels in the clots and correlate these markers with the radiological appearance and recurrence.
2. Methods and Materials/Patients
Patient selection
This was a prospective study done in a series of 80 patients who underwent evacuation of CSDH in our center between 2018 and 2020. Patients underwent imaging in the form of a computed tomography (CT) scan and were grouped based on Nomura’s classification [6], which divided CSDH into high density, iso density, low density, layered, and mixed types. We grouped the former three into the homogeneous group and the latter two into a heterogeneous group.
Inclusion criteria
All patients with a CT or a magnetic resonance imaging scan suggestive of a CSDH who presented with clinical symptoms accounted for by their scan findings-except those excluded.
Exclusion criteria
The exclusion criteria were as follows: Patients on antiplatelets, anticoagulants, anti-inflammatory agents, thrombolytic agents, and those on dialysis; patients with an active septic foci or hematological disorders, or neoplastic disorders, liver dysfunction, or coagulopathy; subjects with a ventriculo-peritoneal shunt.
The above patients were excluded as their conditions could, by themselves, cause a recurrence of the disease anticoagulants and anti-platelets added an independent bleeding risk, as do sepsis and liver diseases, sepsis and neoplasms. Ventriculo-peritoneal shunts can overdrain and precipitate CSDH; hence, they were excluded.
Patients underwent surgical evacuation through a single burr hole or two burr holes, or a mini craniotomy, depending on the clinical condition of the patient and the imaging characteristics of the CSDH.
Sample collection
During the surgery, subdural hematoma fluid was sampled after durotomy. Simultaneously, peripheral venous blood samples were drawn and collected in a tube with sodium citrate. In addition, they were centrifuged at 3000 round per min for 10 min. The supernatant was stored in sealed tubes at −70° Celsius until turbidimetric immunoassay (Sysmex CA7000) was used for quantification, and the reference value was <0.324mg/L.
Data analysis
All statistical analysis was performed using the SPSS software, version 17, for Microsoft Windows. The data were not normally distributed, and therefore, parametric/non-parametric tests were performed. Descriptive statistics were presented as numbers and percentages. The data were expressed as Mean±SD. The Chi-squared test was used for comparison between two attributes. Multiple Logistic regression method used to predict the independent variables. A two-sided P<0.05 was considered statistically significant.
3. Results
Patient demographics
We had a total of 80 patients in this study, with 82 bleeds (2 patients had bilateral bleeds). The average age of our study group was 64.35 years. There were 10 women, and the remaining 70 participants were men. There was no statistically significant correlation between either the biochemical marker or recurrence with the age or gender of the population. The average symptom duration was 13 days. A history of trauma was elicited in 67 patients, and the average time between admission and the trauma was 46 days.
Chronic subdural hematoma characteristics
A total of 43 patients had a left-sided bleed, and 35 patients had a right-sided bleed. Meanwhile, two of them had bilateral CSDH. The average thickness of the CSDH in the present study was 19.93 mm, while the average midline shift was 9.46 mm. We had 13 bleeds, each with iso and low-density subtypes. Additionally, 40 bleeds were mixed subtype, while the remaining 16 were a layered type of CSDH. Figure 1 demonstrates the different types of CSDH (Table 1).
Management
A total of 23 patients underwent evacuation with two burr holes, while 11 patients underwent a mini-craniotomy. The rest of the clots were evacuated by a single burr hole. A total of 63 patients were placed on dexamethasone for one week postoperatively.
D-dimer analysis
All D-dimer values obtained from the CSDH were above the reference range of 0-0.325 mg/L. The mean D-dimer value in the Hypodense group was 454.2 mg/L, while the value in the isodense group was 777.74 mg/L. The highest mean value was noted in the mixed subgroup, where the value was 2370 mg/L. The layered group had a mean value of 1146.85 mg/L. The mean D-dimer value in the homogenous group was 622.48 mg/L, while the mean in the heterogeneous group was 2014.57 mg/L. There was a statistically significant correlation between the imaging subtypes and the D-dimer values (P=0.01). The serum levels of D-dimer were within normal limits in all four groups, and there was no statistically significant correlation.
Recurrences
We had 15 cases of recurrences, of whom 11 had undergone evacuation with a single burr hole, three with two burr holes, and one underwent mini craniotomy. Meanwhile, 11 of these cases were of mixed type, and a further three were layered type. There was a single case of isodense type that recurred.
14 of the 15 recurrences occurred in the heterogeneous group. The mean D-dimer value in this group was 1912.52 mg/L. There was a statistically significant correlation between recurrences and both imaging subtype (P=0.02) and the D-dimer values (P=0.01). There was no correlation between the procedure performed, although a majority had undergone a single burr hole evacuation.
Dexamethasone was administered in only 4 of the patients who had recurrence, against 59 of the 65 cases without recurrence. Given the anti-angiogenic and anti-inflammatory effects of steroids, statistical analysis was carried out, which showed a statistically significant correlation between recurrence and non-usage of steroids in the post-operative period, with a P=0.04.
A logistic regression analysis was done, and the associated significance levels are provided in Table 2.

Accordingly, the radiological subtype, levels of D-dimer, and perioperative steroid use had a statistically significant correlation with recurrences, while the surgical approach neared statistical significance.
4. Discussion
This study notes that a major factor in recurrence is the radiological subtype of the bleed. There is a correlation between the subtype and the D-dimer values, and although not statistically significant, the heterogeneous types and their membranes are factors causing recurrence. Based on the D-dimer values in the CSDH fluid, it may be possible to decide on a more extensive procedure, such as a craniotomy, to address the membrane against the use of two burr holes.
Several hypotheses have been put forth to explain the pathogenesis of CSDH, with the hyperfibrinolysis and re-bleeding theory having gained the widest traction. The presence of a hematoma capsule and inherent hyperfibrinolysis that leads to rebleeding is considered central to the development and maintenance of CSDHs [1, 3, 7]. D-dimer is a by-product of the coagulation cascade and can be measured via analysis of a blood sample. It is a marker with relative sensitivity towards fibrinolysis. This cross-linked fibrin degradation product is released when a blood clot begins to break down [5]. Ito et al. stated that local hyperfibrinolysis prevents complete hemostasis and causes rebleeding into the hematoma cavity [1, 8, 9].
A study was conducted in Brazil involving 778 patients, where 60.4% had a history of trauma. The mean age of that study was 64.3 years, with men accounting for 82.6% of the study population [10]. In this study, about 66% of the patients had a history of trauma, and the average age of our study population was 64.35 years, with a male preponderance of 87.5%. The brain in the elderly weighs roughly 200 g less, allowing for an 11% increase in extra cerebral volume, which may account for the higher incidence in the elderly population [11].
Ducruet et al. stated that burr hole craniotomy is the most performed surgery, and this notion has been reiterated by studies conducted in Canada, the Netherlands, and the United Kingdom [12-14]. It seems to provide the best balance between maximal efficacy and minimal invasiveness [14].
A total of 23 patients underwent evacuation with two burr holes, while 11 patients underwent a mini-craniotomy. The rest of the clots were evacuated by a single burr hole. There were 11 recurrences in the single burr hole group, with three recurrences in the two burr holes group, and one patient who had undergone mini craniotomy had a recurrence. There was no statistically significant correlation between surgery performed and recurrence, even though a higher proportion of recurrences occurred in those who had an evacuation with a single burr hole.
Nomura et al. proposed a system [6] where CSDH was classified based on CT imaging as isodense, hypodense, mixed dense, and layering types of hematoma. They described higher fibrinogen and higher D-dimer levels in the layering and mixed density types of CSDHs compared to the high, iso, and low-density types on CT scans. This was comparable to the findings in the present study, where D-dimer levels were the highest in the mixed group, followed by the Layering type, with the hypodense and isodense groups having lower levels of D-dimer.
A meta-analysis done by Miah et al. where 22 studies were identified involving 5566 patients, mixed density was the strongest prognostic factor of recurrence. Layered type also revealed higher recurrence rates [14, 15]. Similar findings were noted in our study, where the mixed density group had the highest rates of recurrence.
Recurrence rates after surgical evacuation were placed between 4 to 26% by Mori et al. [16]. In our study recurrence rate was 18.29%. Higher recurrence was noted in the mixed and layered types, which had a high D-dimer value. Park et al. concluded that there was a difference in the concentration of D-dimer and fibrinogen between the different types of CSDH on CT, with the heterogeneous group consisting of the layering and mixed types having a higher concentration of both markers when compared to the homogenous group [17]. These are associated intimately with the origins of heterogeneous density, which needs repeated cycles of rebleeding. The findings of our study concur with their inferences. A study on recurrent CSDH concluded that the type of procedure performed doesn’t have a bearing on recurrence [18].
We noted that steroids could be of use in preventing recurrence. Sun et al. [19] suggested that administration of steroids may be associated with a decrease in the tendency of the CSDH to recur, especially in the heterogeneous population. Although statistically significant, given the small sample size, further evaluation needs to be done to ascertain the role of steroids in CSDH, although their role in hemostasis and their anti-fibrinolytic properties may break the cycle of rebleeding, coagulation, and fibrinolysis and prevent recurrence [20].
We noted that all recurrences had a D-dimer value of more than 1000 mg/L in the CSDH fluid. A proof-of-concept analysis after letting out the CSDH may help us decide if a more aggressive approach is needed to tackle the disease. This may be an area of further research. The major limitation of our study was its limited sample size and, conduction in a single center.
5. Conclusion
We noted a higher incidence of D-dimer values in patients with a heterogeneous group of CSDH, which includes the mixed and layering subtypes, compared to the homogenous group consisting of hypo- and isodense groups. Higher D-dimer values and heterogeneous appearance on scan correlated with recurrences, with maximum recurrence noted in the mixed group. Steroids may have a role in the prevention of recurrence in CSDH, although further studies are needed to elucidate their significance in that regard.
Ethical Considerations
Compliance with ethical guidelines
The study received ethical approval from the Institutional Ethics Committee of Sri Ramachandra Institute of Higher Education and Research (Code: CSP-MED/18/SEP46/145). Informed consent was obtained from the participants.
Funding
This study was a part of a Post Graduate Dissertation at Sri Ramachandra Institute of Higher Education and Research.
Authors' contributions
Resources: Visvanathan Krishnaswamy and Vivek Visvesvaran; Writing the original drafts and data analysis: Sangeetha Adhikesavan and Sai Sriram Swamiyappan; Data collection: Sangeetha Adhikesavan and Balasubramanian Kannan; Visualization: Visvanathan Krishnaswamy; Conceptualization, critical reviewand and software: Sai Sriram Swamiyappan; Supervision: Vivek Visvesvaran.
Conflict of interest
The authors declared no conflict of interest.
References
- Ito H, Komai T, Yamamoto S. Fibrinolytic enzyme in the lining walls of chronic subdural hematoma. Journal of Neurosurgery. 1978; 48(2):197-200. [DOI:10.3171/jns.1978.48.2.0197] [PMID]
- Markwalder TM. Chronic subdural hematomas: A review. Journal of Neurosurgery. 1981; 54(5):637-45. [DOI:10.3171/jns.1981.54.5.0637] [PMID]
- Ito H, Saito K, Yamamoto S, Hasegawa T. Tissue-type plasminogen activator in the chronic subdural hematoma. Surgical Neurology. 1988; 30(3):175-9. [DOI:10.1016/0090-3019(88)90269-8] [PMID]
- Weir B, Gordon P. Factors affecting coagulation: Fibrinolysis in chronic subdural fluid collections. Journal of Neurosurgery. 1983; 58(2):242-5. [DOI:10.3171/jns.1983.58.2.0242] [PMID]
- Elms MJ, Bunce IH, Bundesen PG, Rylatt DB, Webber AJ, Masci PP, et al. Rapid detection of cross-linked fibrin degradation products in plasma using monoclonal antibody-coated latex particles. American Journal of Clinical Pathology. 1986; 85(3):360-4. [DOI:10.1093/ajcp/85.3.360] [PMID]
- Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. Journal of Neurosurgery. 1994; 81(6):910-3. [DOI:10.3171/jns.1994.81.6.0910] [PMID]
- Park SH, Lee SH, Park J, Hwang JH, Hwang SK, Hamm IS. Chronic subdural hematoma preceded by traumatic subdural hygroma. Journal of Clinical Neuroscience. 2008; 15(8):868-72. [DOI:10.1016/j.jocn.2007.08.003] [PMID]
- Fujisawa H, Ito H, Saito K, Ikeda K, Nitta H, Yamashita J. Immunohistochemical localization of tissue-type plasminogen activator in the lining wall of chronic subdural hematoma. Surgical Neurology. 1991; 35(6):441-5. [DOI:10.1016/0090-3019(91)90177-B] [PMID]
- Ito H, Yamamoto S, Komai T, Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. Journal of Neurosurgery. 1976; 45(1):26-31. [DOI:10.3171/jns.1976.45.1.0026] [PMID]
- Sousa EB, Brandão LF, Tavares CB, Borges IB, Neto NG, Kessler IM. Epidemiological characteristics of 778 patients who underwent surgical drainage of chronic subdural hematomas in Brasília, Brazil. BMC Surgery. 2013; 13:5. [DOI:10.1186/1471-2482-13-5] [PMID]
- Misra M, Salazar JL, Bloom DM. Subdural-peritoneal shunt: Treatment for bilateral chronic subdural hematoma. Surgical Neurology. 1996; 46(4):378-83. [DOI:10.1016/S0090-3019(96)00188-7] [PMID]
- Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN, et al. The surgical management of chronic subdural hematoma. Neurosurgical Review. 2012; 35(2):155-69. [DOI:10.1007/s10143-011-0349-y] [PMID]
- Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009; 374(9695):1067-73. [DOI:10.1016/S0140-6736(09)61115-6] [PMID]
- Miah IP, Tank Y, Rosendaal FR, Peul WC, Dammers R, Lingsma HF, et al. Radiological prognostic factors of chronic subdural hematoma recurrence: A systematic review and meta-analysis. Neuroradiology. 2021; 63(1):27-40. [DOI:10.1007/s00234-020-02597-4] [PMID]
- Winn HR. Youmans and Winn neurological surgery by Julian R Youmans and H.R. Winn. 7th edition. Philadelphia: Elsevier Health Sciences; 2022. [Link]
- Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurologia Medico-Chirurgica. 2001; 41(8):371-81. [DOI:10.2176/nmc.41.371] [PMID]
- Park SH, Kang DH, Park J, Hwang JH, Hwang SK, Sung JK, et al. Fibrinogen and D-dimer analysis of chronic subdural hematomas and computed tomography findings: A prospective study. Clinical Neurology and Neurosurgery. 2011; 113(4):272-6. [DOI:10.1016/j.clineuro.2010.11.014] [PMID]
- Swamiyappan SS, Krishnaswamy V, Visweswaran V, Bathala RT, Karnati H, Gupta J. (July 27, 2023) recurrent subdural hematoma: An institutional experience. Cureus. 15(7):e42582. [DOI:10.7759/cureus.42582]
- Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: Preliminary results of using dexamethasone. British Journal of Neurosurgery. 2005; 19(4):327-33. [DOI:10.1080/02688690500305332] [PMID]
- Brotman DJ, Girod JP, Posch A, Jani JT, Patel JV, Gupta M, et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thrombosis Research. 2006; 118(2):247-52. [DOI:10.1016/j.thromres.2005.06.006] [PMID]
Type of Study: Research |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |