Sat, Jan 31, 2026
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bagherzadeh S, Jafari M, Ehteshami S, Roohollahi F, Greenberg M, Alikhani P et al . Traumatic Lumbar Fracture in Ankylosing Spondylitis: A Narrative Review with Case Illustration. Iran J Neurosurg 2025; 11 : 16
URL: http://irjns.org/article-1-469-en.html
URL: http://irjns.org/article-1-469-en.html
Sadegh Bagherzadeh1 

 , Mohammad Jafari2
, Mohammad Jafari2 

 , Saeed Ehteshami3
, Saeed Ehteshami3 

 , Faramarz Roohollahi *4
, Faramarz Roohollahi *4 

 , Mark Greenberg5
, Mark Greenberg5 

 , Puya Alikhani5
, Puya Alikhani5 

 , Mohsen Rostami2
, Mohsen Rostami2 




 , Mohammad Jafari2
, Mohammad Jafari2 

 , Saeed Ehteshami3
, Saeed Ehteshami3 

 , Faramarz Roohollahi *4
, Faramarz Roohollahi *4 

 , Mark Greenberg5
, Mark Greenberg5 

 , Puya Alikhani5
, Puya Alikhani5 

 , Mohsen Rostami2
, Mohsen Rostami2 


1- Department of Neurosurgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran & Sports Medicine Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
2- Department of Neurosurgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
3- Orthopedic research center,Mazandaran University of medical sciences,Sari,Iran
4- Department of Neurosurgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran & Sports Medicine Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran ,faramarzroohollahi@yahoo.com
5- Department of Neurosurgery, Brain and Spine, Morsani College of Medicine, University of South Florida, Tampa, United States.
2- Department of Neurosurgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
3- Orthopedic research center,Mazandaran University of medical sciences,Sari,Iran
4- Department of Neurosurgery, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran & Sports Medicine Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran ,
5- Department of Neurosurgery, Brain and Spine, Morsani College of Medicine, University of South Florida, Tampa, United States.
Full Text [PDF 2928 kb]
(1014 Downloads)
| Abstract (HTML) (1364 Views)
Full Text: (865 Views)
1. Introduction
Ankylosing spondylitis (AS) is a rheumatologic condition that affects the spine through chronic inflammation and pathological remodeling of the spine. AS patients have a four-fold risk of spinal fracture compared to a healthy population. Reportedly, 5% to 15% of all AS patients will experience a spinal fracture at some point during their lives [1-3]. In individuals with AS, spinal fractures typically involve all three spinal columns, resulting in a high level of instability. These fractures are closely associated with a significant likelihood of neurological complications, ranging from 33% to 58% for thoracic and lumbar fractures and even higher rates for cervical spine fractures [4-6].
Patients with AS have an increased risk of fracture. Usually, a minor trauma can lead to a spinal fracture [7] due to several reasons, including AS patients who often have osteoporosis [8, 9] Kyphosis is another common feature of AS, which causes limited vision, impaired balance [10], and reduced flexibility of the spine due to a bamboo-like spine in advanced stages of the disease [11].
Timely diagnosis of the spine fracture is difficult in AS patients, particularly in the absence of trauma. Without visible trauma, fracture-related pain may be misinterpreted as inflammatory pain associated with AS, leading patients to avoid seeking medical attention. Some specialists recommend assuming a fracture in any AS patient with an injury, even from minor trauma, unless proven otherwise [12]. Diagnostic delays contribute to a secondary neurological complication rate of up to 15% before fracture treatment [3, 13].
Fractures of the lumbar spine are not frequently observed in patients with AS. At present, no consensus or comprehensive study is available to outline the best approaches for managing this particular type of fracture in AS patients. Our goal is to share our case and thoroughly examine and consolidate the current body of literature relating to the management of lumbar fractures in AS patients.
2. Methods and Materials/Patients
History
A 48-year-old male patient with a history of AS for 20 years, consulted our spine group. The patient had a motorcycle-to-car accident 5 days ago and presented to the Emergency Department with paralysis of both legs. He had no familial history of rheumatologic diseases and did not use alcohol or smoke. He was under treatment with tab methotrexate 7.5 mg once weekly and Tab Naproxen 375 mg in case of pain. The patient had a posterior spinal fusion from T4 to L2 with no clear indication (the patient stated surgery was done to stop the process of progressive kyphosis).
Physical examination
Examination revealed that neck movements were restricted in all directions. The patient exhibited midline tenderness in the upper lumbar region, with a palpable step-off observed upon examination of the spinous process. The motor forces of the upper limbs were normal, and there was no sign of hyperreflexia. Meanwhile, Hoffman’s sign was negative. He had a sensory level below the hypogastric region, and he had a Foley catheter because of urinary retention. Also, the patient had saddle hypoesthesia. His lower limb forces were 1/5, and his sphincter tone was decreased. Lower limb deep tendon reflexes were 1+, Bulbo-cavernous reflexes were normal, and the modified Frankel grade was equal to 3.
Diagnostic assessment
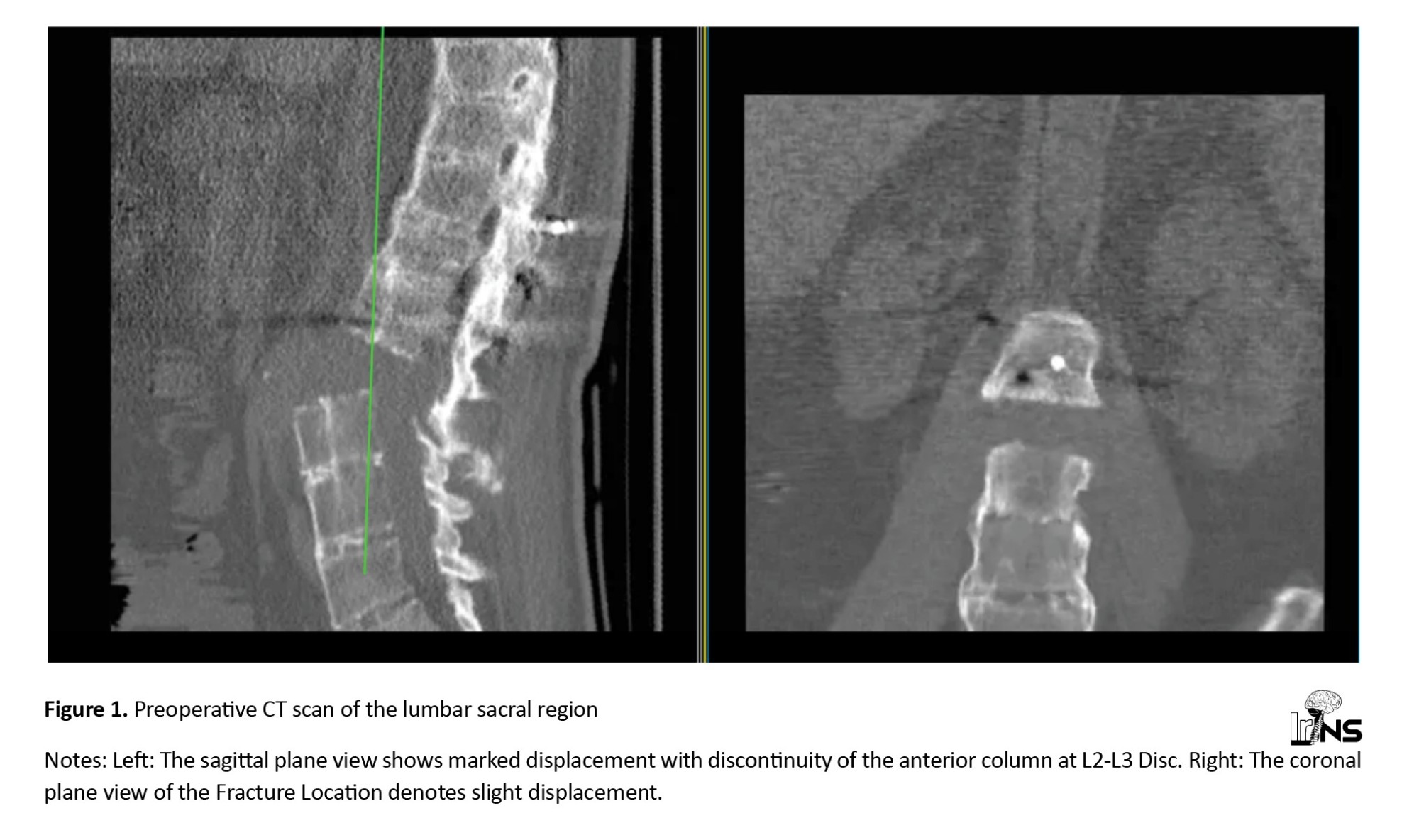
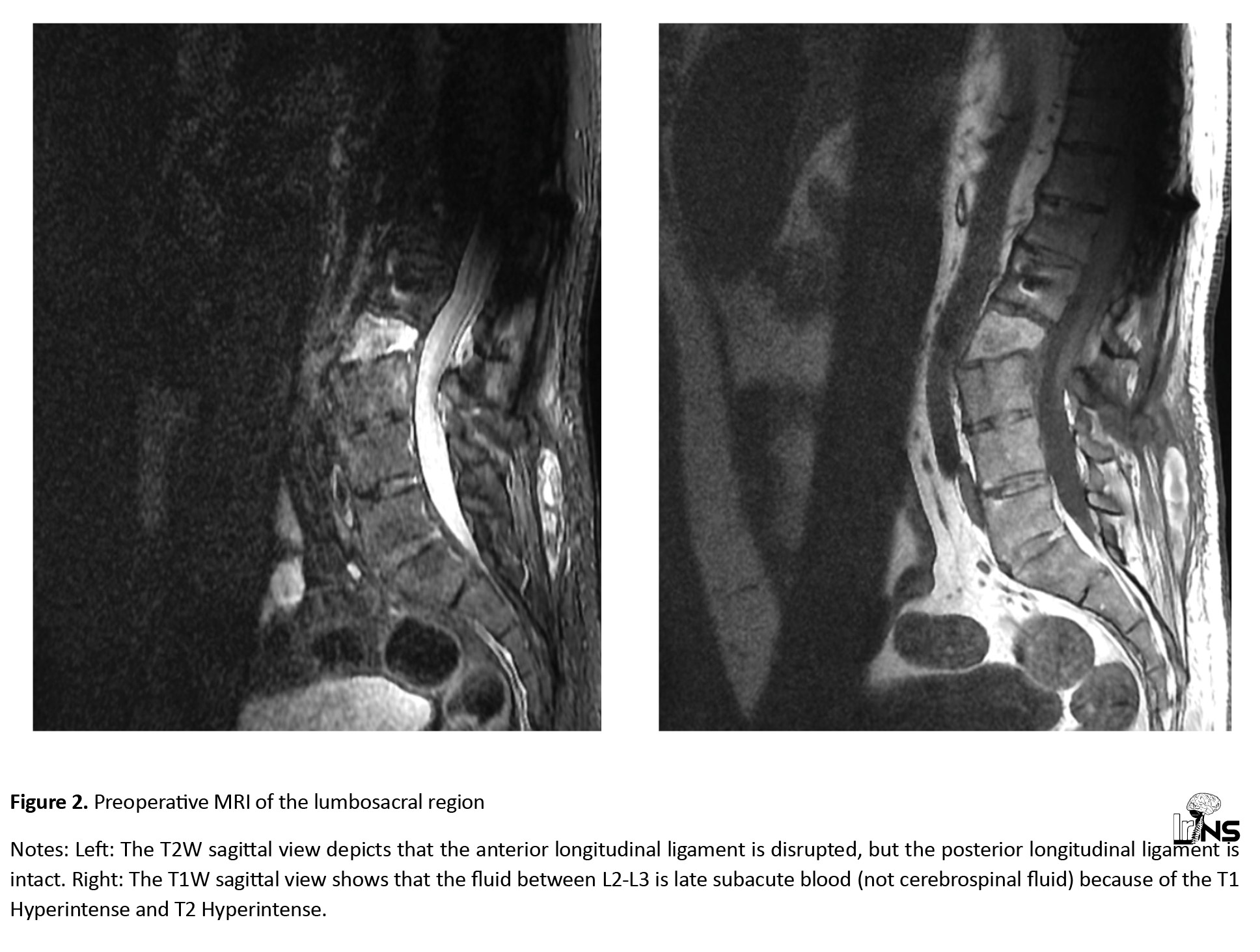
A computed tomography (CT) scan revealed a fracture translation in the L2-L3 intervertebral disc level. The translation was more significant in the sagittal plane than in the coronal plane. According to the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification, this was a type C fracture-dislocation. A whole-spine CT had no other significant findings (Figure 1). A whole-spine magnetic resonance imaging (MRI) was performed, which showed posterior ligamentous complex injury and disruption of the L2-L3 facet. The thecal sac was intact, and there was no intracanalicular fragment. The anterior longitudinal ligament was ossified and disrupted, while the posterior longitudinal ligament was injured but not completely torn (Figure 2).
Therapeutic interventions
According to the thoracolumbar injury classification and severity score, the patient scored 4 points for morphology, 3 points for ligamentous injury, and 3 points for neurological status, totaling 10 points, which indicates the need for surgical intervention. We decided to perform the surgery emergently.
Our planning for surgery consisted of two parts. The first stage involved posterior fixation, while the second stage involved anterior column realignment and stabilization. We used intraoperative neuromonitoring (IONM), which showed a low amplitude with high latency motor evoked potential (MEP) in distal muscles of the lower limb and very weak somatosensory evoked potential (SSEP).
First stage: The patient was positioned prone under IONM. We removed the previously placed construct from T4 to L2. Then, we placed 7.5 mm * 50 mm pedicular screws in L3, L4, L5, and 6.5 mm* 50 mm pedicular screw in T12, L1, and L2. We connected the screws with an appropriately contoured chrome cobalt rod. We did not perform a laminectomy because the preoperative MRI showed no compression in the spinal canal. IONM showed a slight improvement in SSEP after compression between L2 and L3.
Second stage: The patient was placed in a lateral position (left side up) with meticulous caution and under IONM. Next, we localized the incision site under C-arm guidance and used a retroperitoneal corridor to reach the L2-L3 region from the left side. Then we placed an expandable cage in the gap created by trauma between L2 and L3. For further stabilization, we used a 4×25 mm screw and inserted it into the L2 and L3 vertebrae through the lateral side of the body. Then, a rod was placed, further stabilizing the cage in the anterior column. IONM did not show any change in MEP and SSEP.
3. Results
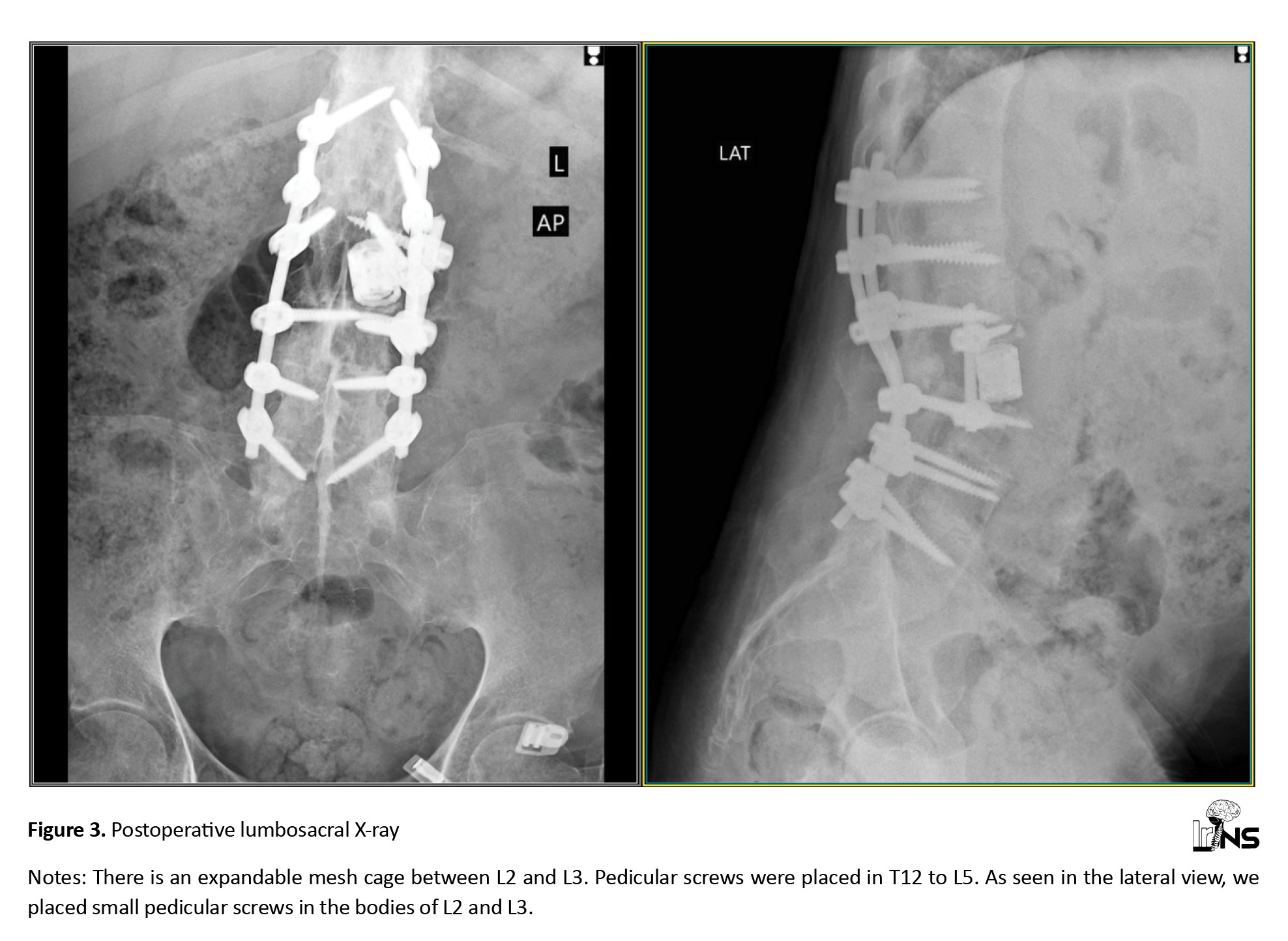
Figure 3 shows the patient’s postoperative image. The overall estimated blood loss was approximately 800 mL, and the total surgery time, divided into two stages, was approximately 7 h. There were no wound infection issues. The patient was in the intensive care unit for 2 days and 5 days in the spine ward. Subsequently, he was discharged to a rehabilitation facility. His examination during discharge revealed that lower limb forces were 2/5, and saddle hypoesthesia improved slightly. He has been trained to perform clean intermittent catheterization due to urinary retention.
The patient was observed by a specialist in physical medicine and rehabilitation. Three months later, he returned to our clinic. He could walk on a crunch and regained his bladder control. Lower limb muscle forces were 4/5, and saddle hypoesthesia was completely gone. The instruments were in place, and no mechanical complications occurred.
4. Discussion
Imaging
The delayed diagnosis of these patients raises significant concerns, primarily due to difficulties in interpreting imaging, which leads to a lack of appropriate spinal immobilization [14]. The initial miss rate for fractures ranges from 19% to 60% [5, 15].
Ossification of spinal ligaments may result in fractures, a notable contributor to spinal instability and persistent back pain. Early diagnosis of these fractures is difficult using X-rays; however, CT and MRI scans play a crucial role in their identification [16, 17]. Fracture-dislocations predominantly occur in the junctional zones of the spine and are challenging to detect on radiographs. CT is regarded as the gold standard for detecting spine fractures in AS [4, 18, 19].
Additionally, an MRI aids in detecting the fracture and offers insights into its timing. Consequently, it is advisable to obtain an MRI alongside a CT [13]. During the acute phase, MRI shows edema in the vertebral body and posterior bony elements. The elevated signal intensity is readily observed in the T2 short tau inversion recovery (STIR) sequence, while the T1 sequence delineates the configuration of the fracture line [18].
Management
Choosing between surgical and non-surgical management
Non-displaced fractures can be treated without surgery; however, there is evidence that thoracolumbar fractures in patients with AS are better treated with surgery than with non-surgical methods.
Bracing intolerance leads to a high failure rate of nonsurgical therapy, which is close to 50% for AS fractures, and the kyphotic deformity frequently makes bracing more difficult [13, 20, 21]. Meanwhile, patients with AS typically have lower bone quality, suggesting that conservative treatment will necessitate a longer duration compared to age-matched persons without AS. Extended bed rest, sometimes necessitated for non-surgical management, can increase the likelihood of problems. While surgical intervention entails specific consequences, the risks associated with non-surgical treatment, including probable deterioration of alignment, non:union:, neurological impairment, and loss of reduction, are comparatively greater [22, 23]. Apart from the reasons mentioned earlier, there is evidence that surgical treatment yields better outcomes.
Prompt surgical intervention can enhance neurological function and diminish the overall complication rate in AS patients [3]. Surgical intervention can markedly enhance the survival of AS patients with spinal fractures [24]. Caron et al. conducted a study in AS patients with thoracolumbar fractures, revealing a 1-year death rate of 51% in non-surgically treated patients, contrasted with 32% in the surgical cohort [25]. Lu et al. [26] indicated that all patients in their case series demonstrated solid fusion and restoration of neurological impairments following surgical intervention. Westerveld et al. [3] additionally verified that surgical intervention while neurologic deficits are present led to no further deterioration in 59% of instances and improvement in 27% of cases.
In conclusion, evidence suggests that surgical intervention is preferable to non-surgical treatment; therefore, unless the surgical risk is excessively high, it is typically recommended to pursue surgical treatment promptly. Lukasiewicz et al. [27] determined that 49.9% of the participants received instrumented fusion surgery. The primary indications for surgery include neurological decline, the existence of an unstable fracture, or the identification of an epidural hematoma.
Non-surgical options
Brace immobilization is a viable alternative for thoracic spine injuries in patients exhibiting more stable damage patterns or possessing comorbidities. It is crucial to recognize that in displaced upper and mid-thoracic AS fractures, the chest wall fails to offer the theoretical support of the fourth column because of its ossified costovertebral connections, resulting in persistent displacement of the fractured region with each breath [28].
Lumbar spine fractures complicate immobilization with a brace because of the cantilever forces applied by the pelvis. Although potentially alleviated by including one leg or the pelvis within the orthosis, the resulting decrease in patient mobility and increased risk of immobility-related complications render this approach unfeasible for these already susceptible patients [21].
Surgical approaches
Multi-segmental fixation is the optimal final intervention for displaced injuries. Three surgical methods exist: The anterior-only approach, the posterior-only approach, and the anterior-posterior approach. The anterior-only approach poses significant challenges due to the proximity of thoracic and abdominal organs, as well as major vessels, anterior to the spine. Moreover, the screws employed in this method may lack adequate stability. The anterior approach is often reserved for instances where the anterior column of the spine has substantially compressed and cannot be sufficiently managed by a posterior approach [20, 29].
The posterior-only technique is favored by many because it utilizes pedicle screws, which provide robust support, mitigate abnormalities, and guarantee stability following surgery. This method results in reduced trauma. In the latter phases of AS, the kyphotic deformity is prevalent, with the posterior column experiencing tension and the anterior column subjected to pressure. Internal fixation exhibits greater stability when positioned on the tension side, rendering the simple posterior method a favored choice for numerous practitioners [20].
When do we need an additional anterior approach?
The single-cortical fixations in both the simple anterior and simple posterior techniques resemble those employed for long bone fractures. Conversely, the combined anterior and posterior surgery offers enhanced reduction and retention strength. However, it is accompanied by the disadvantages of heightened trauma, extended surgical durations, and an elevated chance of complications, especially in senior patients with compromised health. As a result, this method is hardly utilized [29].
In instances of thoracic or lumbar spine fractures in AS, secondary anterior surgery is seldom required, as these patients typically exhibit good bone healing and formation. However, in cases where a significant gap is present in the front of the spine, it is advantageous to consider secondary anterior column reconstruction utilizing a structural graft or cage, as in our case. When there is anterior column compromise, we should use combined approaches.
How many segments do we need to fix?
Werner et al. proposed the fixation of a minimum of three levels above and below the fracture site due to the nature of the fracture in AS patients, which are usually type C AO, and the compromised bone quality in patients with AS [21]. However, we should bear in mind that in the lumbar region, extending three levels below the fracture site may necessitate spino-pelvic fixation.
When should we consider the decompression?
Surgical decompression may be required in instances of an AS-related fracture if there is an epidural hematoma, if fracture displacement is irreducible, and if significant stenosis is inadvertently identified, especially in patients with hyperostotic AS [30].
Can we correct the deformity at the time of trauma surgery?
A few studies have presented simultaneous correction of the kyphosis at the time of AS thoracolumbar fracture surgery [3]. Incorporating an osteotomy during fracture fixation may be a more advantageous choice, yet it is a complex surgery in its own right. Werner et al. advised against attempting deformity correction in the presence of a fracture due to an increased risk of complications [21]. As previously mentioned, experts suggest not attempting to correct sagittal balance during surgery for traumatic fractures, but there is an exception to this rule. Sometimes, like in our case, the trauma gives you the chance of killing two birds with one stone, so when the distraction injury is with the lengthening of the anterior column, use the opportunity.
Is minimally invasive surgery possible for a trauma fracture in AS?
Minimally invasive spine surgery has emerged as a viable alternative for long-segment posterior fixation in thoracolumbar AS fractures, owing to potential complications, such as hemorrhage and infections associated with traditional open spinal surgeries. Percutaneous instrumentation reduces surgical invasiveness, thereby lowering the risk of blood loss and disease transmission. Conversely, minimally invasive techniques limit the ability to correct deformities and perform decompression when necessary [31, 32]. In previous studies, it has been demonstrated that the use of minimally invasive screw fixation reduces surgery-related complications and yields better clinical outcomes [33, 34].
Nuances encountered in management
Positioning: When managing AS patients with a lumbar translational fracture, meticulous attention to positioning is crucial. Many of these individuals exhibit an inherent kyphosis coupled with an extension-type mechanism of the injury. Positioning a thoracolumbar fracture patient with AS in a prone position on a conventional orthopedic table may result in exacerbated uncontrolled extension or translation of the spine within the injury zone, potentially leading to secondary neurological deterioration and vascular compromise. Surgeons should contemplate employing a frame that facilitates regulated kyphosis, such as a Wilson-type frame, for these patients [35].
Instrumentation: Technical difficulties in placing posterior screws sometimes arise from the lack of posterior bone markers in AS, significantly altered facet joints, substantial body size, and spinal abnormalities. Calcification and vertebral rotation change the anatomical landmarks in AS patients, complicating freehand pedicle screw implantation. AS patients encounter a significantly elevated risk of surgical intervention and postoperative complications relative to the general populace [13, 36, 37].
Bone Quality: Bone mineral density is diminished in AS [9], with a 25% incidence of osteoporosis among AS patients over the past decade [38], resulting in a significant implant loosening rate of around 10% to 15% [5, 25]. It is essential to create several anchor points by extending the construct, which may provide improved spinal stability for patients with unstable fractures [39]. Another method to augment implant fixation strength may include cement augmentation of the pedicle screws.
Preoperative issues: Patients frequently exhibit several comorbidities, particularly cardiovascular illness, which exacerbates the mortality risk after a fracture. Commonly administered pharmacological interventions can affect the timing of surgery and the intraoperative difficulties experienced by the patient, including the use of anticoagulants, anti-inflammatories, and pulmonary limitations or hypertension [40]. In these instances, blood loss may exceed typical expectations [41].
Outcome
Rustagi et al. reported that mortality rates one year post-injury varied between 0% and 32%. Complications such as pneumonia, respiratory failure, and pseudoarthrosis occurred in 84% of patients. Neurologic deterioration occurred in 16% of patients, whereas successful fusion rates ranged from 87% to 100%. At the final follow-up, 6% to 66% of patients exhibited improvement in neurologic deficits [29]. Mortality rates were significantly lower in thoracic fractures compared to cervical fractures, while lumbar spine fractures showed no significant difference [25].
The prognosis for patients with ankylosing spine injuries is significantly affected by the status of their neurologic injuries and the severity of their medical comorbidities. Patients with fused spinal columns typically exhibit markedly elevated rates of morbidity and mortality within one year relative to those with non-fused spinal columns. Indications suggest that patients undergoing surgical treatment exhibit longer and improved survival rates compared to those receiving non-surgical intervention for AS fracture-dislocations [29].
5. Conclusion
Lumbar spine fractures in individuals with AS can occur even after minor trauma and may present with minimal symptoms, such as pain. It is crucial to consider this diagnosis. While non-displaced fractures may be treated without surgery, evidence suggests that thoracolumbar fractures in patients with AS may benefit from surgical intervention over non-surgical methods. However, it is important to note that surgery for these individuals requires special considerations, as detailed in our review. The outcome of these types of fractures is significantly influenced by the patient’s neurological injury status and the severity of their medical comorbidities. Favorable results from surgery are often observed, as in our case.
Ethical Considerations
Compliance with ethical guidelines
The Institutional Ethics Committee waived the need for ethical approval for this case report, as it was considered part of standard patient care. The patient gave both verbal and written informed consent for the use of his clinical data and images in this report. The manuscript and images do not reveal the patient’s identity.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Review literature and composition and the execution of the surgery: Mohsen Rostami and Faramarz Roohollahi; Critical analysis: Saeid Ehteshami and Mohammad Jafari; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Ankylosing spondylitis (AS) is a rheumatologic condition that affects the spine through chronic inflammation and pathological remodeling of the spine. AS patients have a four-fold risk of spinal fracture compared to a healthy population. Reportedly, 5% to 15% of all AS patients will experience a spinal fracture at some point during their lives [1-3]. In individuals with AS, spinal fractures typically involve all three spinal columns, resulting in a high level of instability. These fractures are closely associated with a significant likelihood of neurological complications, ranging from 33% to 58% for thoracic and lumbar fractures and even higher rates for cervical spine fractures [4-6].
Patients with AS have an increased risk of fracture. Usually, a minor trauma can lead to a spinal fracture [7] due to several reasons, including AS patients who often have osteoporosis [8, 9] Kyphosis is another common feature of AS, which causes limited vision, impaired balance [10], and reduced flexibility of the spine due to a bamboo-like spine in advanced stages of the disease [11].
Timely diagnosis of the spine fracture is difficult in AS patients, particularly in the absence of trauma. Without visible trauma, fracture-related pain may be misinterpreted as inflammatory pain associated with AS, leading patients to avoid seeking medical attention. Some specialists recommend assuming a fracture in any AS patient with an injury, even from minor trauma, unless proven otherwise [12]. Diagnostic delays contribute to a secondary neurological complication rate of up to 15% before fracture treatment [3, 13].
Fractures of the lumbar spine are not frequently observed in patients with AS. At present, no consensus or comprehensive study is available to outline the best approaches for managing this particular type of fracture in AS patients. Our goal is to share our case and thoroughly examine and consolidate the current body of literature relating to the management of lumbar fractures in AS patients.
2. Methods and Materials/Patients
History
A 48-year-old male patient with a history of AS for 20 years, consulted our spine group. The patient had a motorcycle-to-car accident 5 days ago and presented to the Emergency Department with paralysis of both legs. He had no familial history of rheumatologic diseases and did not use alcohol or smoke. He was under treatment with tab methotrexate 7.5 mg once weekly and Tab Naproxen 375 mg in case of pain. The patient had a posterior spinal fusion from T4 to L2 with no clear indication (the patient stated surgery was done to stop the process of progressive kyphosis).
Physical examination
Examination revealed that neck movements were restricted in all directions. The patient exhibited midline tenderness in the upper lumbar region, with a palpable step-off observed upon examination of the spinous process. The motor forces of the upper limbs were normal, and there was no sign of hyperreflexia. Meanwhile, Hoffman’s sign was negative. He had a sensory level below the hypogastric region, and he had a Foley catheter because of urinary retention. Also, the patient had saddle hypoesthesia. His lower limb forces were 1/5, and his sphincter tone was decreased. Lower limb deep tendon reflexes were 1+, Bulbo-cavernous reflexes were normal, and the modified Frankel grade was equal to 3.
Diagnostic assessment
A computed tomography (CT) scan revealed a fracture translation in the L2-L3 intervertebral disc level. The translation was more significant in the sagittal plane than in the coronal plane. According to the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification, this was a type C fracture-dislocation. A whole-spine CT had no other significant findings (Figure 1). A whole-spine magnetic resonance imaging (MRI) was performed, which showed posterior ligamentous complex injury and disruption of the L2-L3 facet. The thecal sac was intact, and there was no intracanalicular fragment. The anterior longitudinal ligament was ossified and disrupted, while the posterior longitudinal ligament was injured but not completely torn (Figure 2).
Therapeutic interventions
According to the thoracolumbar injury classification and severity score, the patient scored 4 points for morphology, 3 points for ligamentous injury, and 3 points for neurological status, totaling 10 points, which indicates the need for surgical intervention. We decided to perform the surgery emergently.
Our planning for surgery consisted of two parts. The first stage involved posterior fixation, while the second stage involved anterior column realignment and stabilization. We used intraoperative neuromonitoring (IONM), which showed a low amplitude with high latency motor evoked potential (MEP) in distal muscles of the lower limb and very weak somatosensory evoked potential (SSEP).
First stage: The patient was positioned prone under IONM. We removed the previously placed construct from T4 to L2. Then, we placed 7.5 mm * 50 mm pedicular screws in L3, L4, L5, and 6.5 mm* 50 mm pedicular screw in T12, L1, and L2. We connected the screws with an appropriately contoured chrome cobalt rod. We did not perform a laminectomy because the preoperative MRI showed no compression in the spinal canal. IONM showed a slight improvement in SSEP after compression between L2 and L3.
Second stage: The patient was placed in a lateral position (left side up) with meticulous caution and under IONM. Next, we localized the incision site under C-arm guidance and used a retroperitoneal corridor to reach the L2-L3 region from the left side. Then we placed an expandable cage in the gap created by trauma between L2 and L3. For further stabilization, we used a 4×25 mm screw and inserted it into the L2 and L3 vertebrae through the lateral side of the body. Then, a rod was placed, further stabilizing the cage in the anterior column. IONM did not show any change in MEP and SSEP.
3. Results
Figure 3 shows the patient’s postoperative image. The overall estimated blood loss was approximately 800 mL, and the total surgery time, divided into two stages, was approximately 7 h. There were no wound infection issues. The patient was in the intensive care unit for 2 days and 5 days in the spine ward. Subsequently, he was discharged to a rehabilitation facility. His examination during discharge revealed that lower limb forces were 2/5, and saddle hypoesthesia improved slightly. He has been trained to perform clean intermittent catheterization due to urinary retention.
The patient was observed by a specialist in physical medicine and rehabilitation. Three months later, he returned to our clinic. He could walk on a crunch and regained his bladder control. Lower limb muscle forces were 4/5, and saddle hypoesthesia was completely gone. The instruments were in place, and no mechanical complications occurred.
4. Discussion
Imaging
The delayed diagnosis of these patients raises significant concerns, primarily due to difficulties in interpreting imaging, which leads to a lack of appropriate spinal immobilization [14]. The initial miss rate for fractures ranges from 19% to 60% [5, 15].
Ossification of spinal ligaments may result in fractures, a notable contributor to spinal instability and persistent back pain. Early diagnosis of these fractures is difficult using X-rays; however, CT and MRI scans play a crucial role in their identification [16, 17]. Fracture-dislocations predominantly occur in the junctional zones of the spine and are challenging to detect on radiographs. CT is regarded as the gold standard for detecting spine fractures in AS [4, 18, 19].
Additionally, an MRI aids in detecting the fracture and offers insights into its timing. Consequently, it is advisable to obtain an MRI alongside a CT [13]. During the acute phase, MRI shows edema in the vertebral body and posterior bony elements. The elevated signal intensity is readily observed in the T2 short tau inversion recovery (STIR) sequence, while the T1 sequence delineates the configuration of the fracture line [18].
Management
Choosing between surgical and non-surgical management
Non-displaced fractures can be treated without surgery; however, there is evidence that thoracolumbar fractures in patients with AS are better treated with surgery than with non-surgical methods.
Bracing intolerance leads to a high failure rate of nonsurgical therapy, which is close to 50% for AS fractures, and the kyphotic deformity frequently makes bracing more difficult [13, 20, 21]. Meanwhile, patients with AS typically have lower bone quality, suggesting that conservative treatment will necessitate a longer duration compared to age-matched persons without AS. Extended bed rest, sometimes necessitated for non-surgical management, can increase the likelihood of problems. While surgical intervention entails specific consequences, the risks associated with non-surgical treatment, including probable deterioration of alignment, non:union:, neurological impairment, and loss of reduction, are comparatively greater [22, 23]. Apart from the reasons mentioned earlier, there is evidence that surgical treatment yields better outcomes.
Prompt surgical intervention can enhance neurological function and diminish the overall complication rate in AS patients [3]. Surgical intervention can markedly enhance the survival of AS patients with spinal fractures [24]. Caron et al. conducted a study in AS patients with thoracolumbar fractures, revealing a 1-year death rate of 51% in non-surgically treated patients, contrasted with 32% in the surgical cohort [25]. Lu et al. [26] indicated that all patients in their case series demonstrated solid fusion and restoration of neurological impairments following surgical intervention. Westerveld et al. [3] additionally verified that surgical intervention while neurologic deficits are present led to no further deterioration in 59% of instances and improvement in 27% of cases.
In conclusion, evidence suggests that surgical intervention is preferable to non-surgical treatment; therefore, unless the surgical risk is excessively high, it is typically recommended to pursue surgical treatment promptly. Lukasiewicz et al. [27] determined that 49.9% of the participants received instrumented fusion surgery. The primary indications for surgery include neurological decline, the existence of an unstable fracture, or the identification of an epidural hematoma.
Non-surgical options
Brace immobilization is a viable alternative for thoracic spine injuries in patients exhibiting more stable damage patterns or possessing comorbidities. It is crucial to recognize that in displaced upper and mid-thoracic AS fractures, the chest wall fails to offer the theoretical support of the fourth column because of its ossified costovertebral connections, resulting in persistent displacement of the fractured region with each breath [28].
Lumbar spine fractures complicate immobilization with a brace because of the cantilever forces applied by the pelvis. Although potentially alleviated by including one leg or the pelvis within the orthosis, the resulting decrease in patient mobility and increased risk of immobility-related complications render this approach unfeasible for these already susceptible patients [21].
Surgical approaches
Multi-segmental fixation is the optimal final intervention for displaced injuries. Three surgical methods exist: The anterior-only approach, the posterior-only approach, and the anterior-posterior approach. The anterior-only approach poses significant challenges due to the proximity of thoracic and abdominal organs, as well as major vessels, anterior to the spine. Moreover, the screws employed in this method may lack adequate stability. The anterior approach is often reserved for instances where the anterior column of the spine has substantially compressed and cannot be sufficiently managed by a posterior approach [20, 29].
The posterior-only technique is favored by many because it utilizes pedicle screws, which provide robust support, mitigate abnormalities, and guarantee stability following surgery. This method results in reduced trauma. In the latter phases of AS, the kyphotic deformity is prevalent, with the posterior column experiencing tension and the anterior column subjected to pressure. Internal fixation exhibits greater stability when positioned on the tension side, rendering the simple posterior method a favored choice for numerous practitioners [20].
When do we need an additional anterior approach?
The single-cortical fixations in both the simple anterior and simple posterior techniques resemble those employed for long bone fractures. Conversely, the combined anterior and posterior surgery offers enhanced reduction and retention strength. However, it is accompanied by the disadvantages of heightened trauma, extended surgical durations, and an elevated chance of complications, especially in senior patients with compromised health. As a result, this method is hardly utilized [29].
In instances of thoracic or lumbar spine fractures in AS, secondary anterior surgery is seldom required, as these patients typically exhibit good bone healing and formation. However, in cases where a significant gap is present in the front of the spine, it is advantageous to consider secondary anterior column reconstruction utilizing a structural graft or cage, as in our case. When there is anterior column compromise, we should use combined approaches.
How many segments do we need to fix?
Werner et al. proposed the fixation of a minimum of three levels above and below the fracture site due to the nature of the fracture in AS patients, which are usually type C AO, and the compromised bone quality in patients with AS [21]. However, we should bear in mind that in the lumbar region, extending three levels below the fracture site may necessitate spino-pelvic fixation.
When should we consider the decompression?
Surgical decompression may be required in instances of an AS-related fracture if there is an epidural hematoma, if fracture displacement is irreducible, and if significant stenosis is inadvertently identified, especially in patients with hyperostotic AS [30].
Can we correct the deformity at the time of trauma surgery?
A few studies have presented simultaneous correction of the kyphosis at the time of AS thoracolumbar fracture surgery [3]. Incorporating an osteotomy during fracture fixation may be a more advantageous choice, yet it is a complex surgery in its own right. Werner et al. advised against attempting deformity correction in the presence of a fracture due to an increased risk of complications [21]. As previously mentioned, experts suggest not attempting to correct sagittal balance during surgery for traumatic fractures, but there is an exception to this rule. Sometimes, like in our case, the trauma gives you the chance of killing two birds with one stone, so when the distraction injury is with the lengthening of the anterior column, use the opportunity.
Is minimally invasive surgery possible for a trauma fracture in AS?
Minimally invasive spine surgery has emerged as a viable alternative for long-segment posterior fixation in thoracolumbar AS fractures, owing to potential complications, such as hemorrhage and infections associated with traditional open spinal surgeries. Percutaneous instrumentation reduces surgical invasiveness, thereby lowering the risk of blood loss and disease transmission. Conversely, minimally invasive techniques limit the ability to correct deformities and perform decompression when necessary [31, 32]. In previous studies, it has been demonstrated that the use of minimally invasive screw fixation reduces surgery-related complications and yields better clinical outcomes [33, 34].
Nuances encountered in management
Positioning: When managing AS patients with a lumbar translational fracture, meticulous attention to positioning is crucial. Many of these individuals exhibit an inherent kyphosis coupled with an extension-type mechanism of the injury. Positioning a thoracolumbar fracture patient with AS in a prone position on a conventional orthopedic table may result in exacerbated uncontrolled extension or translation of the spine within the injury zone, potentially leading to secondary neurological deterioration and vascular compromise. Surgeons should contemplate employing a frame that facilitates regulated kyphosis, such as a Wilson-type frame, for these patients [35].
Instrumentation: Technical difficulties in placing posterior screws sometimes arise from the lack of posterior bone markers in AS, significantly altered facet joints, substantial body size, and spinal abnormalities. Calcification and vertebral rotation change the anatomical landmarks in AS patients, complicating freehand pedicle screw implantation. AS patients encounter a significantly elevated risk of surgical intervention and postoperative complications relative to the general populace [13, 36, 37].
Bone Quality: Bone mineral density is diminished in AS [9], with a 25% incidence of osteoporosis among AS patients over the past decade [38], resulting in a significant implant loosening rate of around 10% to 15% [5, 25]. It is essential to create several anchor points by extending the construct, which may provide improved spinal stability for patients with unstable fractures [39]. Another method to augment implant fixation strength may include cement augmentation of the pedicle screws.
Preoperative issues: Patients frequently exhibit several comorbidities, particularly cardiovascular illness, which exacerbates the mortality risk after a fracture. Commonly administered pharmacological interventions can affect the timing of surgery and the intraoperative difficulties experienced by the patient, including the use of anticoagulants, anti-inflammatories, and pulmonary limitations or hypertension [40]. In these instances, blood loss may exceed typical expectations [41].
Outcome
Rustagi et al. reported that mortality rates one year post-injury varied between 0% and 32%. Complications such as pneumonia, respiratory failure, and pseudoarthrosis occurred in 84% of patients. Neurologic deterioration occurred in 16% of patients, whereas successful fusion rates ranged from 87% to 100%. At the final follow-up, 6% to 66% of patients exhibited improvement in neurologic deficits [29]. Mortality rates were significantly lower in thoracic fractures compared to cervical fractures, while lumbar spine fractures showed no significant difference [25].
The prognosis for patients with ankylosing spine injuries is significantly affected by the status of their neurologic injuries and the severity of their medical comorbidities. Patients with fused spinal columns typically exhibit markedly elevated rates of morbidity and mortality within one year relative to those with non-fused spinal columns. Indications suggest that patients undergoing surgical treatment exhibit longer and improved survival rates compared to those receiving non-surgical intervention for AS fracture-dislocations [29].
5. Conclusion
Lumbar spine fractures in individuals with AS can occur even after minor trauma and may present with minimal symptoms, such as pain. It is crucial to consider this diagnosis. While non-displaced fractures may be treated without surgery, evidence suggests that thoracolumbar fractures in patients with AS may benefit from surgical intervention over non-surgical methods. However, it is important to note that surgery for these individuals requires special considerations, as detailed in our review. The outcome of these types of fractures is significantly influenced by the patient’s neurological injury status and the severity of their medical comorbidities. Favorable results from surgery are often observed, as in our case.
Ethical Considerations
Compliance with ethical guidelines
The Institutional Ethics Committee waived the need for ethical approval for this case report, as it was considered part of standard patient care. The patient gave both verbal and written informed consent for the use of his clinical data and images in this report. The manuscript and images do not reveal the patient’s identity.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Review literature and composition and the execution of the surgery: Mohsen Rostami and Faramarz Roohollahi; Critical analysis: Saeid Ehteshami and Mohammad Jafari; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Charles YP, Buy X, Gangi A, Steib JP. Fracture in ankylosing spondylitis after minor trauma: Radiological pitfalls and treatment by percutaneous instrumentation. A case report. Orthopaedics & Traumatology, Surgery & Research. 2013; 99(1):115-9. [DOI:10.1016/j.otsr.2012.09.018] [PMID]
- Feldtkeller E, Vosse D, Geusens P, van der Linden S. Prevalence and annual incidence of vertebral fractures in patients with ankylosing spondylitis. Rheumatology International. 2006; 26(3):234-9. [DOI:10.1007/s00296-004-0556-8] [PMID]
- Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: A systematic review of the literature on treatment, neurological status and complications. European Spine Journal. 2009; 18(2):145-56. [DOI:10.1007/s00586-008-0764-0] [PMID]
- Weinstein PR, Karpman RR, Gall EP, Pitt M. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. Journal of Neurosurgery. 1982; 57(5):609-16. [DOI:10.3171/jns.1982.57.5.0609] [PMID]
- Backhaus M, Citak M, Kälicke T, Sobottke R, Russe O, Meindl R, et al. [Spine fractures in patients with ankylosing spondylitis: An analysis of 129 fractures after surgical treatment (German)]. Orthopade. 2011; 40(10):917-22. [DOI:10.1007/s00132-011-1792-8] [PMID]
- Hitchon PW, From AM, Brenton MD, Glaser JA, Torner JC. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. Journal of Neurosurgery. 2002; 97(2 Suppl):218-22. [DOI:10.3171/spi.2002.97.2.0218] [PMID]
- Fatemi G, Gensler LS, Learch TJ, Weisman MH. Spine fractures in ankylosing spondylitis: A case report and review of imaging as well as predisposing factors to falls and fractures. Seminars in Arthritis and Rheumatism. 2014; 44(1):20-4. [DOI:10.1016/j.semarthrit.2014.03.007] [PMID]
- Kim JW, Park S, Jung JY, Kim HA, Kwon SR, Choi ST, et al. Prevalence and factors of osteoporosis and high risk of osteoporotic fracture in patients with ankylosing spondylitis: A multicenter comparative study of bone mineral density and the fracture risk assessment tool. Journal of Clinical Medicine. 2022; 11(10):2830. [DOI:10.3390/jcm11102830] [PMID]
- van der Weijden MA, van der Horst-Bruinsma IE, van Denderen JC, Dijkmans BA, Heymans MW, Lems WF. High frequency of vertebral fractures in early spondylarthropathies. Osteoporosis International. 2012; 23(6):1683-90. [DOI:10.1007/s00198-011-1766-z] [PMID]
- Hu X, Thapa AJ, Cai Z, Wang P, Huang L, Tang Y, et al. Comparison of smith-petersen osteotomy, pedicular subtraction osteotomy, and poly-segmental wedge osteotomy in treating rigid thoracolumbar kyphotic deformity in ankylosing spondylitis a systematic review and meta-analysis. BMC Surgery. 2016; 16:4. [DOI:10.1186/s12893-015-0118-x] [PMID]
- Tu PH, Liu ZH, Yeap MC, Liu YT, Li YC, Huang YC, et al. Spinal cord injury and spinal fracture in patients with ankylosing spondylitis. BMC Emergency Medicine. 2022; 22(1):73. [DOI:10.1186/s12873-022-00635-3] [PMID]
- Zhu R, Song W, Hu W, Jiang Z, Yuan J, Cui Z, et al. The treatment strategies for spine fractures in patients with ankylosing spondylitis: A case report. Medicine. 2017; 96(44):e8462. [DOI:10.1097/MD.0000000000008462] [PMID]
- Chaudhary SB, Hullinger H, Vives MJ. Management of acute spinal fractures in ankylosing spondylitis. ISRN Rheumatology. 2011; 2011:150484. [DOI:10.5402/2011/150484] [PMID]
- Allouch H, Shousha M, Böhm H. [Surgical management of ankylosing spondylitis (Bechterew's disease) (German)]. Zeitschrift fur Rheumatologie. 2017; 76(10):848-59. [DOI:10.1007/s00393-017-0400-7] [PMID]
- Sapkas G, Kateros K, Papadakis SA, Galanakos S, Brilakis E, Machairas G, et al. Surgical outcome after spinal fractures in patients with ankylosing spondylitis. Bmc Musculoskeletal Disorders. 2009; 10:96. [DOI:10.1186/1471-2474-10-96] [PMID]
- Qiao M, Qian BP, Qiu Y, Mao SH, Wang YH. Radiologic and pathological investigation of pseudarthrosis in ankylosing spondylitis: Distinguishing between inflammatory and traumatic etiology. The Journal of Rheumatology. 2019; 46(3):259-65. [DOI:10.3899/jrheum.171249] [PMID]
- Jacobs WB, Fehlings MG. Ankylosing spondylitis and spinal cord injury: Origin, incidence, management, and avoidance. Neurosurgical Focus. 2008; 24(1):E12. [DOI:10.3171/FOC/2008/24/1/E12] [PMID]
- Jurik AG. Imaging the spine in arthritis-A pictorial review. Insights Into Imaging. 2011; 2(2):177-91. [DOI:10.1007/s13244-010-0061-4] [PMID]
- Hendrix RW, Melany M, Miller F, Rogers LF. Fracture of the spine in patients with ankylosis due to diffuse skeletal hyperostosis: Clinical and imaging findings. AJR. American Journal of Roentgenology. 1994; 162(4):899-904. [DOI:10.2214/ajr.162.4.8141015] [PMID]
- Liu R, Sun L, Li CH, Zhai JY, Liu XY. Cause analysis of spinal surgery in ankylosing spondylitis. Journal of Peking University. Health Sciences. 2017; 49(5):835-9. [PMID]
- Werner BC, Samartzis D, Shen FH. Spinal fractures in patients with ankylosing spondylitis: Etiology, diagnosis, and management. The Journal of the American Academy of Orthopaedic Surgeons. 2016; 24(4):241-9. [DOI:10.5435/JAAOS-D-14-00149] [PMID]
- Stenhouse G, Ulbricht C, Khanna M. Spinal injury in ankylosing spondylitis. BMJ. 2014; 348:g3849. [DOI:10.1136/bmj.g3849] [PMID]
- Westerveld LA, van Bemmel JC, Dhert WJ, Oner FC, Verlaan JJ. Clinical outcome after traumatic spinal fractures in patients with ankylosing spinal disorders compared with control patients. The spine Journal. 2014; 14(5):729-40. [DOI:10.1016/j.spinee.2013.06.038] [PMID]
- Robinson Y, Willander J, Olerud C. Surgical stabilization improves survival of spinal fractures related to ankylosing spondylitis. Spine. 2015; 40(21):1697-702. [DOI:10.1097/BRS.0000000000001115] [PMID]
- Caron T, Bransford R, Nguyen Q, Agel J, Chapman J, Bellabarba C. Spine fractures in patients with ankylosing spinal disorders. Spine. 2010; 35(11):E458-64. [DOI:10.1097/BRS.0b013e3181cc764f] [PMID]
- Lu ML, Tsai TT, Lai PL, Fu TS, Niu CC, Chen LH, et al. A retrospective study of treating thoracolumbar spine fractures in ankylosing spondylitis. European Journal of Orthopaedic Surgery & Traumatology. 2014; 24(Suppl 1):S117-23. [DOI:10.1007/s00590-013-1375-y] [PMID]
- Lukasiewicz AM, Bohl DD, Varthi AG, Basques BA, Webb ML, Samuel AM, et al. Spinal fracture in patients with ankylosing spondylitis: Cohort definition, distribution of injuries, and hospital outcomes. Spine. 2016; 41(3):191-6. [DOI:10.1097/BRS.0000000000001190] [PMID]
- Shen FH, Samartzis D. Successful nonoperative treatment of a three-column thoracic fracture in a patient with ankylosing spondylitis: Existence and clinical significance of the fourth column of the spine. Spine. 2007; 32(15):E423-7. [DOI:10.1097/BRS.0b013e318074d59f] [PMID]
- Rustagi T, Drazin D, Oner C, York J, Schroeder GD, Vaccaro AR, et al. Fractures in spinal ankylosing disorders: A narrative review of disease and injury types, treatment techniques, and outcomes. Journal of Orthopaedic Trauma. 2017; 31(Suppl 4):S57-74. [DOI:10.1097/BOT.0000000000000953] [PMID]
- Leroux JL, Legeron P, Moulinier L, Laroche M, Mazieres B, Blotman F, et al. Stenosis of the lumbar spinal canal in vertebral ankylosing hyperostosis. Spine. 1992; 17(10):1213-8. [DOI:10.1097/00007632-199210000-00014] [PMID]
- Blondel B, Fuentes S, Pech-Gourg G, Adetchessi T, Tropiano P, Dufour H. Percutaneous management of thoracolumbar burst fractures: Evolution of techniques and strategy. Orthopaedics & Traumatology. 2011; 97(5):527-32. [DOI:10.1016/j.otsr.2011.03.020] [PMID]
- Charles YP, Zairi F, Vincent C, Fuentes S, Bronsard N, Court C, et al. Minimally invasive posterior surgery for thoracolumbar fractures. New trends to decrease muscle damage. European Journal of Orthopaedic Surgery & Traumatology. 2012; 22:1-7. [DOI:10.1007/s00590-011-0781-2]
- Yeoh D, Moffatt T, Karmani S. Good outcomes of percutaneous fixation of spinal fractures in ankylosing spinal disorders. Injury. 2014; 45(10):1534-8. [DOI:10.1016/j.injury.2014.03.020] [PMID]
- Bredin S, Fabre-Aubrespy M, Blondel B, Falguières J, Schuller S, Walter A, et al. Percutaneous surgery for thoraco-lumbar fractures in ankylosing spondylitis: Study of 31 patients. Orthopaedics & Traumatology, Surgery & Research. 2017; 103(8):1235-9. [DOI:10.1016/j.otsr.2017.07.023] [PMID]
- Whang PG, Goldberg G, Lawrence JP, Hong J, Harrop JS, Anderson DG, et al. The management of spinal injuries in patients with ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis: A comparison of treatment methods and clinical outcomes. Journal of Spinal Disorders & Techniques. 2009; 22(2):77-85. [DOI:10.1097/BSD.0b013e3181679bcb] [PMID]
- El Tecle NE, Abode-Iyamah KO, Hitchon PW, Dahdaleh NS. Management of spinal fractures in patients with ankylosing spondylitis. Clinical Neurology and Neurosurgery. 2015; 139:177-82. [DOI:10.1016/j.clineuro.2015.10.014] [PMID]
- Wang Y, Zhang Y, Mao K, Zhang X, Wang Z, Zheng G, et al. Transpedicular bivertebrae wedge osteotomy and discectomy in lumbar spine for severe ankylosing spondylitis. Journal of Spinal Disorders & Techniques. 2010; 23(3):186-91. [DOI:10.1097/BSD.0b013e3181a5abde] [PMID]
- Davey-Ranasinghe N, Deodhar A. Osteoporosis and vertebral fractures in ankylosing spondylitis. Current Opinion in Rheumatology. 2013; 25(4):509-16. [DOI:10.1097/BOR.0b013e3283620777] [PMID]
- Blondel B, Fuentes S, Tropiano P, Roche P, Métellus P, Dufour H. Systems for long-segment percutaneous spinal fixation: Technical feasibility for various indications. Acta Neurochirurgica. 2011; 153(5):985-91. [DOI:10.1007/s00701-011-0976-7] [PMID]
- Kiltz U, Sieper J, Braun J. [Development of morbidity and mortality in patients with spondyloarthritis (German)]. Zeitschrift fur Rheumatologie. 2011; 70(6):473-9. [DOI:10.1007/s00393-011-0757-y] [PMID]
- Tetzlaff JE, Yoon HJ, Bell G. Massive bleeding during spine surgery in a patient with ankylosing spondylitis. Canadian Journal of Anaesthesia. 1998; 45(9):903-6. [DOI:10.1007/BF03012228] [PMID]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |