Thu, Jan 29, 2026
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Singh M, Raghav A, Kumar S, Amresh Gautam K, Kumar P. Pediatric Head Injury: Analysis, Prognostic Markers, and Its Outcomes. Iran J Neurosurg 2025; 11 : 18
URL: http://irjns.org/article-1-471-en.html
URL: http://irjns.org/article-1-471-en.html
1- Department of Neurosurgery, GSVM Medical College, Kanpur, India. , manishsinghneurosurgery@gmail.com
2- Department of Anatomy and Cell Biology, College of Medicine, Gachon University, Incheon, South Korea. & University Center for Research & Development (UCRD), Chandigarh University, Mohali, India.
3- Department of Biosciences, GD Goenka University, Gurugram, India.
4- Department of Neurosurgery, GSVM Medical College, Kanpur, India.
2- Department of Anatomy and Cell Biology, College of Medicine, Gachon University, Incheon, South Korea. & University Center for Research & Development (UCRD), Chandigarh University, Mohali, India.
3- Department of Biosciences, GD Goenka University, Gurugram, India.
4- Department of Neurosurgery, GSVM Medical College, Kanpur, India.
Keywords: Pediatric, Traumatic brain injury (TBI), Glasgow coma scale (GCS), Pupillary reaction, Injury outcome, Computed tomography (CT)
Full Text [PDF 1135 kb]
(687 Downloads)
| Abstract (HTML) (1185 Views)
Full Text: (233 Views)
Introduction
Traumatic brain injury (TBI) refers to an abnormal change in brain function or anatomy caused by an external force that significantly contributes to the brain injury. According to the Centers for Disease Control and Prevention (CDC) estimates (2002-2006), pediatric TBI caused an average of 2174 deaths per year, 35136 hospitalizations per year, and 473947 emergency department visits in the United States (age 0-14 years) [1]. TBI is a major public health issue among children, causing over 7000 fatalities, approximately 60000 hospital admissions, and nearly 600000 visits to emergency departments each year in the United States alone [2]. This pattern is consistent globally, with TBI significantly impacting pediatric populations across various countries. Research indicates that TBI accounts for over 50% of childhood injuries in Iran, constitutes about one-fifth of trauma-related emergency visits in India, and is responsible for nearly 30% of pediatric trauma cases in Korea [3]. In addition, epidemiological data show that in Australia, TBI affects more than 486 adolescents per 100000 individuals annually, while in the United Kingdom, the incidence is approximately 280 cases per 100000 children. Severe TBI in children is a major cause of morbidity and mortality worldwide [4]. The most common type of injury is a fall, followed by a motor vehicle accident [5]. Child abuse is also a major cause of head trauma in children under the age of two. The percentage of each contributing factor varies across studies, and the distribution varies by age group and gender. Because they rely on adults, infants and young children are more vulnerable to abuse [6, 7].
Considering this emerging need to quantify and describe the clinic-epidemiologic profile of TBI in Indian children, we retrospectively collated the details of children who attended to Trauma Center, GSVM Medical College, Kanpur, India, over 2 years.
2. Methods and Materials/Patients
After obtaining the ethics approval from the Institute Ethics Committee, GSVM Medical College, Kanpur in India, the electronic records were accessed for information on children aged 0-14 years who presented to a tertiary care center with suspected head injury. TBI subjects (n=165) aged up to 14 years managed in the Department of Neurosurgery, GSVM Medical College, Kanpur, were enrolled over the period of 2 years in the present study. The study participants were assessed based on the predetermined proforma. A thorough history of the patients was obtained (including biodata, age, and mode of injury). Patients underwent a thorough general physical examination, systemic examination, and central nervous system examination, which included Glasgow coma scale (GCS), pupil size, and reaction. The patients were divided into three categories based on GCS as follows: Mild head injury (GCS 13-15), moderate head injury (GCS 9-12), and severe head injury (GCS 8). All patients had a plain computed tomography (CT) scan head, and CT findings were recorded. Following the initial resuscitation and workup, the patients were managed conservatively or surgically, depending on the indications. All these patients’ outcomes were graded using the Glasgow outcome scale (GOS) and classified as good (normal, moderate disability) or poor (severe, vegetative, dead).
The outcome was evaluated using age, gender, GCS, pupil size and reaction, CT scan features, intervention, and associated injuries. Multivariate logistic regression analysis of study variables was performed to identify independent prognostic factors.
Results
A total of 165 TBI patients aged up to 14 years, with a Mean±SD age of 9.22±3.16 years. Among recruited study participants, 114(69.09%) patients were males, and 51(30.90%) patients were females, with a male‑to‑female ratio of 2.24:1. Table 1 demonstrated the demographic and injury characteristics of study participants.
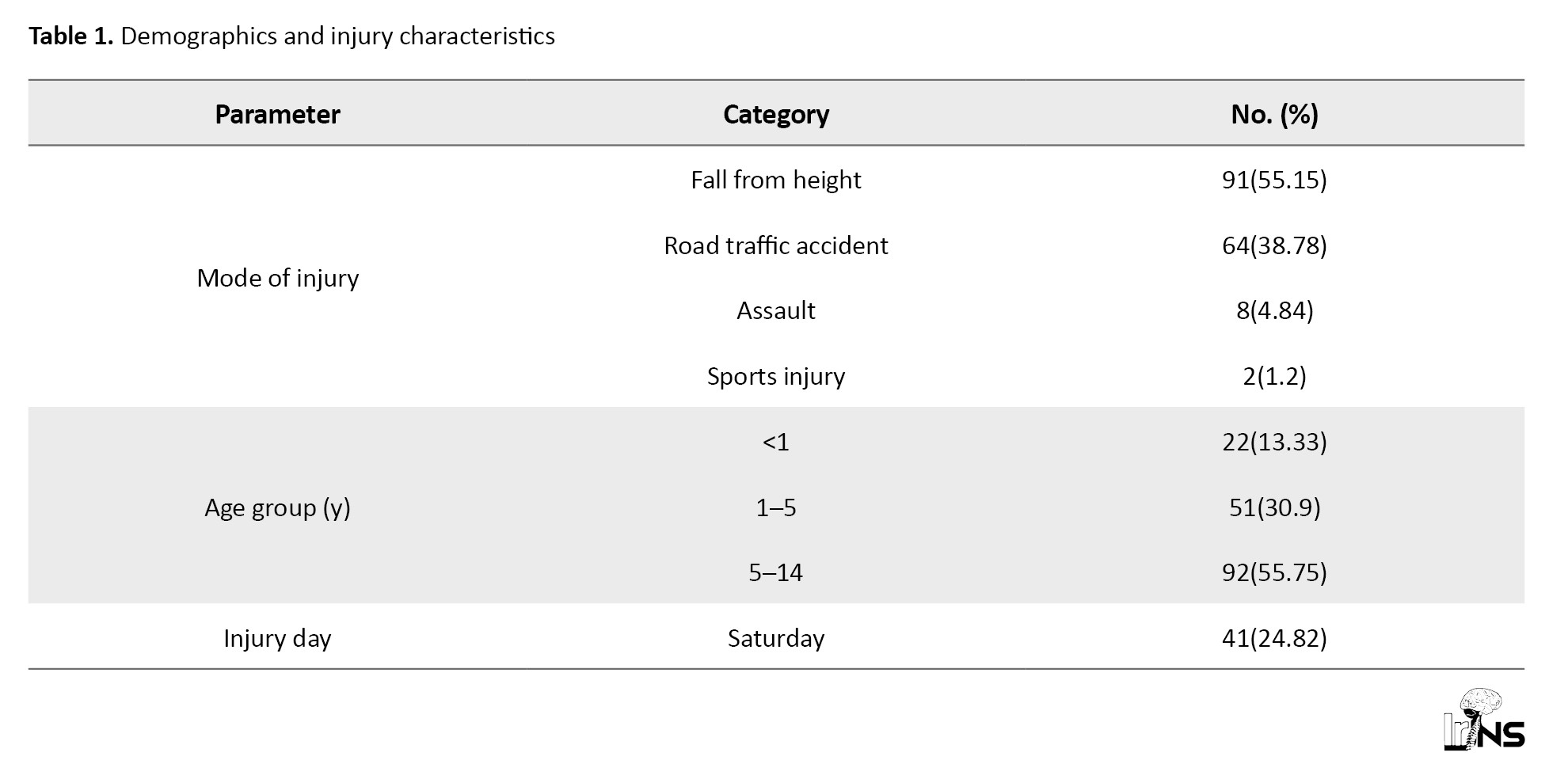
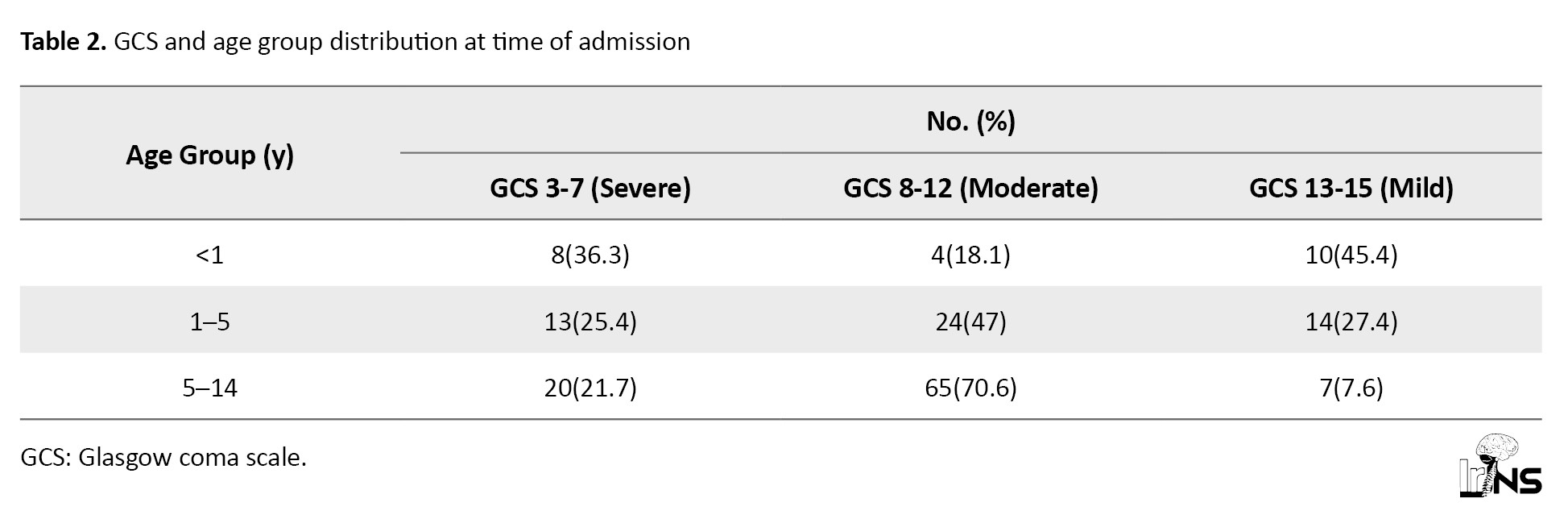
Among 165 pediatric TBI cases analyzed, the most common mechanism of injury was falls (55.15%), followed by road traffic accidents (38.78%), assaults (4.84%), and sports-related injuries (1.2%). Males were predominantly affected across all injury types. Children aged 5–14 years constituted the largest age group (55.75%), followed by 1–5 years (30.90%) and infants <1 year (13.33%). The male-to-female ratio increased with age, from 1.8:1 in subjects under 5 to 3.38:1 in those above 5 years. Injuries were most frequently reported on Saturdays. Injury severity, based on GCS, was categorized as mild in 18.78%, moderate in 56.33%, and severe in 24.84% of cases (Table 2).
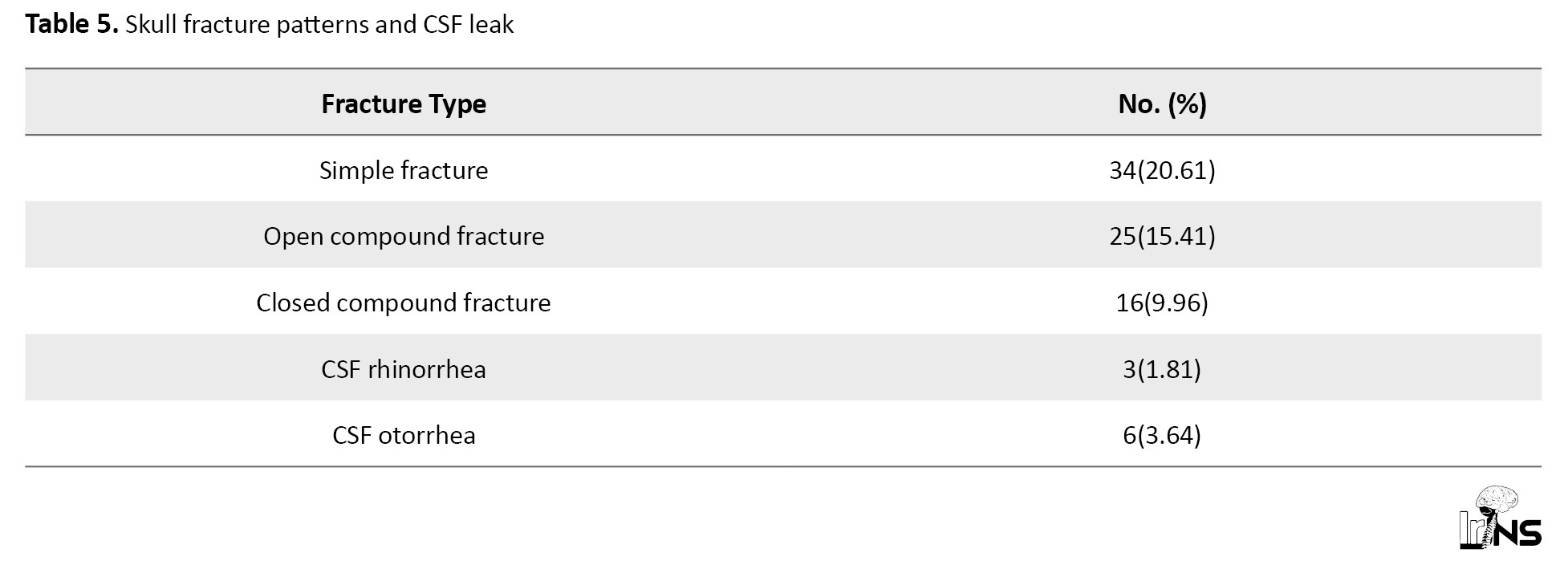
Systemic injuries were present in 20% of patients, commonly involving the chest, maxillofacial region, long bones, abdomen, and spine. A simple-to-compound skull fracture ratio of 1.36:1 was observed, and cases of cerebrospinal fluid (CSF) rhinorrhea and otorrhea often included blood admixture.
Outcomes were favorable in 65.6% of conservatively managed patients. Mortality was highest among infants (<1 year, 18.18%) and patients with severe TBI (GCS 3–7, mortality 36.53%) (Table 3).

Pupillary response at admission was a strong prognostic indicator: Accordingly, 75% of patients with abnormal reactivity had poor outcomes, compared to only 2.8% with normal reactivity (Table 4).
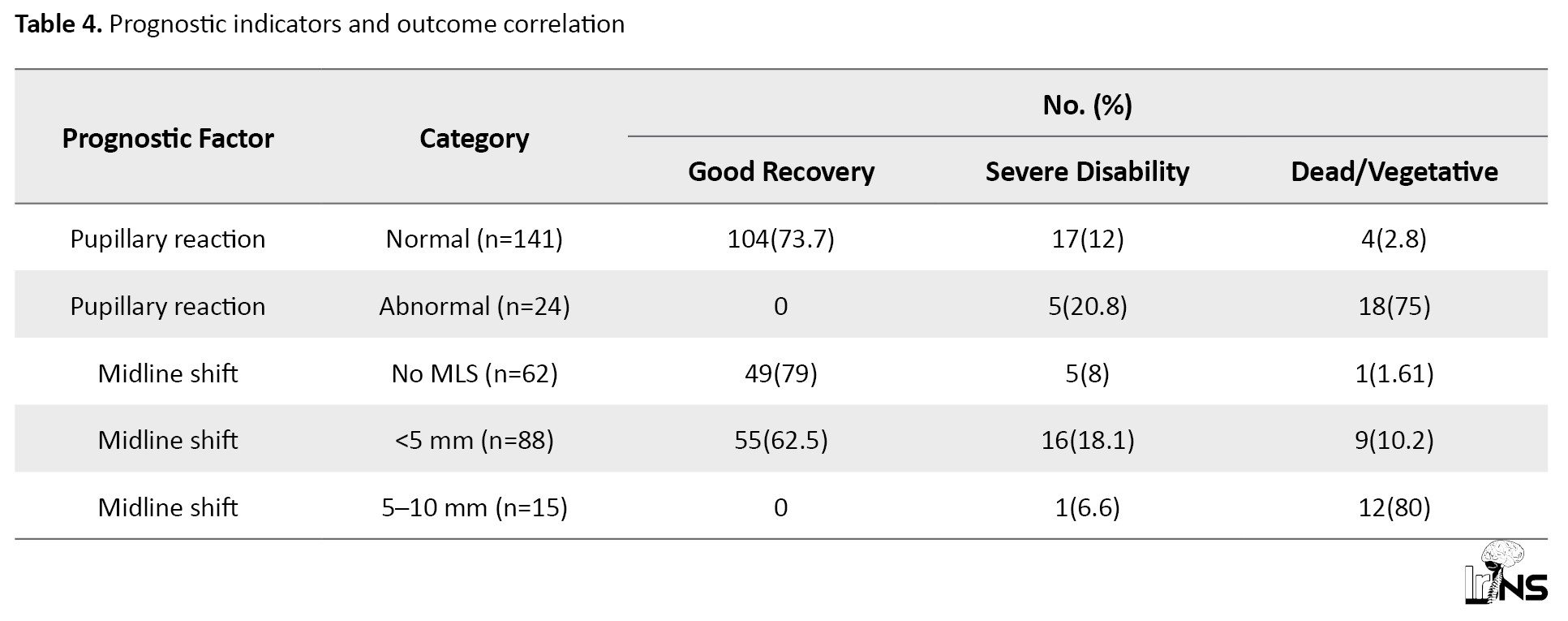
A midline shift >5 mm on CT was associated with an 80% poor outcome rate. Pathologically, patients with both intra- and extra-axial hemorrhages, especially when combined with intraventricular hemorrhage, had significantly worse outcomes. In contrast, isolated extradural hematomas had a favorable prognosis (94.4% recovery; Table 4). The study identified 34 cases (20.61%) of simple skull fractures, 25 cases (15.41%) of open compound fractures, and 16 cases (9.96%) of closed compound fractures. CSF leaks were less common, with 3 cases (1.81%) of CSF rhinorrhea and 6 cases (3.64%) of CSF otorrhea. These findings highlight the prevalence of simple fractures as the most frequent skull fracture type, while CSF leaks were relatively rare complications (Table 5).
Operative treatment was administered to 8 patients, with 50% experiencing poor outcomes (dead/vegetative state), while conservative management was applied to 157 patients, yielding better results (65.6% good recovery). Age-wise analysis demonstrated the highest good recovery rates in the 1–5 years age group (66.67%), followed by the 5–14 years group (61.96%) and the <1 year group (55.09%). Severe disability and mortality were more prevalent in the operative group and the youngest age group (<1 year; Table 6).
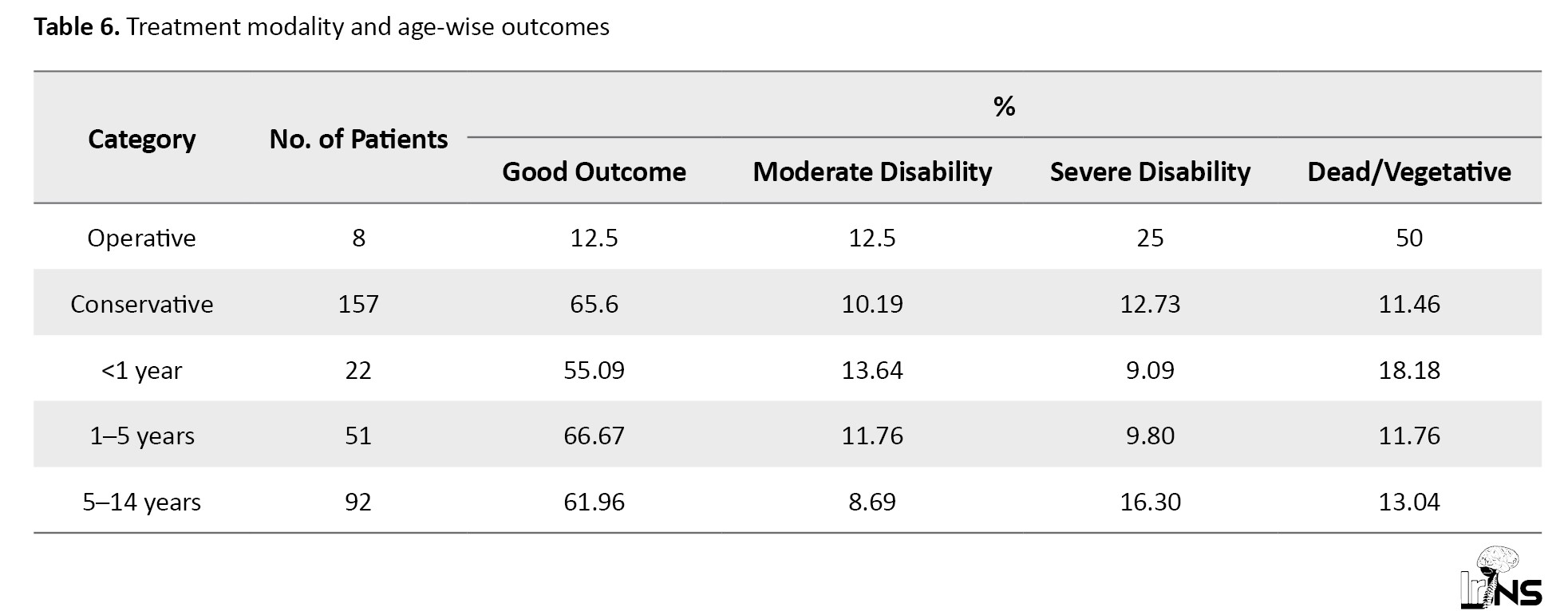
Normal pupillary reaction was strongly associated with good recovery (73.7%), whereas abnormal pupillary reaction correlated with poor outcomes (75% dead/vegetative). Midline shift also influenced outcomes: Patients with no midline shift had the highest good recovery rate (79%), while those with a 5–10 mm shift had the worst prognosis (80% dead/vegetative). These indicators underscore the critical role of neurological and imaging findings in predicting patient outcomes (Table 7).
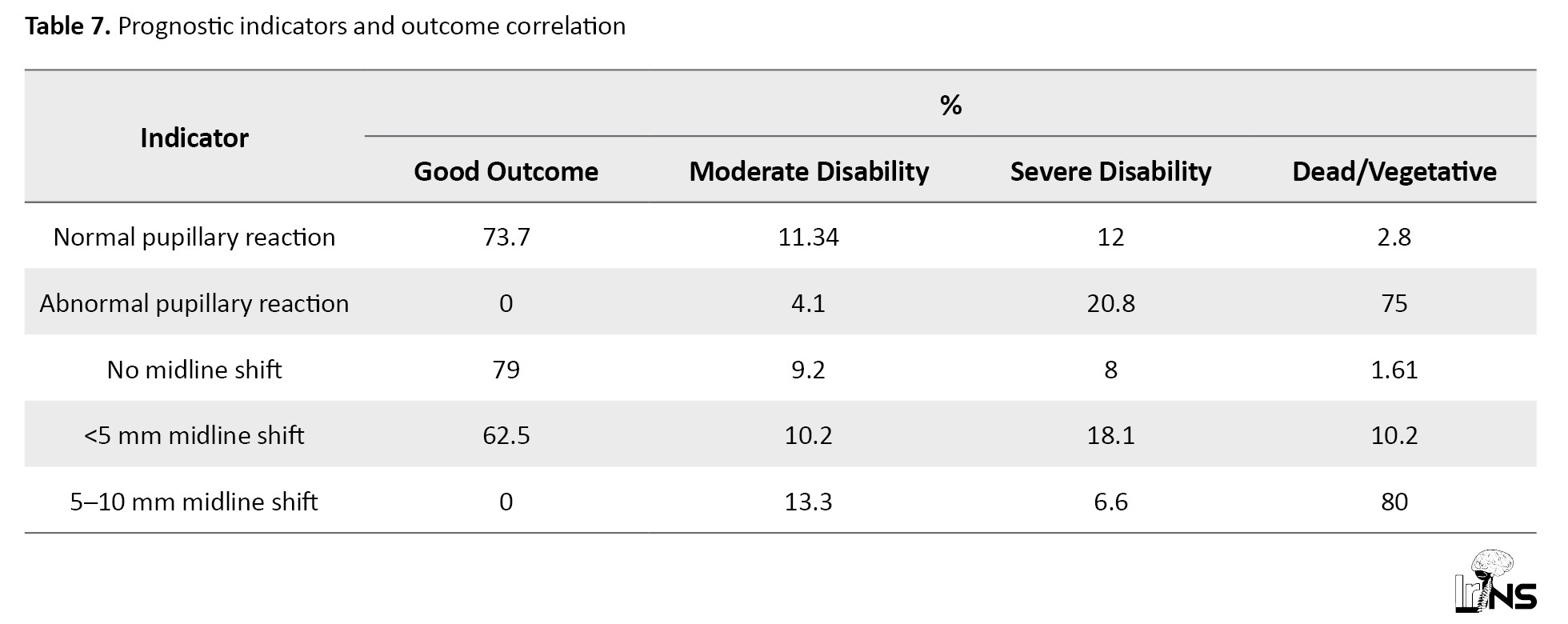
Table 8 reiterates the distribution of skull fractures and CSF leaks, mirroring the data in Table 5.
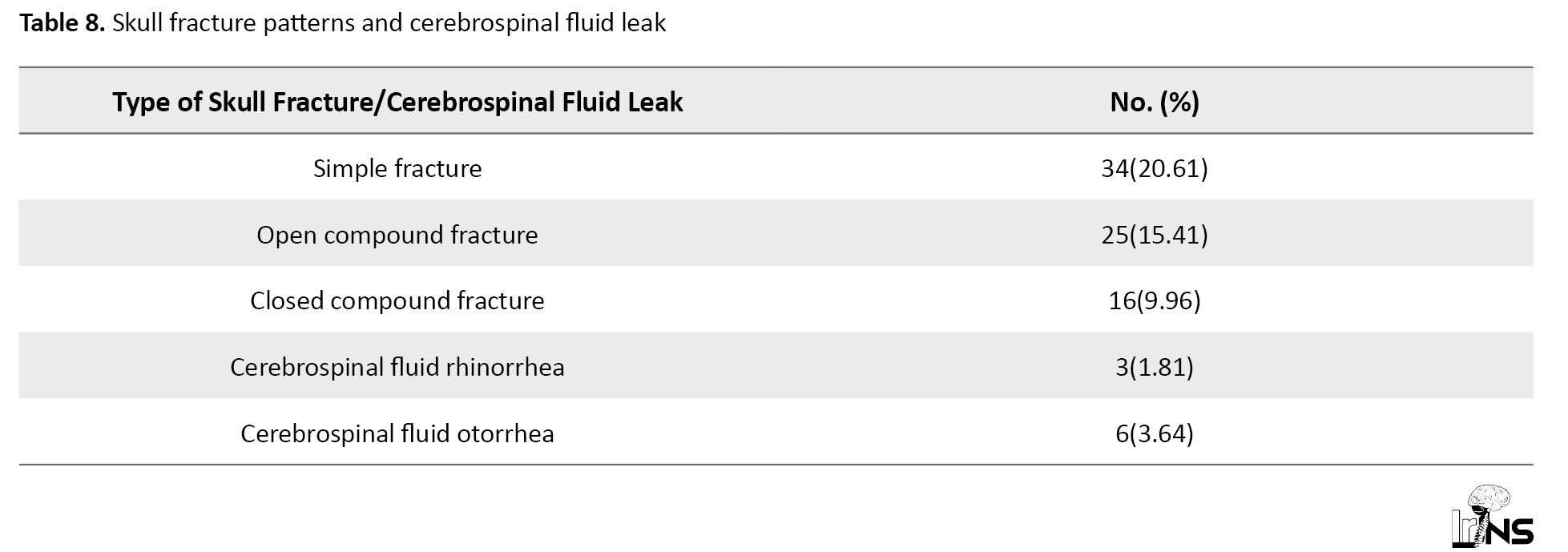
Simple fractures were the most common (20.61%), followed by open compound fractures (15.41%) and closed compound fractures (9.96%). CSF leaks remained rare, with rhinorrhea and otorrhea occurring in 1.81% and 3.64% of cases, respectively. Extra-axial injuries like epidural hematoma had the highest good recovery rate (94.4%), while subdural hematoma with contusion showed the worst outcomes (35.2% good recovery, 35.2% dead/vegetative). Diffuse axonal injury and isolated epidural hematoma were associated with favorable outcomes (84.6% and 94.4% good recovery, respectively). Intraventricular hemorrhage had the highest mortality rate (50%). Combined injuries (e.g. subdural hematoma + contusion) were linked to poorer prognoses compared to isolated injuries. Overall, 63.3% of patients achieved good recovery, while 13.3% had severe disability or died (Table 9).
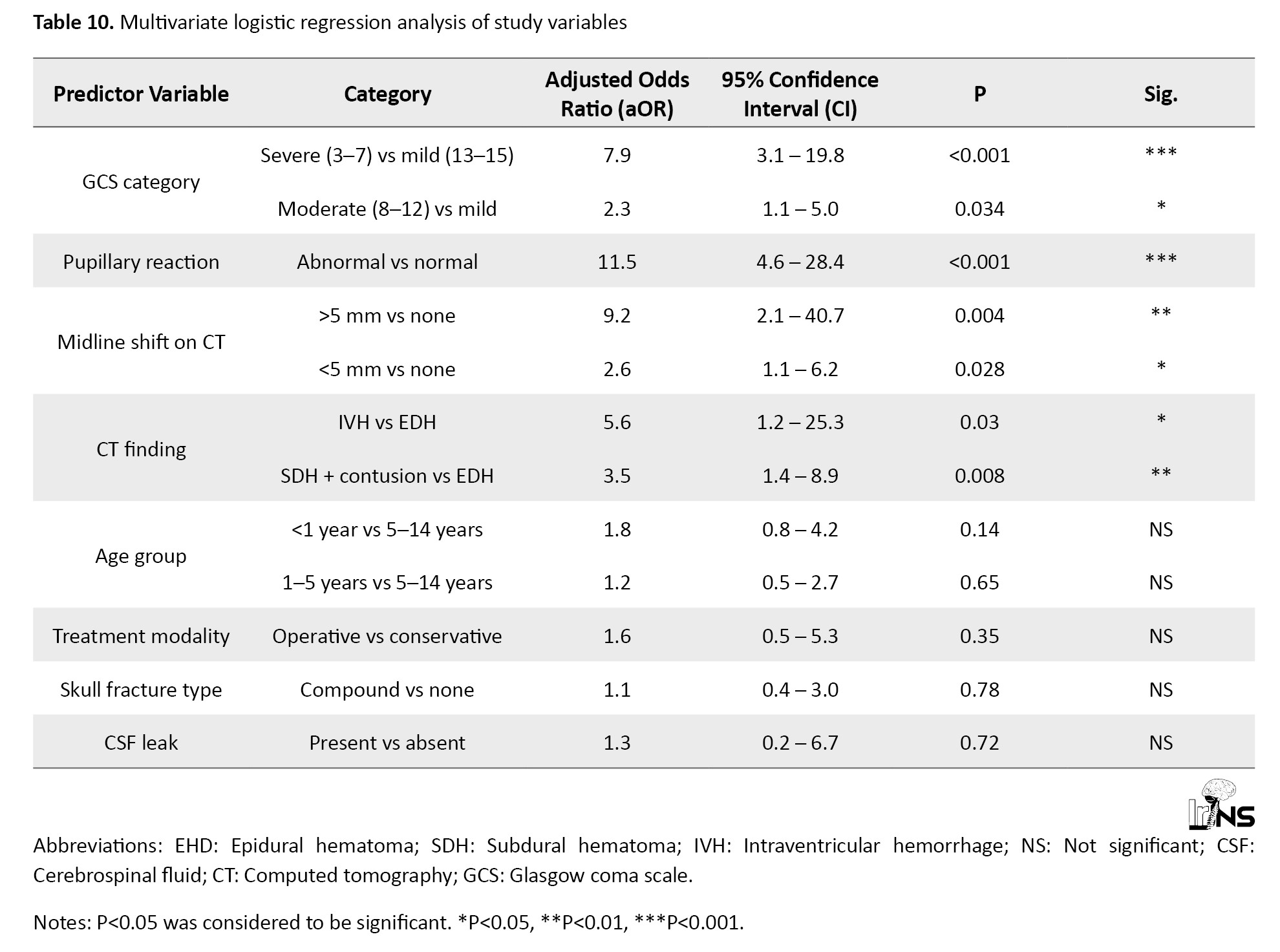
Table 10 demonstrates the multivariate regression analysis of the study variables to identify the independent prognostic factors.
Discussion
Although the outlook for TBI has significantly improved in the developed world due to the development of highly specialised intensive care units and a high degree of interdisciplinary approach, it still presents a significant challenge for the neurosurgery units in our region of the world. Like adult TBIs, juvenile TBIs can happen for a variety of reasons and typically have a positive prognosis. These injuries are typically not isolated, are linked to polytrauma, and are treated mostly following how adults are handled [8-11]. Although this is a significant retrospective study on paediatric TBI, it has a few flaws, such as the lack of availability of details, such as mode of injury, clinical details, and so on, which were only available for admitted patients. There was no information available about post-traumatic seizures, hospitalisation, post-traumatic epilepsy, or long-term neurologic outcomes. These details would have helped us understand the clinic-epidemiologic picture of paediatric TBI in India.
The highest numbers of pediatric head injuries were reported on Saturday. The higher incidence of pediatric head injuries on weekends can be attributed to the fact that primary and middle schools are not functional on weekends in India which make this age group of 5-14 years more susceptible to injuries especially fall from height as they tend to spend more time in indoor or outdoor play activities like running, cycling, jumping etc., which might not be supervised by their caregivers making them prone to head injuries or any other injury. This age group also becomes more vulnerable to head injuries due to risk-taking behavior without parents’ consent or supervision.
When defining the age at which prognosis materially worsens, there are differences in the literature. For instance, there has been debate over the paediatric age range. While some research claims that children under the age of 10 tend to have better outcomes [12, 13], others claim that children under the age of five have a greater mortality rate [14]. As reported by Suresh et al., there was no difference in poor outcomes in children under 5 years old or older than 5 years in our series; however, there was a somewhat higher poor outcome in children over 12 years that was not statistically significant [15]. There has been debate concerning the significance of age as a prognostic indicator. According to Luersson et al. [16] and Braakman et al. [17], age is the main cause of mortality and morbidity. These studies compare adults with children, despite the literature’s assertion that age is a stronger predictor of mortality and morbidity in severe head trauma. In our study, only children were compared, and all types of traumas were considered.
In the present study, the most common mode of injury was falls, 91(55.15%), followed by road traffic accidents 64(38.78%), assault 8(4.84%), and sports 2(1.2%). Previous study have found that falls from a height are among the major causes of head injury in young children compared to older children, where falls are relatively less common causes of head injury despite bicycle accidents, recreational activities, and being struck by a big vehicle [18]. The results of the outcome after treatment are the GOS from a previously published study [19].
The initial GCS score was identified as the single most critical factor determining outcome (P<0.0001) by Beca et al. [20] and Kuday [21]. According to Astrand et al., negative outcomes were observed in GCS 14–15 at 0%, 9–13 at 6.2%, and 8 at 22% [22]. Low GCS did not always reliably predict the outcome in the absence of hypoxia or ischemia, according to Ong et al. [23]. We discovered a sizable effect of GCS on the result in our series. Due to a lack of pre-hospital resuscitation and late presentation to the hospital, developing and underdeveloped communities like ours have worse outcomes.
Pupillary reaction reflects the condition of brain stem compression in a head injury patient. If a patient with TBI has an abnormal pupil, it shows the brain stem is compressed, and the prognosis of the patient is poor. Marmarou et al. [25] in their study concluded that pupillary reaction was an independent parameter to determine the prognosis of patients, they reported that pupillary reactivity was a stable parameter in the early phase of TBI in comparison to the GCS score because it is less affected by sedation and paralysis [24-26].
Toutant et al. conducted a prospective investigation on the absence or compression of basal cisterns on initial CT scan: Concerning indicators of prognosis in severe head injury. Patients without a basal cistern died at a rate of 77%, those with a compressed basal cistern died at a rate of 39%, and those with normal basal cisterns died at a rate of 22%. Midline shift (MLS) of total isolated severe traumatic head injury patients correlated with their GOS extended (GOSE). Most of the patients had MLS >5 mm (553 patients). Out of 553 patients, most patients showed GOSE 1 (83.36%), followed by GOSE 7 (7.23%) and GOSE 8 (2.53%). Statistically, a significant association was observed (P<0.0001) between MLS with outcomes and GOSE [26]. This study supports previous studies showing an increase in mortality with the increase in MLS more than 5 mm.
Around the world, TBI is a significant factor in both death and disability in children and young people [24]. The mechanism, clinical symptoms, and consequences following TBI are still poorly known, even though the condition is on the rise. There has not been much research examining TBI among children, particularly those from the Indian subcontinent, even though they make up a sizable portion of the population who are at risk. Children have a special vulnerability that may be caused by a predisposition to injury, mechanical characteristics like a large head and weak neck muscles, and neurobiological variables like biochemical and molecular pathways important for brain maturation that may further harm the growing brain. Multivariate analysis confirms that neurological status at presentation (GCS, pupil reactivity) and CT imaging findings (midline shift, intraventricular hemorrhage, combined injuries) are independent and statistically significant predictors of poor outcomes in paediatric TBI. These variables should be prioritized in triage, risk stratification, and early clinical decision-making. Demographic variables like age and structural injuries, such as fractures, had no independent prognostic value after adjustment.
Conclusion
Paediatric TBI is a major public health concern with significant clinical and socioeconomic implications. Effective management requires early recognition of prognostic indicators such as level of consciousness and pupillary response, which play a vital role in outcome prediction and clinical decision-making. Timely neuroimaging and appropriate triage are essential for risk stratification and intervention. Beyond acute care, long-term rehabilitation and follow-up are crucial to address the neurodevelopmental impact of TBI in children. The study highlights the importance of integrating standardized neurological assessments and imaging protocols into routine care pathways for paediatric head injury. From a preventive standpoint, public health efforts must focus on education, caregiver awareness, road safety, and childproofing environments to minimize injury risk. Strengthening healthcare infrastructure, especially in resource-limited settings, is also necessary to ensure access to emergency care and rehabilitation services. A multidisciplinary, evidence-based approach is needed to reduce TBI-related mortality and disability in children. Future research should emphasize longitudinal outcomes and the effectiveness of early interventions, contributing to more comprehensive care models. In summary, paediatric TBI demands coordinated clinical, community, and policy responses to mitigate its burden and improve the quality of life for affected children and their families.
Limitations
As the study was retrospective and based solely on hospital records of admitted patients, long-term outcomes, such as neurocognitive function, behavioral sequelae, and post-traumatic seizure or epilepsy data, were not available. These parameters require prospective follow-up and structured neuropsychological assessment, which were beyond the scope and design of this study. We agree that evaluating such outcomes is critical for understanding the full impact of pediatric TBI, and we recommend future prospective studies with long-term follow-up to address these important aspects. Only admitted cases were included, excluding milder injuries managed on an outpatient basis, which introduces selection bias. The absence of long-term follow-up prevented assessment of neurocognitive outcomes, behavioral sequelae, or post-traumatic epilepsy. Data on pre-hospital care, time to intervention, and surgical timing were unavailable. Important confounding factors, such as socioeconomic status, parental supervision, and environmental risks, were not captured. Imaging was limited to CT scans, potentially missing subtle injuries detectable on magnetic resonance imaging. These constraints highlight the need for future prospective, multicenter studies with longitudinal follow-up.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted following ethical principles outlined in the Declaration of Helsinki. Approval was obtained from the Institutional Ethics Committee of GSVM Medical College, Kanpur, India (Code: EC/248/May/2023),
Since the study was retrospective and used hospital records, patient confidentiality was strictly maintained. No personally identifiable information was disclosed. Data were anonymized before analysis to ensure privacy and compliance with ethical standards. No experimental interventions were conducted, and only routine clinical information was used.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
References
Traumatic brain injury (TBI) refers to an abnormal change in brain function or anatomy caused by an external force that significantly contributes to the brain injury. According to the Centers for Disease Control and Prevention (CDC) estimates (2002-2006), pediatric TBI caused an average of 2174 deaths per year, 35136 hospitalizations per year, and 473947 emergency department visits in the United States (age 0-14 years) [1]. TBI is a major public health issue among children, causing over 7000 fatalities, approximately 60000 hospital admissions, and nearly 600000 visits to emergency departments each year in the United States alone [2]. This pattern is consistent globally, with TBI significantly impacting pediatric populations across various countries. Research indicates that TBI accounts for over 50% of childhood injuries in Iran, constitutes about one-fifth of trauma-related emergency visits in India, and is responsible for nearly 30% of pediatric trauma cases in Korea [3]. In addition, epidemiological data show that in Australia, TBI affects more than 486 adolescents per 100000 individuals annually, while in the United Kingdom, the incidence is approximately 280 cases per 100000 children. Severe TBI in children is a major cause of morbidity and mortality worldwide [4]. The most common type of injury is a fall, followed by a motor vehicle accident [5]. Child abuse is also a major cause of head trauma in children under the age of two. The percentage of each contributing factor varies across studies, and the distribution varies by age group and gender. Because they rely on adults, infants and young children are more vulnerable to abuse [6, 7].
Considering this emerging need to quantify and describe the clinic-epidemiologic profile of TBI in Indian children, we retrospectively collated the details of children who attended to Trauma Center, GSVM Medical College, Kanpur, India, over 2 years.
2. Methods and Materials/Patients
After obtaining the ethics approval from the Institute Ethics Committee, GSVM Medical College, Kanpur in India, the electronic records were accessed for information on children aged 0-14 years who presented to a tertiary care center with suspected head injury. TBI subjects (n=165) aged up to 14 years managed in the Department of Neurosurgery, GSVM Medical College, Kanpur, were enrolled over the period of 2 years in the present study. The study participants were assessed based on the predetermined proforma. A thorough history of the patients was obtained (including biodata, age, and mode of injury). Patients underwent a thorough general physical examination, systemic examination, and central nervous system examination, which included Glasgow coma scale (GCS), pupil size, and reaction. The patients were divided into three categories based on GCS as follows: Mild head injury (GCS 13-15), moderate head injury (GCS 9-12), and severe head injury (GCS 8). All patients had a plain computed tomography (CT) scan head, and CT findings were recorded. Following the initial resuscitation and workup, the patients were managed conservatively or surgically, depending on the indications. All these patients’ outcomes were graded using the Glasgow outcome scale (GOS) and classified as good (normal, moderate disability) or poor (severe, vegetative, dead).
The outcome was evaluated using age, gender, GCS, pupil size and reaction, CT scan features, intervention, and associated injuries. Multivariate logistic regression analysis of study variables was performed to identify independent prognostic factors.
Results
A total of 165 TBI patients aged up to 14 years, with a Mean±SD age of 9.22±3.16 years. Among recruited study participants, 114(69.09%) patients were males, and 51(30.90%) patients were females, with a male‑to‑female ratio of 2.24:1. Table 1 demonstrated the demographic and injury characteristics of study participants.

Among 165 pediatric TBI cases analyzed, the most common mechanism of injury was falls (55.15%), followed by road traffic accidents (38.78%), assaults (4.84%), and sports-related injuries (1.2%). Males were predominantly affected across all injury types. Children aged 5–14 years constituted the largest age group (55.75%), followed by 1–5 years (30.90%) and infants <1 year (13.33%). The male-to-female ratio increased with age, from 1.8:1 in subjects under 5 to 3.38:1 in those above 5 years. Injuries were most frequently reported on Saturdays. Injury severity, based on GCS, was categorized as mild in 18.78%, moderate in 56.33%, and severe in 24.84% of cases (Table 2).

Systemic injuries were present in 20% of patients, commonly involving the chest, maxillofacial region, long bones, abdomen, and spine. A simple-to-compound skull fracture ratio of 1.36:1 was observed, and cases of cerebrospinal fluid (CSF) rhinorrhea and otorrhea often included blood admixture.
Outcomes were favorable in 65.6% of conservatively managed patients. Mortality was highest among infants (<1 year, 18.18%) and patients with severe TBI (GCS 3–7, mortality 36.53%) (Table 3).

Pupillary response at admission was a strong prognostic indicator: Accordingly, 75% of patients with abnormal reactivity had poor outcomes, compared to only 2.8% with normal reactivity (Table 4).

A midline shift >5 mm on CT was associated with an 80% poor outcome rate. Pathologically, patients with both intra- and extra-axial hemorrhages, especially when combined with intraventricular hemorrhage, had significantly worse outcomes. In contrast, isolated extradural hematomas had a favorable prognosis (94.4% recovery; Table 4). The study identified 34 cases (20.61%) of simple skull fractures, 25 cases (15.41%) of open compound fractures, and 16 cases (9.96%) of closed compound fractures. CSF leaks were less common, with 3 cases (1.81%) of CSF rhinorrhea and 6 cases (3.64%) of CSF otorrhea. These findings highlight the prevalence of simple fractures as the most frequent skull fracture type, while CSF leaks were relatively rare complications (Table 5).

Operative treatment was administered to 8 patients, with 50% experiencing poor outcomes (dead/vegetative state), while conservative management was applied to 157 patients, yielding better results (65.6% good recovery). Age-wise analysis demonstrated the highest good recovery rates in the 1–5 years age group (66.67%), followed by the 5–14 years group (61.96%) and the <1 year group (55.09%). Severe disability and mortality were more prevalent in the operative group and the youngest age group (<1 year; Table 6).

Normal pupillary reaction was strongly associated with good recovery (73.7%), whereas abnormal pupillary reaction correlated with poor outcomes (75% dead/vegetative). Midline shift also influenced outcomes: Patients with no midline shift had the highest good recovery rate (79%), while those with a 5–10 mm shift had the worst prognosis (80% dead/vegetative). These indicators underscore the critical role of neurological and imaging findings in predicting patient outcomes (Table 7).

Table 8 reiterates the distribution of skull fractures and CSF leaks, mirroring the data in Table 5.

Simple fractures were the most common (20.61%), followed by open compound fractures (15.41%) and closed compound fractures (9.96%). CSF leaks remained rare, with rhinorrhea and otorrhea occurring in 1.81% and 3.64% of cases, respectively. Extra-axial injuries like epidural hematoma had the highest good recovery rate (94.4%), while subdural hematoma with contusion showed the worst outcomes (35.2% good recovery, 35.2% dead/vegetative). Diffuse axonal injury and isolated epidural hematoma were associated with favorable outcomes (84.6% and 94.4% good recovery, respectively). Intraventricular hemorrhage had the highest mortality rate (50%). Combined injuries (e.g. subdural hematoma + contusion) were linked to poorer prognoses compared to isolated injuries. Overall, 63.3% of patients achieved good recovery, while 13.3% had severe disability or died (Table 9).

Table 10 demonstrates the multivariate regression analysis of the study variables to identify the independent prognostic factors.

Discussion
Although the outlook for TBI has significantly improved in the developed world due to the development of highly specialised intensive care units and a high degree of interdisciplinary approach, it still presents a significant challenge for the neurosurgery units in our region of the world. Like adult TBIs, juvenile TBIs can happen for a variety of reasons and typically have a positive prognosis. These injuries are typically not isolated, are linked to polytrauma, and are treated mostly following how adults are handled [8-11]. Although this is a significant retrospective study on paediatric TBI, it has a few flaws, such as the lack of availability of details, such as mode of injury, clinical details, and so on, which were only available for admitted patients. There was no information available about post-traumatic seizures, hospitalisation, post-traumatic epilepsy, or long-term neurologic outcomes. These details would have helped us understand the clinic-epidemiologic picture of paediatric TBI in India.
The highest numbers of pediatric head injuries were reported on Saturday. The higher incidence of pediatric head injuries on weekends can be attributed to the fact that primary and middle schools are not functional on weekends in India which make this age group of 5-14 years more susceptible to injuries especially fall from height as they tend to spend more time in indoor or outdoor play activities like running, cycling, jumping etc., which might not be supervised by their caregivers making them prone to head injuries or any other injury. This age group also becomes more vulnerable to head injuries due to risk-taking behavior without parents’ consent or supervision.
When defining the age at which prognosis materially worsens, there are differences in the literature. For instance, there has been debate over the paediatric age range. While some research claims that children under the age of 10 tend to have better outcomes [12, 13], others claim that children under the age of five have a greater mortality rate [14]. As reported by Suresh et al., there was no difference in poor outcomes in children under 5 years old or older than 5 years in our series; however, there was a somewhat higher poor outcome in children over 12 years that was not statistically significant [15]. There has been debate concerning the significance of age as a prognostic indicator. According to Luersson et al. [16] and Braakman et al. [17], age is the main cause of mortality and morbidity. These studies compare adults with children, despite the literature’s assertion that age is a stronger predictor of mortality and morbidity in severe head trauma. In our study, only children were compared, and all types of traumas were considered.
In the present study, the most common mode of injury was falls, 91(55.15%), followed by road traffic accidents 64(38.78%), assault 8(4.84%), and sports 2(1.2%). Previous study have found that falls from a height are among the major causes of head injury in young children compared to older children, where falls are relatively less common causes of head injury despite bicycle accidents, recreational activities, and being struck by a big vehicle [18]. The results of the outcome after treatment are the GOS from a previously published study [19].
The initial GCS score was identified as the single most critical factor determining outcome (P<0.0001) by Beca et al. [20] and Kuday [21]. According to Astrand et al., negative outcomes were observed in GCS 14–15 at 0%, 9–13 at 6.2%, and 8 at 22% [22]. Low GCS did not always reliably predict the outcome in the absence of hypoxia or ischemia, according to Ong et al. [23]. We discovered a sizable effect of GCS on the result in our series. Due to a lack of pre-hospital resuscitation and late presentation to the hospital, developing and underdeveloped communities like ours have worse outcomes.
Pupillary reaction reflects the condition of brain stem compression in a head injury patient. If a patient with TBI has an abnormal pupil, it shows the brain stem is compressed, and the prognosis of the patient is poor. Marmarou et al. [25] in their study concluded that pupillary reaction was an independent parameter to determine the prognosis of patients, they reported that pupillary reactivity was a stable parameter in the early phase of TBI in comparison to the GCS score because it is less affected by sedation and paralysis [24-26].
Toutant et al. conducted a prospective investigation on the absence or compression of basal cisterns on initial CT scan: Concerning indicators of prognosis in severe head injury. Patients without a basal cistern died at a rate of 77%, those with a compressed basal cistern died at a rate of 39%, and those with normal basal cisterns died at a rate of 22%. Midline shift (MLS) of total isolated severe traumatic head injury patients correlated with their GOS extended (GOSE). Most of the patients had MLS >5 mm (553 patients). Out of 553 patients, most patients showed GOSE 1 (83.36%), followed by GOSE 7 (7.23%) and GOSE 8 (2.53%). Statistically, a significant association was observed (P<0.0001) between MLS with outcomes and GOSE [26]. This study supports previous studies showing an increase in mortality with the increase in MLS more than 5 mm.
Around the world, TBI is a significant factor in both death and disability in children and young people [24]. The mechanism, clinical symptoms, and consequences following TBI are still poorly known, even though the condition is on the rise. There has not been much research examining TBI among children, particularly those from the Indian subcontinent, even though they make up a sizable portion of the population who are at risk. Children have a special vulnerability that may be caused by a predisposition to injury, mechanical characteristics like a large head and weak neck muscles, and neurobiological variables like biochemical and molecular pathways important for brain maturation that may further harm the growing brain. Multivariate analysis confirms that neurological status at presentation (GCS, pupil reactivity) and CT imaging findings (midline shift, intraventricular hemorrhage, combined injuries) are independent and statistically significant predictors of poor outcomes in paediatric TBI. These variables should be prioritized in triage, risk stratification, and early clinical decision-making. Demographic variables like age and structural injuries, such as fractures, had no independent prognostic value after adjustment.
Conclusion
Paediatric TBI is a major public health concern with significant clinical and socioeconomic implications. Effective management requires early recognition of prognostic indicators such as level of consciousness and pupillary response, which play a vital role in outcome prediction and clinical decision-making. Timely neuroimaging and appropriate triage are essential for risk stratification and intervention. Beyond acute care, long-term rehabilitation and follow-up are crucial to address the neurodevelopmental impact of TBI in children. The study highlights the importance of integrating standardized neurological assessments and imaging protocols into routine care pathways for paediatric head injury. From a preventive standpoint, public health efforts must focus on education, caregiver awareness, road safety, and childproofing environments to minimize injury risk. Strengthening healthcare infrastructure, especially in resource-limited settings, is also necessary to ensure access to emergency care and rehabilitation services. A multidisciplinary, evidence-based approach is needed to reduce TBI-related mortality and disability in children. Future research should emphasize longitudinal outcomes and the effectiveness of early interventions, contributing to more comprehensive care models. In summary, paediatric TBI demands coordinated clinical, community, and policy responses to mitigate its burden and improve the quality of life for affected children and their families.
Limitations
As the study was retrospective and based solely on hospital records of admitted patients, long-term outcomes, such as neurocognitive function, behavioral sequelae, and post-traumatic seizure or epilepsy data, were not available. These parameters require prospective follow-up and structured neuropsychological assessment, which were beyond the scope and design of this study. We agree that evaluating such outcomes is critical for understanding the full impact of pediatric TBI, and we recommend future prospective studies with long-term follow-up to address these important aspects. Only admitted cases were included, excluding milder injuries managed on an outpatient basis, which introduces selection bias. The absence of long-term follow-up prevented assessment of neurocognitive outcomes, behavioral sequelae, or post-traumatic epilepsy. Data on pre-hospital care, time to intervention, and surgical timing were unavailable. Important confounding factors, such as socioeconomic status, parental supervision, and environmental risks, were not captured. Imaging was limited to CT scans, potentially missing subtle injuries detectable on magnetic resonance imaging. These constraints highlight the need for future prospective, multicenter studies with longitudinal follow-up.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted following ethical principles outlined in the Declaration of Helsinki. Approval was obtained from the Institutional Ethics Committee of GSVM Medical College, Kanpur, India (Code: EC/248/May/2023),
Since the study was retrospective and used hospital records, patient confidentiality was strictly maintained. No personally identifiable information was disclosed. Data were anonymized before analysis to ensure privacy and compliance with ethical standards. No experimental interventions were conducted, and only routine clinical information was used.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
References
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. The Journal of Head Trauma Rehabilitation. 2006; 21(5):375-8. [DOI:10.1097/00001199-200609000-00001] [PMID]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: Differences by race. The Journal of Head Trauma Rehabilitation. 2005; 20(3):229-38. [DOI:10.1097/00001199-200505000-00006] [PMID]
- Kim HB, Kim DK, Kwak YH, Shin SD, Song KJ, Lee SC, et al. Epidemiology of traumatic head injury in Korean children. Journal of Korean Medical Science. 2012; 27(4):437-42. [DOI:10.3346/jkms.2012.27.4.437] [PMID] [PMCID]
- Jagannathan J, Okonkwo DO, Yeoh HK, Dumont AS, Saulle D, Haizlip J, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. Journal of Neurosurgery. Pediatrics. 2008; 2(4):240-9. [DOI:10.3171/PED.2008.2.10.240] [PMID]
- Adirim TA, Wright JL, Lee E, Lomax TA, Chamberlain JM. Injury surveillance in a pediatric emergency department. The American Journal of Emergency Medicine. 1999; 17(6):499-503. [DOI:10.1016/S0735-6757(99)90184-5] [PMID]
- Kumar R, Mahapatra AK. The changing "epidemiology" of pediatric head injury and its impact on the daily clinical practice. Child'S Nervous System. 2009; 25(7):813-23. [DOI:10.1007/s00381-009-0820-z] [PMID]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; 2(7872):81-4 [DOI:10.1016/S0140-6736(74)91639-0] [PMID]
- Wani AA, Ramzan AU, Malik NK, Qayoom A, Nizami FA, Kirmani AR, et al. Missile injury to the pediatric brain in conflict zones. Journal of Neurosurgery. Pediatrics. 2011; 7(3):276-81. [DOI:10.3171/2010.12.PEDS10241] [PMID]
- Wani AA, Zargar J, Ramzan AU, Malik NK, Qayoom A, Kirmani AR, et al. Head injury caused by tear gas cartridge in teenage population. Pediatric Neurosurgery. 2010; 46(1):25-8. [DOI:10.1159/000314054] [PMID]
- Wani AA, Ramzan AU, Tariq R, Kirmani AR, Bhat AR. Head injury in children due to cricket ball scenario in developing countries. Pediatric Neurosurgery. 2008; 44(3):204-7. [DOI:10.1159/000120151] [PMID]
- Wani AA, Dar TA, Ramzan AU, Kirmani AR, Bhat AR. Craniovertebral junction injuries in children: A review. Indian Journal of Neurotrauma. 2007; 4(2):79-87. [DOI:10.1016/S0973-0508(07)80021-9]
- Carlsson CA, von Essen C, Löfgren J. Factors affecting the clinical corse of patients with severe head injuries. 1. Influence of biological factors. 2. Significance of posttraumatic coma. Journal of Neurosurgery. 1968; 29(3):242-51. [DOI:10.3171/jns.1968.29.3.0242] [PMID]
- Comninos SC. Early prognosis of severe head injuries in children. Acta Neurochirurgica. Supplementum. 1979; 28(1):144-7. [DOI:10.1007/978-3-7091-4088-8_34] [PMID]
- Raimondi AJ, Hirschauer J. Head injury in the infant and toddler. Coma scoring and outcome scale. Childs Brain. 1984; 11(1):12-35. [DOI:10.1159/000120157] [PMID]
- Suresh HS, Praharaj SS, Devi BI, Shukla D, Kolluri VS. Prognosis in children with head injury: An analysis of 340 patients. Neurology India. 2003; 51(1):16-8. [Link]
- Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient's age. A longitudinal prospective study of adult and pediatric head injury. Journal of Neurosurgery. 1988; 68(3):409-16. [DOI:10.3171/jns.1988.68.3.0409] [PMID]
- Braakman R, Gelpke GJ, Habbema JD, Maas AI, Minderhoud JM. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery. 1980; 6(4):362-370. [DOI:10.1227/00006123-198004000-00002] [PMID]
- Tarlov E. Neurological surgery: A comprehensive reference guide to the diagnosis and management of neurosurgical problems. JAMA. 1983; 249(6):832-3. [DOI:10.1001/jama.1983.03330300090054]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1(7905):480-4. [DOI:10.1016/S0140-6736(75)92830-5] [PMID]
- Beca J, Cox PN, Taylor MJ, Bohn D, Butt W, Logan WJ, et al. Somatosensory evoked potentials for prediction of outcome in acute severe brain injury. The Journal of Pediatrics. 1995; 126(1):44-9. [DOI:10.1016/S0022-3476(95)70498-1] [PMID]
- Kuday C, Uzan M, Hanci M. Statistical analysis of the factors affecting the outcome of extradural haematomas: 115 cases. Acta Neurochirurgica. 1994; 131(3-4):203-6. [DOI:10.1007/BF01808613] [PMID]
- Astrand R, Undén J, Hesselgard K, Reinstrup P, Romner B. Clinical factors associated with intracranial complications after pediatric traumatic head injury: an observational study of children submitted to a neurosurgical referral unit. Pediatric Neurosurgery. 2010; 46(2):101-9. [DOI:10.1159/000319006] [PMID]
- Ong L, Selladurai BM, Dhillon MK, Atan M, Lye MS. The prognostic value of the glasgow coma scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatric Neurosurgery. 1996; 24(6):285-91. [DOI:10.1159/000121057] [PMID]
- Ritter AM, Muizelaar JP, Barnes T, Choi S, Fatouros P, Ward J, Bullock MR. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 1999 May;44(5):941-8. [DOI: 10.1097/00006123-199905000-00005]
- Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, et al. Prognostic value of the glasgow coma scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: An IMPACT analysis. Journal of Neurotrauma. 2007; 24(2):270-80. [DOI:10.1089/neu.2006.0029] [PMID]
- Toutant SM, Klauber MR, Marshall LF, Toole BM, Bowers SA, Seelig JM, et al. Absent or compressed basal cisterns on first CT scan: Ominous predictors of outcome in severe head injury. Journal of Neurosurgery. 1984; 61(4):691-4. [DOI:10.3171/jns.1984.61.4.0691] [PMID]
Type of Study: Research |
Subject:
Pediatric Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






