Wed, Jul 16, 2025
Volume 4, Issue 3 (Summer 2018)
Iran J Neurosurg 2018, 4(3): 125-138 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ramezani S, Yousefzadeh-Chabok S. Identification of Imaging and Clinical Markers Predicting Chronic Sleep Disturbances After Traumatic Brain Injury in Adults. Iran J Neurosurg 2018; 4 (3) :125-138
URL: http://irjns.org/article-1-141-en.html
URL: http://irjns.org/article-1-141-en.html
1- Neuroscience Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
2- Guilan Road Trauma Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran ,sh.yousefzadeh@gmail.com
2- Guilan Road Trauma Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran ,
Keywords: Chronic sleep disturbance, Traumatic Brain Injury, Executive function, neuropsychiatric deficit
Full Text [PDF 818 kb]
(1719 Downloads)
| Abstract (HTML) (5244 Views)
Full Text: (2338 Views)
Highlights
• The prevalence of post-traumatic sleep-wake disorders is about 35%.
• Insomnia is the commonest sleep problem after Traumatic Brain Injury (TBI) and co-occurs with fatigue.
• Chronic insomnia was followed by mild TBI, acute severe headache, and frontal lesion.
• Predictors of excessive daytime sleepiness were diffuse lesions of right hemisphere.
• Executive function was poorer in patients with sleep problem.
Plain Language Summary
Circadian Rhythm Sleep Disorders (CRSDs) are among common post-traumatic complications which may be persistent in most cases. These disorders may differ in affected patients. The injured area in the brain has a role in developing a specific type of sleep disorder. CRSDs is following a trauma-induced event, which disrupt the active participation of patients during rehabilitation interventions and interfere or delay their neurological and functional improvement. Considering the patients with traumatic brain injury (TBI), as population with heterogeneous physical, cognitive, and psychological complications, the aim of this study is to identify the severity and location of lesions related to TBI and clinical factors associated with imaging findings in them in order to predict the occurrence of CRSDs after trauma. In this regard, 165 patients with TBI admitted to the hospital were enrolled in the study, and their demographic, clinical and para-clinical data were recorded using a questionnaire. The patients were examined for cognitive, psychological, and physical function through standard tests during discharge. The follow-up evaluation for a variety of CRSDs was conducted six months later. 146 of 165 patients were participated in the follow-up examinations, and 35% of them had a variety of CRSDs six months after TBI, where the most common types were “insomnia” and “daytime sleepness”. Those with a poorer cognitive function during discharge were more prone to sleep disorders. The hypersomnia was found to be more frequent in patients with bilateral cerebral lesions. Insomnia was associated with chronic fatigue, and was found mostly in people with mild localized lesions in the frontal cortex and severe headaches during the hospitalization period. On the other hand, daytime sleepness, mostly accompanied by aggressive behaviors, was more common in patients with disseminated lesions in the right-brain hemisphere, especially in the brainstem. In general, cognitive and psychological disorders and headache happening immediately after TBI in hospitalized patients as well as the type, severity and location of brain injuries affect the occurrence of sleep disorders and their types. Therefore, identifying the population exposed to risk factors and early interventions to remove them is a vital priority in the treatment of sleep disorders after TBI.
1. Introduction
Traumatic Brain Injury (TBI) mainly results in various sensorimotor, cognitive, psychological, and emotional disabilities in survivors. Some complications of TBI remain consistent in the longterm [1-6]. Post-Traumatic Sleep-Wake Disturbances (PTSWDs) are among the most common undesirable outcomes of TBI, with various prevalence rates reported by different studies [7-12]. Various categories of PTSWDs, including insomnia, hypersomnia, Excessive Daytime Sleepiness (EDS), parasomnia, obstructive sleep apnea, and Periodic Limb Movement Disorder (PLMD) and Circadian Rhythm Disorder (CRD), have been observed with varying frequency in the TBI population [7, 8, 10, 13, 14]. Obviously, a normal sleep-wake cycle involves a balance between arousal stimulatory and inhibitory systems and functional coordination between them. Therefore, the disruption of the neural circuits regulating sleep-wake cycle caused by TBI creates sleep disorders, and a specific type of PTSWDs occurs depending on the affected area of brain [15, 16].
The clinical manifestations of PTSWDs may appear in different phases of the disease [17]. The occurrence of PTSWDs in the acute phase is harmful to the nervous system restoration and interferes with primary neural improvement and may prolong neuroplasticity [15]. Also, sleep problems occurring in subacute and chronic phases prevent the active participation of the patients in the rehabilitation program and delay their functional recovery [15, 17]. Additionally, it negatively affects the various aspects of health and quality of life, and challenges return to healthy lifestyle [7, 13, 18, 19]. Thus, better understanding of the factors involved in the onset or durability of PTSWDs is of importance in preventing the persistence of this complication, making decision for an early therapeutic intervention, and accelerating and facilitating optimal recovery in patients’ daily activities.
Previous studies reported a clear relationship between the occurrence of PTSWDs, mood disorders, daytime fatigue and nighttime pain [20-22]. Some studies have supported the association between chronic insomnia and headache, anxiety and depression following TBI. Likewise, TBI severity could be associated with the risk of chronic hypersomnia [10]. However, there are contradictory findings on the prognostic role of severity, location, and type of lesion in the occurrence of new-onset sleep-wake cycle problems in TBI [7, 8, 10, 13, 18, 22, 23]. In addition, there is little information available on neuropathological and neuropsychological factors involved in the development of different types of PTSWDs.
The clinical and paraclinical characteristics in patients prone to chronic PTSWDs remain undiscovered. These information gaps demand further prospective investigations. Furthermore, the relationship between Post-Taumatic Psychiatric Disorders (PTPDs) and the various types of consistent PTSWDs is unclear. Moreover, post-TBI sleep disorders negatively impact the cognitive and emotional functions [17, 24]. As one of the risk factors for chronic traumatic encephalopathy, PTSWDs contribute to the onset and exacerbation of symptoms, including poor cognition, aggression, and impulsivity [25, 26]. Sleep disorders may be the cause of cognitive impairment and Alzheimer disease [27, 28]. Therefore, it is useful to clarify whether the early post-TBI executive and cognitive functions evaluated at the discharge are associated with disrupted sleep in the chronic phase.
In this regard, the present study aimed to determine the clinical and imaging markers predicting the occurrence of various types of PTSWDs. We also explored the role of early post-TBI executive and cognitive functions in developing various categories of PTSWDs, in particular. Furthermore, the prevalence of PTPDs was investigated in the different types of post-traumatic sleep disorders.
2. Methods and Materials/Patients
In this prospective longitudinal study, 165 qualified patients with closed TBI participated. The subjects aged between 18 and 65 years and were admitted to the Neurosurgery ward of Poursina Hospital in Rasht City, Iran. They were enrolled in the study after providing a written consent. The inclusion criteria were as follows: intracranial injuries determined in primary neuroimaging findings in adult patients with primary TBI, experiencing amnesia for 20 minutes or more, being conscious during neuropsychological examinations (discharge phase), and having a functional outcome score of 3 or higher according to Glasgow Outcome Scale (GOS) [29], having at least 4 years of education. Exclusion criteria were the patients in a vegetative state, or with severe loss of consciousness at discharge who were unable to perform the cognitive tasks, those with normal neuroimaging findings but a history of TBI and previous sleep and psychiatric disorders, patients with preexisting cognitive dysfunctions, substance abuse, neurological or orthopedic disorders, patients with malignant diseases, intellectual disability, or sensory disabilities, as well as individuals receiving sedative medications.
The demographic, clinical and paraclinical characteristics of patients were recorded by a separate questionnaire. The family history of sleep and psychiatric disorders were identified by evaluating the presence of these disorders in first-degree relatives of patients. Type, size and location of lesions were determined using neuroimaging techniques within 24 hours after brain trauma. The obtained data were reported by a neuroradiologist unaware of the neurological and neuropsychological outcomes. The data about the brain lesion side, site and type were recorded. The injury types were classified according to lesion morphology [29], including hematoma, contusion, edema, pneumocephalus and Diffuse Axonal Injury (DAI). Lesion site consists of the cortex, subcortex, epidural, subdural, subarachnoid spaces, ventricles, and brain stem. For the multifocal damages, the most intense and wide site on brain scans were considered as an analyzable lesion site. TBI severity was ranked according to the post-resuscitation Glasgow Coma Scale (GCS) score and the duration of Post-Traumatic Amnesia (PTA) [29].
• The prevalence of post-traumatic sleep-wake disorders is about 35%.
• Insomnia is the commonest sleep problem after Traumatic Brain Injury (TBI) and co-occurs with fatigue.
• Chronic insomnia was followed by mild TBI, acute severe headache, and frontal lesion.
• Predictors of excessive daytime sleepiness were diffuse lesions of right hemisphere.
• Executive function was poorer in patients with sleep problem.
Plain Language Summary
Circadian Rhythm Sleep Disorders (CRSDs) are among common post-traumatic complications which may be persistent in most cases. These disorders may differ in affected patients. The injured area in the brain has a role in developing a specific type of sleep disorder. CRSDs is following a trauma-induced event, which disrupt the active participation of patients during rehabilitation interventions and interfere or delay their neurological and functional improvement. Considering the patients with traumatic brain injury (TBI), as population with heterogeneous physical, cognitive, and psychological complications, the aim of this study is to identify the severity and location of lesions related to TBI and clinical factors associated with imaging findings in them in order to predict the occurrence of CRSDs after trauma. In this regard, 165 patients with TBI admitted to the hospital were enrolled in the study, and their demographic, clinical and para-clinical data were recorded using a questionnaire. The patients were examined for cognitive, psychological, and physical function through standard tests during discharge. The follow-up evaluation for a variety of CRSDs was conducted six months later. 146 of 165 patients were participated in the follow-up examinations, and 35% of them had a variety of CRSDs six months after TBI, where the most common types were “insomnia” and “daytime sleepness”. Those with a poorer cognitive function during discharge were more prone to sleep disorders. The hypersomnia was found to be more frequent in patients with bilateral cerebral lesions. Insomnia was associated with chronic fatigue, and was found mostly in people with mild localized lesions in the frontal cortex and severe headaches during the hospitalization period. On the other hand, daytime sleepness, mostly accompanied by aggressive behaviors, was more common in patients with disseminated lesions in the right-brain hemisphere, especially in the brainstem. In general, cognitive and psychological disorders and headache happening immediately after TBI in hospitalized patients as well as the type, severity and location of brain injuries affect the occurrence of sleep disorders and their types. Therefore, identifying the population exposed to risk factors and early interventions to remove them is a vital priority in the treatment of sleep disorders after TBI.
1. Introduction
Traumatic Brain Injury (TBI) mainly results in various sensorimotor, cognitive, psychological, and emotional disabilities in survivors. Some complications of TBI remain consistent in the longterm [1-6]. Post-Traumatic Sleep-Wake Disturbances (PTSWDs) are among the most common undesirable outcomes of TBI, with various prevalence rates reported by different studies [7-12]. Various categories of PTSWDs, including insomnia, hypersomnia, Excessive Daytime Sleepiness (EDS), parasomnia, obstructive sleep apnea, and Periodic Limb Movement Disorder (PLMD) and Circadian Rhythm Disorder (CRD), have been observed with varying frequency in the TBI population [7, 8, 10, 13, 14]. Obviously, a normal sleep-wake cycle involves a balance between arousal stimulatory and inhibitory systems and functional coordination between them. Therefore, the disruption of the neural circuits regulating sleep-wake cycle caused by TBI creates sleep disorders, and a specific type of PTSWDs occurs depending on the affected area of brain [15, 16].
The clinical manifestations of PTSWDs may appear in different phases of the disease [17]. The occurrence of PTSWDs in the acute phase is harmful to the nervous system restoration and interferes with primary neural improvement and may prolong neuroplasticity [15]. Also, sleep problems occurring in subacute and chronic phases prevent the active participation of the patients in the rehabilitation program and delay their functional recovery [15, 17]. Additionally, it negatively affects the various aspects of health and quality of life, and challenges return to healthy lifestyle [7, 13, 18, 19]. Thus, better understanding of the factors involved in the onset or durability of PTSWDs is of importance in preventing the persistence of this complication, making decision for an early therapeutic intervention, and accelerating and facilitating optimal recovery in patients’ daily activities.
Previous studies reported a clear relationship between the occurrence of PTSWDs, mood disorders, daytime fatigue and nighttime pain [20-22]. Some studies have supported the association between chronic insomnia and headache, anxiety and depression following TBI. Likewise, TBI severity could be associated with the risk of chronic hypersomnia [10]. However, there are contradictory findings on the prognostic role of severity, location, and type of lesion in the occurrence of new-onset sleep-wake cycle problems in TBI [7, 8, 10, 13, 18, 22, 23]. In addition, there is little information available on neuropathological and neuropsychological factors involved in the development of different types of PTSWDs.
The clinical and paraclinical characteristics in patients prone to chronic PTSWDs remain undiscovered. These information gaps demand further prospective investigations. Furthermore, the relationship between Post-Taumatic Psychiatric Disorders (PTPDs) and the various types of consistent PTSWDs is unclear. Moreover, post-TBI sleep disorders negatively impact the cognitive and emotional functions [17, 24]. As one of the risk factors for chronic traumatic encephalopathy, PTSWDs contribute to the onset and exacerbation of symptoms, including poor cognition, aggression, and impulsivity [25, 26]. Sleep disorders may be the cause of cognitive impairment and Alzheimer disease [27, 28]. Therefore, it is useful to clarify whether the early post-TBI executive and cognitive functions evaluated at the discharge are associated with disrupted sleep in the chronic phase.
In this regard, the present study aimed to determine the clinical and imaging markers predicting the occurrence of various types of PTSWDs. We also explored the role of early post-TBI executive and cognitive functions in developing various categories of PTSWDs, in particular. Furthermore, the prevalence of PTPDs was investigated in the different types of post-traumatic sleep disorders.
2. Methods and Materials/Patients
In this prospective longitudinal study, 165 qualified patients with closed TBI participated. The subjects aged between 18 and 65 years and were admitted to the Neurosurgery ward of Poursina Hospital in Rasht City, Iran. They were enrolled in the study after providing a written consent. The inclusion criteria were as follows: intracranial injuries determined in primary neuroimaging findings in adult patients with primary TBI, experiencing amnesia for 20 minutes or more, being conscious during neuropsychological examinations (discharge phase), and having a functional outcome score of 3 or higher according to Glasgow Outcome Scale (GOS) [29], having at least 4 years of education. Exclusion criteria were the patients in a vegetative state, or with severe loss of consciousness at discharge who were unable to perform the cognitive tasks, those with normal neuroimaging findings but a history of TBI and previous sleep and psychiatric disorders, patients with preexisting cognitive dysfunctions, substance abuse, neurological or orthopedic disorders, patients with malignant diseases, intellectual disability, or sensory disabilities, as well as individuals receiving sedative medications.
The demographic, clinical and paraclinical characteristics of patients were recorded by a separate questionnaire. The family history of sleep and psychiatric disorders were identified by evaluating the presence of these disorders in first-degree relatives of patients. Type, size and location of lesions were determined using neuroimaging techniques within 24 hours after brain trauma. The obtained data were reported by a neuroradiologist unaware of the neurological and neuropsychological outcomes. The data about the brain lesion side, site and type were recorded. The injury types were classified according to lesion morphology [29], including hematoma, contusion, edema, pneumocephalus and Diffuse Axonal Injury (DAI). Lesion site consists of the cortex, subcortex, epidural, subdural, subarachnoid spaces, ventricles, and brain stem. For the multifocal damages, the most intense and wide site on brain scans were considered as an analyzable lesion site. TBI severity was ranked according to the post-resuscitation Glasgow Coma Scale (GCS) score and the duration of Post-Traumatic Amnesia (PTA) [29].
Persian version of the Hospital Anxiety and Depression Scale (HADS) [30] was applied to assess self-reported mood status. The presence and severity of headache was subjectively evaluated using the Numerical Pain Rating Scale (NPRS), which assigns numbers 0-10 to the amount of pain. According to the NPRS, zero means no pain. Mild pain is specified by 1-3, moderate pain by 4-6, and severe pain by 7-10. The executive functioning was measured by verbal fluency test as previously described [6]. Cognitive impairment was objectively diagnosed using the standard Persian version of Mini-Mental State Examination (MMSE) after the patient’s discharge [31].
HADS, NPRS, MMSE and verbal fluency test were performed after discharge. The duration of post injury when patients were synchronically evaluated with the duration of hospitalization was 10 days on average. All examinations of discharge phase were performed by a trained research assistant. Six months after the TBI occurrence, the samples were divided into 2 groups of with and without chronic PTSWDs. A differential diagnosis of PTSWDs and psychiatric disorders was conducted through a structured interview using a clinical checklist by a psychiatrist and according to the Diagnostic and Statistical Manual of Mental Disorders-V [32].
Subjective standard evaluations were also performed to determine the nature and severity of PTSWDs. For this purpose, the Persian version of Pittsburgh Sleep Quality Index (PSQI) [33], the Insomnia Severity Index (ISI) [34], and the Epworth Sleepiness Scale (ESS) [35] were used. In this study, daytime fatigue is reported in individual interviews with an ESS score of more than 10 as fatigue, scores less than 10 in the ESS as EDS, scores less than 7 in the ISI as insomnia, and an increased need for sleep (at least 2 hours more than before TBI occurrence) per 24 hours as hypersomnia. Psychiatric symptoms developed 6 months after the occurrence of TBI were considered as chronic PTPDs. In the discharge assessments, early cognitive impairments were diagnosed with a score of less than 23 in MMSE.
Acute anxiety and depression were determined with a score of more than 7 in the HADS. The obtained data were analyzed using SPSS. After evaluating the normal distribution of quantitative variables by Kolmogorov-Smirnov test, Independent Samples t test and Chi-squared test were applied to analyze the quantitative and qualitative demographic, clinical and paraclinical data between the study groups. The scores of sleep indexes, HADS anxiety and depression subscales, cognitive and executive functions in various types of PTSWDs and non-PTSWDs were compared using One-way ANOVA (analysis of variance) and Scheffe post hoc test. To determine clinical and imaging markers predicting the PTSWDs at 6 months after the TBI onset, a multiple logistic regression using Backward method was used in a multivariate analysis. Two-tailed test was used and 3 significance levels including P<0.05, P<0.01 and P<0.001 were considered.
3. Results
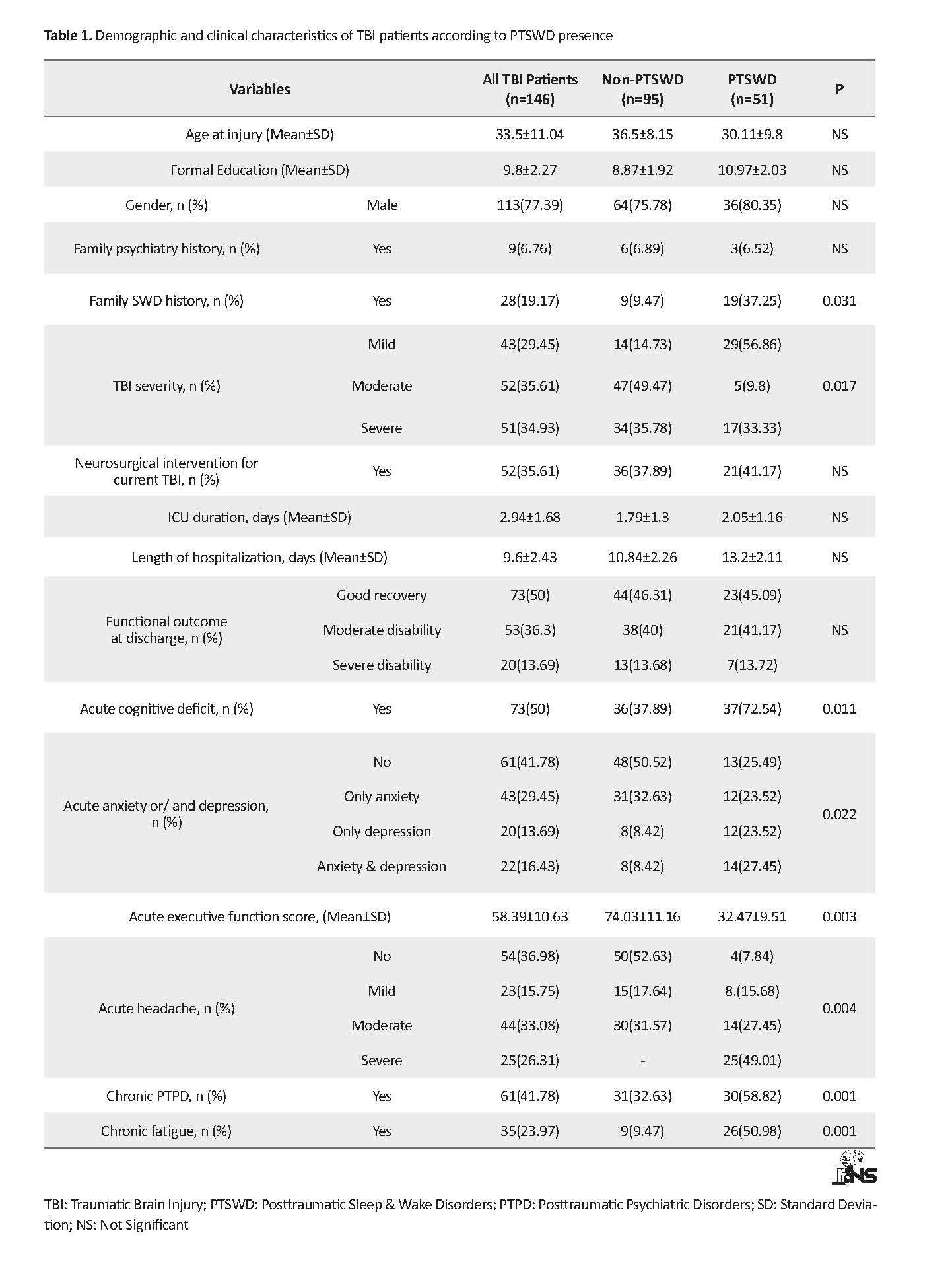
Of 165 patients enrolled in the primary sampling, 146 patients were remained in follow-up assessments, of whom 51 (34.93%) patients had PTSWDs. According to Table 1, the Mean±SD age of the subjects was 35.5±11.04 years. The ratio of male to female was approximately 3.4:1. Their Mean±SD GCS score was 9.4±2.13. About 73% of TBI patients reported a good recovery according to GOS. Moderate and severe disabilities were observed in about 36% and 13% of all TBI patients, respectively. According to neuroimaging findings, hematoma and pneumocephalus were the most frequent lesion types in the studied TBI patients.
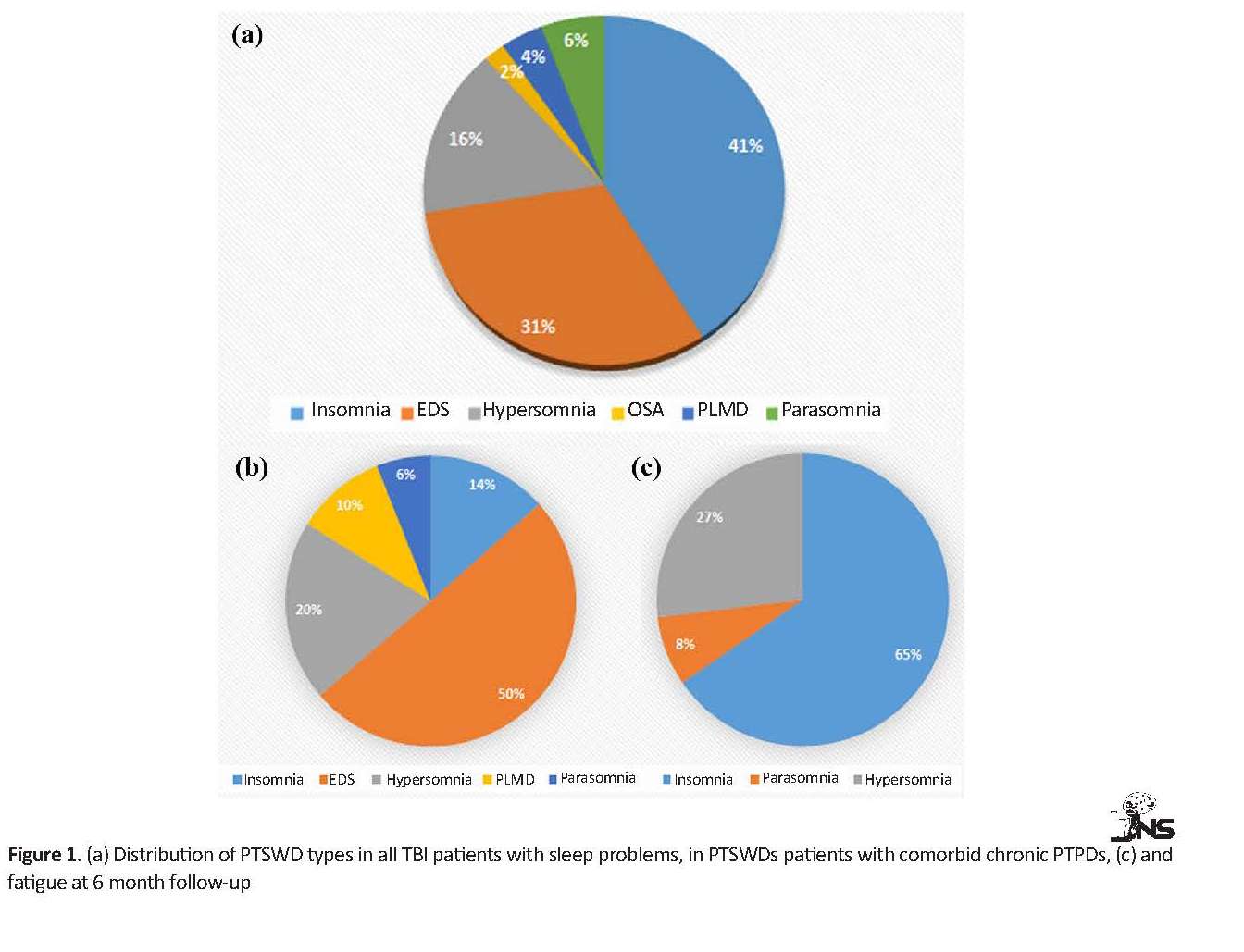
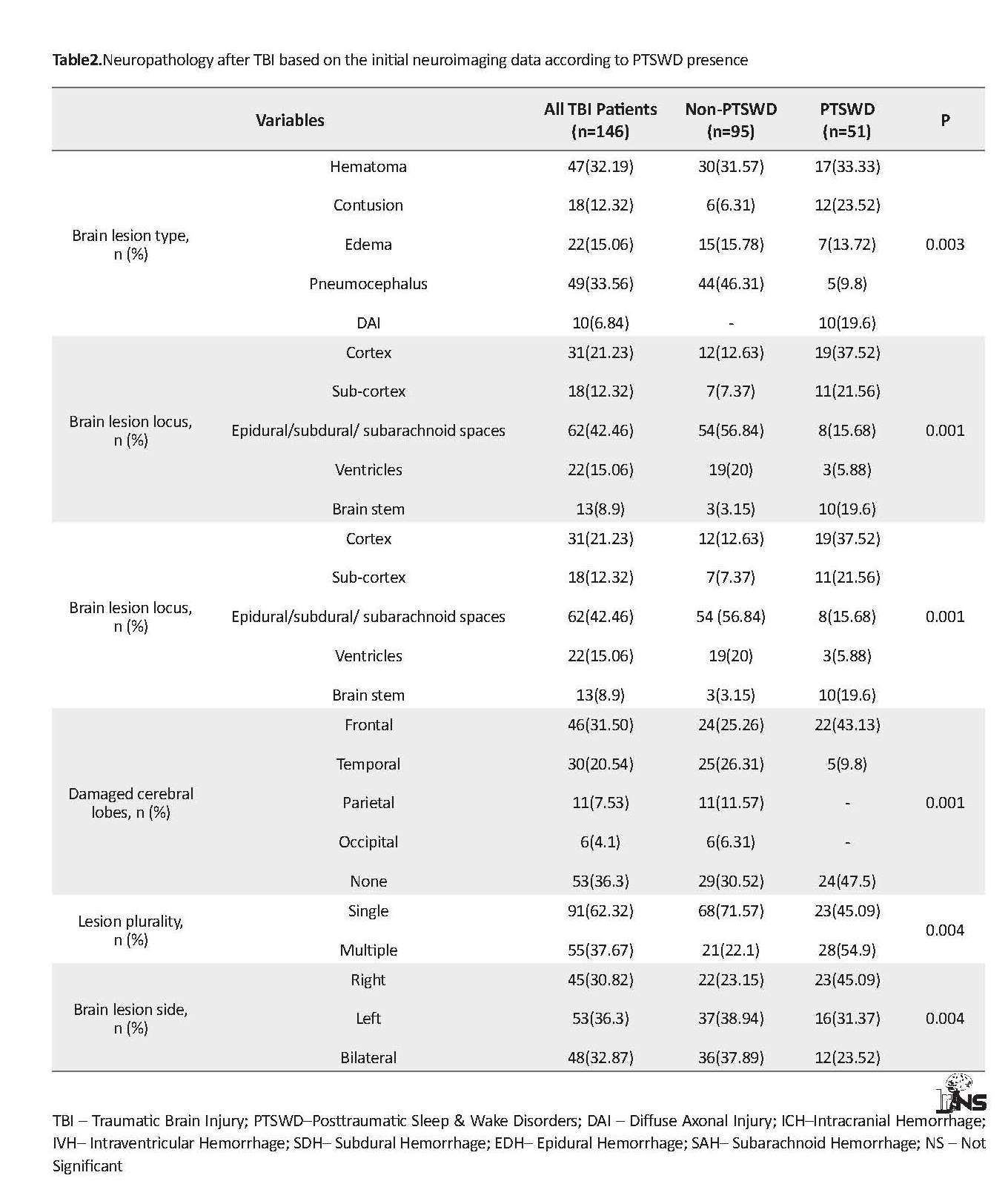
The occurrence of lesion in subdural or epidural or subarachnoid spaces were more than other lesion sites. About 62% and 37% of TBI patients represented single and multiple lesions on brain scans, respectively. About 30%, 36% and 32% of TBI patients had right, left and bilateral brain damage, respectively. More details are presented in Table 2. Figure 1 A shows the distribution of different types of PTSWDs at 6 months after the onset of a lesion in people with closed TBI. Insomnia (41%) and EDS (31%) were the most common chronic sleep disorders in the subjects. About 16% of the study participants experienced hypersomnia. Parasomnia, PLMD, and OSA composed a low proportion of PTSWDs.
HADS, NPRS, MMSE and verbal fluency test were performed after discharge. The duration of post injury when patients were synchronically evaluated with the duration of hospitalization was 10 days on average. All examinations of discharge phase were performed by a trained research assistant. Six months after the TBI occurrence, the samples were divided into 2 groups of with and without chronic PTSWDs. A differential diagnosis of PTSWDs and psychiatric disorders was conducted through a structured interview using a clinical checklist by a psychiatrist and according to the Diagnostic and Statistical Manual of Mental Disorders-V [32].
Subjective standard evaluations were also performed to determine the nature and severity of PTSWDs. For this purpose, the Persian version of Pittsburgh Sleep Quality Index (PSQI) [33], the Insomnia Severity Index (ISI) [34], and the Epworth Sleepiness Scale (ESS) [35] were used. In this study, daytime fatigue is reported in individual interviews with an ESS score of more than 10 as fatigue, scores less than 10 in the ESS as EDS, scores less than 7 in the ISI as insomnia, and an increased need for sleep (at least 2 hours more than before TBI occurrence) per 24 hours as hypersomnia. Psychiatric symptoms developed 6 months after the occurrence of TBI were considered as chronic PTPDs. In the discharge assessments, early cognitive impairments were diagnosed with a score of less than 23 in MMSE.
Acute anxiety and depression were determined with a score of more than 7 in the HADS. The obtained data were analyzed using SPSS. After evaluating the normal distribution of quantitative variables by Kolmogorov-Smirnov test, Independent Samples t test and Chi-squared test were applied to analyze the quantitative and qualitative demographic, clinical and paraclinical data between the study groups. The scores of sleep indexes, HADS anxiety and depression subscales, cognitive and executive functions in various types of PTSWDs and non-PTSWDs were compared using One-way ANOVA (analysis of variance) and Scheffe post hoc test. To determine clinical and imaging markers predicting the PTSWDs at 6 months after the TBI onset, a multiple logistic regression using Backward method was used in a multivariate analysis. Two-tailed test was used and 3 significance levels including P<0.05, P<0.01 and P<0.001 were considered.
3. Results
Of 165 patients enrolled in the primary sampling, 146 patients were remained in follow-up assessments, of whom 51 (34.93%) patients had PTSWDs. According to Table 1, the Mean±SD age of the subjects was 35.5±11.04 years. The ratio of male to female was approximately 3.4:1. Their Mean±SD GCS score was 9.4±2.13. About 73% of TBI patients reported a good recovery according to GOS. Moderate and severe disabilities were observed in about 36% and 13% of all TBI patients, respectively. According to neuroimaging findings, hematoma and pneumocephalus were the most frequent lesion types in the studied TBI patients.
The occurrence of lesion in subdural or epidural or subarachnoid spaces were more than other lesion sites. About 62% and 37% of TBI patients represented single and multiple lesions on brain scans, respectively. About 30%, 36% and 32% of TBI patients had right, left and bilateral brain damage, respectively. More details are presented in Table 2. Figure 1 A shows the distribution of different types of PTSWDs at 6 months after the onset of a lesion in people with closed TBI. Insomnia (41%) and EDS (31%) were the most common chronic sleep disorders in the subjects. About 16% of the study participants experienced hypersomnia. Parasomnia, PLMD, and OSA composed a low proportion of PTSWDs.
Based on Table 1, there was no significant difference between the groups with and without PTSWDs in terms of age, gender, educational level, family history of psychiatric disorders, and functional outcomes at discharge (P>0.05). Neurosurgical intervention for managing brain lesions in TBI patients did not have a significant effect on PTSWDs appearance (P>0.05). Furthermore, an identical percentage of people who underwent neurosurgery was observed between PTSWDs and non-PTSWDs groups. However, the family history of sleep disorders seems to increase the risk of PTSWDs occurrence (P<0.03).
The obtained results suggested that TBI severity was significantly different between the 2 groups (P<0.01). Moreover, a high percentage (72.54%) of people with sleep problems showed early cognitive deficits after TBI, while about 37% of non-PTSWDs had a cognitive impairment. This difference was statistically significant between the 2 groups (P<0.01). In addition, the PTSWDs group had significantly lower executive functioning scores than non-PTSWDs group at the discharge (P<0.003). There was mostly anxiety alone, and mild or moderate headache in half of non-PTSWDs that was significantly different from the prevalence of acute anxiety and or depression (75%) and headache (92%) in the people with PTSWDs. Most patients with PTSWDs experienced severe headache during the acute phase of TBI, while none of the patients without Chronic Sleep Disturbances demonstrated severe headaches during the same phase (Table 1).
The results revealed a significant difference in chronic fatigue between the 2 groups (P<0.001). A high percentage (about 90%) of people without sleep difficulties did not complain of fatigue, but half of the patients with PTSWDs suffered from chronic fatigue. Also, the occurrence of chronic PTPDs in the PTSWDs group was significantly more than that in non-PTSWDs group (P<0.001). Half of the patients with PTSWDs that had comorbid PTPDs were classified in the EDS category (Figure 1 B).
According to Figure 1 C, among the types of PTSWDs, chronic fatigue was more prevalent in patients with insomnia, ranked after TBI (65%). In patients with insomnia, comorbid PTPDs were not very common (20%); however, insomnia patients with comorbid PTPD showed more PTSD symptoms than the other types of PTPDs. Moreover, about 93% of patients with EDS suffered from PTPDs, including aggression, as the most common symptom (56.25%). In total, 75% of patients with hypersomnia had comorbid PTPDs, predominantly with symptomatic depression (87.5%). PTPDs were concurrently observed in all patients with PLMD and parasomnia, which were mainly manifested with symptoms of PTSD and aggression (Figure 2).
The results revealed a significant difference in chronic fatigue between the 2 groups (P<0.001). A high percentage (about 90%) of people without sleep difficulties did not complain of fatigue, but half of the patients with PTSWDs suffered from chronic fatigue. Also, the occurrence of chronic PTPDs in the PTSWDs group was significantly more than that in non-PTSWDs group (P<0.001). Half of the patients with PTSWDs that had comorbid PTPDs were classified in the EDS category (Figure 1 B).
According to Figure 1 C, among the types of PTSWDs, chronic fatigue was more prevalent in patients with insomnia, ranked after TBI (65%). In patients with insomnia, comorbid PTPDs were not very common (20%); however, insomnia patients with comorbid PTPD showed more PTSD symptoms than the other types of PTPDs. Moreover, about 93% of patients with EDS suffered from PTPDs, including aggression, as the most common symptom (56.25%). In total, 75% of patients with hypersomnia had comorbid PTPDs, predominantly with symptomatic depression (87.5%). PTPDs were concurrently observed in all patients with PLMD and parasomnia, which were mainly manifested with symptoms of PTSD and aggression (Figure 2).
Based on the data presented in Table 2, there was a significant difference between the 2 groups regarding the brain injury type (P<0.003), locus (P<0.001), lobe (P<0.001), side and plurality. All TBI patients diagnosed with DAI according to primary brain scans represented the chronic PTSWDs. Among all types of brain lesions, pneumocephalus was more prevalent in the group without sleep problems. In addition, PTSWDs patients demonstrated a high frequency of cortical (37.52%), subcortical (21.56%) and brain stem (19.6%) injuries compared to those without PTSWDs. Furthermore, in 50% the PTSWDs that displayed cerebral lobe damages, the most commonly affected lobe was frontal. Frontal lobe lesions were more prevalent in the group with sleep problems (43.13%), than those without sleep problems after TBI (25.26%). The multiple lesions and right side damage of brain were more frequent in PTSWDs group, compared to non-PTSWDs ones (P<0.004).
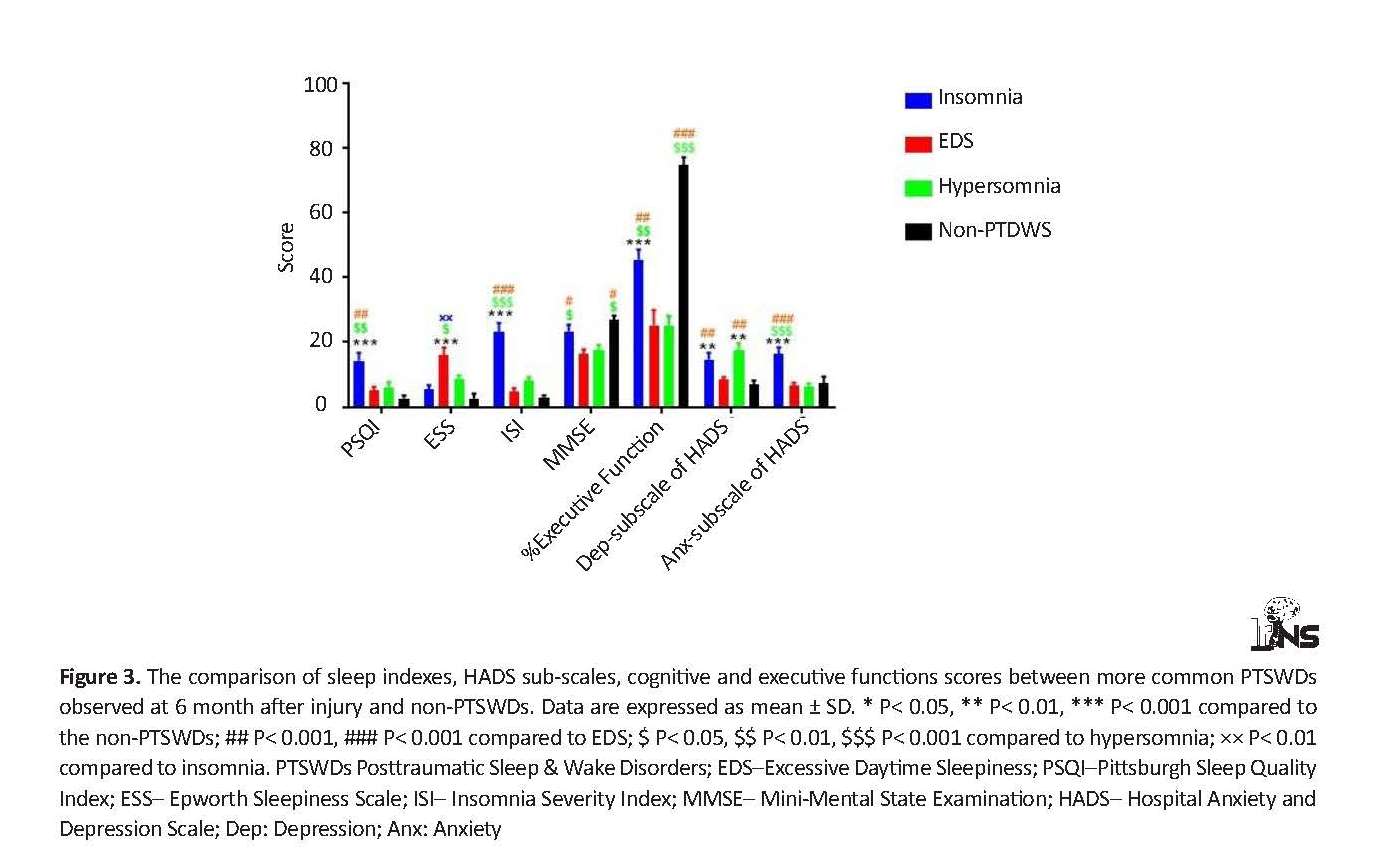
Figure 3 shows the ANOVA results. Based on the obtained data, the mean score of PSQI in insomnia group was significantly higher than that in non-PTSWDs (P<0.001), EDS and hypersomnia groups (P<0.01). Patients with EDS obtained a higher mean ESS score than other groups. The highest mean ISI score was observed in the insomnia group, which was statistically significant (P<0.001). The mean MMSE and executive function scores in TBI patients with insomnia and those without PTSWDs were higher than those in EDS and hypersomnia (P<0.01). There was no significant difference in mean MMSE and executive function scores between the EDS and hypersomnia groups. Although mean MMSE score was not different between the 2 groups of insomnia and non-PTSWDs, the mean score of executive function was significantly higher in non-PTSWDs, compared to insomnia patients (P<0.001). Similarly, the mean depression subscale score was significantly higher in insomnia and hypersomnia groups compared to other groups (P<0.01). There was no significant difference in the mean depression subscale score between individuals with EDS and non-PTSW. However, insomnia patients obtained higher anxiety subscale scores than the other groups (P<0.001).
To determine the predictors of common chronic PTSWDs types of our study, all significant clinical and imaging variables were included in multiple logistic regression model. In the final modeling processing step (Table 3), mild TBI (OR=8.38; 95% confidence interval=3.19-13.14), cortical damage (OR=5.96; 95% CI=2.02-9.05), acute severe headache (OR=4.59; 95% CI=1.27-6.53), and frontal lobe damage (OR=2.04; 95% CI=1.07-4.15) were considered as strong insomnia predictors. Interestingly, severe TBI was the first variable to remain in the final step of modeling to predict EDS (OR=12.73; 95% CI =2.64-15.6). After that, DAI (OR=9.91; 95% CI=2.18-13.59), brainstem lesion (OR=9.78; 95% CI=2.03-12.75), and right side damage (OR=8.41; 95% CI=1.48-10.58) predicted EDS. In addition, bilateral lesion was identified as the first hypersomnia predicting factor (OR=9.85; 95% CI=2.07-13.03). Acute depression and contusion were the second (OR=8.62; 95% CI=1.25-10.5) and third predictors (OR=3.27; 95% CI=1.04-5.33) of hypersomnia.
4. Discussion
The overall prevalence of new-onset sleep disturbances in the current study was estimated about 35%, consistent with some previous reports and inconsistent with some others [7, 10-12]. Apparently, insomnia is the most common post-TBI sleep problem, and is strongly associated with mild brain lesions, cortical lesions of frontal lobe, and severe headaches. According to the obtained results, most patients who suffered from PTSWDs had mild brain damages, and experienced frontal lobe cortical hematomas within 6 months after the lesion occurrence. Therefore, a possible reason for the higher incidence of insomnia in our study may be the high prevalence of mild TBI and cortical lesions of frontal lobe in all subjects with PTSWDs. Although high prevalence of TBI-induced insomnia found in our study was consistent with the results of Hou and colleagues [10], it did not confirm Baumann et al. study reports [7].
We also realized that a high frequency of sleep disruptions after insomnia belonged to EDS. Severe TBI strongly predicts the risk of developing EDS, and the frequency of severe injuries in people with PTSWDs is less than mild TBI. Thus, perhaps this is the reason why EDS was the second most commonly occurring type of PTSWDs in this study. The heterogeneity of TBI population is considered as one of the possible explanation for the discrepancy between the current and previous studies in terms of the prevalence of post-TBI sleep problems and its different types. We used self-reported questionnaires and subjective evaluations instead of objective tests such as polysomnography and multiple sleep latency tests to detect PTSWDs. Such methods might have led to underestimating the occurrence of PTSWDs, especially in people with DAI and severe brain damages who possibly experience an impaired self-awareness [31, 32].
Patients with insomnia obtained higher scores in PSQI and ISI than others, and ESS scores in patients with EDS were more than others. These findings may be due to the high sensitivity of PSQI and ISI for screening insomnia as well as ESS for diagnosing EDS.
Our study revealed that PTSWD patients suffered from acute headache, acute anxiety and depression (separately or together), early poor executive and cognitive functions, as well as chronic fatigue and PTPDs, more than non-PTSWDs ones. These sequels after TBI appear to be a sequence of destructive neurobiological processes resulted from secondary insults, including inflammation, excitotoxicity, abnormal perfusion, ischemia, and apoptotic or necrotic cell death. Additionally, its occurrence is inevitable at all degrees of TBI, although it is found in more severe lesions with worse clinical manifestations [11, 36].
These secondary neural injuries are associated with biomechanical, structural and functional changes in the damaged brain regions and corresponding homologous regions structurally or functionally connected with them [37, 38]. Subsequently, they cause the inefficiency of neural networks and imbalance of neurotransmitter systems regulating affective and cognitive behaviors [39, 40], that seem to contribute in the occurrence of sleep disorders [41, 42]. The patients with chronic sleep problems in comparison with non-PTSWDs ones had DAI and contusion especially in cortical, subcortical and brainstem regions. Therefore, it is believed that the early disruption of neural circuits caused by brain’s white and gray matter lesions negatively impacts cognitive and emotional processing [40, 43, 44], and leads to psychiatric [6] and sleep-wake cycle disturbances in the long term.
We also found that chronic comorbid PTPDs are more prevalent in EDS than the other categories of TBI-induced sleep disorders. However, all patients with PLMD and parasomnia and the majority of hypersomnia ones suffered from chronic PTPDs. A possibility to explain this result may be that the total number of patients with EDS is higher than the other types of sleep disorders except for insomnia. Aggression and depression were more prevalent than other PTPD types in patients with EDS and hypersomnia, respectively. Our prior study has highlighted that the most common chronic PTPDs, including aggression, depression, and PTSD, are associated with DAI, severe brain damages, and TBI, respectively [6]. Similarly, the current study revealed that factors affecting the appearance of EDS accompanied with PTPDs, included severe TBI, DAI and brain stem lesions.
The paucity of comorbid chronic PTPDs in insomnia patients may be attributed to the differences in the TBI severity and neuropathology of insomnia, which include mild TBI and cortical lesions. Furthermore, post-TBI new-onset executive dysfunction may trigger aggression, impulsivity and antisocial behavior [45, 46] and could be associated with PTPDs occurrence [6]. Here, a weaker executive dysfunction was also beholden in those having EDS and hypersomnia. Therefore, it is possible that post-TBI early executive dysfunction predisposes the comorbidity of PTPDs and chronic EDS or hypersomnia.
Specifically, it appears that the simultaneous occurrence of PTPDs and EDS or hypersomnia following TBI and during its chronic phase is probably due to the overlap between the centers controlling cognitive and sleep behaviors and their associated neuropathology. This means that severe brain stem lesions and DAI, especially in axon projections targeting the limbic system and diencephalon, may disrupt the inhibitory projections of monoaminergic nuclei of Arousal-Reticular Ascending System (ARAS), such as raphe nucleus, locus coeruleus on the Ventrolateral Preoptic nucleus (VLPO) of the hypothalamus and create hypersomnolence [16, 47].
Cognitive behaviors are regulated by mesencephalic subdivision of the limbic network, including dorsal raphe nucleus and locus coeruleus projecting the axonal fibers to cortical and subcortical centers of the limbic circuit. Thus, it is conceivable that the lesion of these neural structures and the axonal sharing in relevant white matter tracts lead to PTPDs occurrence [16, 48, 49]. In EDS, aggression is prevalent. Based on evidence, the dorsal raphe nucleus located in the brain stem has serotonergic transmission in the projections areas of prefrontal cortex, a main aggression control site [50], that plays an important role in the management of aggressive behaviors, and a lesion occurring in this nucleus causes antisocial behaviors [51]. Therefore, in patients with EDS, who mainly have brain stem lesions, damages of dorsal raphe nucleus or its serotonergic projections may take part in the occurrence of aggressive behaviors. Furthermore, acute depression was considered as a clinical marker predicting hypersomnia and turned to be chronic in more than half of the patients with hypersomnia.
Depression is associated with a decreased level of norepinephrine and central serotonin that their main sources are locus coeruleus nuclei and dorsal raphe in brainstem, respectively [52, 53]. The frontothalamic and frontostriatal circuits complicated in the modulation of executive functions of frontal lobe, emotional processing, and sleep-wake cycles, are highly vulnerable to TBI [39, 54]. The noradrenergic projections of locus coeruleus in brain stem towards neocortex and the limbic system modulate motivational, cognitive and goal-directed responses [55, 56].
Accordingly, it is believed that following long-lasting secondary neuronal insults caused by DAI and brain stem lesions, an abnormality occurs in the function of common neural networks regulating cognition, emotion, mood and wakefulness. The possibility of aggressive and depressive and hypersomnolence states increases by early cognitive and executive dysfunctions.
Another important result of our study was that the patients with insomnia showed weaker early executive functioning than non-PTSWD group. However, it was significantly better than the executive functioning of the patients with EDS and hypersomnia. This may be due to the higher frequency of mild lesions in insomnia patients, compared to EDS ones. The frontal lobe, especially DLPFC, is involved in the mental processes of executive function [57]. Therefore, executive dysfunction (mostly frontal lobe lesions) is possible in the insomnia group. Many of the white matter microstructural lesions associated with executive dysfunction following mild trauma are not visible on CT scans and conventional MR imaging used in our study [58, 59].
People with insomnia experienced a mild lesion. Thus, they were probably aware of their sustained disabilities and personal, professional and social failures. As a result, they were mainly prone to depression, anxiety, and nocturnal sleep problems. We also found that a high proportion of people with chronic fatigue experienced insomnia. It is evident that adenosine accumulation in the basal forebrain region occurs after prolonged periods of sleeplessness or sleep deprivation [60, 61]. Furthermore, reports indicate that increased plasma adenosine is associated with chronic fatigue syndrome [62]. Therefore, the activation of central adenosine receptors or elevation of its ligand in the basal forebrain of insomnia patients may be a possible mechanism for the occurrence of chronic fatigue in these patients.
Studies on the intrinsic architecture of brain white matter and individual sleep patterns have reported that poorer sleep duration and quality are associated with subtle white matter microstructural changes [60]. Therefore, it is hypothesized that individuals with a family history of sleep disorders are more likely to have an inherited susceptibility of the white matter architecture and susceptible to develop PTSWDs.
5. Conclusion
In this prospective study, we identified the risk factors that predispose the development of chronic PTSWDs. Based on the current results, mild TBI, the presence of cortical lesions, especially in the frontal lobe, and severe acute phase headaches can predict chronic insomnia, the most prevalent type of PTSWDs in the study, which was accompanied by fatigue more than others. Interestingly, the occurrence of EDS, which was mainly associated with chronic PTPDs, and especially aggression, was directly related to the lesion itself. Right lesions, especially DAI and brainstem lesions, were the most prominent markers of imaging. Additionally, severe brain injury was the most important clinical prognostic marker of chronic EDS.
Patients with acute depression and bilateral contusion were more susceptible to hypersomnia, which was associated with chronic depression. The prominent role of early executive dysfunction was evident in the development of common types of chronic PTSWDs. Investigating the efficacy of early neuro-cognitive rehabilitation in TBI patients to reduce the odds of chronic PTSWDs occurrence is recommended for the future studies. Furthermore, the early detection of neural circuits and network dysfunctions provoking various types of PTSWDs using advanced modalities of MRI such as functional MRI is a critical priority for early diagnosis and proper therapeutic interventions in these patients. Importantly, understanding of the neuropathogenesis of all types of PTSWDs may help in applying the noninvasive brain stimulation techniques modulating the pathological neural circuits involved in sleep-wake cycle disorders.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; They were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization, investigation, writing of original draft: Sara Ramezani; Critical review: Shahrokh Yousefzadeh, Sara Ramezani; and Editing of the work: Shahrokh Yousefzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the assistance of Guilan Road Trauma Research Center at Guilan University of Medical Sciences.
References
Chabok SY, Kapourchali SR, Leili EK, Saberi A, Mohtasham-Amiri Z. Effective factors on linguistic disorder during acute phase following Traumatic Brain Injury in adults. Neuropsychologia. 2012; 50(7):1444-50. [DOI:10.1016/j.neuropsychologia.2012.02.029] [PMID]
Chabok SY, Kapourchali SR, Saberi A, Mohtasham-Amiri Z. Operative and nonoperative linguistic outcomes in brain injury patients. Journal of the Neurological Sciences. 2012; 317(1-2):130-6. [DOI:10.1016/j.jns.2012.02.009] [PMID]
McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild Traumatic Brain Injury (MTBI) and chronic cognitive impairment: A scoping review. PLOS One. 2017; 12(4):e0174847 [DOI:10.1371/journal.pone.0174847]
Stocchetti N, Zanier ER. Chronic impact of Traumatic Brain Injury on outcome and quality of life: A narrative review. Critical Care. 2016; 20(1):148-51. [DOI:10.1186/s13054-016-1318-1]
Whitnall L, McMillan T, Murray G, Teasdale G. Disability in young people and adults after head injury: 5–7 year follow up of a prospective cohort study. Journal of Neurology, Neurosurgery & Psychiatry. 2006; 77(5):640-5. [DOI:10.1136/jnnp.2005.078246] [PMID] [PMCID]
Yousefzadeh-Chabok S, Ramezani S, Reihanian Z, Safaei M, Alijani B, Amini N. The role of early posttraumatic neuropsychological outcomes in the appearance of latter psychiatric disorders in adults with brain trauma. Asian Journal of Neurosurgery. 2015; 10(3):173-80. [DOI:10.4103/1793-5482.161165] [PMID] [PMCID]
Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep–wake disturbances 6 months after Traumatic Brain Injury: A prospective study. Brain. 2007; 130(7):1873-83. [DOI:10.1093/brain/awm109] [PMID]
Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in Traumatic Brain Injury. Journal of Clinical Sleep Medicine. 2007; 3(4):349-56. [PMID] [PMCID]
Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in Traumatic Brain Injury: A meta-analysis. Journal of Clinical Sleep Medicine. 2016; 12(3):419-28. [DOI:10.5664/jcsm.5598] [PMID] [PMCID]
Hou L, Han X, Sheng P, Tong W, Li Z, Xu D, et al. Risk factors associated with sleep disturbance following Traumatic Brain Injury: Clinical findings and questionnaire based study. PLoS One. 2013; 8(10):e76087. [DOI:10.1371/journal.pone.0076087]
Mathias J, Alvaro P. Prevalence of sleep disturbances, disorders, and problems following Traumatic Brain Injury: A meta-analysis. Sleep Medicine. 2012; 13(7):898-905. [DOI:10.1016/j.sleep.2012.04.006] [PMID]
Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation. 2006; 87(2):278-85. [DOI:10.1016/j.apmr.2005.10.024] [PMID]
Makley M, English J, Drubach D, Kreuz A, Celnik P, Tarwater P. Prevalence of sleep disturbance in closed head injury patients in a rehabilitation unit. Neurorehabilitation and Neural Repair. 2008; 22(4):341-7. [DOI:10.1177/1545968308315598] [PMID]
Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Archives of Physical Medicine and Rehabilitation. 2001; 82(11):1526-32. [DOI:10.1053/apmr.2001.26093] [PMID]
Rao V, Neubauer D, Vaishnavi S. Sleep disturbances after Traumatic Brain Injury. Psychiatric Times. 2015; 32(9):30-5. [DOI:10.1176/appi.psy.50.3.198]
Viola-Saltzman M, Musleh C. Traumatic Brain Injury-induced sleep disorders. Neuropsychiatric Disease And Treatment. 2016; 12:339-48. [DOI:10.2147/NDT.S69105] [PMID] [PMCID]
Zuzuárregui JRP, Bickart K, Kutscher SJ. A review of sleep disturbances following Traumatic Brain Injury. Sleep Science and Practice. 2018; 2(1):2. [DOI:10.1186/s41606-018-0020-4]
Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep–wake disturbances 3 years after Traumatic Brain Injury. Journal of Neurology, Neurosurgery & Psychiatry. 2010; 81(12):1402-5. [DOI:10.1136/jnnp.2009.201913] [PMID]
Orff HJ, Ayalon L, Drummond SP. Traumatic Brain Injury and sleep disturbance: A review of current research. The Journal of Head Trauma Rehabilitation. 2009; 24(3):155-65. [DOI:10.1097/HTR.0b013e3181a0b281] [PMID]
Bushnik T, Caplan B, Bogner J, Brenner L, Ponsford J, Schönberger M, et al. A model of fatigue following Traumatic Brain Injury. Journal of Head Trauma Rehabilitation. 2015; 30(4):277-82. [DOI:10.1097/HTR.0000000000000049] [PMID]
Guilleminault C, Yuen K, Gulevich M, Karadeniz D, Leger D, Philip P. Hypersomnia after head–neck trauma a medicolegal dilemma. Neurology. 2000; 54(3):653-9. [DOI:10.1212/WNL.54.3.653]
Ouellet MC, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with Traumatic Brain Injury: Frequency, characteristics, and risk factors. The Journal of Head Trauma Rehabilitation. 2006; 21(3):199-212. [DOI:10.1097/00001199-200605000-00001] [PMID]
Makley MJ, Johnson-Greene L, Tarwater PM, Kreuz AJ, Spiro J, Rao V, et al. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabilitation and Neural Repair. 2009; 23(4):320-6. [DOI:10.1177/1545968308325268] [PMID]
Shay N, Yeates KO, Walz NC, Stancin T, Taylor HG, Beebe DW, et al. Sleep problems and their relationship to cognitive and behavioral outcomes in young children with Traumatic Brain Injury. Journal of Neurotrauma. 2014; 31(14):1305-12. [DOI:10.1089/neu.2013.3275] [PMID] [PMCID]
Asken BM, Sullan MJ, Snyder AR, Houck ZM, Bryant VE, Hizel LP, et al. Factors influencing clinical correlates of Chronic Traumatic Encephalopathy (CTE): A review. Neuropsychology Review. 2016; 26(4):340-63. [DOI:10.1007/s11065-016-9327-z] [PMID] [PMCID]
Quan SF. Are sleep disturbances a risk for chronic traumatic encephalopathy? Only the shadow knows. Journal of Clinical Sleep Medicine. 2014; 10(3):241-2. [DOI:10.5664/jcsm.3516]
Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. The American Journal of Geriatric Psychiatry. 2014; 22(11):1262-71. [DOI:10.1016/j.jagp.2013.04.015] [PMID]
Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s Disease and cognitive decline in older persons. Sleep. 2013; 36(7):1027-32. [DOI:10.5665/sleep.2802]
Sturges BK, Dickinson PJ. Principles of neurosurgery. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. BSAVA manual of canine and feline neurology. Birmingham: British Small Animal Veterinary Association; 2014.
Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): Translation and validation study of the Iranian version. Health and Quality of Life Outcomes. 2003; 1(1):14. [DOI:10.1186/1477-7525-1-19] [PMID] [PMCID]
Foroughan M, Jafari Z, Shirin BP, Ghaem MFZ, Rahgozar M. [Validation of Mini-Mental State Examination (MMSE) in the elderly population of Tehran (Persian.)]. Advances in Cognitive Science. 2008; 10(2):29-37.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Philadelphia: American Psychiatric; 2013. [DOI:10.1176/appi.books.9780890425596]
Moghaddam JF, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep and Breathing. 2012; 16(1):79-82. [DOI:10.1007/s11325-010-0478-5] [PMID]
Yazdi Z, Sadeghniiat-Haghighi K, Zohal MA, Elmizadeh K. Validity and reliability of the Iranian version of the insomnia severity index. The Malaysian Journal of Medical Sciences. 2012; 19(4):31-6. [PMID] [PMCID]
Haghighi KS, Montazeri A, Mehrizi AK, Aminian O, Golkhandan AR, Saraei M, et al. The Epworth Sleepiness Scale: Tanslation and validation study of the Iranian version. Sleep and Breathing. 2013; 17(1):419-26. [DOI:10.1007/s11325-012-0646-x] [PMID]
Werner C, Engelhard K. Pathophysiology of Traumatic Brain Injury. British Journal of Anesthesia. 2007; 99(1):4-9. [DOI:10.1093/bja/aem131] [PMID]
Gilbert N, Bernier RA, Calhoun VD, Brenner E, Grossner E, Rajtmajer SM, et al. Diminished neural network dynamics after moderate and severe Traumatic Brain Injury. PLos One. 2018; 13(6):e0197419. [DOI:10.1371/journal.pone.0197419]
Van Der Horn HJ, Liemburg EJ, Scheenen ME, De Koning ME, Spikman JM, Van Der Naalt J. Graph analysis of functional brain networks in patients with mild Traumatic Brain Injury. PLoS One. 2017; 12(1):e0171031. [DOI:10.1371/journal.pone.0171031]
McAllister TW. Neurobiological consequences of Traumatic Brain Injury. Dialogues in Clinical Neuroscience. 2011; 13(3):287-300. [PMID] [PMCID]
Sharp DJ, Scott G, Leech R. Network dysfunction after Traumatic Brain Injury. Nature Reviews Neurology. 2014; 10(3):156-66. [DOI:10.1038/nrneurol.2014.15]
Klumpp H, Hosseini B, Phan KL. Self-reported sleep quality modulates amygdala resting-state functional connectivity in anxiety and depression. Frontiers in Psychiatry. 2018; 9:220. [DOI:10.3389/fpsyt.2018.00220] [PMID] [PMCID]
Pang R, Zhan Y, Zhang Y, Guo R, Wang J, Guo X, et al. Aberrant functional connectivity architecture in participants with chronic insomnia disorder accompanying cognitive dysfunction: A whole-brain, data-driven analysis. Frontiers in Neuroscience. 2017; 11(259):1-12. [DOI:10.3389/fnins.2017.00259] [PMID] [PMCID]
Wolf JA, Koch PF. Disruption of network synchrony and cognitive dysfunction after Traumatic Brain Injury. Frontiers in Systems Neuroscience. 2016; 10:43. [DOI:10.3389/fnsys.2016.00043] [PMID] [PMCID]
Xiao H, Yang Y, Xi JH, Chen ZQ. Structural and functional connectivity in Traumatic Brain Injury. Neural Regeneration Research. 2015; 10(12):2062-71. [DOI:10.4103/1673-5374.172328] [PMID] [PMCID]
Tlustos SJ, Peter Chiu C, Walz NC, Wade SL. Neural substrates of inhibitory and emotional processing in adolescents with Traumatic Brain Injury. Journal of Pediatric Rehabilitation Medicine. 2015; 8(4):321-33. [DOI:10.3233/PRM-150350] [PMID] [PMCID]
Wood RL, Worthington A. Neurobehavioral abnormalities associated with executive dysfunction after Traumatic Brain Injury. Frontiers in Behavioral Neuroscience. 2017; 11(43):195. [DOI:10.3389/fnbeh.2017.00195] [PMID] [PMCID]
Hauw JJ, Hausser Hauw C, De Girolami U, Hasboun D, Seilhean D. Neuropathology of sleep disorders: A review. Journal of Neuropathology & Experimental Neurology. 2011; 70(4):243-52. [DOI:10.1097/NEN.0b013e318211488e] [PMID]
Morse A, Garner D. Traumatic Brain Injury, sleep disorders, and psychiatric disorders: An under recognized relationship. Medical Sciences. 2018; 6(1):3-16. [DOI:10.3390/medsci6010015]
Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Research Reviews. 2009; 61(2):281-306. [DOI:10.1016/j.brainresrev.2009.08.001] [PMID]
Balázsfi D, Zelena D, Demeter K, Miskolczi C, Varga ZK, Nagyváradi Á, et al. Differential roles of the two raphe nuclei in amiable social behavior and aggression-an optogenetic study. Frontiers in Behavioral Neuroscience. 2018; 12:163. [DOI:10.3389/fnbeh.2018.00163] [PMID] [PMCID]
File SE, Deakin J. Chemical lesions of both dorsal and median raphe nuclei and changes in social and aggressive behaviour in rats. Pharmacology Biochemistry and Behavior. 1980; 12(6):855-9. [DOI:10.1016/0091-3057(80)90444-X]
Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric Disease and Treatment. 2011; 7(1):9-13. [PMID] [PMCID]
Schatzberg AF, Garlow SJ, Nemeroff CB. Molecular and cellular mechanisms in depression. Neuropsychopharmacology the Fifth Generation of Progress. 2002; 45(2):1039-50. [PMID]
McDonald BC, Flashman LA, Saykin AJ. Executive dysfunction following Traumatic Brain Injury: Neural substrates and treatment strategies. Neurorehabilitation. 2002; 17(4):333-44. [PMID]
Samuels E, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Current Neuropharmacology. 2008; 6(3):254-85. [DOI:10.2174/157015908785777193] [PMID] [PMCID]
Sara SJ, Bouret S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. 2012; 76(1):130-41. [DOI:10.1016/j.neuron.2012.09.011] [PMID]
Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function. 2010; 214(5-6):655-67. [DOI:10.1007/s00429-010-0262-0] [PMID] [PMCID]
Metting Z, Rödiger LA, De Keyser J, van der Naalt J. Structural and functional neuroimaging in mild-to-moderate head injury. The Lancet Neurology. 2007; 6(8):699-710. [DOI:10.1016/S1474-4422(07)70191-6]
Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT. Imaging evidence and recommendations for Traumatic Brain Injury: Advanced neuro-and neurovascular imaging techniques. American Journal of Neuroradiology. 2015; 36(2):1-11. [DOI:10.3174/ajnr.A4181] [PMID]
Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007; 18(18):1895-9. [DOI:10.1097/WNR.0b013e3282f262f6] [PMID]
Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, et al. Adenosine and sleep homeostasis in the basal forebrain. Journal of Neuroscience. 2006; 26(31):8092-100. [DOI:10.1523/JNEUROSCI.2181-06.2006] [PMID]
Duley JA, Garrick DP, Pratt DA. Raised plasma adenosine associated with chronic fatigue syndrome: A preliminary study. Journal of Chronic Fatigue Syndrome. 2000; 7(3):77-85. [DOI:10.1300/J092v07n03_07]
4. Discussion
The overall prevalence of new-onset sleep disturbances in the current study was estimated about 35%, consistent with some previous reports and inconsistent with some others [7, 10-12]. Apparently, insomnia is the most common post-TBI sleep problem, and is strongly associated with mild brain lesions, cortical lesions of frontal lobe, and severe headaches. According to the obtained results, most patients who suffered from PTSWDs had mild brain damages, and experienced frontal lobe cortical hematomas within 6 months after the lesion occurrence. Therefore, a possible reason for the higher incidence of insomnia in our study may be the high prevalence of mild TBI and cortical lesions of frontal lobe in all subjects with PTSWDs. Although high prevalence of TBI-induced insomnia found in our study was consistent with the results of Hou and colleagues [10], it did not confirm Baumann et al. study reports [7].
We also realized that a high frequency of sleep disruptions after insomnia belonged to EDS. Severe TBI strongly predicts the risk of developing EDS, and the frequency of severe injuries in people with PTSWDs is less than mild TBI. Thus, perhaps this is the reason why EDS was the second most commonly occurring type of PTSWDs in this study. The heterogeneity of TBI population is considered as one of the possible explanation for the discrepancy between the current and previous studies in terms of the prevalence of post-TBI sleep problems and its different types. We used self-reported questionnaires and subjective evaluations instead of objective tests such as polysomnography and multiple sleep latency tests to detect PTSWDs. Such methods might have led to underestimating the occurrence of PTSWDs, especially in people with DAI and severe brain damages who possibly experience an impaired self-awareness [31, 32].
Patients with insomnia obtained higher scores in PSQI and ISI than others, and ESS scores in patients with EDS were more than others. These findings may be due to the high sensitivity of PSQI and ISI for screening insomnia as well as ESS for diagnosing EDS.
Our study revealed that PTSWD patients suffered from acute headache, acute anxiety and depression (separately or together), early poor executive and cognitive functions, as well as chronic fatigue and PTPDs, more than non-PTSWDs ones. These sequels after TBI appear to be a sequence of destructive neurobiological processes resulted from secondary insults, including inflammation, excitotoxicity, abnormal perfusion, ischemia, and apoptotic or necrotic cell death. Additionally, its occurrence is inevitable at all degrees of TBI, although it is found in more severe lesions with worse clinical manifestations [11, 36].
These secondary neural injuries are associated with biomechanical, structural and functional changes in the damaged brain regions and corresponding homologous regions structurally or functionally connected with them [37, 38]. Subsequently, they cause the inefficiency of neural networks and imbalance of neurotransmitter systems regulating affective and cognitive behaviors [39, 40], that seem to contribute in the occurrence of sleep disorders [41, 42]. The patients with chronic sleep problems in comparison with non-PTSWDs ones had DAI and contusion especially in cortical, subcortical and brainstem regions. Therefore, it is believed that the early disruption of neural circuits caused by brain’s white and gray matter lesions negatively impacts cognitive and emotional processing [40, 43, 44], and leads to psychiatric [6] and sleep-wake cycle disturbances in the long term.
We also found that chronic comorbid PTPDs are more prevalent in EDS than the other categories of TBI-induced sleep disorders. However, all patients with PLMD and parasomnia and the majority of hypersomnia ones suffered from chronic PTPDs. A possibility to explain this result may be that the total number of patients with EDS is higher than the other types of sleep disorders except for insomnia. Aggression and depression were more prevalent than other PTPD types in patients with EDS and hypersomnia, respectively. Our prior study has highlighted that the most common chronic PTPDs, including aggression, depression, and PTSD, are associated with DAI, severe brain damages, and TBI, respectively [6]. Similarly, the current study revealed that factors affecting the appearance of EDS accompanied with PTPDs, included severe TBI, DAI and brain stem lesions.
The paucity of comorbid chronic PTPDs in insomnia patients may be attributed to the differences in the TBI severity and neuropathology of insomnia, which include mild TBI and cortical lesions. Furthermore, post-TBI new-onset executive dysfunction may trigger aggression, impulsivity and antisocial behavior [45, 46] and could be associated with PTPDs occurrence [6]. Here, a weaker executive dysfunction was also beholden in those having EDS and hypersomnia. Therefore, it is possible that post-TBI early executive dysfunction predisposes the comorbidity of PTPDs and chronic EDS or hypersomnia.
Specifically, it appears that the simultaneous occurrence of PTPDs and EDS or hypersomnia following TBI and during its chronic phase is probably due to the overlap between the centers controlling cognitive and sleep behaviors and their associated neuropathology. This means that severe brain stem lesions and DAI, especially in axon projections targeting the limbic system and diencephalon, may disrupt the inhibitory projections of monoaminergic nuclei of Arousal-Reticular Ascending System (ARAS), such as raphe nucleus, locus coeruleus on the Ventrolateral Preoptic nucleus (VLPO) of the hypothalamus and create hypersomnolence [16, 47].
Cognitive behaviors are regulated by mesencephalic subdivision of the limbic network, including dorsal raphe nucleus and locus coeruleus projecting the axonal fibers to cortical and subcortical centers of the limbic circuit. Thus, it is conceivable that the lesion of these neural structures and the axonal sharing in relevant white matter tracts lead to PTPDs occurrence [16, 48, 49]. In EDS, aggression is prevalent. Based on evidence, the dorsal raphe nucleus located in the brain stem has serotonergic transmission in the projections areas of prefrontal cortex, a main aggression control site [50], that plays an important role in the management of aggressive behaviors, and a lesion occurring in this nucleus causes antisocial behaviors [51]. Therefore, in patients with EDS, who mainly have brain stem lesions, damages of dorsal raphe nucleus or its serotonergic projections may take part in the occurrence of aggressive behaviors. Furthermore, acute depression was considered as a clinical marker predicting hypersomnia and turned to be chronic in more than half of the patients with hypersomnia.
Depression is associated with a decreased level of norepinephrine and central serotonin that their main sources are locus coeruleus nuclei and dorsal raphe in brainstem, respectively [52, 53]. The frontothalamic and frontostriatal circuits complicated in the modulation of executive functions of frontal lobe, emotional processing, and sleep-wake cycles, are highly vulnerable to TBI [39, 54]. The noradrenergic projections of locus coeruleus in brain stem towards neocortex and the limbic system modulate motivational, cognitive and goal-directed responses [55, 56].
Accordingly, it is believed that following long-lasting secondary neuronal insults caused by DAI and brain stem lesions, an abnormality occurs in the function of common neural networks regulating cognition, emotion, mood and wakefulness. The possibility of aggressive and depressive and hypersomnolence states increases by early cognitive and executive dysfunctions.
Another important result of our study was that the patients with insomnia showed weaker early executive functioning than non-PTSWD group. However, it was significantly better than the executive functioning of the patients with EDS and hypersomnia. This may be due to the higher frequency of mild lesions in insomnia patients, compared to EDS ones. The frontal lobe, especially DLPFC, is involved in the mental processes of executive function [57]. Therefore, executive dysfunction (mostly frontal lobe lesions) is possible in the insomnia group. Many of the white matter microstructural lesions associated with executive dysfunction following mild trauma are not visible on CT scans and conventional MR imaging used in our study [58, 59].
People with insomnia experienced a mild lesion. Thus, they were probably aware of their sustained disabilities and personal, professional and social failures. As a result, they were mainly prone to depression, anxiety, and nocturnal sleep problems. We also found that a high proportion of people with chronic fatigue experienced insomnia. It is evident that adenosine accumulation in the basal forebrain region occurs after prolonged periods of sleeplessness or sleep deprivation [60, 61]. Furthermore, reports indicate that increased plasma adenosine is associated with chronic fatigue syndrome [62]. Therefore, the activation of central adenosine receptors or elevation of its ligand in the basal forebrain of insomnia patients may be a possible mechanism for the occurrence of chronic fatigue in these patients.
Studies on the intrinsic architecture of brain white matter and individual sleep patterns have reported that poorer sleep duration and quality are associated with subtle white matter microstructural changes [60]. Therefore, it is hypothesized that individuals with a family history of sleep disorders are more likely to have an inherited susceptibility of the white matter architecture and susceptible to develop PTSWDs.
5. Conclusion
In this prospective study, we identified the risk factors that predispose the development of chronic PTSWDs. Based on the current results, mild TBI, the presence of cortical lesions, especially in the frontal lobe, and severe acute phase headaches can predict chronic insomnia, the most prevalent type of PTSWDs in the study, which was accompanied by fatigue more than others. Interestingly, the occurrence of EDS, which was mainly associated with chronic PTPDs, and especially aggression, was directly related to the lesion itself. Right lesions, especially DAI and brainstem lesions, were the most prominent markers of imaging. Additionally, severe brain injury was the most important clinical prognostic marker of chronic EDS.
Patients with acute depression and bilateral contusion were more susceptible to hypersomnia, which was associated with chronic depression. The prominent role of early executive dysfunction was evident in the development of common types of chronic PTSWDs. Investigating the efficacy of early neuro-cognitive rehabilitation in TBI patients to reduce the odds of chronic PTSWDs occurrence is recommended for the future studies. Furthermore, the early detection of neural circuits and network dysfunctions provoking various types of PTSWDs using advanced modalities of MRI such as functional MRI is a critical priority for early diagnosis and proper therapeutic interventions in these patients. Importantly, understanding of the neuropathogenesis of all types of PTSWDs may help in applying the noninvasive brain stimulation techniques modulating the pathological neural circuits involved in sleep-wake cycle disorders.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; They were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization, investigation, writing of original draft: Sara Ramezani; Critical review: Shahrokh Yousefzadeh, Sara Ramezani; and Editing of the work: Shahrokh Yousefzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the assistance of Guilan Road Trauma Research Center at Guilan University of Medical Sciences.
References
Chabok SY, Kapourchali SR, Leili EK, Saberi A, Mohtasham-Amiri Z. Effective factors on linguistic disorder during acute phase following Traumatic Brain Injury in adults. Neuropsychologia. 2012; 50(7):1444-50. [DOI:10.1016/j.neuropsychologia.2012.02.029] [PMID]
Chabok SY, Kapourchali SR, Saberi A, Mohtasham-Amiri Z. Operative and nonoperative linguistic outcomes in brain injury patients. Journal of the Neurological Sciences. 2012; 317(1-2):130-6. [DOI:10.1016/j.jns.2012.02.009] [PMID]
McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild Traumatic Brain Injury (MTBI) and chronic cognitive impairment: A scoping review. PLOS One. 2017; 12(4):e0174847 [DOI:10.1371/journal.pone.0174847]
Stocchetti N, Zanier ER. Chronic impact of Traumatic Brain Injury on outcome and quality of life: A narrative review. Critical Care. 2016; 20(1):148-51. [DOI:10.1186/s13054-016-1318-1]
Whitnall L, McMillan T, Murray G, Teasdale G. Disability in young people and adults after head injury: 5–7 year follow up of a prospective cohort study. Journal of Neurology, Neurosurgery & Psychiatry. 2006; 77(5):640-5. [DOI:10.1136/jnnp.2005.078246] [PMID] [PMCID]
Yousefzadeh-Chabok S, Ramezani S, Reihanian Z, Safaei M, Alijani B, Amini N. The role of early posttraumatic neuropsychological outcomes in the appearance of latter psychiatric disorders in adults with brain trauma. Asian Journal of Neurosurgery. 2015; 10(3):173-80. [DOI:10.4103/1793-5482.161165] [PMID] [PMCID]
Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep–wake disturbances 6 months after Traumatic Brain Injury: A prospective study. Brain. 2007; 130(7):1873-83. [DOI:10.1093/brain/awm109] [PMID]
Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in Traumatic Brain Injury. Journal of Clinical Sleep Medicine. 2007; 3(4):349-56. [PMID] [PMCID]
Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in Traumatic Brain Injury: A meta-analysis. Journal of Clinical Sleep Medicine. 2016; 12(3):419-28. [DOI:10.5664/jcsm.5598] [PMID] [PMCID]
Hou L, Han X, Sheng P, Tong W, Li Z, Xu D, et al. Risk factors associated with sleep disturbance following Traumatic Brain Injury: Clinical findings and questionnaire based study. PLoS One. 2013; 8(10):e76087. [DOI:10.1371/journal.pone.0076087]
Mathias J, Alvaro P. Prevalence of sleep disturbances, disorders, and problems following Traumatic Brain Injury: A meta-analysis. Sleep Medicine. 2012; 13(7):898-905. [DOI:10.1016/j.sleep.2012.04.006] [PMID]
Parcell DL, Ponsford JL, Rajaratnam SM, Redman JR. Self-reported changes to nighttime sleep after Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation. 2006; 87(2):278-85. [DOI:10.1016/j.apmr.2005.10.024] [PMID]
Makley M, English J, Drubach D, Kreuz A, Celnik P, Tarwater P. Prevalence of sleep disturbance in closed head injury patients in a rehabilitation unit. Neurorehabilitation and Neural Repair. 2008; 22(4):341-7. [DOI:10.1177/1545968308315598] [PMID]
Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Archives of Physical Medicine and Rehabilitation. 2001; 82(11):1526-32. [DOI:10.1053/apmr.2001.26093] [PMID]
Rao V, Neubauer D, Vaishnavi S. Sleep disturbances after Traumatic Brain Injury. Psychiatric Times. 2015; 32(9):30-5. [DOI:10.1176/appi.psy.50.3.198]
Viola-Saltzman M, Musleh C. Traumatic Brain Injury-induced sleep disorders. Neuropsychiatric Disease And Treatment. 2016; 12:339-48. [DOI:10.2147/NDT.S69105] [PMID] [PMCID]
Zuzuárregui JRP, Bickart K, Kutscher SJ. A review of sleep disturbances following Traumatic Brain Injury. Sleep Science and Practice. 2018; 2(1):2. [DOI:10.1186/s41606-018-0020-4]
Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep–wake disturbances 3 years after Traumatic Brain Injury. Journal of Neurology, Neurosurgery & Psychiatry. 2010; 81(12):1402-5. [DOI:10.1136/jnnp.2009.201913] [PMID]
Orff HJ, Ayalon L, Drummond SP. Traumatic Brain Injury and sleep disturbance: A review of current research. The Journal of Head Trauma Rehabilitation. 2009; 24(3):155-65. [DOI:10.1097/HTR.0b013e3181a0b281] [PMID]
Bushnik T, Caplan B, Bogner J, Brenner L, Ponsford J, Schönberger M, et al. A model of fatigue following Traumatic Brain Injury. Journal of Head Trauma Rehabilitation. 2015; 30(4):277-82. [DOI:10.1097/HTR.0000000000000049] [PMID]
Guilleminault C, Yuen K, Gulevich M, Karadeniz D, Leger D, Philip P. Hypersomnia after head–neck trauma a medicolegal dilemma. Neurology. 2000; 54(3):653-9. [DOI:10.1212/WNL.54.3.653]
Ouellet MC, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with Traumatic Brain Injury: Frequency, characteristics, and risk factors. The Journal of Head Trauma Rehabilitation. 2006; 21(3):199-212. [DOI:10.1097/00001199-200605000-00001] [PMID]
Makley MJ, Johnson-Greene L, Tarwater PM, Kreuz AJ, Spiro J, Rao V, et al. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabilitation and Neural Repair. 2009; 23(4):320-6. [DOI:10.1177/1545968308325268] [PMID]
Shay N, Yeates KO, Walz NC, Stancin T, Taylor HG, Beebe DW, et al. Sleep problems and their relationship to cognitive and behavioral outcomes in young children with Traumatic Brain Injury. Journal of Neurotrauma. 2014; 31(14):1305-12. [DOI:10.1089/neu.2013.3275] [PMID] [PMCID]
Asken BM, Sullan MJ, Snyder AR, Houck ZM, Bryant VE, Hizel LP, et al. Factors influencing clinical correlates of Chronic Traumatic Encephalopathy (CTE): A review. Neuropsychology Review. 2016; 26(4):340-63. [DOI:10.1007/s11065-016-9327-z] [PMID] [PMCID]
Quan SF. Are sleep disturbances a risk for chronic traumatic encephalopathy? Only the shadow knows. Journal of Clinical Sleep Medicine. 2014; 10(3):241-2. [DOI:10.5664/jcsm.3516]
Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. The American Journal of Geriatric Psychiatry. 2014; 22(11):1262-71. [DOI:10.1016/j.jagp.2013.04.015] [PMID]
Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s Disease and cognitive decline in older persons. Sleep. 2013; 36(7):1027-32. [DOI:10.5665/sleep.2802]
Sturges BK, Dickinson PJ. Principles of neurosurgery. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. BSAVA manual of canine and feline neurology. Birmingham: British Small Animal Veterinary Association; 2014.
Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): Translation and validation study of the Iranian version. Health and Quality of Life Outcomes. 2003; 1(1):14. [DOI:10.1186/1477-7525-1-19] [PMID] [PMCID]
Foroughan M, Jafari Z, Shirin BP, Ghaem MFZ, Rahgozar M. [Validation of Mini-Mental State Examination (MMSE) in the elderly population of Tehran (Persian.)]. Advances in Cognitive Science. 2008; 10(2):29-37.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Philadelphia: American Psychiatric; 2013. [DOI:10.1176/appi.books.9780890425596]
Moghaddam JF, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep and Breathing. 2012; 16(1):79-82. [DOI:10.1007/s11325-010-0478-5] [PMID]
Yazdi Z, Sadeghniiat-Haghighi K, Zohal MA, Elmizadeh K. Validity and reliability of the Iranian version of the insomnia severity index. The Malaysian Journal of Medical Sciences. 2012; 19(4):31-6. [PMID] [PMCID]
Haghighi KS, Montazeri A, Mehrizi AK, Aminian O, Golkhandan AR, Saraei M, et al. The Epworth Sleepiness Scale: Tanslation and validation study of the Iranian version. Sleep and Breathing. 2013; 17(1):419-26. [DOI:10.1007/s11325-012-0646-x] [PMID]
Werner C, Engelhard K. Pathophysiology of Traumatic Brain Injury. British Journal of Anesthesia. 2007; 99(1):4-9. [DOI:10.1093/bja/aem131] [PMID]
Gilbert N, Bernier RA, Calhoun VD, Brenner E, Grossner E, Rajtmajer SM, et al. Diminished neural network dynamics after moderate and severe Traumatic Brain Injury. PLos One. 2018; 13(6):e0197419. [DOI:10.1371/journal.pone.0197419]
Van Der Horn HJ, Liemburg EJ, Scheenen ME, De Koning ME, Spikman JM, Van Der Naalt J. Graph analysis of functional brain networks in patients with mild Traumatic Brain Injury. PLoS One. 2017; 12(1):e0171031. [DOI:10.1371/journal.pone.0171031]
McAllister TW. Neurobiological consequences of Traumatic Brain Injury. Dialogues in Clinical Neuroscience. 2011; 13(3):287-300. [PMID] [PMCID]
Sharp DJ, Scott G, Leech R. Network dysfunction after Traumatic Brain Injury. Nature Reviews Neurology. 2014; 10(3):156-66. [DOI:10.1038/nrneurol.2014.15]
Klumpp H, Hosseini B, Phan KL. Self-reported sleep quality modulates amygdala resting-state functional connectivity in anxiety and depression. Frontiers in Psychiatry. 2018; 9:220. [DOI:10.3389/fpsyt.2018.00220] [PMID] [PMCID]
Pang R, Zhan Y, Zhang Y, Guo R, Wang J, Guo X, et al. Aberrant functional connectivity architecture in participants with chronic insomnia disorder accompanying cognitive dysfunction: A whole-brain, data-driven analysis. Frontiers in Neuroscience. 2017; 11(259):1-12. [DOI:10.3389/fnins.2017.00259] [PMID] [PMCID]
Wolf JA, Koch PF. Disruption of network synchrony and cognitive dysfunction after Traumatic Brain Injury. Frontiers in Systems Neuroscience. 2016; 10:43. [DOI:10.3389/fnsys.2016.00043] [PMID] [PMCID]
Xiao H, Yang Y, Xi JH, Chen ZQ. Structural and functional connectivity in Traumatic Brain Injury. Neural Regeneration Research. 2015; 10(12):2062-71. [DOI:10.4103/1673-5374.172328] [PMID] [PMCID]
Tlustos SJ, Peter Chiu C, Walz NC, Wade SL. Neural substrates of inhibitory and emotional processing in adolescents with Traumatic Brain Injury. Journal of Pediatric Rehabilitation Medicine. 2015; 8(4):321-33. [DOI:10.3233/PRM-150350] [PMID] [PMCID]
Wood RL, Worthington A. Neurobehavioral abnormalities associated with executive dysfunction after Traumatic Brain Injury. Frontiers in Behavioral Neuroscience. 2017; 11(43):195. [DOI:10.3389/fnbeh.2017.00195] [PMID] [PMCID]
Hauw JJ, Hausser Hauw C, De Girolami U, Hasboun D, Seilhean D. Neuropathology of sleep disorders: A review. Journal of Neuropathology & Experimental Neurology. 2011; 70(4):243-52. [DOI:10.1097/NEN.0b013e318211488e] [PMID]
Morse A, Garner D. Traumatic Brain Injury, sleep disorders, and psychiatric disorders: An under recognized relationship. Medical Sciences. 2018; 6(1):3-16. [DOI:10.3390/medsci6010015]
Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Research Reviews. 2009; 61(2):281-306. [DOI:10.1016/j.brainresrev.2009.08.001] [PMID]
Balázsfi D, Zelena D, Demeter K, Miskolczi C, Varga ZK, Nagyváradi Á, et al. Differential roles of the two raphe nuclei in amiable social behavior and aggression-an optogenetic study. Frontiers in Behavioral Neuroscience. 2018; 12:163. [DOI:10.3389/fnbeh.2018.00163] [PMID] [PMCID]
File SE, Deakin J. Chemical lesions of both dorsal and median raphe nuclei and changes in social and aggressive behaviour in rats. Pharmacology Biochemistry and Behavior. 1980; 12(6):855-9. [DOI:10.1016/0091-3057(80)90444-X]
Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric Disease and Treatment. 2011; 7(1):9-13. [PMID] [PMCID]
Schatzberg AF, Garlow SJ, Nemeroff CB. Molecular and cellular mechanisms in depression. Neuropsychopharmacology the Fifth Generation of Progress. 2002; 45(2):1039-50. [PMID]
McDonald BC, Flashman LA, Saykin AJ. Executive dysfunction following Traumatic Brain Injury: Neural substrates and treatment strategies. Neurorehabilitation. 2002; 17(4):333-44. [PMID]
Samuels E, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Current Neuropharmacology. 2008; 6(3):254-85. [DOI:10.2174/157015908785777193] [PMID] [PMCID]
Sara SJ, Bouret S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. 2012; 76(1):130-41. [DOI:10.1016/j.neuron.2012.09.011] [PMID]
Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function. 2010; 214(5-6):655-67. [DOI:10.1007/s00429-010-0262-0] [PMID] [PMCID]
Metting Z, Rödiger LA, De Keyser J, van der Naalt J. Structural and functional neuroimaging in mild-to-moderate head injury. The Lancet Neurology. 2007; 6(8):699-710. [DOI:10.1016/S1474-4422(07)70191-6]
Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT. Imaging evidence and recommendations for Traumatic Brain Injury: Advanced neuro-and neurovascular imaging techniques. American Journal of Neuroradiology. 2015; 36(2):1-11. [DOI:10.3174/ajnr.A4181] [PMID]
Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007; 18(18):1895-9. [DOI:10.1097/WNR.0b013e3282f262f6] [PMID]
Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, et al. Adenosine and sleep homeostasis in the basal forebrain. Journal of Neuroscience. 2006; 26(31):8092-100. [DOI:10.1523/JNEUROSCI.2181-06.2006] [PMID]
Duley JA, Garrick DP, Pratt DA. Raised plasma adenosine associated with chronic fatigue syndrome: A preliminary study. Journal of Chronic Fatigue Syndrome. 2000; 7(3):77-85. [DOI:10.1300/J092v07n03_07]
Type of Study: Review |
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |