Thu, Jul 3, 2025
Volume 4, Issue 4 (Autumn 2018)
Iran J Neurosurg 2018, 4(4): 219-224 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Behzadnia H, Hoseinzadeh J, Heydari T, Andalib S. Evaluation of Prevalence and Risk Factors of Post-Craniotomy Meningitis in Non-Emergency Patients. Iran J Neurosurg 2018; 4 (4) :219-224
URL: http://irjns.org/article-1-151-en.html
URL: http://irjns.org/article-1-151-en.html
1- Department of Neurosurgery, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
2- Georgia Institute of Technology, College of Sciences, Atlanta, Georgia, USA
3- Neuroscience Research Center, Department of Neurosurgery, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran; Guilan Road Trauma Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran ,sasan.andalib@yahoo.co.uk
2- Georgia Institute of Technology, College of Sciences, Atlanta, Georgia, USA
3- Neuroscience Research Center, Department of Neurosurgery, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran; Guilan Road Trauma Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran ,
Keywords: Craniotomy, Meningitis, Glasgow Comma Scale (GCS), External Ventricular Drainage(EVD), Hydrocephalus, Prevalence, Risk factors
Full Text [PDF 469 kb]
(1585 Downloads)
| Abstract (HTML) (5309 Views)
Full Text: (1854 Views)
1. Introduction
raniotomy is a surgical procedure in which a part of the skull is removed to reveal the meninges and brain tissue. According to previous studies, the occurrence of meningitis increases after craniotomy, but this rate varies due to different risk factors [1-7]. Meningitis increases the morbidity and mortality of patients [1-3]. Therefore, identifying risk factors and finding solutions to prevent and control them can reduce the incidence of Post-Craniotomy Meningitis (PCM), and thus decrease its resulting complications. Identifying the Post-Craniotomy Meningitis (PCM) microorganisms involved and the risk factors including diabetes, a peri-craniotomy External Ventricular Drain (EVD), duration of surgery, and pre-operative corticosteroid use can help physicians accurately control them and ultimately reduce the occurrence of meningitis and its resulting complications. The aim of the present study is to evaluate the clinical parameters that lead to meningitis in patients who have underwent non-emergency craniotomy in the neurosurgical operation rooms of Poursina Hospital in Rasht, Iran.
2. Materials and Methods/ Patients
Subsequent to reviewing the medical records of patients operated on by the neurosurgery department of Poursina Hospital of the Guilan University of Medical Sciences in Rasht from September 23, 2016 to September 22, 2017, the patient information for this study was collected.
The data collected includes the duration of hospitalization, the presence of underlying diseases (heart failure, chronic obstructive pulmonary disease, diabetes, chronic renal failure, cirrhosis, tissue disease and cancer), Glasgow Coma Scale (GCS), previous use of corticosteroid, preoperative antibiotic use, duration of surgery, indications for the surgery (brain tumor, trauma, brain cerebrovascular disease, and hydrocephalus among others),type of anesthesia (general, epidural, and local),synchronous infection (lower respiratory tract infection, urinary tract infection, gastrointestinal, blood, and wound infections), an External Ventricular Drain (EVD), time of death, post-surgical complications,microorganisms involved in meningitis, and other noteworthy information.
All of these patients underwent a non-emergency craniotomy and survived at least 7 days after surgery. Exclusion criteria included traumatic surgery, burr hole surgery, stereotactic surgery, and trans-sphenoidal surgery. Due to the retrospective nature of the study, the data was recorded without obtaining any consent forms, so it was presented anonymously.
The data was analyzed using SPSS software, Version 23.0. Categorical variables were compared by using a chi-square test analysis.Odds Ratio (OR) was also calculated with a 95% confidence interval (95% CI).
3. Results
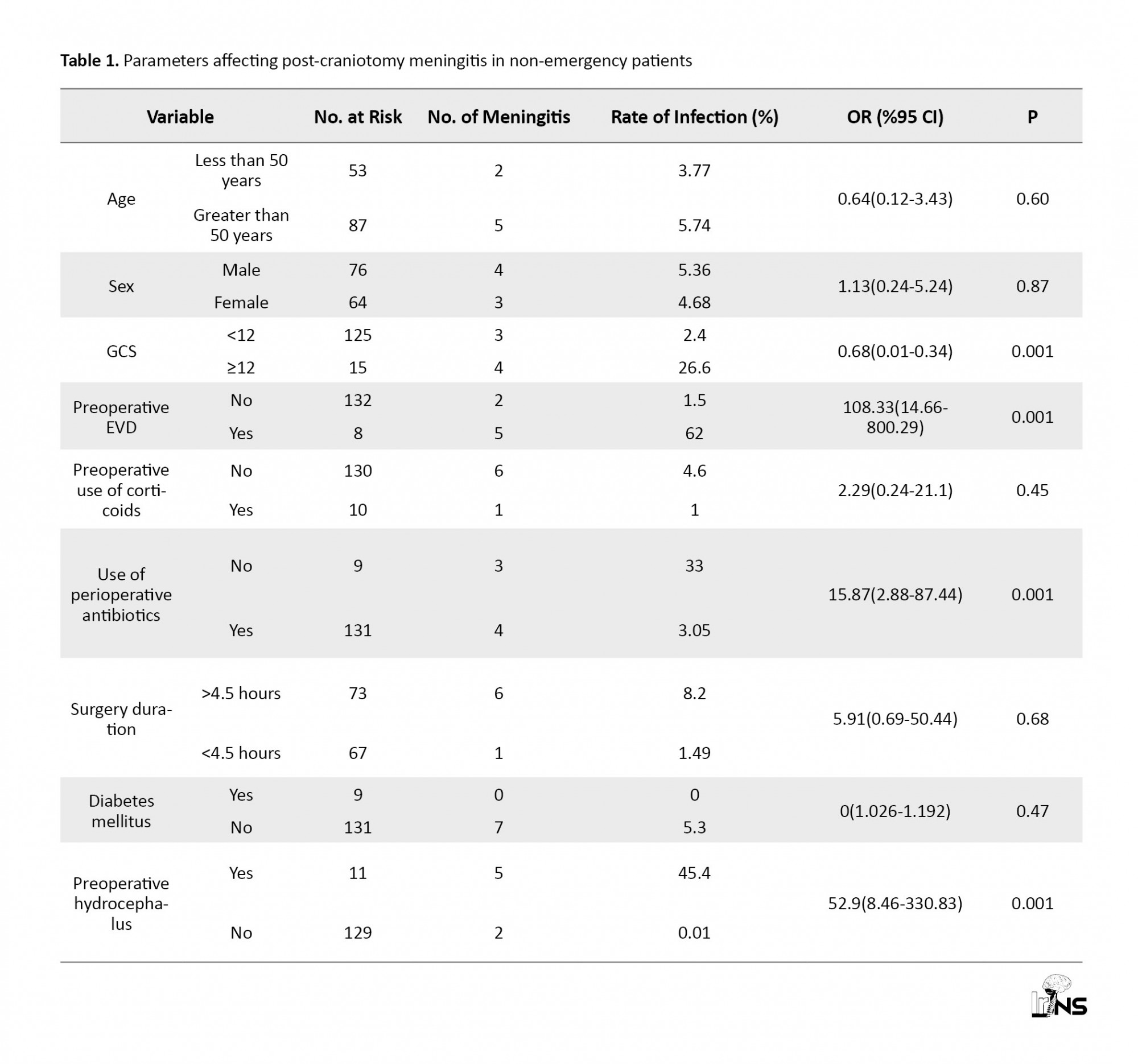
In the present study, of 986 non-emergency patients, 140 cases (14.2%) underwent craniotomy.After craniotomy, none of the patients died within 7 days, and 7 patients (5%) suffered from meningitis. Table 1 illustrates parameters affecting Post-Craniotomy Meningitis in non-emergency (PCM) patients. The prevalence of meningitis after non-emergency craniotomy for those younger than 50 years old was higher than that of those who were older than 50 years (5.74% vs. 3.77%). 54% of the patients were male, and 4 of 7 patients with meningitis were male (57%). 15 patients (10.7%) had a GCS <12, and 4 of them (26.6%) suffered from meningitis (p=0.001).8 patients (5.7 %) had an EVD before craniotomy, and 5 of 8 cases suffered from PCM.
raniotomy is a surgical procedure in which a part of the skull is removed to reveal the meninges and brain tissue. According to previous studies, the occurrence of meningitis increases after craniotomy, but this rate varies due to different risk factors [1-7]. Meningitis increases the morbidity and mortality of patients [1-3]. Therefore, identifying risk factors and finding solutions to prevent and control them can reduce the incidence of Post-Craniotomy Meningitis (PCM), and thus decrease its resulting complications. Identifying the Post-Craniotomy Meningitis (PCM) microorganisms involved and the risk factors including diabetes, a peri-craniotomy External Ventricular Drain (EVD), duration of surgery, and pre-operative corticosteroid use can help physicians accurately control them and ultimately reduce the occurrence of meningitis and its resulting complications. The aim of the present study is to evaluate the clinical parameters that lead to meningitis in patients who have underwent non-emergency craniotomy in the neurosurgical operation rooms of Poursina Hospital in Rasht, Iran.
2. Materials and Methods/ Patients
Subsequent to reviewing the medical records of patients operated on by the neurosurgery department of Poursina Hospital of the Guilan University of Medical Sciences in Rasht from September 23, 2016 to September 22, 2017, the patient information for this study was collected.
The data collected includes the duration of hospitalization, the presence of underlying diseases (heart failure, chronic obstructive pulmonary disease, diabetes, chronic renal failure, cirrhosis, tissue disease and cancer), Glasgow Coma Scale (GCS), previous use of corticosteroid, preoperative antibiotic use, duration of surgery, indications for the surgery (brain tumor, trauma, brain cerebrovascular disease, and hydrocephalus among others),type of anesthesia (general, epidural, and local),synchronous infection (lower respiratory tract infection, urinary tract infection, gastrointestinal, blood, and wound infections), an External Ventricular Drain (EVD), time of death, post-surgical complications,microorganisms involved in meningitis, and other noteworthy information.
All of these patients underwent a non-emergency craniotomy and survived at least 7 days after surgery. Exclusion criteria included traumatic surgery, burr hole surgery, stereotactic surgery, and trans-sphenoidal surgery. Due to the retrospective nature of the study, the data was recorded without obtaining any consent forms, so it was presented anonymously.
The data was analyzed using SPSS software, Version 23.0. Categorical variables were compared by using a chi-square test analysis.Odds Ratio (OR) was also calculated with a 95% confidence interval (95% CI).
3. Results
In the present study, of 986 non-emergency patients, 140 cases (14.2%) underwent craniotomy.After craniotomy, none of the patients died within 7 days, and 7 patients (5%) suffered from meningitis. Table 1 illustrates parameters affecting Post-Craniotomy Meningitis in non-emergency (PCM) patients. The prevalence of meningitis after non-emergency craniotomy for those younger than 50 years old was higher than that of those who were older than 50 years (5.74% vs. 3.77%). 54% of the patients were male, and 4 of 7 patients with meningitis were male (57%). 15 patients (10.7%) had a GCS <12, and 4 of them (26.6%) suffered from meningitis (p=0.001).8 patients (5.7 %) had an EVD before craniotomy, and 5 of 8 cases suffered from PCM.
Eleven patients (7.8%) had hydrocephalus before the operation, and 5 of those patients (45.5%) suffered from PCM (p=0.001). For all patients, prophylactic antibiotics were injected 1 hour before the craniotomy; however, 5% (7 cases) suffered from meningitis. The operation lasted longer than 4.5 hours in 73 patients (52.1%); 6 cases of them (8.2%) suffered from PCM (P=0.68). 1 of 10 patients with the use of corticosteroids suffered from PCM. 9 patients had diabetes, but none of them suffered from meningitis. 6 of 81 patients with brain tumors (7.4%) and 1 of 29 patients with aneurysms (3.4%) suffered from PCM. Three patients died from the 8th to 30th day after surgery, and 2 of them had meningitis. None of the patients with cranioplasty, or craniosynostosis surgery, and Chiari malformation surgery suffered from PCM. The unilateral analysis of the risk factors for PCM and the variables involved them are presented in Table 1.Factors with a P value less than 0.05 in a uniform analysis include: a GCS <12, preoperative EVD, perioperative antibiotics, and preoperative hydrocephalus. There were 81 patients with brain tumors of which patients (6.2%) experienced meningitis. One (3.4%) out of the 29 patients with a brain vascular aneurysm had meningitis. There were 23 undergoing cranioplasty of which 1 (4.3%) experienced meningitis. There were 4 patients with Craniosynostosis and 1 with Chiari malformation, but none of them had meningitis.
Seven CSF samples were sent for detection of pathogens in our study. Positive cultures were only observed in 3 patients (42.9%). All three organisms were Acinetobacter spp (100%).
4. Discussion
The occurrence of PCM has been reported to be approximately 0.3-8.9% in the literature [3-8, 5-15], but 8.6% in the Chen et al. study [1]. In our study, the prevalence of meningitis was 5%. The lower PCM level may be due to the non-emergency aspect of our surgeries.
In the literature, gram-negative rods and Enterobacteriaceae have had an important role in the incidence of PCM [1-4, 8-10, 13-19]. The rates of positive cultures in the studies of Chen [1] and Kourbeti [2] were 10.4 %, and 100%, respectively. The most common microorganisms in the Chen [1] and Kourbeti [2] studies were Acinetobacter spp, with rates accounting for 40% and 45%, respectively. Seven CSF samples were sent for detection of pathogens in our study. Positive cultures were only observed in 3 patients (42.9%). All three microorganisms were Acinetobacter spp (100%).
Diabetic patients were at a higher risk of developing meningitis than those without diabetes. The occurrence of PCM in diabetic patients was 18.5% in Chen’s study and 4.1% in Kourbe’s study, but it was 0% in our study. The lack of meningitis in our study may be due to the precise control of blood glucose levels prior to our non-emergency craniotomy or the absence of patients who required emergency attention.Therefore, we suggest further studies to prove these predictions.
Of the 131 patients in our study who received prophylactic antibiotics an hour before surgery, 3% of them (4 patients) suffered from meningitis. In the Chen et al. study [1], 10.8% of the patients suffered from meningitis. Barker’s study [20] documents the benefits of antibiotics to prevent PCM. In our study, a significant relationship between the preoperative antibiotics and the prevention of PCM was found (P=0.001).
The rate of the effect of steroids on the occurrence of PCM varied throughout the mentioned studies [7, 21, 22]. In the Chen [1] and Kourbeti [2] studies, a 5% and 7.10% rate was found, and the relationship was statistically significant in the Kourbeti study [2]. Among our patients with a history of corticosteroid use, no significant relationship was found (P=0.45).
In our study, 8.2% of patients with a craniotomy lasting longer than 4.5 hours had meningitis (6 off 7 patients with PCM). Despite this observation, there was no significant relationship between craniotomy duration and meningitis (P=0.68), as in previous studies [1, 2].
According to previous studies, craniotomy in patients with EVD increases the occurrence of meningitis [1-27]. In our study, 57% of the patients who had EVD (4 cases) suffered from PCM. To prevent PCM, we recommend that an EVD is avoided in unnecessary conditions, and if it is present, it should be removed as soon as possible.
Eleven patients (7.8%) had hydrocephalus prior to craniotomy, and 5 patients (45.4%) suffered from PCM, resulting in a significant relationship between hydrocephalus prior to craniotomy and PCM (P=0.001). Therefore, it is recommended that special attention be paid to this significant relationship in future studies. In our study, the greatest limitations were the fact that it was retrospective and dependent on the accuracy of the data in the clinical charts.
5. Conclusion
Unlike many studies, this study was conducted on non-emergency patients. We determined the prevalence, risk factors, and microbiology that can lead to PCM in our patients.We were able to confirm the importance of perioperative hydrocephalus, perioperative ventricular drains, perioperative antibiotics, and a GCS <12 in the development of PCM. The perioperative steroids, diabetes mellitus, duration of surgery, gender, and age were not significant risk factors for PCM. From the retrospective surveillance of infections in non-emergency patients, the offending pathogen was identified as Acinetobacter spp, which highlights another important aspect of this study. All in all, we believe that this finding will help physicians select the best empirical antibiotics for these patients.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declare no conflict of interest.
References
Seven CSF samples were sent for detection of pathogens in our study. Positive cultures were only observed in 3 patients (42.9%). All three organisms were Acinetobacter spp (100%).
4. Discussion
The occurrence of PCM has been reported to be approximately 0.3-8.9% in the literature [3-8, 5-15], but 8.6% in the Chen et al. study [1]. In our study, the prevalence of meningitis was 5%. The lower PCM level may be due to the non-emergency aspect of our surgeries.
In the literature, gram-negative rods and Enterobacteriaceae have had an important role in the incidence of PCM [1-4, 8-10, 13-19]. The rates of positive cultures in the studies of Chen [1] and Kourbeti [2] were 10.4 %, and 100%, respectively. The most common microorganisms in the Chen [1] and Kourbeti [2] studies were Acinetobacter spp, with rates accounting for 40% and 45%, respectively. Seven CSF samples were sent for detection of pathogens in our study. Positive cultures were only observed in 3 patients (42.9%). All three microorganisms were Acinetobacter spp (100%).
Diabetic patients were at a higher risk of developing meningitis than those without diabetes. The occurrence of PCM in diabetic patients was 18.5% in Chen’s study and 4.1% in Kourbe’s study, but it was 0% in our study. The lack of meningitis in our study may be due to the precise control of blood glucose levels prior to our non-emergency craniotomy or the absence of patients who required emergency attention.Therefore, we suggest further studies to prove these predictions.
Of the 131 patients in our study who received prophylactic antibiotics an hour before surgery, 3% of them (4 patients) suffered from meningitis. In the Chen et al. study [1], 10.8% of the patients suffered from meningitis. Barker’s study [20] documents the benefits of antibiotics to prevent PCM. In our study, a significant relationship between the preoperative antibiotics and the prevention of PCM was found (P=0.001).
The rate of the effect of steroids on the occurrence of PCM varied throughout the mentioned studies [7, 21, 22]. In the Chen [1] and Kourbeti [2] studies, a 5% and 7.10% rate was found, and the relationship was statistically significant in the Kourbeti study [2]. Among our patients with a history of corticosteroid use, no significant relationship was found (P=0.45).
In our study, 8.2% of patients with a craniotomy lasting longer than 4.5 hours had meningitis (6 off 7 patients with PCM). Despite this observation, there was no significant relationship between craniotomy duration and meningitis (P=0.68), as in previous studies [1, 2].
According to previous studies, craniotomy in patients with EVD increases the occurrence of meningitis [1-27]. In our study, 57% of the patients who had EVD (4 cases) suffered from PCM. To prevent PCM, we recommend that an EVD is avoided in unnecessary conditions, and if it is present, it should be removed as soon as possible.
Eleven patients (7.8%) had hydrocephalus prior to craniotomy, and 5 patients (45.4%) suffered from PCM, resulting in a significant relationship between hydrocephalus prior to craniotomy and PCM (P=0.001). Therefore, it is recommended that special attention be paid to this significant relationship in future studies. In our study, the greatest limitations were the fact that it was retrospective and dependent on the accuracy of the data in the clinical charts.
5. Conclusion
Unlike many studies, this study was conducted on non-emergency patients. We determined the prevalence, risk factors, and microbiology that can lead to PCM in our patients.We were able to confirm the importance of perioperative hydrocephalus, perioperative ventricular drains, perioperative antibiotics, and a GCS <12 in the development of PCM. The perioperative steroids, diabetes mellitus, duration of surgery, gender, and age were not significant risk factors for PCM. From the retrospective surveillance of infections in non-emergency patients, the offending pathogen was identified as Acinetobacter spp, which highlights another important aspect of this study. All in all, we believe that this finding will help physicians select the best empirical antibiotics for these patients.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declare no conflict of interest.
References
- Chen C, Zhang B, Yu S, Sun F, Ruan Q, Zhang W, et al. The Incidence and Risk Factors of Meningitis after Major Craniotomy in China: A Retrospective Cohort Study. PLoS ONE. 2014; 9(7):e101961. [DOI:10.1371/journal.pone.0101961] [PMID] [PMCID]
- Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, et al. Infections in patients undergoing craniotomy: Risk factors associated with post-craniotomy meningitis. Journal of Neurosurgery. 2015; 122(5):1113-9. [DOI:10.3171/2014.8.JNS132557] [PMID]
- Reichert MC, Medeiros EA, Ferraz FA. Hospital-acquired meningitis in patients undergoing craniotomy: incidence, evolution, and risk factors. American Journal of Infection Control. 2002; 30(3):158-64. [DOI:10.1067/mic.2002.119925] [PMID]
- Lietard C, Thebaud V, Besson G, Lejeune B. Risk factors for neurosurgical site infections: An 18-month prospective survey. Journal of Neurosurgery. 2008; 109(4):729-34. [DOI:10.3171/JNS/2008/109/10/0729] [PMID]
- Blomstedt GC. Infections in neurosurgery: A retrospective study of 1143 patients and 1517 operations. Acta Neurochirurgica. 2008; 78(3-4):81-90. [DOI:10.1007/BF01808684] [PMID]
- Federico G, Tumbarello M, Spanu T, Rosell R, Iacoangeli M, et al. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scandinavian Journal of Infectious Diseases. 2001; 33(7):533-7 [DOI:10.1080/00365540110026557] [PMID]
- Erdem I, Hakan T, Ceran N, Metin F, Akcay SS, Kucukercan M, et al. Clinical features, laboratory data, management and the risk factors that affect the mortality in patients with postoperative meningitis. Neurology India. 2008; 56(4):433-7. [DOI:10.4103/0028-3886.44629] [PMID]
- Wright RL. A survey of possible etiologic agents in postoperative craniotomy infections. Journal of Neurosurgery. 1966; 25(2):125-32. [DOI:10.3171/jns.1966.25.2.0125] [PMID]
- Buckwold FJ, Hand R, Hansebout RR. Hospital-acquired bacterial meningitis in neurosurgical patients. Journal of Neurosurgery. 1977; 46(4):494-500. [DOI:10.3171/jns.1977.46.4.0494] [PMID]
- McClelland S, Hall WA. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clinical Infectious Diseases. 2007; 45(1):55-9. [DOI:10.1086/518580] [PMID]
- Sneh-Arbib O, Shiferstein A, Dagan N, Fein S, Telem L, Muchtar N, et al. Surgical site infections following craniotomy focusing on possible post-operative acquisition of infection: Prospective cohort study. European Journal of Clinical Microbiology & Infectious Diseases. 2013; 32:1511-6. [DOI:10.1007/s10096-013-1904-y] [PMID]
- Srinivas D, Veena Kumari HB, Somanna S, Bhagavatula I, Anandappa CB. The incidence of postoperative meningitis in neurosurgery: An institutional experience. Neurology India. 2011; 59(2):195-8. [DOI:10.4103/0028-3886.79136] [PMID]
- Kourbeti IS, Jacobs AV, Koslow M, Karabetsos D, Holzman RS. Risk factors associated with postcraniotomy meningitis. Neurosurgery. 2007; 60(2):317-25. [DOI:10.1227/01.NEU.0000249266.26322.25] [PMID]
- Patir R, Mahapatra AK, Banerji AK. Risk factors in postoperative neurosurgical infection. A prospective study. Acta Neurochirurgica. 1992; 119(1-4):80-4. [DOI:10.1007/BF01541786] [PMID]
- Korinek AM, Golmard JL, Elcheick A, Bismuth R, van Effenterre R, Coriat P, et al. Risk factors for neurosurgical site infections after craniotomy: A critical reappraisal of antibiotic prophylaxis on 4578 patients. British Journal of Neurosurgery. 2005; 19(2):155-62. [DOI:10.1080/02688690500145639] [PMID]
- Briggs S, Ellis-Pegler R, Raymond N, Thomas M, Wilkinson L. Gramnegative bacillary meningitis after cranial surgery or trauma in adults. Scandinavian Journal of Infectious Diseases. 2004; 36:165-73. [DOI:10.1080/00365540410027193] [PMID]
- Huang CR, Lu CH, Chang WN. Adult Enterobacter meningitis: A high incidence of coinfection with other pathogens and frequent association with neurosurgical procedures. Infection. 2001; 29(2):75-9.[DOI:10.1007/s15010-001-0087-0] [PMID]
- Agarwal M, Thomas P. Prevalence of post-op. nosocomial infection in neurosurgical patients and associated risk factors-a prospective study of 2441 patients. The Nursing Journal of India. 2003; 94(9):197-8.
- Erman T, Demirhindi H, Gocer AI, Tuna M, Ildan F, Boyar B. Risk factors for surgical site infections in neurosurgery patients with antibiotic prophylaxis. Surgical Neurology. 2005; 63(2):107-12. [PMID]
- Barker FG. Efficacy of prophylactic antibiotic therapy in spinal surgery: A meta-analysis. Neurosurgery. 2002; 51(2):391-400. [DOI:10.1097/00006123-200208000-00017] [PMID]
- Kourbeti IS, Vakis AF, Papadakis JA, Karabetsos DA, Bertsias G, Filippou M, et al. Infections in traumatic brain injury patients. Clinical Microbiology and Infection . 2012; 18(4):359-64.[DOI:10.1111/j.1469-0691.2011.03625.x] [PMID]
- Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr. Ventriculostomy-related infections: A critical review of the literature. Neurosurgery. 2002; 51(1):170-82 [DOI:10.1097/00006123-200207000-00024] [PMID]
- Mahé V, Kermarrec N, Ecoffey C. [Infections related to external ventricular drainage (French)]. Annales Francaises D'anesthesie et de Reanimation. 1995; 14(1):8-12.[DOI:10.1016/S0750-7658(05)80144-3]
- Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. A prospective epidemiologic study. The New England Journal of Medicine. 1984; 310(9):553-9. [DOI:10.1056/NEJM198403013100903] [PMID]
- McPhee IB, Williams RP, Swanson CE. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1998; 23(6):726-33.[DOI:10.1097/00007632-199803150-00015] [PMID]
- Narotam PK, van Dellen JR, du Trevou MD, Gouws E. Operative sepsis in neurosurgery: A method of classifying surgical cases. Neurosurgery. 1994; 34(3):409-16[DOI:10.1097/00006123-199403000-00004]
- Reichert MCF, Medeiros EAS, Ferraz FAP. Hospitalacquired meningitis in patients undergoing craniotomy: Incidence, evolution, and risk factors. American Journal of Infection Control. 2002; 30(3):158-64. [DOI:10.1067/mic.2002.119925] [PMID]
Type of Study: Research |
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |