Fri, Jul 4, 2025
Volume 6, Issue 4 (Autumn 2020)
Iran J Neurosurg 2020, 6(4): 203-210 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Safari H, Bagheri S, Ahmadi Halili B. Cosmetic Outcomes of Scalp in Standard Reverse Question Mark Incision and L.G. Kempe Incision in Large Craniotomies. Iran J Neurosurg 2020; 6 (4) :203-210
URL: http://irjns.org/article-1-251-en.html
URL: http://irjns.org/article-1-251-en.html
1- Department of Neurological Surgery, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Department of Pathology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3- Department of Neurological Surgery, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. ,Behnia.1994@gmail.com
2- Department of Pathology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3- Department of Neurological Surgery, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. ,
Full Text [PDF 1026 kb]
(1174 Downloads)
| Abstract (HTML) (3616 Views)
Full Text: (3819 Views)
1. Introduction
Craniotomy is a surgery in which a flap of the skull is opened to access intracranial pathologies. This surgery leaves a scar, the size of which varies from patient to patient according to the size of the scalp incision. Specifically, in large-size craniotomies, cosmetic problems are the common concern for both patient and surgeon. Patients may experience alopecia and whitening of the scalp, which may eventually begin to fall out. This is especially true for patients undergoing radiation therapy after craniotomy surgery. Coping with hair loss after brain surgery is difficult for some patients, especially young ones. The hair comes back after the brain surgery and the situation improves. However, the process is slow and may take several months [1].
A certain amount of hair loss along the scalp incision is inevitable after craniotomy surgery. However, some techniques in regular scalp incisions exacerbate hair loss. In Extracranial and Intracranial (EC-IC) bypass surgery, the vessels that feed the scalp are removed and anastomosed in the cerebral arteries, resulting in more pronounced hair loss compared to other neurosurgical procedures [2]. Today, surgical interventions should be performed with care and consideration of the cosmetic effect on patients because the craniofacial region is probably the most important area of the body in terms of aesthetics [3]. Modern neurosurgery should use precautions and surgical techniques to reduce the poor aesthetic consequences of patients. Therefore, a surgical protocol, regardless of the location and nature of the lesion, should be used. It is recommended to minimize the aesthetic effect with a step-by-step method [4-8]. Since paying attention to the aesthetic aspects of the patient’s head after surgery is very important for the patient’s mental health and social life, surgeons should try to adopt a method that minimizes damage to the scalp and follicles, heals the wound as soon as possible and reduces the extent of the incision area [9-11]. The aim of this study was to compare the 2 incision methods mentioned in terms of aesthetics and patient satisfaction and also their effect on the density of hair follicles of surgical site scalp.
L.G. Kempe incision
For the LG Kepmpe incision, the scalp on the sagittal suture from the top of the window’s peak to the inion with a “T-Bar” is cut from 1 to 2 centimeters anterior to the tragus at the temporal root of the zygoma and ascending to the midline. The sagittal incision is one centimeter behind the coronal suture (Figure 1).

Closure involves holding a suture at the top of the wound, followed by suturing and stapling Galea, and then removing the suture at the top of the wound [12].
Standard reverse question mark incision
The large reverse question mark scalp incision starts 1-2 cm anterior to the tragus at the root of the zygoma, curving posteriorly above and gently behind the ear toward the asterion. This incision then gently curves around the parietal boss to the midline and forward to the widow’s peak [13]. The scalp is incised and reflected anteriorly as a myocutaneous flap of the scalp and temporalis muscle (Figure 1).
2. Methods and Materials/Patients
After obtaining necessary permissions including the ethical code of the ethical committee of Golestan hospital (Code: IR.AJUMS.HGOLESTAN.REC.1399.004) and clinical trial (Code: IRCT20180906040962N1). Patients who were candidate for large frontotemporaneopranital craniotomy were selected after examining the inclusion and exclusion criteria.
Patients who were candidate for large frontotemporaneopranital craniectomy or craniotomy aged 18-65 years were included in the study. Exclusion criteria were as follows; age over 65 or under 18 years, presence of trauma wounds at the patient’s craniotomy site, history of radiotherapy, diabetes mellitus, vascular diseases, use of immunosuppressive drugs, history of previous skin problems, history of psoriasis, the presence of androgenic baldness in the patient, and presence of non-androgenic blisters in the patient.
Thirty-one patients were included in the study in 2 groups of 14 and 17 people. In this study, selected patients were randomly divided into 2 groups by the permutation block method.
Before starting the surgery in the operating room, a biopsy was taken from the scalp of the affected area in the frontoparietal area (1 cm in front of the coronal suture at a distance of 6 cm from the sagittal suture line). For evaluation of hair follicle density, scalp biopsy was sent to the pathology laboratory and evaluated for the number of hair follicles in each Low Power Field (LPF). In each group, patients underwent craniotomy with mentioned incisions. Six months after the date of initial sampling, patients were called in for follow-up and evaluated the aesthetics of the wound scar and the second scalp sampling. The aesthetic evaluation was performed by asking the patient to score the appearance and density of hair in the craniotomy area as one of the desirable, moderate, and poor results. Independent evaluation of the appearance of scarring was also performed by the researchers through examining the scar using SBSES scoring (Stony brook scar evaluation score) (Table 1) [14].
.PNG)
Also, the second biopsy was taken from the scalp (parietal) from the same area under sterile conditions and sent to the pathology laboratory for re-evaluation of the number of hair follicles in each LPF. Unfortunately, 8 cases expired and lost their follow-up. Finally, we managed to follow up the 23 patients, 11 patients in the standard reverse question mark incision group and 12 patients in the L.G. Kempe incision group. The results were compared with the previous sample of the patients and also between the 2 groups. SBSES score was measured and recorded by a single person in the 6th month. The patient’s information was recorded in the designed questionnaire from the beginning.
3. Results
The definitive collected samples through the follow-up process consisted of 2 groups: a 12-member intervention group with L.G. Kempe incision and an 11-member control group with standard reverse question mark incision. In the collected samples, the subjects were divided by sex, including 14 males (9 in the LG Kempe section and 5 in the reverse question mark section) and 9 females (3 in the L.G. Kempe section and 6 in the reverse question mark section).
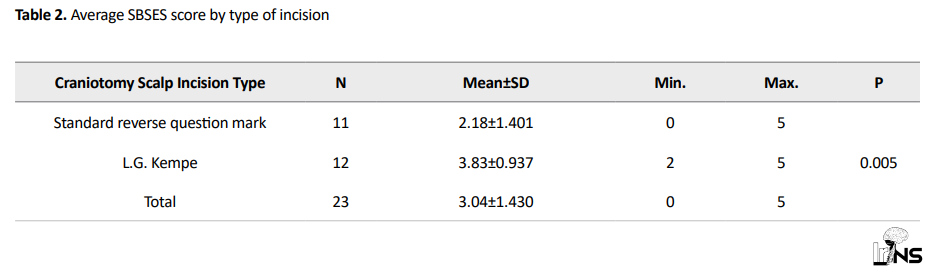
The results obtained from SBSES score evaluations are as follows: SBSES scores were compared between the intervention and control groups using the independent sample t-test. The results of comparing the SBSES score between the 2 groups indicated that the mean SBSES score in the intervention group (mean=3.83) was higher than that of the control group (mean=2.18) (p=0.005). There was a significant relationship (p<0.05) between the type of incision and the SBSES score (Table 2).
The results of the data were as follows: in the intervention group (L.G. Kempe) 6 people had a good evaluation (50%), 4 people had a moderate evaluation (33%) and 2 people had a poor evaluation (17%). Also in the control group, 4 people reported good evaluation (36%), 4 people reported moderate evaluation (37%) and 3 people reported poor evaluation (27%).
Also, the result of patients’ satisfaction with the effect of residual scar area in the 2 groups was evaluated by the chi-square test, but no significant relationship was found (P=0.75).
In the results of biopsies obtained at the follow-up period of 6 months from the patients’ scalp, the obtained follicular examinations demonstrated the mean of the intervention group was 8.92 and the mean of the control group was 9.18 in each LPF.
Comparison of mean follicular density between the intervention and control groups with independent sample t-test displayed no significant relationship between the type of surgical incision and follicular hair density after surgery between the 2 groups (P=0.910).
Studying the changes of hair follicle density showed that the mean changes of hair follicles were generally 1.09, in the intervention group 0.83 and, in the control group 1.36. Using paired sample t-test, the mean after surgery was not significantly different from before surgery (P=0.657).
Also, in the results of comparing the mean changes in hair follicle density between the control and intervention groups, which was performed by 1-way ANOVA statistical test, no significant relationship was found between the type of surgical incision and the mean changes in follicular density (P=0.137).
4. Discussion
Today, surgical interventions must be treated with care for their aesthetic impact on the patient. The cranium area and the scalp also have a special place in terms of beauty and psychosocial effects, and the head and neck are probably the most important anatomical areas in the human body. Neurosurgeons, in addition to trying to acquire modern neurosurgery skills, should also use precautionary measures and reliable surgical techniques to reduce the poor aesthetic consequences in patients. This should be a fundamental approach even if more aesthetic considerations are experienced by young people, women, and bald people. The adoption of a common protocol for the proper treatment of all patients seems absolutely necessary. Thus, neurosurgeons should get used to considering the aesthetic aspect as a necessity for all patients, even those with undesirable injuries. Therefore, a surgical protocol, regardless of the location and nature of the lesion, should be used to minimize the aesthetic effect with a step-by-step method [4-8]. One of the inevitable complications of scalp incision is scarring and loss of hair follicles in the cut area. Since paying attention to the aesthetic aspects of the patient’s head after surgery is very important for the patient’s mental health and social life, surgeons should try to adopt a method that minimizes damage to the scalp and follicles, heals the wound in the fastest and most possible way and minimizes the extent of the incision area [9, 10].
In a retrospective study, Ragel et al. compared the results of 3 craniotomies in 90 patients. They pointed to the need to use alternative methods of scalp incision, including the LG Kemp incision, due to the unacceptably high rate of reverse question mark incision wound failure in patients requiring large craniotomies or patients with complex scalp injuries. The advantages of this incision (L.G. Kemp) included the preservation of the scalp arterial blood supply source by the occipital and superficial temporal arteries and the restriction of the incision on the lambdoid suture, which has been subjected to prolonged pressure. The authors recommended L.G. Kepme incision to maintain blood flow in large craniectomies to prevent brain strangulation on bone margins, minimal brain debridement, adequate brain decompression, and dural substitute dural closure.
In the results of this study, after the examinations and evaluation of the scar with the SBSES scoring system, , there was a significant relationship (P>0.05) between the type of incision and the SBSES score between the intervention and control groups. The result can indicate that the scalp blood supply of patients in L.G. Kempe craniotomy was less affected and the affected areas in the incision area had higher blood flow.
The results of the study on patient satisfaction as a direct question to the patient about the condition of the operation area and the scar indicate no significant relationship (P=0.75). A prospective study by Diccini et al. found that most patients did not report a change in self-esteem due to hair loss. Self-esteem was the same among patients who were told about the process of hair loss. The majority of patients used accessories as a strategy to hide shaved areas and believed that hair loss did not harm their quality of life or their social relations [15].
Therefore, in line with the research mentioned, the findings of the researchers of this study illustrated that the type of operation incision did not have much effect on the patient’s satisfaction or dissatisfaction with the scar. One of the reasons for this can be attributed to the socio-cultural conditions of patients, which can be a good topic for future research. Moreover, due to the limited number of sample size and the existing cultural and economic diversities, no definite conclusion can be reached and the rejection or confirmation of the results requires further investigation.
In the results of biopsies obtained at the follow-up period of 6 months after the operation, by comparing the mean follicular density, no significant relationship was found between the type of surgical incision and follicular hair density after surgery between the 2 groups (P=0.910). The results also suggest that the mean hair follicle density after surgery was not significantly different from before surgery (P=0.657). Our perception was that LGkempe incision, because of maintaining better blood supply for scalp, leads to better results in terms of follicular density. It may be because of the small number of our cases.
A certain amount of hair loss along the scalp incision is inevitable after craniotomy surgery. However, some techniques in regular scalp incisions exacerbate hair loss. In Extracranial and Intracranial (EC-IC) bypass surgery, the vessels that feed the scalp are removed and anastomosed in the cerebral arteries, resulting in more pronounced hair loss compared with other neurosurgical procedures [2]. However, the findings of the researchers in this study did not reveal a clue of follicular damage which could lead to a decrease in follicular density and hair loss due to damage to the hair follicles. The apparent hair loss can be caused by telegenic effluvium, which is a temporary process triggered by anesthesia, stress, and hormonal changes after surgery, from which the hair follicle is not damaged and the lost hair is repaired after a while [16].
5. Conclusion
The results of the study showed that patients who underwent craniotomy with L.G. Kempe incision scored significantly higher mean scores in SBSES scoring evaluations. Although the 2 surgical incisions were not significantly superior in maintaining density and preventing follicular damage, because of its better SBSES score L.G. Kempe incision can be considered an aesthetically superior method that could prevent the formation of undesirable scar.
Ethical Considerations
Compliance with ethical guidelines
The study has been reviewed by the Ethical Committee of Golestan hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz (Code: IR.AJUMS.HGOLESTAN.REC.1399.004). The Clinical Trial Registration Code: IRCT20180906040962N1.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors had equal contributions in performing all stages of the study.
Conflict of interest
The authors declared no conflicts of interest.
Craniotomy is a surgery in which a flap of the skull is opened to access intracranial pathologies. This surgery leaves a scar, the size of which varies from patient to patient according to the size of the scalp incision. Specifically, in large-size craniotomies, cosmetic problems are the common concern for both patient and surgeon. Patients may experience alopecia and whitening of the scalp, which may eventually begin to fall out. This is especially true for patients undergoing radiation therapy after craniotomy surgery. Coping with hair loss after brain surgery is difficult for some patients, especially young ones. The hair comes back after the brain surgery and the situation improves. However, the process is slow and may take several months [1].
A certain amount of hair loss along the scalp incision is inevitable after craniotomy surgery. However, some techniques in regular scalp incisions exacerbate hair loss. In Extracranial and Intracranial (EC-IC) bypass surgery, the vessels that feed the scalp are removed and anastomosed in the cerebral arteries, resulting in more pronounced hair loss compared to other neurosurgical procedures [2]. Today, surgical interventions should be performed with care and consideration of the cosmetic effect on patients because the craniofacial region is probably the most important area of the body in terms of aesthetics [3]. Modern neurosurgery should use precautions and surgical techniques to reduce the poor aesthetic consequences of patients. Therefore, a surgical protocol, regardless of the location and nature of the lesion, should be used. It is recommended to minimize the aesthetic effect with a step-by-step method [4-8]. Since paying attention to the aesthetic aspects of the patient’s head after surgery is very important for the patient’s mental health and social life, surgeons should try to adopt a method that minimizes damage to the scalp and follicles, heals the wound as soon as possible and reduces the extent of the incision area [9-11]. The aim of this study was to compare the 2 incision methods mentioned in terms of aesthetics and patient satisfaction and also their effect on the density of hair follicles of surgical site scalp.
L.G. Kempe incision
For the LG Kepmpe incision, the scalp on the sagittal suture from the top of the window’s peak to the inion with a “T-Bar” is cut from 1 to 2 centimeters anterior to the tragus at the temporal root of the zygoma and ascending to the midline. The sagittal incision is one centimeter behind the coronal suture (Figure 1).

Closure involves holding a suture at the top of the wound, followed by suturing and stapling Galea, and then removing the suture at the top of the wound [12].
Standard reverse question mark incision
The large reverse question mark scalp incision starts 1-2 cm anterior to the tragus at the root of the zygoma, curving posteriorly above and gently behind the ear toward the asterion. This incision then gently curves around the parietal boss to the midline and forward to the widow’s peak [13]. The scalp is incised and reflected anteriorly as a myocutaneous flap of the scalp and temporalis muscle (Figure 1).
2. Methods and Materials/Patients
After obtaining necessary permissions including the ethical code of the ethical committee of Golestan hospital (Code: IR.AJUMS.HGOLESTAN.REC.1399.004) and clinical trial (Code: IRCT20180906040962N1). Patients who were candidate for large frontotemporaneopranital craniotomy were selected after examining the inclusion and exclusion criteria.
Patients who were candidate for large frontotemporaneopranital craniectomy or craniotomy aged 18-65 years were included in the study. Exclusion criteria were as follows; age over 65 or under 18 years, presence of trauma wounds at the patient’s craniotomy site, history of radiotherapy, diabetes mellitus, vascular diseases, use of immunosuppressive drugs, history of previous skin problems, history of psoriasis, the presence of androgenic baldness in the patient, and presence of non-androgenic blisters in the patient.
Thirty-one patients were included in the study in 2 groups of 14 and 17 people. In this study, selected patients were randomly divided into 2 groups by the permutation block method.
Before starting the surgery in the operating room, a biopsy was taken from the scalp of the affected area in the frontoparietal area (1 cm in front of the coronal suture at a distance of 6 cm from the sagittal suture line). For evaluation of hair follicle density, scalp biopsy was sent to the pathology laboratory and evaluated for the number of hair follicles in each Low Power Field (LPF). In each group, patients underwent craniotomy with mentioned incisions. Six months after the date of initial sampling, patients were called in for follow-up and evaluated the aesthetics of the wound scar and the second scalp sampling. The aesthetic evaluation was performed by asking the patient to score the appearance and density of hair in the craniotomy area as one of the desirable, moderate, and poor results. Independent evaluation of the appearance of scarring was also performed by the researchers through examining the scar using SBSES scoring (Stony brook scar evaluation score) (Table 1) [14].
.PNG)
Also, the second biopsy was taken from the scalp (parietal) from the same area under sterile conditions and sent to the pathology laboratory for re-evaluation of the number of hair follicles in each LPF. Unfortunately, 8 cases expired and lost their follow-up. Finally, we managed to follow up the 23 patients, 11 patients in the standard reverse question mark incision group and 12 patients in the L.G. Kempe incision group. The results were compared with the previous sample of the patients and also between the 2 groups. SBSES score was measured and recorded by a single person in the 6th month. The patient’s information was recorded in the designed questionnaire from the beginning.
3. Results
The definitive collected samples through the follow-up process consisted of 2 groups: a 12-member intervention group with L.G. Kempe incision and an 11-member control group with standard reverse question mark incision. In the collected samples, the subjects were divided by sex, including 14 males (9 in the LG Kempe section and 5 in the reverse question mark section) and 9 females (3 in the L.G. Kempe section and 6 in the reverse question mark section).
The results obtained from SBSES score evaluations are as follows: SBSES scores were compared between the intervention and control groups using the independent sample t-test. The results of comparing the SBSES score between the 2 groups indicated that the mean SBSES score in the intervention group (mean=3.83) was higher than that of the control group (mean=2.18) (p=0.005). There was a significant relationship (p<0.05) between the type of incision and the SBSES score (Table 2).

The results of the data were as follows: in the intervention group (L.G. Kempe) 6 people had a good evaluation (50%), 4 people had a moderate evaluation (33%) and 2 people had a poor evaluation (17%). Also in the control group, 4 people reported good evaluation (36%), 4 people reported moderate evaluation (37%) and 3 people reported poor evaluation (27%).
Also, the result of patients’ satisfaction with the effect of residual scar area in the 2 groups was evaluated by the chi-square test, but no significant relationship was found (P=0.75).
In the results of biopsies obtained at the follow-up period of 6 months from the patients’ scalp, the obtained follicular examinations demonstrated the mean of the intervention group was 8.92 and the mean of the control group was 9.18 in each LPF.
Comparison of mean follicular density between the intervention and control groups with independent sample t-test displayed no significant relationship between the type of surgical incision and follicular hair density after surgery between the 2 groups (P=0.910).
Studying the changes of hair follicle density showed that the mean changes of hair follicles were generally 1.09, in the intervention group 0.83 and, in the control group 1.36. Using paired sample t-test, the mean after surgery was not significantly different from before surgery (P=0.657).
Also, in the results of comparing the mean changes in hair follicle density between the control and intervention groups, which was performed by 1-way ANOVA statistical test, no significant relationship was found between the type of surgical incision and the mean changes in follicular density (P=0.137).
4. Discussion
Today, surgical interventions must be treated with care for their aesthetic impact on the patient. The cranium area and the scalp also have a special place in terms of beauty and psychosocial effects, and the head and neck are probably the most important anatomical areas in the human body. Neurosurgeons, in addition to trying to acquire modern neurosurgery skills, should also use precautionary measures and reliable surgical techniques to reduce the poor aesthetic consequences in patients. This should be a fundamental approach even if more aesthetic considerations are experienced by young people, women, and bald people. The adoption of a common protocol for the proper treatment of all patients seems absolutely necessary. Thus, neurosurgeons should get used to considering the aesthetic aspect as a necessity for all patients, even those with undesirable injuries. Therefore, a surgical protocol, regardless of the location and nature of the lesion, should be used to minimize the aesthetic effect with a step-by-step method [4-8]. One of the inevitable complications of scalp incision is scarring and loss of hair follicles in the cut area. Since paying attention to the aesthetic aspects of the patient’s head after surgery is very important for the patient’s mental health and social life, surgeons should try to adopt a method that minimizes damage to the scalp and follicles, heals the wound in the fastest and most possible way and minimizes the extent of the incision area [9, 10].
In a retrospective study, Ragel et al. compared the results of 3 craniotomies in 90 patients. They pointed to the need to use alternative methods of scalp incision, including the LG Kemp incision, due to the unacceptably high rate of reverse question mark incision wound failure in patients requiring large craniotomies or patients with complex scalp injuries. The advantages of this incision (L.G. Kemp) included the preservation of the scalp arterial blood supply source by the occipital and superficial temporal arteries and the restriction of the incision on the lambdoid suture, which has been subjected to prolonged pressure. The authors recommended L.G. Kepme incision to maintain blood flow in large craniectomies to prevent brain strangulation on bone margins, minimal brain debridement, adequate brain decompression, and dural substitute dural closure.
In the results of this study, after the examinations and evaluation of the scar with the SBSES scoring system, , there was a significant relationship (P>0.05) between the type of incision and the SBSES score between the intervention and control groups. The result can indicate that the scalp blood supply of patients in L.G. Kempe craniotomy was less affected and the affected areas in the incision area had higher blood flow.
The results of the study on patient satisfaction as a direct question to the patient about the condition of the operation area and the scar indicate no significant relationship (P=0.75). A prospective study by Diccini et al. found that most patients did not report a change in self-esteem due to hair loss. Self-esteem was the same among patients who were told about the process of hair loss. The majority of patients used accessories as a strategy to hide shaved areas and believed that hair loss did not harm their quality of life or their social relations [15].
Therefore, in line with the research mentioned, the findings of the researchers of this study illustrated that the type of operation incision did not have much effect on the patient’s satisfaction or dissatisfaction with the scar. One of the reasons for this can be attributed to the socio-cultural conditions of patients, which can be a good topic for future research. Moreover, due to the limited number of sample size and the existing cultural and economic diversities, no definite conclusion can be reached and the rejection or confirmation of the results requires further investigation.
In the results of biopsies obtained at the follow-up period of 6 months after the operation, by comparing the mean follicular density, no significant relationship was found between the type of surgical incision and follicular hair density after surgery between the 2 groups (P=0.910). The results also suggest that the mean hair follicle density after surgery was not significantly different from before surgery (P=0.657). Our perception was that LGkempe incision, because of maintaining better blood supply for scalp, leads to better results in terms of follicular density. It may be because of the small number of our cases.
A certain amount of hair loss along the scalp incision is inevitable after craniotomy surgery. However, some techniques in regular scalp incisions exacerbate hair loss. In Extracranial and Intracranial (EC-IC) bypass surgery, the vessels that feed the scalp are removed and anastomosed in the cerebral arteries, resulting in more pronounced hair loss compared with other neurosurgical procedures [2]. However, the findings of the researchers in this study did not reveal a clue of follicular damage which could lead to a decrease in follicular density and hair loss due to damage to the hair follicles. The apparent hair loss can be caused by telegenic effluvium, which is a temporary process triggered by anesthesia, stress, and hormonal changes after surgery, from which the hair follicle is not damaged and the lost hair is repaired after a while [16].
5. Conclusion
The results of the study showed that patients who underwent craniotomy with L.G. Kempe incision scored significantly higher mean scores in SBSES scoring evaluations. Although the 2 surgical incisions were not significantly superior in maintaining density and preventing follicular damage, because of its better SBSES score L.G. Kempe incision can be considered an aesthetically superior method that could prevent the formation of undesirable scar.
Ethical Considerations
Compliance with ethical guidelines
The study has been reviewed by the Ethical Committee of Golestan hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz (Code: IR.AJUMS.HGOLESTAN.REC.1399.004). The Clinical Trial Registration Code: IRCT20180906040962N1.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors had equal contributions in performing all stages of the study.
Conflict of interest
The authors declared no conflicts of interest.
- 018 [2019 october 8]. Available from: https://medigence.com/blog/dealing-with-hair-loss-after-craniotomy/
- Sanada Y, Yabuuchi T, Yoshioka H, Kubota H, Kato A. Zigzag skin incision effectively camouflages the scar and alopecia for moyamoya disease: Technical note. Neurologia Medico-Chirurgica. 2015; 55(3):210-3. [DOI:10.2176/nmc.tn.2014-0193] [PMID] [PMCID]
- Frati A, Pichierri A, Esposito V, Frati R, Delfini R, Cantore G, et al. Aesthetic issues in neurosurgery: A protocol to improve cosmetic outcome in cranial surgery. Neurosurgical Review. 2007; 30(1):69-76. [DOI:10.1007/s10143-006-0050-8] [PMID]
- Missori P, Polli FM, Fontana E, Delfini R. Closure of skin or scalp with absorbable sutures. Plastic and ReconStructive Surgery. 2003; 112(3):924-5. [DOI:10.1097/01.PRS.0000074497.87004.2D] [PMID]
- Matsumoto K, Akagi K, Abekura M, Ohkawa M, Tasaki O, Tomishima T. Cosmetic and functional reconstruction achieved using a split myofascial bone flap for pterional craniotomy. Technical Note. Journal of Neurosurgery. 2001; 94(4):667-70. [DOI:10.3171/jns.2001.94.4.0667] [PMID]
- Bogaev CA. Cosmetic considerations in cranial base surgery. Neurosurgery Clinics. 2002; 13(4):421-41. [DOI:10.1016/S1042-3680(02)00027-X]
- Sato S, Sato M, Nishizawa M, Oizumi T, Hiwatari M, Kajiwara T, et al. Method to improve cosmetic outcome following craniotomy. Neurological Research. 2001; 23(4):339-42. [DOI:10.1179/016164101101198721] [PMID]
- Sekhar LN. The cosmetic aspects of neurosurgery. Neurosurgery Clinics of North America. 2002; 13(4):401-3. [DOI:10.1016/S1042-3680(02)00026-8]
- Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005; 331(7522):951-3. [DOI:10.1136/bmj.331.7522.951] [PMID] [PMCID]
- Gharehdaghi J, Jafari-Marandi H, Faress F, Zeinali M, Safari H. Morphology of asterion and its proximity to deep vein sinuses in Iranian adult skull. British Journal of Neurosurgery. 2020; 34(1):55-8. [DOI:10.1080/02688697.2019.1687846] [PMID]
- Winn HR. Youmans and Winn Neurological Surgery. Neurological Surgery. Amsterdam: Elsevier; 2017. https://www.google.com/books/edition/Youmans_and_Winn_Neurological_Surgery/z3q7wAEACAAJ?hl=en
- Kempe LG. Hemispherectomy. In Operative Neurosurgery. New York: Springer-Verlag; 1968. [DOI:10.1007/978-3-662-12634-9_17]
- Ragel BT, Klimo P Jr, Martin JE, Teff RJ, Bakken HE, Armonda RA. Wartime decompressive craniectomy: Technique and lessons learned. Neurosurgical Focus. 2010; 28(5):E2. [DOI:10.3171/2010.3.FOCUS1028] [PMID]
- Vercelli S, Ferriero G, Sartorio F, Stissi V, Franchignoni F. How to assess postsurgical scars: A review of outcome measures. Disability and Rehabilitation. 2009; 31(25):2055-63. [DOI:10.3109/09638280902874196a] [PMID]
- Diccini S, Yoshinaga SN, Marcolan JF. [Hair removal repercussions on patient’s self-esteem in craniotomy (Portuguese)]. Revista da Escola de Enfermagem da U S P. 2009; 43(3):596-601. [DOI:10.1590/S0080-62342009000300014] [PMID]
- Nichter L. Hair loss after surgery explained [Internet]. 2021 [Updated 2021]. Available from: https://drnichter.com/hair-loss-after-surgery-explained/
Type of Study: Clinical Trial |
Subject:
Basic Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






