Wed, Jul 16, 2025
Volume 9 - Continuous Publishing
Iran J Neurosurg 2023, 9 - Continuous Publishing: 72-81 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haddadi K, Alaee A, Ghafari A, khademloo M. Single Surgeon Experience of Adjacent Segment Disease and Related Risk Factors Following Posterior Decompression and Fusion in Lumbar Degenerative Disorders. Iran J Neurosurg 2023; 9 : 10
URL: http://irjns.org/article-1-329-en.html
URL: http://irjns.org/article-1-329-en.html
1- Department of Neurosurgery, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran
2- Department of Radiology, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3- Department of Neurosurgery, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
4- Department of Community Medicine, School of Medicine, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran ,paper87@yahoo.com
2- Department of Radiology, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3- Department of Neurosurgery, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
4- Department of Community Medicine, School of Medicine, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran ,
Full Text [PDF 1111 kb]
(598 Downloads)
| Abstract (HTML) (2298 Views)
Full Text: (854 Views)
1. Introduction
2. Methods and Materials/Patients
Protocol review
Subjects
3. Results

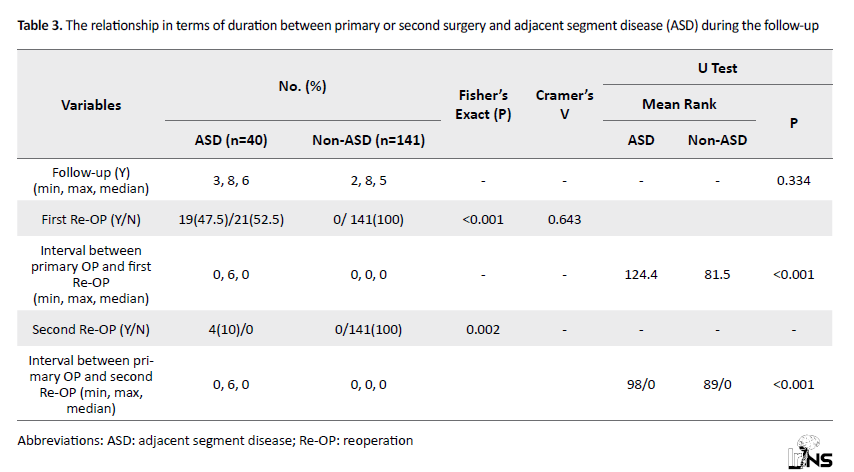
Timing: No significant relationship was observed in terms of duration between primary surgery and ASD during the follow-up of the patients (P=0.334). A significant relationship was found between a history of reoperation and ASD. Given Cramer’s V value (0.643), this relationship was strong. Also, a significant relationship was observed between ASD and the interval between the primary surgery and the first reoperation (P<0.05). The interval between primary surgery and the first reoperation was higher in the group of patients with ASD with a mean rank of 124.4 than in the group of patients without ASD, with an average rank of 81.5. A significant relationship was present between the history of two reoperations and ASD (P=0.002). According to Cramer’s V value (0.282), this relationship was moderate. A significant relationship was seen between ASD and the interval between the first surgery and the second reoperation (P<0.05). The interval between the first surgery and the second reoperation was higher in the group of patients with ASD with a mean rank of 98 than in the group of patients without ASD with a mean rank of 89 (Table 3).
Fusion level: In the group with ASD, the median number of lumbar fusion levels was 2 and the median number of fusion levels in the group without ASD was also not significantly related between ASD and the number of fusion levels (P=0.110).
Laminectomy: A significant relationship was observed between laminectomy at a high fusion level and ASD (P<0.05). According to Cramer’s V value (0.346), this relationship was strong.
Fusion termination: A significant relationship was observed between fusion terminations in the L1 vertebra and ASD. According to Cramer’s V value (0.148), this relationship was weak. Nonetheless, no significant relationship was observed between the two groups in terms of fusion terminating in L5 (P=0.11) and S1 (P=0.522) (Figure 2).
.png)
The graphs show a significant relationship between laminectomy at high fusion level, terminations in L1 vertebra, cross-linking tools and
ASD (P<0.05).
4. Discussion
5. Conclusion
Ethical Considerations
Compliance with ethical guidelines
Funding
This study was extracted from a thesis in neurosurgery specialty by Anousheh Ghaffari funded by Mazandaran University of Medical Sciences, Iran.
Authors' contributions
Conflict of interest
The authors declared no potential conflict of interest.
Acknowledgements
The authors express their gratitude to the volunteers who participated in the research.
References
Degeneration occurring in the moving segments above or below the fused part of the spine is known as Adjacent Segment Disease (ASD). About five decades ago, some case studies were published on ASD as a relatively uncommon complication of lumbar and sacroiliac fusion [1, 2].
ASD is a broad term referring to any abnormal process taking place in the moving segment next to a fusion. Disc herniation is one of the most common forms of ASD [3-6]. Listhesis (anterolisthesis or retrolisthesis), instability, stenosis, hypertrophic facet arthritis, and osteophyte formation have been reported frequently in the literature [7-15]. Scoliosis and compression fractures of the vertebrae are less common [10]. Although many studies have shown extensive biomechanical changes in ASD after fusion, relatively few studies have examined the biomechanical changes leading to ASD. A recent in-vivo study on animal models showed that biomechanical changes can initially lead to ASD [16-32]. In the literature, the incidence of ASD varied in a wide range of ASD incidence is due to the retrospective nature of some of the studies, different research methodologies, ASD definitions, and patient populations. In many of these studies, the criteria used to determine ASD were based solely on radiographic findings rather than the patient’s clinical symptoms [3-6, 8, 11-13]. Therefore, asymptomatic disc degeneration, canal stenosis, disc herniation, spondylolisthesis, or instability at an adjacent surface have all been considered ASD. With radiographic criteria alone, the incidence of ASD is between 8% and 100% [15]. In contrast, studies reporting the incidence of ASD based on clinical symptoms have yielded rates of 5.2% to 18.5% [33-44]. This study was conducted to evaluate the frequency of adjacent segment disease and its risk factors following posterior decompression and fusion in degenerative lumbar disorders.
ASD is a broad term referring to any abnormal process taking place in the moving segment next to a fusion. Disc herniation is one of the most common forms of ASD [3-6]. Listhesis (anterolisthesis or retrolisthesis), instability, stenosis, hypertrophic facet arthritis, and osteophyte formation have been reported frequently in the literature [7-15]. Scoliosis and compression fractures of the vertebrae are less common [10]. Although many studies have shown extensive biomechanical changes in ASD after fusion, relatively few studies have examined the biomechanical changes leading to ASD. A recent in-vivo study on animal models showed that biomechanical changes can initially lead to ASD [16-32]. In the literature, the incidence of ASD varied in a wide range of ASD incidence is due to the retrospective nature of some of the studies, different research methodologies, ASD definitions, and patient populations. In many of these studies, the criteria used to determine ASD were based solely on radiographic findings rather than the patient’s clinical symptoms [3-6, 8, 11-13]. Therefore, asymptomatic disc degeneration, canal stenosis, disc herniation, spondylolisthesis, or instability at an adjacent surface have all been considered ASD. With radiographic criteria alone, the incidence of ASD is between 8% and 100% [15]. In contrast, studies reporting the incidence of ASD based on clinical symptoms have yielded rates of 5.2% to 18.5% [33-44]. This study was conducted to evaluate the frequency of adjacent segment disease and its risk factors following posterior decompression and fusion in degenerative lumbar disorders.
2. Methods and Materials/Patients
Protocol review
The present retrospective cohort was conducted on a study population consisting of patients with degenerative spinal disease who underwent lumbar fusion surgery and needed reoperation. This study was approved by the institutional review board of the Orthopedic Research Center of Mazandaran University of Medical Science in Iran (IR.MAZUMS.RIB.REC.1400.023).
Subjects
The study population involved subjects who were admitted directly to our Neuro-Spine Department between 2013 and 2019 with an indication of degenerative disease based on the inclusion criteria. All the patients were operated on by the same spine surgeon. Out of the total of 277 candidates, 181 eligible patients were enrolled in the study. The radiological examination helped identify the patients with the adjacent segmental disease after clinical fusion surgery without any clinical signs, the patients with ASD and clinical symptoms, and the patients without ASD but with surgical indications and meeting the inclusion criteria.
Inclusion criteria
The inclusion criteria included patients who had clinical manifestations of lumbar ASD with the following; the patient’s informed consent, degenerative or isthmic spondylolisthesis, lumbar spinal canal stenosis, degenerative scoliosis, herniated disc, and the possibility of instability.
Exclusion criteria
The exclusion criteria included the patient’s lack of consent to participate in the study, a history of surgery indication other than spinal degenerative diseases, such as trauma, infection, tumor, deformity, or inflammatory diseases, having underlying medical conditions, such as diabetes, rheumatoid arthritis, kidney disease, chemoradiotherapy, cancer, corticosteroid use, and osteoporosis.
Outcome variables
Radiological examination
The presence of lumbar disc herniation, listhesis, spinal canal stenosis, vertebral fracture, and facet disorder in the segment adjacent to the fusion were some of the points of interest in the radiological examinations. The number of fusion levels, laminectomy above the fusion surface, damage to the upper facet above the fusion, and the presence of a cross-link were recorded in the fusion and instrumentation complex (Figure 1).
.png)
The patients were also asked about any contributing risk factors, such as smoking and osteoporosis, which were recorded in their designated history checklist.
Statistical methodsInclusion criteria
The inclusion criteria included patients who had clinical manifestations of lumbar ASD with the following; the patient’s informed consent, degenerative or isthmic spondylolisthesis, lumbar spinal canal stenosis, degenerative scoliosis, herniated disc, and the possibility of instability.
Exclusion criteria
The exclusion criteria included the patient’s lack of consent to participate in the study, a history of surgery indication other than spinal degenerative diseases, such as trauma, infection, tumor, deformity, or inflammatory diseases, having underlying medical conditions, such as diabetes, rheumatoid arthritis, kidney disease, chemoradiotherapy, cancer, corticosteroid use, and osteoporosis.
Outcome variables
Radiological examination
The presence of lumbar disc herniation, listhesis, spinal canal stenosis, vertebral fracture, and facet disorder in the segment adjacent to the fusion were some of the points of interest in the radiological examinations. The number of fusion levels, laminectomy above the fusion surface, damage to the upper facet above the fusion, and the presence of a cross-link were recorded in the fusion and instrumentation complex (Figure 1).
.png)
The patients were also asked about any contributing risk factors, such as smoking and osteoporosis, which were recorded in their designated history checklist.
Quantitative variables were presented as Mean±SD and qualitative variables as numbers (percentage, frequency, mean, minimum, and maximum). The t-test was used for the comparative analysis of the independent quantitative variables for normal distributions of the data and Mann-Whitney’s U test was used for abnormal distributions. The statistical significance level was considered 0.05 and all statistical analyses were performed with SPSS software, version 24 and Stata, version 14.
3. Results
Forty patients (22.1%) out of 181 patients in this study had ASD, 21 patients (11.6%) had just ASD with radiographic findings and 19 patients (10.5%) had clinical adjacent segments. Out of the 40 patients with ASD, 17 patients (42.5%) were male and 33 (57.5%) were female. Out of 141 patients who did not have ASD, 61 patients (43.3%) were male and 80 (56.7%) were female. In addition, no significant relationship was observed in terms of gender between the two groups with ASD and non-ASD (P=0.932). The median age in the ASD group was 54 years and the median age in the non-ASD group was 48 years. Also, a significant relationship was observed between the patient’s age and ASD (P=0.045).
Body mass index (BMI): The mean BMI in the group with ASD was 28 Kg/m2 and the median BMI in the group without ASD was 25.9 Kg/m2. Also, a significant relationship was observed between the patient’s BMI and ASD (P=0.003), as the group of patients with ASD had a higher BMI with a mean rank of 114.4 than the non-ASD group, with a mean rank of 84.9.
Smoking: Out of the 40 patients with ASD, 11 patients (27.5%) had a history of smoking and 29 (72.5%) had no history of smoking. Similarly, in the group without ASD, 25 patients (17.7%) had a history of smoking and 116 (82.3%) had no history of smoking. No significant relationship was observed between ASD and a history of smoking (P=0.172). Table 1 presents the relationship between sex, age, smoking, and BMI with ASD.
Indications: In both groups with and without ASD, the highest frequency of spinal canal stenosis surgery (45%) pertained to ischemic or degenerative isthmic spondylolisthesis or potential instability. A significant relationship was observed between surgical indications and ASD (P=0.002). Patients with lumbar spinal canal stenosis were more likely to develop ASD. According to Cramer’s V value (0.311), this relationship was strong (Table 2).

Timing: No significant relationship was observed in terms of duration between primary surgery and ASD during the follow-up of the patients (P=0.334). A significant relationship was found between a history of reoperation and ASD. Given Cramer’s V value (0.643), this relationship was strong. Also, a significant relationship was observed between ASD and the interval between the primary surgery and the first reoperation (P<0.05). The interval between primary surgery and the first reoperation was higher in the group of patients with ASD with a mean rank of 124.4 than in the group of patients without ASD, with an average rank of 81.5. A significant relationship was present between the history of two reoperations and ASD (P=0.002). According to Cramer’s V value (0.282), this relationship was moderate. A significant relationship was seen between ASD and the interval between the first surgery and the second reoperation (P<0.05). The interval between the first surgery and the second reoperation was higher in the group of patients with ASD with a mean rank of 98 than in the group of patients without ASD with a mean rank of 89 (Table 3).

Fusion level: In the group with ASD, the median number of lumbar fusion levels was 2 and the median number of fusion levels in the group without ASD was also not significantly related between ASD and the number of fusion levels (P=0.110).
Laminectomy: A significant relationship was observed between laminectomy at a high fusion level and ASD (P<0.05). According to Cramer’s V value (0.346), this relationship was strong.
Fusion termination: A significant relationship was observed between fusion terminations in the L1 vertebra and ASD. According to Cramer’s V value (0.148), this relationship was weak. Nonetheless, no significant relationship was observed between the two groups in terms of fusion terminating in L5 (P=0.11) and S1 (P=0.522) (Figure 2).
.png)
The graphs show a significant relationship between laminectomy at high fusion level, terminations in L1 vertebra, cross-linking tools and
ASD (P<0.05).
Cross-links: A significant relationship was found between cross-linking tools and ASD (P<0.05). According to Cramer’s V value (0.351), this relationship was strong (Figure 2).
4. Discussion
Other similar studies [46, 47] found no significant relationship between gender and the incidence of ASD. Okuda et al. conducted a study on ASD after lumbar intervertebral fusion in 1000 patients and showed no relationship between the incidence of ASD (total 9%) and the patient’s age but ASD was more in patients over 60 years of age [45] in the present study, the median age was 54 years in the ASD group and 48 years in the non-ASD group. Also, a significant relationship was observed between the patient’s age and ASD in both groups (P=0.045). The group of patients with ASD with an average rank of 105.6 was older than the group of patients without ASD with an average rank of 86.8.
Body mass index (BMI) is a universally accepted and simple indicator defined by the World Health Organization, with values above 25 Kg/m2 suggesting overweight and above 30 Kg/m2 obesity [48]. In the study conducted by Simmons et al. [49] on women aged 45 to 64 years and an average of nine-year follow-up, increased BMI was one of the risk factors for disc degeneration, and a BMI above 25 increased the risk of lumbar disc degeneration. In the present study, the median BMI was 28 Kg/m2 in the group with ASD and 25.9 Kg/m2 in the group without ASD. Also, a significant relationship existed between the patient’s BMI and ASD (P=0.003).
The group with ASD had a higher BMI with an average rank of 114.4 than the group without ASD with an average rank of 84.9. In addition, paraspinal muscle strength is not very good in overweight or obese patients compared with normal-weight people. During surgery, the removal of these muscles is necessary to expose the spine and lamina appendages. Furthermore, paraspinal muscle traction is inevitable in this operation for decompression and fusion, which can reduce muscle function after surgery. If the paraspinal muscles are not strong enough to maintain a standing position, the degeneration of the intervertebral disc of the articular appendage may be accelerated, especially in the segment above the fusion surface [50, 51]. Therefore, a BMI greater than 25 is not only a risk factor associated with normal spinal degeneration in healthy individuals, but can also play a crucial role in the development of ASD. Accordingly, preoperative and postoperative weight control can reduce the incidence of ASD and improve treatment outcomes and patient satisfaction [52]. In the study conducted by Bagheri et al. [53], no relationship was found between smoking and the incidence of ASD. In a meta-analysis by Wang et al. [47], the history of smoking was evaluated in the groups with and without ASD. History of smoking was found to have a significant relationship with the incidence of ASD. In the present study, out of 40 patients with ASD, 11(27.5%) had a previous history of smoking and 29(72.5%) had no history. Similarly, in the group without ASD, 25 patients (17.7%) had a smoking history and 116 patients (82.3%) had no history. No significant relationship was observed between ASD and history of smoking (P=0.172). In the study conducted by Okuda et al. [45], a significant relationship was found between the length of fusion and the incidence of ASD. In addition, in the study conducted by Bagheri et al. [53], a significant relationship was observed between the incidence of ASD and having a fusion level above four. Wang et al. [54] found no significant relationship between the incidence of ASD and the fusion level. In our study, the minimum fusion level was one in both groups, the maximum level was four in the group with ASD and five in the non-ASD group, and the median was two in both groups. No significant relationship was found between ASD and fusion level (P=0.110). In our study, the highest surgical indication in patients with ASD was stenosis (45%), while in the non-ASD group, spondylolisthesis and the possibility of instability (24.1%) were the surgical indications. A significant relationship was also found between surgical indications and ASD (P=0.002). Patients with lumbar spinal canal stenosis were more likely to develop ASD, and according to Cramer’s V value, which was 0.311, this relationship was strong. In the study conducted by Wang et al. [47] on patients with disc herniation, canal stenosis, and spondylolisthesis, no significant relationship existed between surgical indication and the incidence of ASD. The maximum follow-up period of patients from the time of initial surgery and their current resource was 6 years (at least 3 years and 6 years in the group of patients with ASD). No significant relationship was observed in terms of follow-up time between primary surgery and second operation and the presence or absence of ASD during the follow-up of the patients (P=0.334). In the group with ASD, 47.5% of the patients had a history of one reoperation. A total of 52.5% of the patients had no history of reoperation. In the non-ASD group, none of the patients had a history of reoperation. A significant relationship also existed between the history of reoperation and ASD. Given Cramer’s V value of 0.643, this correlation was found to be strong.
Although, only 10% of these patients had two reoperations, a significant relationship was observed between the history of two reoperations and ASD. Furthermore, the time interval between primary surgery and the first reoperation was significantly related to ASD (P<0.05).
The time interval between the first surgery and the second reoperation was also significantly related to ASD (P<0.05). Masevnin et al. [46] showed no significant relationship between the follow-up time of the patients and the incidence of ASD. This finding is also true for the study by Bagheri et al. [53].
In the present study, a significant relationship was observed between laminectomy at a high fusion level and ASD. Given Cramer’s V value of 0.346, this correlation was deemed relatively strong. A significant relationship was found between cross-linking tools and ASD. Given Cramer’s V value obtained, i.e. 0.351, this correlation was also deemed strong. The imaging findings of the patients, obtained by plain X-ray, MRI, and CT scan, included herniated disc, lumbar spinal canal stenosis, kyphosis, radial fracture, and spondylolisthesis. Most findings belonged to the group of patients with ASD spinal canal stenosis (50%). In the non-ASD group, 133 patients had no findings in the imaging studies. A significant relationship also existed between the imaging findings and ASD (P<0.05). Patients with lumbar spinal canal stenosis are more likely to develop ASD.
Considering Cramer’s V value obtained, i.e. 0.964, this correlation was deemed strong. In the present study, a significant relationship was observed between the terminations of fusion to the L1 vertebra and ASD. Given Cramer’s V value of 0.148, this correlation was found to be poor. Meanwhile, no significant relationship was observed between fusion terminations to L5 or S1 vertebrae. In the study by Masevnin et al. [46], where fusion levels included L2-L3, L3-L4, and L4-L5, no significant relationship existed between ASD and fusion levels. Also, in the study by Wang et al. [47], in which fusion levels included L4-L5, L5-S1, and L4-S1, no significant relationship was found with ASD. In the meta-analysis by Wang et al. [54], no significant relationship was observed between fusion termination to S1 and ASD.
Although, only 10% of these patients had two reoperations, a significant relationship was observed between the history of two reoperations and ASD. Furthermore, the time interval between primary surgery and the first reoperation was significantly related to ASD (P<0.05).
The time interval between the first surgery and the second reoperation was also significantly related to ASD (P<0.05). Masevnin et al. [46] showed no significant relationship between the follow-up time of the patients and the incidence of ASD. This finding is also true for the study by Bagheri et al. [53].
In the present study, a significant relationship was observed between laminectomy at a high fusion level and ASD. Given Cramer’s V value of 0.346, this correlation was deemed relatively strong. A significant relationship was found between cross-linking tools and ASD. Given Cramer’s V value obtained, i.e. 0.351, this correlation was also deemed strong. The imaging findings of the patients, obtained by plain X-ray, MRI, and CT scan, included herniated disc, lumbar spinal canal stenosis, kyphosis, radial fracture, and spondylolisthesis. Most findings belonged to the group of patients with ASD spinal canal stenosis (50%). In the non-ASD group, 133 patients had no findings in the imaging studies. A significant relationship also existed between the imaging findings and ASD (P<0.05). Patients with lumbar spinal canal stenosis are more likely to develop ASD.
Considering Cramer’s V value obtained, i.e. 0.964, this correlation was deemed strong. In the present study, a significant relationship was observed between the terminations of fusion to the L1 vertebra and ASD. Given Cramer’s V value of 0.148, this correlation was found to be poor. Meanwhile, no significant relationship was observed between fusion terminations to L5 or S1 vertebrae. In the study by Masevnin et al. [46], where fusion levels included L2-L3, L3-L4, and L4-L5, no significant relationship existed between ASD and fusion levels. Also, in the study by Wang et al. [47], in which fusion levels included L4-L5, L5-S1, and L4-S1, no significant relationship was found with ASD. In the meta-analysis by Wang et al. [54], no significant relationship was observed between fusion termination to S1 and ASD.
5. Conclusion
The present research identified a significant correlation between the incidence of ASD and the following risk factors; BMI, older age, lumbar spinal canal stenosis, reoperation, high fusion laminectomy, crosslinking, lumbar imaging stenosis, and end of fusion to L1 vertebrae. Spine surgeons need to be aware of these possible risk factors in all related procedures, including pedicle screw insertion to decompression procedures.
Unfortunately, one of the limitations of the present study was the failure to measure spinopelvic parameters in the patients’ graphs and examine its relationship with the incidence of ASD.
Unfortunately, one of the limitations of the present study was the failure to measure spinopelvic parameters in the patients’ graphs and examine its relationship with the incidence of ASD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the institutional review board of the Orthopedic Research Center of Mazandaran University of Medical Sciences in Iran, under the ethics code IR.MAZUMS.RIB.REC.1400.023. Before beginning the test and after gaining familiarity with the surgery method and the potential risks and benefits of the procedure, the legally certified representative of the patient signed an informed consent form.
Funding
This study was extracted from a thesis in neurosurgery specialty by Anousheh Ghaffari funded by Mazandaran University of Medical Sciences, Iran.
Authors' contributions
Conception and design: Kaveh Haddadi, Abdolrasool Alaee, Mohammad Khademloo; Data collection: Kaveh Haddadi, Anoushe Ghafari; Data analysis and interpretation: Mohammad Khademloo; Drafting the article: All authors; Critically revising the article: All authors; Reviewing the submitted version of the manuscript: All authors; Approving the final version of the manuscript: All authors.
Conflict of interest
The authors declared no potential conflict of interest.
Acknowledgements
The authors express their gratitude to the volunteers who participated in the research.
References
- Anderson CE. Spondyloschisis following spine fusion. The Journal of Bone & Joint Surgery. 1956; 38(5):1142-6. [DOI:10.2106/00004623-195638050-00019]
- Unander-Scharin L. A case of spondylolisthesis lumbalis acquisita. Acta Orthopaedica Scandinavica. 1950; 19(4):536-44. [DOI:10.3109/17453675008991107] [PMID]
- Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996; 21(8):970-81. [DOI:10.1097/00007632-199604150-00013] [PMID]
- Ishihara H, Osada R, Kanamori M, Kawaguchi Y, Ohmori K, Kimura T, et al. Minimum 10-year follow-up study of anterior lumbar interbody fusion for isthmic spondylolisthesis. Journal of Spinal Disorders. 2001; 14(2):91-9. [DOI:10.1097/00002517-200104000-00001] [PMID]
- Cannizzaro D, Anania CD, Safa A, Zaed I, Morenghi M, Riva M, et al. Lumbar adjacent segment degeneration after spinal fusion surgery. A systematic review and meta-analysis. Journal of Neurosurgical Sciences. 2022. [DOI:10.23736/S0390-5616.22.05891-X]
- Miyakoshi N, Abe E, Shimada Y, Okuyama K, Suzuki T, Sato K. Outcome of one-level posterior lumbar interbody fusion for spondylolisthesis and postoperative intervertebral disc degeneration adjacent to the fusion. Spine. 2000; 25(14):1837-42. [DOI:10.1097/00007632-200007150-00016] [PMID]
- Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine. 1995; 20(6):743-7. [DOI:10.1097/00007632-199503150-00018] [PMID]
- Dong S, Li J, Jia X, Zhu J, Chen Y, Yuan B. Analysis of risk factors for adjacent segment degeneration after minimally invasive transforaminal interbody fusion at lumbosacral spine. Computational Intelligence and Neuroscience. 2022; 2022:4745534. [DOI:10.1155/2022/4745534] [PMID] [PMCID]
- Brodsky AE. Post-laminectomy and post-fusion stenosis of the lumbar spine. Clinical Orthopaedics and Related Research. 1976; 115:130-9. [DOI:10.1097/00003086-197603000-00022]
- Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. Journal of Neurosurgery. 1999; 90(2 Suppl):163-9. [DOI:10.3171/spi.1999.90.2.0163] [PMID]
- Hambly MF, Wiltse LL, Raghavan N, Schneiderman G, Koenig C. The transition zone above a lumbosacral fusion. Spine. 1998; 23(16):1785-92. [DOI:10.1097/00007632-199808150-00012] [PMID]
- Hsu KY, Zucherman J, White A. The long-term effect of lumbar spine fusion: Deterioration of adjacent motion segments. In: Yonenobu K, Ono K, Takemitsu Y, editors. Lumbar fusion and stabilization. Tokyo: Springer; 1993. [Link]
- Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. European Spine Journal. 2001; 10(4):309-13. [DOI:10.1007/s005860000207] [PMID] [PMCID]
- Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988; 13(3):375-7. [DOI:10.1097/00007632-198803000-00029] [PMID]
- Wimmer C, Gluch H, Krismer M, Ogon M, Jesenko R. AP-translation in the proximal disc adjacent to lumbar spine fusion: A retrospective comparison of mono-and polysegmental fusion in 120 patients. Acta Orthopaedica Scandinavica. 1997; 68(3):269-72. [DOI:10.3109/17453679708996699] [PMID]
- Lee CK, Langrana NA. Lumbosacral spinal fusion. A biomechanical study. Spine. 1984; 9(6):574-81. [DOI:10.1097/00007632-198409000-00007] [PMID]
- Ha KY, Schendel MJ, Lewis JL, Ogilvie JW. Effect of immobilization and configuration on lumbar adjacent-segment biomechanics. Journal of Spinal Disorders. 1993; 6(2):99-105. [DOI:10.1097/00002517-199304000-00002] [PMID]
- Bastian L, Lange U, Knop C, Tusch G, Blauth M. Evaluation of the mobility of adjacent segments after posterior thoracolumbar fixation: A biomechanical study. European Spine Journal. 2001; 10(4):295-300. [DOI:10.1007/s005860100278] [PMID] [PMCID]
- Chow DH, Luk KD, Evans JH, Leong JC. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine. 1996; 21(5):549-55. [DOI:10.1097/00007632-199603010-00004] [PMID]
- Wu J, Yang D, Han Y, Xu H, Wen W, Xu H, et al. Application of dual-trajectory screws in revision surgery for lumbar adjacent segment disease: A finite element study. Journal of Orthopaedic Surgery and Research. 2022; 17(1):427.[DOI:10.1186/s13018-022-03317-9] [PMID] [PMCID]
- Nagata H, Schendel MJ, Transfeldt EE, Lewis JL. The effects of immobilization of long segments of the spine on the adjacent and distal facet force and lumbosacral motion. Spine. 1993; 18(16):2471-9. [DOI:10.1097/00007632-199312000-00017] [PMID]
- Quinnell RC, Stockdale HR. Some experimental observations of the influence of a single lumbar floating fusion on the remaining lumbar spine. Spine. 1981; 6(3):263-7. [DOI:10.1097/00007632-198105000-00008] [PMID]
- Dekutoski MB, Schendel MJ, Ogilvie JW, Olsewski JM, Wallace LJ, Lewis JL. Comparison of in vivo and in vitro adjacent segment motion after lumbar fusion. Spine. 1994; 19(15):1745-51. [DOI:10.1097/00007632-199408000-00015] [PMID]
- Frymoyer JW, Hanley EN Jr, Howe J, Kuhlmann D, Matteri RE. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine. 1979; 4(5):435-40. [DOI:10.1097/00007632-197909000-00008] [PMID]
- Toivonen LA, Mäntymäki H, Häkkinen A, Kautiainen H, Neva MH. Postoperative sagittal balance has only a limited role in the development of adjacent segment disease after lumbar spine fusion for degenerative lumbar spine disorders: A subanalysis of the 10-year follow-up study. Spine. 2022; 47(19):1357-61. [DOI:10.1097/BRS.0000000000004400] [PMID] [PMCID]
- Axelsson P, Johnsson R, Strömqvist B. The spondylolytic vertebra and its adjacent segment. Mobility measured before and after posterolateral fusion. Spine. 1997; 22(4):414-7. [DOI:10.1097/00007632-199702150-00012] [PMID]
- Chen CS, Cheng CK, Liu CL. A biomechanical comparison of posterolateral fusion and posterior fusion in the lumbar spine. Journal of Spinal Disorders & Techniques. 2002; 15(1):53-63. [DOI:10.1097/00024720-200202000-00010] [PMID]
- Chen CS, Cheng CK, Liu CL, Lo WH. Stress analysis of the disc adjacent to interbody fusion in lumbar spine. Medical Engineering & Physics. 2001; 23(7):485-93. [DOI:10.1016/S1350-4533(01)00076-5] [PMID]
- Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995; 20(5):526-31. [DOI:10.1097/00007632-199503010-00004] [PMID]
- Cunningham BW, Kotani Y, McNulty PS, Cappuccino A, McAfee PC. The effect of spinal destabilization and instrumentation on lumbar intradiscal pressure: An in vitro biomechanical analysis. Spine. 1997; 22(22):2655-63. [DOI:10.1097/00007632-199711150-00014] [PMID]
- Kim YE, Goel VK, Weinstein JN, Lim TH. Effect of disc degeneration at one level on the adjacent level in axial mode. Spine. 1991; 16(3):331-5. [DOI:10.1097/00007632-199103000-00013] [PMID]
- Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: An in vivo mouse model and finite-element study. Spine. 1998; 23(23):2493-506. [DOI:10.1097/00007632-199812010-00004] [PMID]
- Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. Journal of Spinal Disorders. 1995; 8(6):464-73. [DOI:10.1097/00002517-199512000-00008] [PMID]
- Duan PG, Mummaneni PV, Berven SH, Mayer R, Ruan HB, Chang CC, et al. Revision surgery for adjacent segment degeneration after fusion for lumbar spondylolisthesis is there a correlation with roussouly type? Spine. 2022; 47(1):E10-5. [DOI:10.1097/BRS.0000000000003708] [PMID]
- Mesregah MK, Yoshida B, Lashkari N, Abedi A, Meisel HJ, Diwan A, et al. Demographic, clinical, and operative risk factors associated with postoperative adjacent segment disease in patients undergoing lumbar spine fusions: A systematic review and meta-analysis. The Spine Journal. 2022; 22(6):1038-69. [PMID]
- Kumar M, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. European Spine Journal. 2001; 10(4):314-9. [DOI:10.1007/s005860000239] [PMID] [PMCID]
- Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, el-Khoury GY, et al. Long-term follow-up of lower lumbar fusion patients. Spine. 1987; 12(2):97-104. [DOI:10.1097/00007632-198703000-00004] [PMID]
- Nakai S, Yoshizawa H, Kobayashi S. Long-term follow-up study of posterior lumbar interbody fusion. Journal of Spinal Disorders. 1999; 12(4):293-9. [DOI:10.1097/00002517-199908000-00004] [PMID]
- Pihlajamäki H, Böstman O, Ruuskanen M, Myllynen P, Kinnunen J, Karaharju E. Posterolateral lumbosacral fusion with transpedicular fixation: 63 consecutive cases followed for 4(2-6) years. Acta Orthopaedica Scandinavica. 1996; 67(1):63-8. [DOI:10.3109/17453679608995612] [PMID]
- Rahm MD, Hall BB. Adjacent-segment degeneration after lumbar fusion with instrumentation: A retrospective study. Journal of Spinal Disorders. 1996; 9(5):392-400. [DOI:10.1097/00002517-199610000-00005] [PMID]
- Wiltse LL, Radecki SE, Biel HM, DiMartino PP, Oas RA, Farjalla G, et al. Comparative study of the incidence and severity of degenerative change in the transition zones after instrumented versus noninstrumented fusions of the lumbar spine. Journal of Spinal Disorders. 1999; 12(1):27-33. [DOI:10.1097/00002517-199902000-00004] [PMID]
- Guigui P, Lambert P, Lassale B, Deburge A. [Long-term outcome at adjacent levels of lumbar arthrodesis (French)]. Revue de Chirurgie Orthopedique et Reparatrice de L'appareil Moteur. 1997; 83(8):685-96. [PMID]
- Kanayama M, Hashimoto T, Shigenobu K, Harada M, Oha F, Ohkoshi Y, et al. Adjacent-segment morbidity after Graf ligamentoplasty compared with posterolateral lumbar fusion. Journal of Neurosurgery. 2001; 95(1 Suppl):5–10. [DOI:10.3171/spi.2001.95.1.0005] [PMID]
- Kuslich SD, Danielson G, Dowdle JD, Sherman J, Fredrickson B, Yuan H, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine. 2000; 25(20):2656-62. [DOI:10.1097/00007632-200010150-00018] [PMID]
- Okuda S, Yamashita T, Matsumoto T, Nagamoto Y, Sugiura T, Takahashi Y, et al. Adjacent segment disease after posterior lumbar interbody fusion: A case series of 1000 patients. Global Spine Journal. 2018; 8(7):722-7. [DOI:10.1177/2192568218766488] [PMID] [PMCID]
- Masevnin S, Ptashnikov D, Mikhaylov D, Smekalenkov O, Zaborovskii N, Lapaeva O. Early adjacent segment degeneration after short lumbar fusion. Global Spine Journal. 2016; 6(1_suppl):s-0036-1582968-s-0036-. [DOI:10.1055/s-0036-1582968]
- Wang H, Ma L, Yang D, Wang T, Liu S, Yang S, et al. Incidence and risk factors of adjacent segment disease following posterior decompression and instrumented fusion for degenerative lumbar disorders. Medicine. 2017; 96(5):e6032.[DOI:10.1097/MD.0000000000006032] [PMID] [PMCID]
- WHO Consultation on Obesity (1999: Geneva, Switzerland), World Health Organization. Obesity: Preventing and managing the global epidemic: Report of a WHO consultation. Geneva: World Health Organization; 2000. [Link]
- Symmons DP, van Hemert AM, Vandenbroucke JP, Valkenburg HA. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women. II. Radiographic findings. Annals of the Rheumatic Diseases. 1991; 50(3):162-6. [DOI:10.1136/ard.50.3.162] [PMID] [PMCID]
- Ranger TA, Newell N, Grant CA, Barker PJ, Pearcy MJ. Role of the middle lumbar fascia on spinal mechanics: A human biomechanical assessment. Spine. 2017; 42(8):E459-65. [DOI:10.1097/BRS.0000000000001854] [PMID]
- Ge W, Cao DY, Long CR, Pickar JG. Plane of vertebral movement eliciting muscle lengthening history in the low back influences the decrease in muscle spindle responsiveness of the cat. Journal of Applied Physiology. 2011; 111(6):1735-43. [DOI:10.1152/japplphysiol.00059.2011] [PMID] [PMCID]
- Wang H, Ma L, Yang D, Wang T, Yang S, Wang Y, et al. Incidence and risk factors for the progression of proximal junctional kyphosis in degenerative lumbar scoliosis following long instrumented posterior spinal fusion. Medicine. 2016; 95(32):e4443. [DOI:10.1097/MD.0000000000004443] [PMID] [PMCID]
- Bagheri SR, Alimohammadi E, Zamani Froushani A, Abdi A. Adjacent segment disease after posterior lumbar instrumentation surgery for degenerative disease: Incidence and risk factors. Journal of Orthopaedic Surgery. 2019; 27(2):2309499019842378. [DOI:10.1177/2309499019842378] [PMID]
- Wang H, Zhang D, Ma L, Shen Y, Ding W. Factors predicting patient dissatisfaction 2 years after discectomy for lumbar disc herniation in a Chinese older cohort: A prospective study of 843 cases at a single institution. Medicine. 2015; 94(40):e1584. [DOI:10.1097/MD.0000000000001584] [PMID] [PMCID]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







