Thu, Jul 3, 2025
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Noman Abdullah Alborihi S, Abdullah Al-Awadi A, Noman Abdullah Alborihi E. Parasagittal and parafalcine cystic meningiomas, as well as other cystic meningiomas case series and systematic literature review.. Iran J Neurosurg 2024; 10 : 3
URL: http://irjns.org/article-1-365-en.html
URL: http://irjns.org/article-1-365-en.html
1- Department of Neurosurgery, Al-Thawra Modern General Hospital (TMGH), Sana’a, Yemen and Department of Neurosurgery, Uni-Max International Hospital, Sana’a, Yemen. , drsoliman3@gmail.com
2- Department of Neurosurgery, Al-Thawra Modern General Hospital (TMGH), Sana’a, Yemen.
3- Department of Neurosurgery, A-Jumhairi Teaching Hospital, Sana’a, Yemen AND Department of Neurosurgery, Saudi German Hospital Sana’a, Sana’a, Yemen
2- Department of Neurosurgery, Al-Thawra Modern General Hospital (TMGH), Sana’a, Yemen.
3- Department of Neurosurgery, A-Jumhairi Teaching Hospital, Sana’a, Yemen AND Department of Neurosurgery, Saudi German Hospital Sana’a, Sana’a, Yemen
Full Text [PDF 1951 kb]
(822 Downloads)
| Abstract (HTML) (4017 Views)
Full Text: (501 Views)
1. Background and Importance
Cysts can form within meningiomas either intratumorally (central or peripheral to the tumor) or as peritumoral arachnoid cysts (adjacent to the parenchyma and meningioma) (Figure 1). Cystic meningioma (CM) is rare, accounting for 2% to 7% of all intracranial meningiomas [1-3], and the location of cystic and solid components within the tumor can pose challenges in diagnosis. Preoperative misdiagnosis was reported in 62% of cases with computed tomography (CT) scans and in 25% of cases with angiography [4]. Magnetic resonance imaging (MRI) is more accurate and has a diagnostic error rate of 20% in these cases [5, 6].
After convexity CMs, the second most common location for CM is parasagittal [7], accounting for 26% of cases [8]. Parafalcine meningioma constitutes only 2% of all CMs.
In this report, we present two rare cases of CM, one case of parasagittal CM, one case of parafalcine CM, and two cases of convexity CM.
A total of 15% of meningiomas demonstrate atypical MRI features, such as cystic and necrotic areas, ring-like enhancement, and invasion of surrounding brain tissue. These characteristics can resemble malignant brain tumors, such as gliomas or metastatic tumors, leading to inaccurate radiological reports and misinterpretation of treatment decisions [9, 10].
What distinguishes the current research is that the tumor in the first case exhibited malignant traits, including brain edema and a shift in the midline of more than 5 cm, despite being a benign CM.
Previous studies did not mention a CM located in the parafalcine region with these specific histopathological characteristics. The third case occurred post-delivery, and the fourth case involved an older patient who experienced improvement following surgery. Excising a CM may yield a favorable prognosis.
Treatment: The National Comprehensive Cancer Network® (NCCN®) suggestions summarize the present-day method of meningioma management (NCCN®, 2022) visible set of rules meningiomas (MEN)-1 and for the observe-up visible set of rules meningiomas (MEN)-2 [11] (Figure 2).
2. Case Presentation
Case series
A prospective evaluation of four cases of CM was conducted at Al-Thawra Model General Hospital and Uni-Max International Hospital, in Sana’a City, Yemen, from June 2020 to June 2023. The evaluation included the assessment of the patient’s age, gender, clinical presentation, neurological deficits, radiological site, morphological characteristics of the cyst, and histopathological type.
During the study period, our hospital treated four patients diagnosed with CM out of 134 meningiomas. These diagnoses were confirmed through surgical procedures and pathological examination. The prevalence of CM among the meningioma cases was found to be 3%. Among the four patients with CM, one case was parafalcine, one case was parasagittal, and two cases were convexity CM.
During the one-year follow-up period, the four cases of CM were closely monitored and evaluated at Al-Thawra Model General Hospital in Sana’a and Uni-Max International Hospital, Yemen, from June 2020 to June 2023.
This case series presents four cases of CMs, each with different locations and clinical presentations.
Case 1: Parasagittal CM (extratumoral cyst)
A 40-year-old right-handed woman presented with recurrent severe headaches for one month and left-sided paresthesia. She had no known medical conditions and no relevant family history. The patient resided in a mountainous village in Al Mahwit Governorate, Yemen City. On examination, she exhibited intact cognition without any language or speech abnormalities. However, she had a left-sided weakness with a power grade of 4, hyperreflexia in deep tendon reflexes, and an extensor plantar reflex. Mild sensation deficits were also observed on the left side.
Investigation
In routine tests, including complete blood count (CBC), chest x-ray, serum electrolytes, kidney function tests (KFT), liver function tests (LFT), abdominal ultrasonography, electrocardiogram, and random blood sugar, all yielded normal results. A contrast-enhanced CT scan of the brain revealed a solid mass with peritumoral cystic components and surrounding edema in the right frontoparietal region. Subsequent brain MRI with gadolinium detected a homogeneously enhanced extra-axial mass with a dural tail, peripheral cystic components, and underlying brain edema (Figure 3).
Management and outcome
A surgical resection of the tumor was performed at Simpson’s grade one (Figures 3C, E, and F), and histopathological analysis revealed a syncytial World Health Organization (WHO) grade one meningioma with no significant evidence of mitosis or cellular necrosis (Figure 3G). Following a 7-day hospitalization, the patient was discharged on oral levetiracetam 500 mg (twice daily) and panadol extra 500 mg (as needed) for 30 days. One month post-surgery, the patient exhibited improvement in all symptoms.
Case 2:Parafalcine CM (intratumoral cyst)
A 60-year-old right-handed woman had been suffering from recurrent severe headaches, right-sided paresthesia, and urinary and fecal incontinence for a month. She had no history of high blood pressure, diabetes, head injuries, or substance abuse. The patient resided in a mountain village in Yemen’s Haji Province. On examination, she exhibited intact perception with no speech or language abnormalities. However, she had grade 0 weakness in the right upper arm, grade 3 weakness in the right lower limb, and normal deep tendon reflexes and extensor plantar reflexes. The sensation was normal on both sides (Figure 4).
Investigation
A series of routine CBC, chest x-rays, serum electrolytes, KFT, LFT, and abdominal ultrasound, all yielded normal results, except for a random blood sugar level of 12.5 millimoles per liter (mmol). Brain CT showed a solid mass in the right frontoparietal region with intratumoral cystic components and edema surrounding the lesion. Brain MRI with gadolinium revealed a heterogeneously enhancing mass with a dural base, intratumoral cysts, and surrounding edema. The presence of a dural tail was also noted.
Management and outcome:
The patient underwent surgery to remove Simpson’s grade I tumor. A histopathological examination showed grade 3 rhabdoid meningioma with high levels of mitotic activity and areas of necrosis [6]. After 10 days, the patient was released and given a prescription for oral levetiracetam (500 mg twice a day) for one month, dexamethasone (5 mg twice a day) for three days, and pantoprazole (40 mg once a day) for 10 days. Following the surgery, the patient’s symptoms gradually improved.
Case 3: Convexity CM in pregnant women
A 42-year-old right-handed female patient post-delivery complained of a decreased level of consciousness Glasgow coma scale (GCS)=E2 V2 M4 and seizures and right-sided paresthesia, as well as the history of progressive headaches for the past six months. He had no significant medical or family history of neurological disorders. The patient lived in a rural area in Yemen’s Amran governorate. During the examination, he showed intact cognition, normal language and speech, and no focal neurological deficits.
Investigation:
Routine tests, including CBC, chest x-ray, serum electrolytes, kFT, LFT, and abdominal ultrasonography, were within normal limits. Brain MRI with gadolinium revealed a homogeneously enhancing extra-axial mass with peripheral cystic components and underlying brain edema in the right parietal region.
Management and outcome
The patient underwent surgical resection of the tumor at grade one (Simpson’s grade). Histopathological examination confirmed a syncytial WHO grade one meningioma without significant mitotic activity or cellular necrosis. The patient was discharged after 5 days and prescribed oral levetiracetam for 60 days. Postoperatively, the patient’s symptoms resolved completely.
Case 4: Convexity CM in older age
A 79-year-old right-handed man presented with a history of seizures, progressive headaches, and intermittent right-sided weakness over the past four months. He had no significant medical or family history of neurological disorders. The patient resided in a rural area in Yemen’s Hodeidah governorate. During the examination, he displayed intact cognition, normal language, and speech. However, the affected individual had grade 4 weakness in his right upper arm and grade 2 weakness in his right lower limb.
Investigation
Routine tests, such as a CBC, chest x-ray, serum electrolyte test, kidney function test, liver function test, and abdominal ultrasonography, showed normal results. A brain MRI with gadolinium revealed a homogeneous enhancing extra-axial mass with intratumoral cystic components and underlying brain edema in the left parietal region.
Management and outcome
The patient underwent surgical resection of the tumor at a grade one level (according to Simpson’s grading system). A histopathological examination was conducted, which confirmed the presence of syncytial meningioma with a WHO grade of one and minimal mitotic activity or cellular necrosis. The patient was discharged from the hospital after six days and was prescribed an oral medication called levetiracetam 500 mg bd for 60 days. Following the surgery, the patient’s symptoms showed a significant improvement.
Differential diagnosis
The differential diagnosis for CMs includes various primary malignancies, abscesses [12], and other tumor types. The following conditions should be considered:
1. Extra-axial primary malignancies: High-grade meningiomas can mimic CMs and should be considered as a potential differential diagnosis.
2. Glioblastoma: Glioblastomas are aggressive brain tumors that can present with cystic components. They should be considered in the differential diagnosis, especially in cases where the radiological features and clinical presentation are atypical for meningiomas.
3. Hemangiopericytoma: Hemangiopericytomas are rare tumors that can occur in the central nervous system. They can have cystic components and should be considered in the differential diagnosis of CMs.
4. Hemangioblastoma: Hemangioblastomas are vascular tumors that can present with cystic features. They are more commonly associated with the posterior fossa but can occur in other locations as well.
5. Solitary fibrous tumor: Solitary fibrous tumors can occur in various locations, including the central nervous system. They can have cystic components and may resemble CMs.
6. Metastases: Metastatic tumors of the brain, particularly those with cystic features, should be considered in the differential diagnosis.
7. Sarcoma: Rarely, primary intracranial sarcomas can present with cystic features and should be considered in the differential diagnosis.
8. Polycystic astrocytoma or other bone tumors: In rare cases, polycystic astrocytoma or other bone tumors can present with cystic components and may mimic CMs.
3. Discussion
Meningiomas constitute 20% of all central nervous system tumors. They are typically solid tumors. Despite the presence of clear radiographic signs, the presence of cystic lesions in meningiomas can lead surgeons to consider alternative tumor diagnoses. CMs, which account for approximately 3%-7% of meningiomas in adults and are less common in women than men [15], often occur in the frontoparietal areas [8, 12]. While CMs are most frequently found in parasagittal and convexity regions, they can occur in any location within the brain [16].
The formation of cysts in meningiomas may be attributed to cellular necrosis, hemorrhage, ischemia, or the secretion of fluid by functional tumor cells. Glial cells may proliferate in response to the tumor, leading to the elaboration of fluid within the cysts [2, 17, 18].
Amin et al [1]. classified CMs into four patterns or subtypes based on the location of the cyst around the meningioma and brain. These patterns include centrally positioned intratumoral cysts (type 1), peripherally positioned intratumoral cysts (type 2), cysts positioned in the adjoining parenchyma (type 3), and arachnoid cysts located between the brain and meningioma (type 4). Worthington et al. added a fifth type, purely CM, which involves the entrapped cerebrospinal fluid [20]. Additionally, Weber et al. conducted a study that categorized peritumoral CMs according to tumor invasion in the cystic wall [21].
Cyst type 1 differs from other lesions producing a typical ring uptake image, which is also observed in high-grade primary neoplasms and metastases. Cyst types 2 and 3 can be confusing and often occur with another cystic masses that have mural nodules [15] and dural bases, such as pleomorphic xanthoastrocytoma and desmoplastic primary neoplasms. If it develops in posterior fossa may be misdiagnosed with hemangioblastomas. Cyst type 4 is easily detectable in radiological images [22].
Approximately 54.6% of CMs are associated with prominent vasogenic edema, leading to symptomatic intracranial hypertension and a more rapid clinical course in patients with large-volume cysts [22]. Types 2 and 3 cysts tend to produce greater vasogenic edema, therefore, an effect of mass disproportionate to the size of the tumor [17, 22].
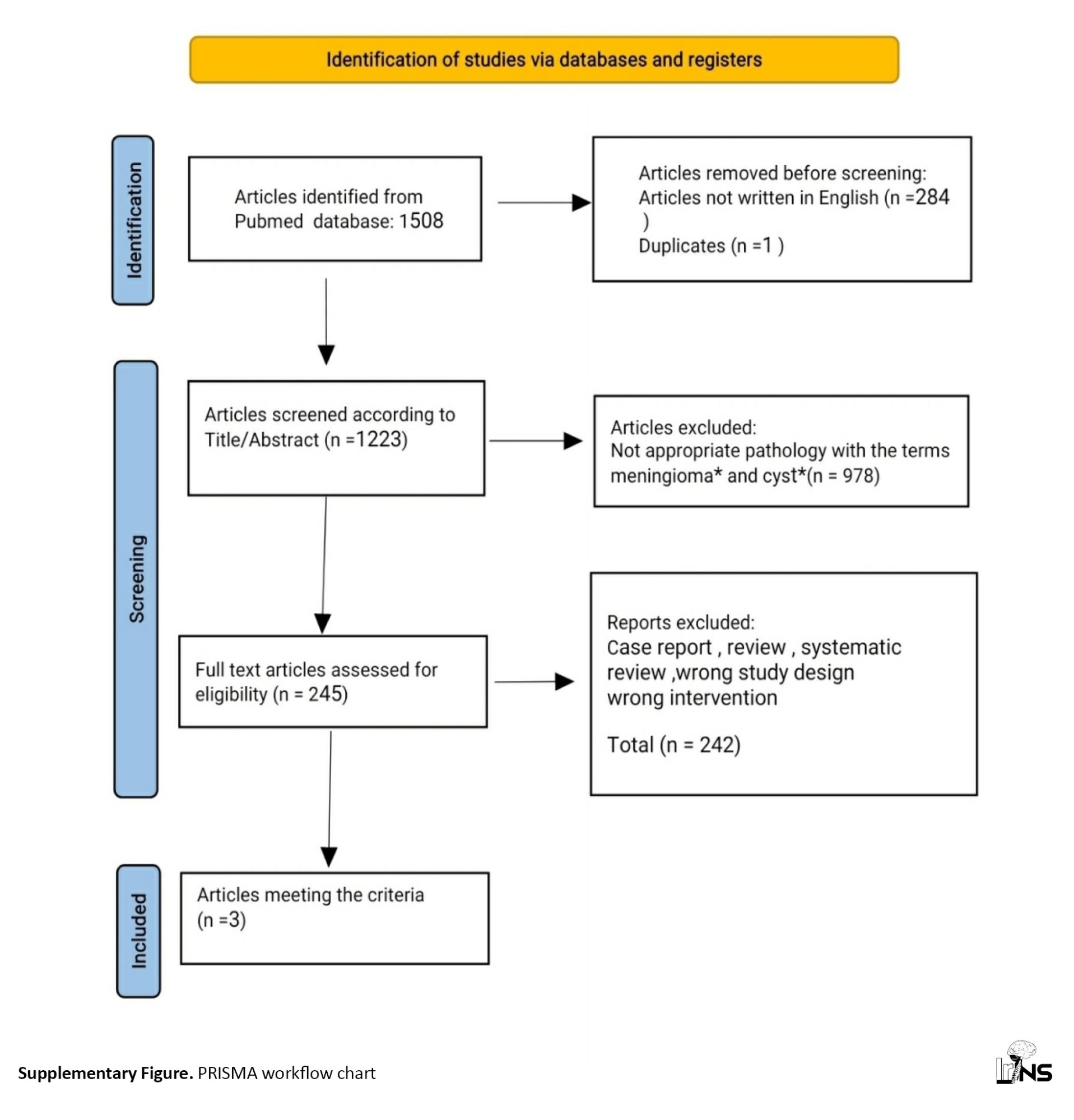
The systematic literature review was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Articles on CMs were searched in the PubMed electronic database from the existence of the database until July 2023.
After searching the Pubmed database, an initial pool of 1508 articles was identified. Subsequently, 284 non-English articles and 1 duplicate were excluded, resulting in 1223 manuscripts remaining.
To further refine the selection, we scrutinized the titles and abstracts of these articles. Through this analysis, it was determined that 978 articles did not pertain to the targeted pathology indicated by the specified keywords.
Next, the full texts of 245 articles were evaluated for eligibility. Regrettably, 242 of these articles were found unsuitable for our intended purposes. The reasons for their exclusion encompassed being case reports, reviews, systematic reviews, or possessing flawed study designs or interventions.
Ultimately, only three articles fulfilled our criteria and stand as valuable sources for subsequent scholarly investigation. The PRISMA chart visually represents this process (Supplementary and Supplementary Figure).
The search strategy used the following truncation: (Supplementary).
After applying the search strategy, 9 articles were obtained. These articles were manually checked, and 6 studies were excluded because they either included only one case, were case reports, or were review studies. The remaining three studies were analyzed and presented in Table 1.

Three significant studies were identified, and their findings were summarized: (Supplementary).
In the first case, the symptoms were more aggressive despite the small size of the tumor, as observed in the CT scan (Figure 3B). A distinctive appearance of an arachnoid cyst, secondary to the trapping of cerebrospinal fluid areas and widening of the subarachnoid space, was observed below the tumor. This appearance corresponds to the fourth type described by Nauta in 1979. This observation prompted consideration of other possible diagnoses for this cystic tumor, such as polycystic astrocytomas and hemangioblastomas. High-grade gliomas cannot be excluded due to the presence of severe edema and midline shift in the radiological images. Similar results were observed in the third and fourth cases.
In the second case, the patient had a cystic type 2 meningioma with intratumoral cysts of Nauta’s type, which may be secondary to central necrosis or intratumoral hemorrhage [23]. The mass effect was mild brain edema without midline shift, indicating a benign cystic tumor. However, a definitive diagnosis of high-grade astrocytomas cannot be ruled out based on CT alone. The presence of microvascular proliferation and or necrosis would indicate a grade 4 tumor, corresponding to a WHO grade 4 astrocytoma isocitrate dehydrogenase (IDH)-mutant [12, 24].
Angiography plays a crucial role in the diagnosis of CM. Meningiomas typically exhibit a blush that persists beyond the venous phase in the early arterial phase of angiography. Successful surgery relies on identifying and reducing the hemorrhage-related arterial feeders for meningiomas and evaluating the patency of the superior sagittal sinus and other dural venous sinuses, particularly for parasagittal or falcine meningiomas. Angiography can also aid in preoperative embolization and the diagnosis of prolonged homogeneous tumor blush. However, angiography misdiagnoses meningiomas in more than 25% of cases [20].
Angiography in the first case revealed a prolonged homogeneous tumor blush and Sindou grade 3, indicating invasion of the lateral wall of the superior sagittal sinus [25]. The feeder artery was the medial meningeal artery, emphasizing the importance of angiography in diagnosing CMs.
In the second case, angiography revealed an abnormal vascular blush in the early arterial phase and was Sindou type 2 without invasion to the medial segment of the superior sagittal sinus (SSS). The feeder artery was from both the external and internal carotid arteries. Therefore, other tumors, such as glioma, hemangiopericytoma, hemangioblastomas, and glioblastoma multiform, can be diagnostic dispersion.
Surgery is the primary treatment for CMs. The goal is to achieve complete resection while preserving neurological function. In some cases, preoperative embolization may be performed to reduce blood supply to the tumor and minimize intraoperative bleeding. The surgical approach depends on the location and size of the tumor. For CMs, a craniotomy is usually performed to access and remove the tumor. In cases where critical structures or venous sinuses are involved, a multidisciplinary team approach may be required to plan the surgery and ensure optimal outcomes.
In our study, MRI was more accurate for differentiating extraxial tumors from intra-axial tumors, such as hemangiopericytoma, hemangioblastomas, solitary fibrous tumors, and meningioma. Hemangiopericytoma and solitary fibrous tumors mirror meningiomas [26].
Go et al [4] reported an infratentorial mass that was more likely to be a hemangioblastoma in radiological images. However, the intraoperative appearance was similar to pilocytic astrocytoma. Finally, a histopathological exam for a biopsy taken from the mass and cyst showed that the mass became WHO grade 1 meningioma (syncytical type).
Cystic regions were stated in as much as 3% of combined series [27], even though their presence in pediatric meningiomas is stated to be higher [28]. In a blended collection, the article and colleagues [29] discovered 25 CMs in 210 stated instances, representing 12% of those tumors.
Some factors that facilitate the operation and results of the operation, including the location of the tumor, involvement of neurovascular tissue and venous sinuses, and other factors that also assist surgeons in the discussion and surgical approach for gross total resection [25].
In all meningiomas, gross total resection (GTR) is possible in 70%–80% of patients [30]. Tang et al. reported that gross total resection (GTR) in CMs was 84.2% [31].
When we operate on patients, it is easier to take the whole tumor, but it was much easier in the first case, therefore, peripheral arachnoid cysts should be a priori to facilitate surgery, while multiple, peripheral, thin-walled cysts should represent a surgical challenge. Otherwise, the fluid in type 2 CM can contain islets of neoplastic cells.
In the first case, the operation was performed under anesthesia and aseptic conditions, and the skin incision appeared laterally. Four bone boreholes were observed (two left para medline 2.5 and other two burr holes left away 8 cm from the left burr holes and craniotomy performed 7 cm anterior to the posterior ear line). The devascularization starts with coagulation of the medial meningeal artery (posterior branch) and dura then the open dura and is carefully reflected. Coagulation of the tumor surface and separation of the SSS from the tumor under microscopic surgery compared to tumor debulking and excision as grade one in Simpson’s grade (Figure 3C). The arachnoid cyst opened (Figure 3E) and was empty. Finally, occlusion layer-by-layer with drainage and postoperative CT scans were performed (Figure 3C).
In case (case 2), the ipsilateral paramedian approach was selected under anesthesia and aseptic conditions. The skin incision appeared and was refracted anteriorly. Six bone boreholes were performed (two left para medline 2.5 cm and two right para medline 2.5 cm, two other burr holes 8 cm from the left burr holes, and craniotomy in the median of the nasal-onion line). Devascularization starts with coagulation of the medial meningeal artery and dura, and the open dura presents a U shape (Figure 4D), which is carefully reflected. Coagulation tumor surface and separation of the SSS from the tumor under microscopic surgery then debulking of the tumor and intratumoral cyst were opened (Figure 4E), and biopsy was taken from the tumor and the cyst.
The excision of the tumor was grade one in Simpson’s grade (Figure 4F) then closer layer by layer with drainage, and a postoperative CT scan was performed (Figure 4C).
Regarding the third and fourth cases, they were both characterized by a convexity tumor with cystic features. The removal of these tumors was very easy and straightforward, and it was revealed that they were syncytial meningiomas.
The prognosis of CMs depends on various factors, including the extent of tumor resection, histological subtype, and presence of residual tumor or recurrence. CMs have a favorable prognosis compared to other types of brain tumors. However, the presence of high-grade features, such as increased mitotic activity or necrosis, may indicate a more aggressive tumor behavior and poorer prognosis.
Histopathological grading is a crucial factor for prognosis and follow-up regimens of meningiomas. Parasagittal and falcine meningiomas are more commonly associated with higher-grade tumors.
In all cases, the histopathological evaluation revealed pathognomonic features of meningiomas, such as whorls, intranuclear cytoplasmic pseudo inclusions, and psammoma bodies.
Our study demonstrated remarkable histopathological results in all cases. The radiographic imaging and tumor size in the first case contradicted malignant behavior, and the examination revealed a benign tumor containing meningothelial whorls and syncytial cells, identified as a syncytial meningioma (Figure 3G). In the second case, despite appearing benign on the CT scan, the patient was recommended for radiation therapy due to the presence of oval eccentric nuclei with amphophilic cytoplasm in both the tumor biopsy sample (Figure 4G) and the cyst sample (Figure 4H). In this particular case, the nuclear features were not consistent with a rhabdoid tumor, as rhabdoid tumors typically exhibit enlarged, bizarre, lobulated nuclei with huge and eosinophilic nucleoli. Considering the high frequency of atypical meningiomas, cystic changes indicate a greater histological aggressiveness.
After following four cases for a year, only one had a tumor recurrence, which was a parafalcine CM.
4. Conclusion
CMs, though rare, should be considered when diagnosing cystic intracranial tumors with a dural base or enhanced nodules. These tumors commonly occur in the parasagittal or parafalcine locations and may invade the superior sagittal sinus, making complete excision difficult. Benign CMs can cause greater brain swelling compared to high-grade tumors, especially when they invade the sinus. The presence of the cyst facilitates the excision of meningiomas and reduces the risks.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted by the ethical principles of the Helsinki Declaration and was approved by the Institutional Review Board (IRB) of the Al-Thawra Modern General Hospital (TMGH) and Uni-Max International Hospital in Sana’a City, Yemen (Code: 18320201632021). Informed consent was obtained from all patients before participating in the study.
Funding
The study was funded by Basim Al-Amiri, the businessman and director of Uni Max International Hospital, and Samah Ghubash, the Deputy General Manager and specialist in Obstetrics and Gynecology.
Authors' contributions
Data collection: Soliman Noman Abdullah Alborihi; Conceptualization, study design, data analysis, data interpretation, writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are also grateful to Uni-Max International Hospital in Sana’a City, Yemen, and all the medical teams and staff in the Neurosurgery Unit of this critical healthcare institution. Additionally, the authors thank the Ministry of Health and Population, represented by Taha Al-Mutawakel, for their support. Furthermore, their gratitude extends to Mujahid Ma’sar, President of the Yemeni Medical Council. Finally, the authors express their appreciation to the management of Al-Thawra Modern General Hospital in Sana’a City, Yemen, for their continuous support in all aspects, which greatly contributed to the success of this study.
References
Supplementary
Systematic literature review
The search strategy used the following truncation:
(“Cystic meningioma*” (title/abstract) not “case report*” (title/abstract) and address (filter) or autobiography (filter) or bibliography (filter) or biography (filter) or booksdocs (filter) or classicalarticle (filter) or clinicalconference (filter) or clinicalstudy (filter) or clinicaltrial (filter) or clinicaltrialprotocol (filter) or clinicaltrialphasei (filter) or clinicaltrialphaseii (filter) or clinicaltrialphaseiii (filter) or clinicaltrialphaseiv (filter) or veterinaryclinicaltrial (filter) or comment (filter) or comparativestudy (filter) or congress (filter) or consensusdevelopmentconference (filter) or consensusdevelopmentconferencenih (filter) or controlledclinicaltrial (filter) or correctedandrepublishedarticle (filter) or dataset (filter) or dictionary (filter) or directory (filter) or duplicatepublication (filter) or editorial (filter) or electronicsupplementarymaterials (filter) or Englishabstract (Filter) or evaluationstudy (filter) or festschrift (filter) or governmentpublication (filter) or guideline (filter) or historicalarticle (filter) or interactivetutorial (filter) or interview (filter) or introductoryjournalarticle (filter) or lecture (filter) or legalcase (filter) or legislation (filter) or letter (filter) or meta-analysis (filter) or multicenterstudy (filter) or news (filter) or newspaperarticle (filter) or observationalstudy (filter) or veterinaryobservationalstudy (filter) or overall (filter) or patienteducationhandout (filter) or periodicalindex (filter) or personalnarrative (filter) or portrait (filter) or practiceguideline (filter) or pragmaticclinicaltrial (filter) or preprint (filter) or publishederratum (filter) or randomizedcontrolledtrial (filter) or researchsupportamericanrecoveryandreinvestmentact (filter) or researchsupportnihextramural (filter) or researchsupportnihintramural (filter) or researchsupportnonusgovt (filter) or researchsupportusgovtnonphs (filter) or researchsupportusgovtphs (filter) or researchsupportusgovernment (filter) or retractedpublication (filter) or retractionofpublication (filter) or scientificintegrityreview (filter) or technicalreport (filter) or twinstudy (filter) or validationstudy (filter) or videoaudiomedia (filter) or webcast (filter) and (humans [filter]) and (English [filter]).
Three significant studies were identified, and their findings were summarized:
In the first study [13]
In a retrospective analysis, a group of 21 patients diagnosed with CMs were examined. Among these patients, 14 had cysts inside the tumor (intratumoral cysts), while 7 had cysts next to the tumor (peritumoral cysts). Out of the 14 intratumoral cysts, 6 were classified as Nauta type I and 8 as type II. Most patients with intratumoral cysts were women, and most tumors were located in the convexity region of the brain, specifically 9 cases in this region.
The results from CT and MRI differed between Nauta type I and type II cysts. Regarding the 7 peritumoral cysts, most of them were also located in the convexity region of the brain, specifically five cases in this region. Among the peritumoral cysts, 5 were classified as Nauta type IV, while 2 were classified as type III. The imaging characteristics of peritumoral cysts were similar to type II cysts, except they did not show enhancement of the cyst wall.
Concerning the distribution of CMs, the intratumoral cysts were found in various locations:
- Parietal convexity: 4 cases
- Frontal convexity: 3 cases
- Frontotemporal convexity: 1 case
- Parietooccipital convexity: 1 case
- Sphenoid ridge: 4 cases
- Petrous ridge/posterior fossa: 1 case
As for the peritumoral cysts, their distribution was as follows:
- Parietal convexity: 3 cases
- Frontal convexity: 2 cases
- Tuberculum sella: 1 case
- Olfactory groove: 1 case
The histopathological subtypes of the CMs in the study 1 were as follows:
For the intratumoral cysts:
- Meningothelial: 6 case
- Angiomatous: 4 cases
- Fibroblastic: 3 cases
- Anaplastic: 1 case
And for the peritumoral cysts:
- Meningothelial: 5 cases
- Fibroblastic: 2 cases
All surgical patients in the study underwent intervention, and complete resection was achieved in 19 cases. However, in 2 cases where the tumors were located in challenging areas, only partial resection was possible. The histopathological results varied depending on the Nauta type, with tumor involvement observed in type II cysts.
The patients were followed up for a period ranging from 1 to 12 years. During the follow-up period, one patient had tumor recurrence, which required further surgical intervention.
In the second study [14]
The study included 21 cases, with a male-to-female ratio of 1.1 to 1 and an average age of 53 years (ranging from 26 to 68). The most common symptoms observed before surgery were headaches in 8 patients, hemiparesis in three patients, dizziness in 3 patients, and slurred speech in 3 patients. The distribution of tumors was as follows, 8 tumors in the convexity (38%), 4 in the parasagittal region (19%), 3 in the tentorium (14%), and 2 in the sphenoid ridge (10%). Additionally, in one case each of the tumors was located in the cerebellopontine angle, olfactory groove, falx, and the third ventricle. On average, the duration of symptoms was 1.6 months, ranging from one day to five months. Preoperative imaging correctly identified 19 out of 21 cases as meningiomas (90%), but in two cases, they were initially misdiagnosed as oligodendroglioma and glioblastoma multiforme.
Based on the classifications by Nauta et al. and Rengachary et al., the study identified intratumoral cysts in 13 tumors (eight cases of type I and five cases of type II), peritumoral cysts in five tumors (four cases of type III and one case of type IV), and a combination of intratumoral and peritumoral cysts (type I and III) in three cases. In MRI scans, cysts of type I and II showed enhanced cyst walls with gadolinium, while type III and IV cysts showed no enhancement. Histopathological analysis revealed the presence of seven fibroblastic meningiomas (38%), six transitional meningiomas (29%), five atypical meningiomas (24%), as well as one meningothelial, one angiomatous, and one lipomatous meningioma. Intratumoral cysts were commonly found in atypical and transitional meningiomas, whereas peritumoral cysts were associated with fibroblastic meningiomas.
In the third study [7]
This study reviewed the clinical features and management outcomes of 15 patients with CMs. The tumor locations were as follows, convexity (10), falx (2), pterion (2), and lateral ventricle (1). The cystic lesions found were categorized into type I (3), type II (3), type III (3), type IV (1), and type V (5). Histopathologically, 6 atypical, 4 meningothelial, 2 malignant, 1 fibroblastic, 1 angiomatous, and 1 transitional were observed. Intratumoral CMs were more common in atypical types, while peritumoral CMs were more common in meningothelial and atypical types. The cystic portion of the three CMs appeared hypointense or mildly hyperintense on diffusion-weighted imaging (DWI). The apparent diffusion coefficient (ADC) ratio (ADCR) of diffusion-weighted imaging (DWI) for the cystic part of two type I CMs was 1.25 and 0.82, and for the cystic part of one type III CM, it was 4.04. The tumor was surgically removed in all patients.
Cysts can form within meningiomas either intratumorally (central or peripheral to the tumor) or as peritumoral arachnoid cysts (adjacent to the parenchyma and meningioma) (Figure 1). Cystic meningioma (CM) is rare, accounting for 2% to 7% of all intracranial meningiomas [1-3], and the location of cystic and solid components within the tumor can pose challenges in diagnosis. Preoperative misdiagnosis was reported in 62% of cases with computed tomography (CT) scans and in 25% of cases with angiography [4]. Magnetic resonance imaging (MRI) is more accurate and has a diagnostic error rate of 20% in these cases [5, 6].
After convexity CMs, the second most common location for CM is parasagittal [7], accounting for 26% of cases [8]. Parafalcine meningioma constitutes only 2% of all CMs.
In this report, we present two rare cases of CM, one case of parasagittal CM, one case of parafalcine CM, and two cases of convexity CM.
A total of 15% of meningiomas demonstrate atypical MRI features, such as cystic and necrotic areas, ring-like enhancement, and invasion of surrounding brain tissue. These characteristics can resemble malignant brain tumors, such as gliomas or metastatic tumors, leading to inaccurate radiological reports and misinterpretation of treatment decisions [9, 10].
What distinguishes the current research is that the tumor in the first case exhibited malignant traits, including brain edema and a shift in the midline of more than 5 cm, despite being a benign CM.
Previous studies did not mention a CM located in the parafalcine region with these specific histopathological characteristics. The third case occurred post-delivery, and the fourth case involved an older patient who experienced improvement following surgery. Excising a CM may yield a favorable prognosis.
Treatment: The National Comprehensive Cancer Network® (NCCN®) suggestions summarize the present-day method of meningioma management (NCCN®, 2022) visible set of rules meningiomas (MEN)-1 and for the observe-up visible set of rules meningiomas (MEN)-2 [11] (Figure 2).
2. Case Presentation
Case series
A prospective evaluation of four cases of CM was conducted at Al-Thawra Model General Hospital and Uni-Max International Hospital, in Sana’a City, Yemen, from June 2020 to June 2023. The evaluation included the assessment of the patient’s age, gender, clinical presentation, neurological deficits, radiological site, morphological characteristics of the cyst, and histopathological type.
During the study period, our hospital treated four patients diagnosed with CM out of 134 meningiomas. These diagnoses were confirmed through surgical procedures and pathological examination. The prevalence of CM among the meningioma cases was found to be 3%. Among the four patients with CM, one case was parafalcine, one case was parasagittal, and two cases were convexity CM.
During the one-year follow-up period, the four cases of CM were closely monitored and evaluated at Al-Thawra Model General Hospital in Sana’a and Uni-Max International Hospital, Yemen, from June 2020 to June 2023.
This case series presents four cases of CMs, each with different locations and clinical presentations.
Case 1: Parasagittal CM (extratumoral cyst)
A 40-year-old right-handed woman presented with recurrent severe headaches for one month and left-sided paresthesia. She had no known medical conditions and no relevant family history. The patient resided in a mountainous village in Al Mahwit Governorate, Yemen City. On examination, she exhibited intact cognition without any language or speech abnormalities. However, she had a left-sided weakness with a power grade of 4, hyperreflexia in deep tendon reflexes, and an extensor plantar reflex. Mild sensation deficits were also observed on the left side.
Investigation
In routine tests, including complete blood count (CBC), chest x-ray, serum electrolytes, kidney function tests (KFT), liver function tests (LFT), abdominal ultrasonography, electrocardiogram, and random blood sugar, all yielded normal results. A contrast-enhanced CT scan of the brain revealed a solid mass with peritumoral cystic components and surrounding edema in the right frontoparietal region. Subsequent brain MRI with gadolinium detected a homogeneously enhanced extra-axial mass with a dural tail, peripheral cystic components, and underlying brain edema (Figure 3).
Management and outcome
A surgical resection of the tumor was performed at Simpson’s grade one (Figures 3C, E, and F), and histopathological analysis revealed a syncytial World Health Organization (WHO) grade one meningioma with no significant evidence of mitosis or cellular necrosis (Figure 3G). Following a 7-day hospitalization, the patient was discharged on oral levetiracetam 500 mg (twice daily) and panadol extra 500 mg (as needed) for 30 days. One month post-surgery, the patient exhibited improvement in all symptoms.
Case 2:Parafalcine CM (intratumoral cyst)
A 60-year-old right-handed woman had been suffering from recurrent severe headaches, right-sided paresthesia, and urinary and fecal incontinence for a month. She had no history of high blood pressure, diabetes, head injuries, or substance abuse. The patient resided in a mountain village in Yemen’s Haji Province. On examination, she exhibited intact perception with no speech or language abnormalities. However, she had grade 0 weakness in the right upper arm, grade 3 weakness in the right lower limb, and normal deep tendon reflexes and extensor plantar reflexes. The sensation was normal on both sides (Figure 4).
Investigation
A series of routine CBC, chest x-rays, serum electrolytes, KFT, LFT, and abdominal ultrasound, all yielded normal results, except for a random blood sugar level of 12.5 millimoles per liter (mmol). Brain CT showed a solid mass in the right frontoparietal region with intratumoral cystic components and edema surrounding the lesion. Brain MRI with gadolinium revealed a heterogeneously enhancing mass with a dural base, intratumoral cysts, and surrounding edema. The presence of a dural tail was also noted.
Management and outcome:
The patient underwent surgery to remove Simpson’s grade I tumor. A histopathological examination showed grade 3 rhabdoid meningioma with high levels of mitotic activity and areas of necrosis [6]. After 10 days, the patient was released and given a prescription for oral levetiracetam (500 mg twice a day) for one month, dexamethasone (5 mg twice a day) for three days, and pantoprazole (40 mg once a day) for 10 days. Following the surgery, the patient’s symptoms gradually improved.
Case 3: Convexity CM in pregnant women
A 42-year-old right-handed female patient post-delivery complained of a decreased level of consciousness Glasgow coma scale (GCS)=E2 V2 M4 and seizures and right-sided paresthesia, as well as the history of progressive headaches for the past six months. He had no significant medical or family history of neurological disorders. The patient lived in a rural area in Yemen’s Amran governorate. During the examination, he showed intact cognition, normal language and speech, and no focal neurological deficits.
Investigation:
Routine tests, including CBC, chest x-ray, serum electrolytes, kFT, LFT, and abdominal ultrasonography, were within normal limits. Brain MRI with gadolinium revealed a homogeneously enhancing extra-axial mass with peripheral cystic components and underlying brain edema in the right parietal region.
Management and outcome
The patient underwent surgical resection of the tumor at grade one (Simpson’s grade). Histopathological examination confirmed a syncytial WHO grade one meningioma without significant mitotic activity or cellular necrosis. The patient was discharged after 5 days and prescribed oral levetiracetam for 60 days. Postoperatively, the patient’s symptoms resolved completely.
Case 4: Convexity CM in older age
A 79-year-old right-handed man presented with a history of seizures, progressive headaches, and intermittent right-sided weakness over the past four months. He had no significant medical or family history of neurological disorders. The patient resided in a rural area in Yemen’s Hodeidah governorate. During the examination, he displayed intact cognition, normal language, and speech. However, the affected individual had grade 4 weakness in his right upper arm and grade 2 weakness in his right lower limb.
Investigation
Routine tests, such as a CBC, chest x-ray, serum electrolyte test, kidney function test, liver function test, and abdominal ultrasonography, showed normal results. A brain MRI with gadolinium revealed a homogeneous enhancing extra-axial mass with intratumoral cystic components and underlying brain edema in the left parietal region.
Management and outcome
The patient underwent surgical resection of the tumor at a grade one level (according to Simpson’s grading system). A histopathological examination was conducted, which confirmed the presence of syncytial meningioma with a WHO grade of one and minimal mitotic activity or cellular necrosis. The patient was discharged from the hospital after six days and was prescribed an oral medication called levetiracetam 500 mg bd for 60 days. Following the surgery, the patient’s symptoms showed a significant improvement.
Differential diagnosis
The differential diagnosis for CMs includes various primary malignancies, abscesses [12], and other tumor types. The following conditions should be considered:
1. Extra-axial primary malignancies: High-grade meningiomas can mimic CMs and should be considered as a potential differential diagnosis.
2. Glioblastoma: Glioblastomas are aggressive brain tumors that can present with cystic components. They should be considered in the differential diagnosis, especially in cases where the radiological features and clinical presentation are atypical for meningiomas.
3. Hemangiopericytoma: Hemangiopericytomas are rare tumors that can occur in the central nervous system. They can have cystic components and should be considered in the differential diagnosis of CMs.
4. Hemangioblastoma: Hemangioblastomas are vascular tumors that can present with cystic features. They are more commonly associated with the posterior fossa but can occur in other locations as well.
5. Solitary fibrous tumor: Solitary fibrous tumors can occur in various locations, including the central nervous system. They can have cystic components and may resemble CMs.
6. Metastases: Metastatic tumors of the brain, particularly those with cystic features, should be considered in the differential diagnosis.
7. Sarcoma: Rarely, primary intracranial sarcomas can present with cystic features and should be considered in the differential diagnosis.
8. Polycystic astrocytoma or other bone tumors: In rare cases, polycystic astrocytoma or other bone tumors can present with cystic components and may mimic CMs.
3. Discussion
Meningiomas constitute 20% of all central nervous system tumors. They are typically solid tumors. Despite the presence of clear radiographic signs, the presence of cystic lesions in meningiomas can lead surgeons to consider alternative tumor diagnoses. CMs, which account for approximately 3%-7% of meningiomas in adults and are less common in women than men [15], often occur in the frontoparietal areas [8, 12]. While CMs are most frequently found in parasagittal and convexity regions, they can occur in any location within the brain [16].
The formation of cysts in meningiomas may be attributed to cellular necrosis, hemorrhage, ischemia, or the secretion of fluid by functional tumor cells. Glial cells may proliferate in response to the tumor, leading to the elaboration of fluid within the cysts [2, 17, 18].
Amin et al [1]. classified CMs into four patterns or subtypes based on the location of the cyst around the meningioma and brain. These patterns include centrally positioned intratumoral cysts (type 1), peripherally positioned intratumoral cysts (type 2), cysts positioned in the adjoining parenchyma (type 3), and arachnoid cysts located between the brain and meningioma (type 4). Worthington et al. added a fifth type, purely CM, which involves the entrapped cerebrospinal fluid [20]. Additionally, Weber et al. conducted a study that categorized peritumoral CMs according to tumor invasion in the cystic wall [21].
Cyst type 1 differs from other lesions producing a typical ring uptake image, which is also observed in high-grade primary neoplasms and metastases. Cyst types 2 and 3 can be confusing and often occur with another cystic masses that have mural nodules [15] and dural bases, such as pleomorphic xanthoastrocytoma and desmoplastic primary neoplasms. If it develops in posterior fossa may be misdiagnosed with hemangioblastomas. Cyst type 4 is easily detectable in radiological images [22].
Approximately 54.6% of CMs are associated with prominent vasogenic edema, leading to symptomatic intracranial hypertension and a more rapid clinical course in patients with large-volume cysts [22]. Types 2 and 3 cysts tend to produce greater vasogenic edema, therefore, an effect of mass disproportionate to the size of the tumor [17, 22].
The systematic literature review was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Articles on CMs were searched in the PubMed electronic database from the existence of the database until July 2023.
After searching the Pubmed database, an initial pool of 1508 articles was identified. Subsequently, 284 non-English articles and 1 duplicate were excluded, resulting in 1223 manuscripts remaining.
To further refine the selection, we scrutinized the titles and abstracts of these articles. Through this analysis, it was determined that 978 articles did not pertain to the targeted pathology indicated by the specified keywords.
Next, the full texts of 245 articles were evaluated for eligibility. Regrettably, 242 of these articles were found unsuitable for our intended purposes. The reasons for their exclusion encompassed being case reports, reviews, systematic reviews, or possessing flawed study designs or interventions.
Ultimately, only three articles fulfilled our criteria and stand as valuable sources for subsequent scholarly investigation. The PRISMA chart visually represents this process (Supplementary and Supplementary Figure).
The search strategy used the following truncation: (Supplementary).
After applying the search strategy, 9 articles were obtained. These articles were manually checked, and 6 studies were excluded because they either included only one case, were case reports, or were review studies. The remaining three studies were analyzed and presented in Table 1.

Three significant studies were identified, and their findings were summarized: (Supplementary).
In the first case, the symptoms were more aggressive despite the small size of the tumor, as observed in the CT scan (Figure 3B). A distinctive appearance of an arachnoid cyst, secondary to the trapping of cerebrospinal fluid areas and widening of the subarachnoid space, was observed below the tumor. This appearance corresponds to the fourth type described by Nauta in 1979. This observation prompted consideration of other possible diagnoses for this cystic tumor, such as polycystic astrocytomas and hemangioblastomas. High-grade gliomas cannot be excluded due to the presence of severe edema and midline shift in the radiological images. Similar results were observed in the third and fourth cases.
In the second case, the patient had a cystic type 2 meningioma with intratumoral cysts of Nauta’s type, which may be secondary to central necrosis or intratumoral hemorrhage [23]. The mass effect was mild brain edema without midline shift, indicating a benign cystic tumor. However, a definitive diagnosis of high-grade astrocytomas cannot be ruled out based on CT alone. The presence of microvascular proliferation and or necrosis would indicate a grade 4 tumor, corresponding to a WHO grade 4 astrocytoma isocitrate dehydrogenase (IDH)-mutant [12, 24].
Angiography plays a crucial role in the diagnosis of CM. Meningiomas typically exhibit a blush that persists beyond the venous phase in the early arterial phase of angiography. Successful surgery relies on identifying and reducing the hemorrhage-related arterial feeders for meningiomas and evaluating the patency of the superior sagittal sinus and other dural venous sinuses, particularly for parasagittal or falcine meningiomas. Angiography can also aid in preoperative embolization and the diagnosis of prolonged homogeneous tumor blush. However, angiography misdiagnoses meningiomas in more than 25% of cases [20].
Angiography in the first case revealed a prolonged homogeneous tumor blush and Sindou grade 3, indicating invasion of the lateral wall of the superior sagittal sinus [25]. The feeder artery was the medial meningeal artery, emphasizing the importance of angiography in diagnosing CMs.
In the second case, angiography revealed an abnormal vascular blush in the early arterial phase and was Sindou type 2 without invasion to the medial segment of the superior sagittal sinus (SSS). The feeder artery was from both the external and internal carotid arteries. Therefore, other tumors, such as glioma, hemangiopericytoma, hemangioblastomas, and glioblastoma multiform, can be diagnostic dispersion.
Surgery is the primary treatment for CMs. The goal is to achieve complete resection while preserving neurological function. In some cases, preoperative embolization may be performed to reduce blood supply to the tumor and minimize intraoperative bleeding. The surgical approach depends on the location and size of the tumor. For CMs, a craniotomy is usually performed to access and remove the tumor. In cases where critical structures or venous sinuses are involved, a multidisciplinary team approach may be required to plan the surgery and ensure optimal outcomes.
In our study, MRI was more accurate for differentiating extraxial tumors from intra-axial tumors, such as hemangiopericytoma, hemangioblastomas, solitary fibrous tumors, and meningioma. Hemangiopericytoma and solitary fibrous tumors mirror meningiomas [26].
Go et al [4] reported an infratentorial mass that was more likely to be a hemangioblastoma in radiological images. However, the intraoperative appearance was similar to pilocytic astrocytoma. Finally, a histopathological exam for a biopsy taken from the mass and cyst showed that the mass became WHO grade 1 meningioma (syncytical type).
Cystic regions were stated in as much as 3% of combined series [27], even though their presence in pediatric meningiomas is stated to be higher [28]. In a blended collection, the article and colleagues [29] discovered 25 CMs in 210 stated instances, representing 12% of those tumors.
Some factors that facilitate the operation and results of the operation, including the location of the tumor, involvement of neurovascular tissue and venous sinuses, and other factors that also assist surgeons in the discussion and surgical approach for gross total resection [25].
In all meningiomas, gross total resection (GTR) is possible in 70%–80% of patients [30]. Tang et al. reported that gross total resection (GTR) in CMs was 84.2% [31].
When we operate on patients, it is easier to take the whole tumor, but it was much easier in the first case, therefore, peripheral arachnoid cysts should be a priori to facilitate surgery, while multiple, peripheral, thin-walled cysts should represent a surgical challenge. Otherwise, the fluid in type 2 CM can contain islets of neoplastic cells.
In the first case, the operation was performed under anesthesia and aseptic conditions, and the skin incision appeared laterally. Four bone boreholes were observed (two left para medline 2.5 and other two burr holes left away 8 cm from the left burr holes and craniotomy performed 7 cm anterior to the posterior ear line). The devascularization starts with coagulation of the medial meningeal artery (posterior branch) and dura then the open dura and is carefully reflected. Coagulation of the tumor surface and separation of the SSS from the tumor under microscopic surgery compared to tumor debulking and excision as grade one in Simpson’s grade (Figure 3C). The arachnoid cyst opened (Figure 3E) and was empty. Finally, occlusion layer-by-layer with drainage and postoperative CT scans were performed (Figure 3C).
In case (case 2), the ipsilateral paramedian approach was selected under anesthesia and aseptic conditions. The skin incision appeared and was refracted anteriorly. Six bone boreholes were performed (two left para medline 2.5 cm and two right para medline 2.5 cm, two other burr holes 8 cm from the left burr holes, and craniotomy in the median of the nasal-onion line). Devascularization starts with coagulation of the medial meningeal artery and dura, and the open dura presents a U shape (Figure 4D), which is carefully reflected. Coagulation tumor surface and separation of the SSS from the tumor under microscopic surgery then debulking of the tumor and intratumoral cyst were opened (Figure 4E), and biopsy was taken from the tumor and the cyst.
The excision of the tumor was grade one in Simpson’s grade (Figure 4F) then closer layer by layer with drainage, and a postoperative CT scan was performed (Figure 4C).
Regarding the third and fourth cases, they were both characterized by a convexity tumor with cystic features. The removal of these tumors was very easy and straightforward, and it was revealed that they were syncytial meningiomas.
The prognosis of CMs depends on various factors, including the extent of tumor resection, histological subtype, and presence of residual tumor or recurrence. CMs have a favorable prognosis compared to other types of brain tumors. However, the presence of high-grade features, such as increased mitotic activity or necrosis, may indicate a more aggressive tumor behavior and poorer prognosis.
Histopathological grading is a crucial factor for prognosis and follow-up regimens of meningiomas. Parasagittal and falcine meningiomas are more commonly associated with higher-grade tumors.
In all cases, the histopathological evaluation revealed pathognomonic features of meningiomas, such as whorls, intranuclear cytoplasmic pseudo inclusions, and psammoma bodies.
Our study demonstrated remarkable histopathological results in all cases. The radiographic imaging and tumor size in the first case contradicted malignant behavior, and the examination revealed a benign tumor containing meningothelial whorls and syncytial cells, identified as a syncytial meningioma (Figure 3G). In the second case, despite appearing benign on the CT scan, the patient was recommended for radiation therapy due to the presence of oval eccentric nuclei with amphophilic cytoplasm in both the tumor biopsy sample (Figure 4G) and the cyst sample (Figure 4H). In this particular case, the nuclear features were not consistent with a rhabdoid tumor, as rhabdoid tumors typically exhibit enlarged, bizarre, lobulated nuclei with huge and eosinophilic nucleoli. Considering the high frequency of atypical meningiomas, cystic changes indicate a greater histological aggressiveness.
After following four cases for a year, only one had a tumor recurrence, which was a parafalcine CM.
4. Conclusion
CMs, though rare, should be considered when diagnosing cystic intracranial tumors with a dural base or enhanced nodules. These tumors commonly occur in the parasagittal or parafalcine locations and may invade the superior sagittal sinus, making complete excision difficult. Benign CMs can cause greater brain swelling compared to high-grade tumors, especially when they invade the sinus. The presence of the cyst facilitates the excision of meningiomas and reduces the risks.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted by the ethical principles of the Helsinki Declaration and was approved by the Institutional Review Board (IRB) of the Al-Thawra Modern General Hospital (TMGH) and Uni-Max International Hospital in Sana’a City, Yemen (Code: 18320201632021). Informed consent was obtained from all patients before participating in the study.
Funding
The study was funded by Basim Al-Amiri, the businessman and director of Uni Max International Hospital, and Samah Ghubash, the Deputy General Manager and specialist in Obstetrics and Gynecology.
Authors' contributions
Data collection: Soliman Noman Abdullah Alborihi; Conceptualization, study design, data analysis, data interpretation, writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors are also grateful to Uni-Max International Hospital in Sana’a City, Yemen, and all the medical teams and staff in the Neurosurgery Unit of this critical healthcare institution. Additionally, the authors thank the Ministry of Health and Population, represented by Taha Al-Mutawakel, for their support. Furthermore, their gratitude extends to Mujahid Ma’sar, President of the Yemeni Medical Council. Finally, the authors express their appreciation to the management of Al-Thawra Modern General Hospital in Sana’a City, Yemen, for their continuous support in all aspects, which greatly contributed to the success of this study.
References
- Amin OS. Two supratentorial meningiomas: Are they different? BMJ Case Reports. 2012; 2012:bcr2012007177. [DOI:10.1136/bcr-2012-007177] [PMID]
- Maj B, Łatka D, Kita P. [Cystic meningioma: Report of three cases (Polish)]. Neurologia i Neurochirurgia Polska. 2002; 36(1):199-206. [PMID]
- Bonnal J, Brotchi J. Surgery of the superior sagittal sinus in parasagittal meningiomas. Journal of Neurosurgery. 1978; 48(6):935-45. [DOI:10.3171/jns.1978.48.6.0935] [PMID]
- Go KO, Lee K, Heo W, Lee YS, Park YS, Kim SK, et al. Cystic Meningiomas: Correlation between radiologic and histopathologic features. Brain Tumor Research and Treatment. 2018; 6(1):13-21. [DOI:10.14791/btrt.2018.6.e3] [PMID]
- Ferrante L, Acqui M, Lunardi P, Qasho R, Fortuna A. MRI in the diagnosis of cystic meningiomas: Surgical implications. Acta Neurochirurgica. 1997; 139(1):8-11. [DOI:10.1007/BF01850861] [PMID]
- Lahkim M, Andour H, Laamrani FZ, Nouali HE, Fenni JE. Cystic meningioma: A case report with a literature review. Radiology Case Reports. 2021; 16(10):2958-61. [DOI:10.1016/j.radcr.2021.07.016] [PMID]
- Chen TY, Lai PH, Ho JT, Wang JS, Chen WL, Pan HB, et al. Magnetic resonance imaging and diffusion-weighted images of cystic meningioma: Correlating with histopathology. Clinical Imaging. 2004; 28(1):10-9. [DOI:10.1016/S0899-7071(03)00032] [PMID]
- Fortuna A, Ferrante L, Acqui M, Guglielmi G, Mastronardi L. Cystic meningiomas. Acta Neurochirurgica. 1988; (1-2):23-30. [DOI:10.1007/BF01541262] [PMID]
- Hakyemez B, Yildirim N, Erdoğan C, Kocaeli H, Korfali E, Parlak M. Meningiomas with conventional MRI findings resembling intraaxial tumors: Can perfusion-weighted MRI be helpful in differentiation? Neuroradiology. 2006; 48(10):695-702. [DOI:10.1007/s00234-006-0115-y] [PMID]
- Harting I, Hartmann M, Bonsanto MM, Sommer C, Sartor K. Characterization of necrotic meningioma using diffusion MRI, perfusion MRI, and MR spectroscopy: Case report and review of the literature. Neuroradiology. 2004; 46(3):189-93. [DOI:10.1007/s00234-003-1144-4] [PMID]
- Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncology (London, England). 2018; 14(21):2161-77. [DOI:10.2217/fon-2018-0006] [PMID]
- Amin OS, Shwani SS, Khalifa F. Cystic meningioma. BMJ Case Report. 2015; 2015:bcr2014207690. [DOI: 10.1136/bcr-2014-207690] [PMID]
- Liu M, Liu Y, Li X, Zhu S, Wu C. Cystic meninigioma. Journal of Clinical Neuroscience. 2007; 14(9):856-9. [DOI:10.1016/j.jocn.2006.06.003] [PMID]
- Jung TY, Jung S, Shin SR, Moon KS, Kim IY, Park SJ, et al. Clinical and histopathological analysis of cystic meningiomas. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia. 2005; 12(6):651-5. [DOI:10.1016/j.jocn.2004.09.020] [PMID]
- Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991; 11(6):1087-106. [DOI:10.1148/radiographics.11.6.1749851] [PMID]
- Dietemann JL, Eid MA, Soares IM, Bogorin A, Boyer P, Draghici S. [Tumeurs cranioencephaliques: Tumeurs extra-axiales (French)]. In: Dietemann JL, editor. Neuro-imagerie diagnostique (3e édition). Amsterdam: Elsevier; 2018. [DOI:10.1016/B978-2-294-75394-7.00008-4]
- Meningiomas. Their Classification, Regional Behaviour, Life History, and Surgical End Results. Bull Med Libr Assoc. 1938;27(2):185. [PMID]
- Guan TK, Pancharatnam D, Chandran H, Hooi TK, Kumar G, Ganesan D. Infratentorial benign cystic meningioma mimicking a hemangioblastoma radiologically and a pilocytic astrocytoma intraoperatively: A case report. Journal of Medical Case Reports. 2013; 7:87. [DOI:10.1186/1752-1947-7-87] [PMID]
- Nauta HJ, Tucker WS, Horsey WJ, Bilbao JM, Gonsalves C. Xanthochromic cysts associated with meningioma. Journal of Neurology, Neurosurgery. 1979; 42(6):529-35. [DOI:10.1136/jnnp.42.6.529] [PMID]
- Worthington C, Caron JL, Melanson D, Leblanc R. Meningioma cysts. Neurology. 1985; 35(12):1720-4. [DOI:10.1212/WNL.35.12.1720] [PMID]
- Weber J, Gassel AM, Hoch A, Kilisek L, Spring A. Intraoperative management of cystic meningiomas. Neurosurgical Review. 2003; 26(1):62-6. [DOI:10.1007/s10143-002-0228-7] [PMID]
- Carrasco Moro R, Jiménez Zapata HD, Pian Arias H, Martínez San Millán JS, Martínez Rodrigo MA, Pascual Garvi JM. Cystic meningiomas: Radiological and pathological correlation with surgical implications. Neurocirugia (English Edition). 2019; 30(1):1-10. [DOI:10.1016/j.neucir.2018.08.002] [PMID]
- Pereira BJA, Oba-Shinjo SM, de Almeida AN, Marie SKN. Molecular alterations in meningiomas: Literature review. Clinical Neurology and Neurosurgery. 2019; 176:89-96. [DOI:10.1016/j.clineuro.2018.12.004] [PMID]
- Torp SH, Solheim O, Skjulsvik AJ. The WHO 2021 classification of central nervous system tumours: A practical update on what neurosurgeons need to know-a minireview. Acta Neurochirurgica. 2022; 164(9):2453-64. [DOI:10.1007/s00701-022-05301-y] [PMID]
- Ricci A, Di Vitantonio H, De Paulis D, Del Maestro M, Gallieni M, Dechcordi SR, et al. Parasagittal meningiomas: Our surgical experience and the reconstruction technique of the superior sagittal sinus. Surgical Neurology International. 2017; 8:1. [DOI:10.4103/2152-7806.198728] [PMID]
- Abd El-Bary TH, Samir MA, Elgaddar AA, El-Shiekh MO. Brief insight about management of meningioma. Journal of Pharmaceutical Negative Results. 2023; 14 Special:610-8. [DOI:10.47750/pnr.2023.14.S02.74]
- Osborn AG. Diagnostic neuroradiology: A text/atlas. St. Louis, Mosby; 1994. [Link]
- Gauchotte G, Peyre M, Pouget C, Cazals-Hatem D, Polivka M, Rech F, et al. Prognostic value of histopathological features and loss of H3K27me3 immunolabeling in anaplastic meningioma: A multicenter retrospective study. Journal of Neuropathology and Experimental Neurology. 2020; 79(7):754-62. [DOI:10.1093/jnen/nlaa038] [PMID]
- Artico M, Ferrante L, Cervoni L, Colonnese C, Fortuna A. Pediatric cystic meningioma: Report of three cases. Child's nervous system: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 1995; 11(3):137-40. [DOI:10.1007/BF00570253] [PMID]
- Ogasawara C, Philbrick BD, Adamson DC. Meningioma: A review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. 2021; 9(3):319. [DOI:10.3390/biomedicines9030319] [PMID]
- Tang AR, Chotai S, Grisham CJ, Guidry BS, McDermott JR, Le CH, et al. Outcomes following surgical resection of cystic intracranial meningiomas. Journal of Neuro-Oncology. 2022; 160(1):33-40. [DOI:10.1007/s11060-022-04096-3] [PMID]
- Gkasdaris G, Vasiljevic A, Cartalat S, Pelissou-Guyotat I, Guyotat J, Dumot C, et al. Purely cystic meningioma: Case report and systematic review of the literature. Clinical Neurology and Neurosurgery. 2022; 223:107498. [DOI:10.1016/j.clineuro.2022.107498] [PMID]
- Horbinski C, Nabors LB, Portnow J, Baehring J, Bhatia A, Bloch O, et al. NCCN Guidelines® insights: Central nervous system cancers, version 2.2022. Journal of the National Comprehensive Cancer Network: JNCCN. 2023; 21(1):12-20. [DOI:10.6004/jnccn.2023.0002] [PMID]
Supplementary
Systematic literature review
The search strategy used the following truncation:
(“Cystic meningioma*” (title/abstract) not “case report*” (title/abstract) and address (filter) or autobiography (filter) or bibliography (filter) or biography (filter) or booksdocs (filter) or classicalarticle (filter) or clinicalconference (filter) or clinicalstudy (filter) or clinicaltrial (filter) or clinicaltrialprotocol (filter) or clinicaltrialphasei (filter) or clinicaltrialphaseii (filter) or clinicaltrialphaseiii (filter) or clinicaltrialphaseiv (filter) or veterinaryclinicaltrial (filter) or comment (filter) or comparativestudy (filter) or congress (filter) or consensusdevelopmentconference (filter) or consensusdevelopmentconferencenih (filter) or controlledclinicaltrial (filter) or correctedandrepublishedarticle (filter) or dataset (filter) or dictionary (filter) or directory (filter) or duplicatepublication (filter) or editorial (filter) or electronicsupplementarymaterials (filter) or Englishabstract (Filter) or evaluationstudy (filter) or festschrift (filter) or governmentpublication (filter) or guideline (filter) or historicalarticle (filter) or interactivetutorial (filter) or interview (filter) or introductoryjournalarticle (filter) or lecture (filter) or legalcase (filter) or legislation (filter) or letter (filter) or meta-analysis (filter) or multicenterstudy (filter) or news (filter) or newspaperarticle (filter) or observationalstudy (filter) or veterinaryobservationalstudy (filter) or overall (filter) or patienteducationhandout (filter) or periodicalindex (filter) or personalnarrative (filter) or portrait (filter) or practiceguideline (filter) or pragmaticclinicaltrial (filter) or preprint (filter) or publishederratum (filter) or randomizedcontrolledtrial (filter) or researchsupportamericanrecoveryandreinvestmentact (filter) or researchsupportnihextramural (filter) or researchsupportnihintramural (filter) or researchsupportnonusgovt (filter) or researchsupportusgovtnonphs (filter) or researchsupportusgovtphs (filter) or researchsupportusgovernment (filter) or retractedpublication (filter) or retractionofpublication (filter) or scientificintegrityreview (filter) or technicalreport (filter) or twinstudy (filter) or validationstudy (filter) or videoaudiomedia (filter) or webcast (filter) and (humans [filter]) and (English [filter]).
Three significant studies were identified, and their findings were summarized:
In the first study [13]
In a retrospective analysis, a group of 21 patients diagnosed with CMs were examined. Among these patients, 14 had cysts inside the tumor (intratumoral cysts), while 7 had cysts next to the tumor (peritumoral cysts). Out of the 14 intratumoral cysts, 6 were classified as Nauta type I and 8 as type II. Most patients with intratumoral cysts were women, and most tumors were located in the convexity region of the brain, specifically 9 cases in this region.
The results from CT and MRI differed between Nauta type I and type II cysts. Regarding the 7 peritumoral cysts, most of them were also located in the convexity region of the brain, specifically five cases in this region. Among the peritumoral cysts, 5 were classified as Nauta type IV, while 2 were classified as type III. The imaging characteristics of peritumoral cysts were similar to type II cysts, except they did not show enhancement of the cyst wall.
Concerning the distribution of CMs, the intratumoral cysts were found in various locations:
- Parietal convexity: 4 cases
- Frontal convexity: 3 cases
- Frontotemporal convexity: 1 case
- Parietooccipital convexity: 1 case
- Sphenoid ridge: 4 cases
- Petrous ridge/posterior fossa: 1 case
As for the peritumoral cysts, their distribution was as follows:
- Parietal convexity: 3 cases
- Frontal convexity: 2 cases
- Tuberculum sella: 1 case
- Olfactory groove: 1 case
The histopathological subtypes of the CMs in the study 1 were as follows:
For the intratumoral cysts:
- Meningothelial: 6 case
- Angiomatous: 4 cases
- Fibroblastic: 3 cases
- Anaplastic: 1 case
And for the peritumoral cysts:
- Meningothelial: 5 cases
- Fibroblastic: 2 cases
All surgical patients in the study underwent intervention, and complete resection was achieved in 19 cases. However, in 2 cases where the tumors were located in challenging areas, only partial resection was possible. The histopathological results varied depending on the Nauta type, with tumor involvement observed in type II cysts.
The patients were followed up for a period ranging from 1 to 12 years. During the follow-up period, one patient had tumor recurrence, which required further surgical intervention.
In the second study [14]
The study included 21 cases, with a male-to-female ratio of 1.1 to 1 and an average age of 53 years (ranging from 26 to 68). The most common symptoms observed before surgery were headaches in 8 patients, hemiparesis in three patients, dizziness in 3 patients, and slurred speech in 3 patients. The distribution of tumors was as follows, 8 tumors in the convexity (38%), 4 in the parasagittal region (19%), 3 in the tentorium (14%), and 2 in the sphenoid ridge (10%). Additionally, in one case each of the tumors was located in the cerebellopontine angle, olfactory groove, falx, and the third ventricle. On average, the duration of symptoms was 1.6 months, ranging from one day to five months. Preoperative imaging correctly identified 19 out of 21 cases as meningiomas (90%), but in two cases, they were initially misdiagnosed as oligodendroglioma and glioblastoma multiforme.
Based on the classifications by Nauta et al. and Rengachary et al., the study identified intratumoral cysts in 13 tumors (eight cases of type I and five cases of type II), peritumoral cysts in five tumors (four cases of type III and one case of type IV), and a combination of intratumoral and peritumoral cysts (type I and III) in three cases. In MRI scans, cysts of type I and II showed enhanced cyst walls with gadolinium, while type III and IV cysts showed no enhancement. Histopathological analysis revealed the presence of seven fibroblastic meningiomas (38%), six transitional meningiomas (29%), five atypical meningiomas (24%), as well as one meningothelial, one angiomatous, and one lipomatous meningioma. Intratumoral cysts were commonly found in atypical and transitional meningiomas, whereas peritumoral cysts were associated with fibroblastic meningiomas.
In the third study [7]
This study reviewed the clinical features and management outcomes of 15 patients with CMs. The tumor locations were as follows, convexity (10), falx (2), pterion (2), and lateral ventricle (1). The cystic lesions found were categorized into type I (3), type II (3), type III (3), type IV (1), and type V (5). Histopathologically, 6 atypical, 4 meningothelial, 2 malignant, 1 fibroblastic, 1 angiomatous, and 1 transitional were observed. Intratumoral CMs were more common in atypical types, while peritumoral CMs were more common in meningothelial and atypical types. The cystic portion of the three CMs appeared hypointense or mildly hyperintense on diffusion-weighted imaging (DWI). The apparent diffusion coefficient (ADC) ratio (ADCR) of diffusion-weighted imaging (DWI) for the cystic part of two type I CMs was 1.25 and 0.82, and for the cystic part of one type III CM, it was 4.04. The tumor was surgically removed in all patients.

Type of Study: Case report |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |










