Mon, Jul 21, 2025
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rohilla S, Yadav S, Singh I. Predicting the Consistency of Meningiomas
Preoperatively Using Magnetic Resonance Imaging. Iran J Neurosurg 2024; 10 : 18
URL: http://irjns.org/article-1-409-en.html
URL: http://irjns.org/article-1-409-en.html
1- Department of Radiodiagnosis, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India. , seemarohilla@yahoo.co.in
2- Department of Radiodiagnosis, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India.
3- Department of Neurosurgery, Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India.
2- Department of Radiodiagnosis, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India.
3- Department of Neurosurgery, Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India.
Keywords: Meningioma consistency, Tumor/cerebellar peduncle, T2-weighted imaging intensity
(TCTI) ratio, Diffusion tensor
imaging (DTI), Magnetization
transfer ratio (MTR), Fractional
anisotropy (FA)
Full Text [PDF 1987 kb]
(305 Downloads)
| Abstract (HTML) (1319 Views)
Full Text: (111 Views)
1. Introduction
Meningiomas account for 16 to 20% of all cerebral tumors and are the most prevalent non-glial tumors of the central nervous system (CNS) [1]. The extent of resection and the surgical method can be affected by tumor vascularization, consistency, and surgical plane. In meningiomas of the skull base and those near neurovascular systems, these variables are more important. Hard tumors that are adherent to neighboring vital structures, such as the internal carotid artery, optic chiasm, and cranial nerves, carry a higher risk of surgical morbidity and necessitate meticulous dissection procedures for removal [2]. Surgical resection is accomplished by first devascularizing the tumor from the surrounding area of the capsule and then debulking it from the center [3]. One of the key factors in removing the entire tumor while avoiding neurological deficits is the consistency of meningiomas [4]. The surgical approach chosen may be partially determined by the ability to accurately predict the consistency of a meningioma preoperatively based on neuroimaging studies. This ability may also provide additional information about the need for staged resection, expected subtotal resection, or other challenges related to consistency and/or vascularity [5].
Most of the studies conducted previously indicate the role of T2-weighted imaging in predicting the firmness of meningiomas preoperatively. The purpose of our study was to demonstrate the effectiveness of tumor/cerebellar peduncle T2-weighted imaging intensity (TCTI) ratio, diffusion tensor imaging (DTI), and magnetization transfer ratio (MTR) in predicting the consistency of tumors in the North Indian population in an objective manner.
2. Methods and Materials/Patients
Subjects
Twenty-five patients with meningiomas were studied, and those with previous cranial surgery/radiotherapy, as well as cases where the surgeon did not define the consistency of the tumor, were excluded.
Imaging
All patients were subjected to magnetic resonance imaging (MRI) on a 3T MRI system (GE, Discovery 750w with GEM Suite, Milwaukee, WI, USA) using a dedicated head coil with 32 channels.
The following sequences were performed: T1W (Time to echo [TE]=24 ms, Time to repitition (TR)=2671.1 ms, field of view (FOV)=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=320, bandwidth=41.67 kHz); T2W (TE=114 ms, TR=8901 ms, FOV=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=512, bandwidth=50 kHz); T2W/FLAIR (TE=120 ms, TR=10000 ms, FOV=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=320, bandwidth=31.25 kHz); DWI (TE=83 ms, TR=261 ms, FOV=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=192, bandwidth=166.7 kHz, b=1000 s/mm²); Diffusion tensor imaging (DTI) (TE=78 ms, TR=6413 ms, FOV=22 cm, slice thickness=4.0 mm, slice spacing=1.0 mm, frequency=128, bandwidth=250 kHz, b=1000 s/mm², diffusion directions=30); and MT (TE=15 ms, TR=1300 ms, FOV=24 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=256, bandwidth=14.71 kHz).
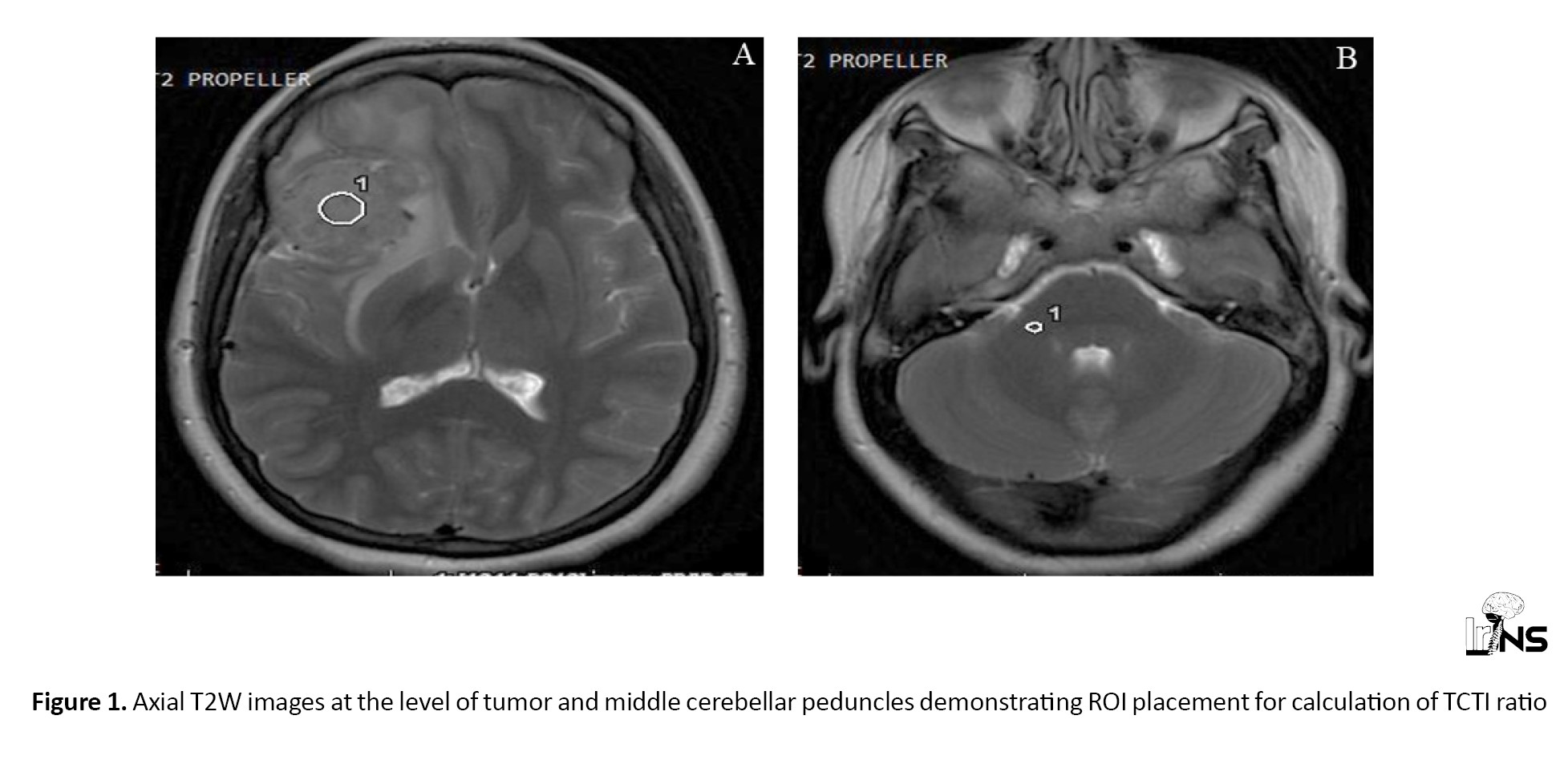
ROIs were placed on both the tumor and the cerebellar peduncle on T2W images, and the ratio of signal intensities was calculated (TCTI ratio) (Figure 1). The ROIs did not have any preset dimensions, as the size of meningioma was variable. In general, ROIs were made as large as possible to obtain the most representative sample of tumors on the axial sequence. In meningiomas with heterogeneous signal intensity, separate ROIs were placed within the tumor, and an average value was calculated. Likewise, ROIs were placed on the MT on and MT off images of the MT sequence, and the MT ratio was calculated (Figure 2). DTI data were transferred to ReadyView software, on AWS server version 3.2, where the placement of the ROI on the tumor allowed for the calculation of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) by averaging the two minor eigenvalues (Figure 3). Various parameters obtained were correlated with the intraoperative consistency of meningiomas.
Operative procedure
During surgery, the consistency of the tumor was assessed and classified as soft for tumors that could be completely removed easily with a suction cannula, and hard for those requiring the use of surgical scissors, a scalpel blade, or a cavitron ultrasonic surgical aspirator (CUSA) set at a high amplitude, or for tumors that could not be removed by gentle manipulation. After exposure of the tumor, specimens were taken from different areas of the tumor and sent for histopathological examination to confirm the diagnosis.
Statistical analysis:
Data were analyzed using SPSS software, version 23. Mean±SD, as well as medians and interquartile ranges (IQRs), were calculated for continuous variables, while frequencies and percentages were calculated for categorical variables. Group comparisons for continuously distributed data were made using an independent samples t-test when comparing two groups. In cases of non-normally distributed data, appropriate non-parametric tests, specifically the Wilcoxon test, were used. The chi-squared test was used for group comparisons of categorical data. Linear correlation between two continuous variables was explored using Pearson’s correlation (for normally distributed data) and Spearman’s correlation (for non-normally distributed data). Statistical significance was set at P<0.05. For MRI findings showing a significant correlation with tumor consistency, receiver operator characteristic (ROC) analysis was performed to predict an optimal cut-off for a continuous predictor of a binary outcome. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were calculated to assess the diagnostic performance of the predictors by creating a 2×2 cross-table with the outcome.
3. Results
There were 11 males and 14 females, ranging in age from 18 to 68 years. There was no correlation between consistency and the size or location of the tumor, except for skull base tumors, where the majority (6 out of 7) of tumors were firm. There was no significant difference between soft and firm meningiomas in terms of T1, T2, and fluid attenuated inversion recovery (FLAIR) signal intensity. However, all tumors that were hyperintense on T2W/FLAIR images were soft, while all tumors that were hypointense on T2W/FLAIR images were firm.
There was a significant difference in the TCTI ratio (P=0.036) between soft and firm tumors. Table 1 shows the consistency of meningioma with respect to the TCTI ratio.
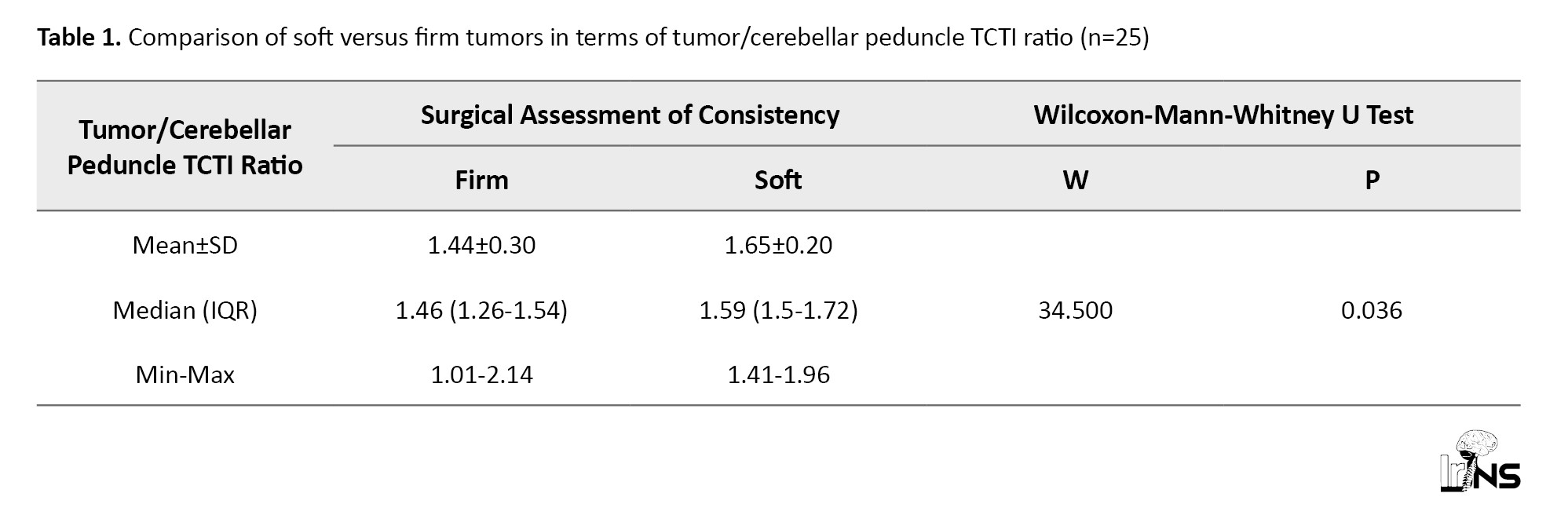
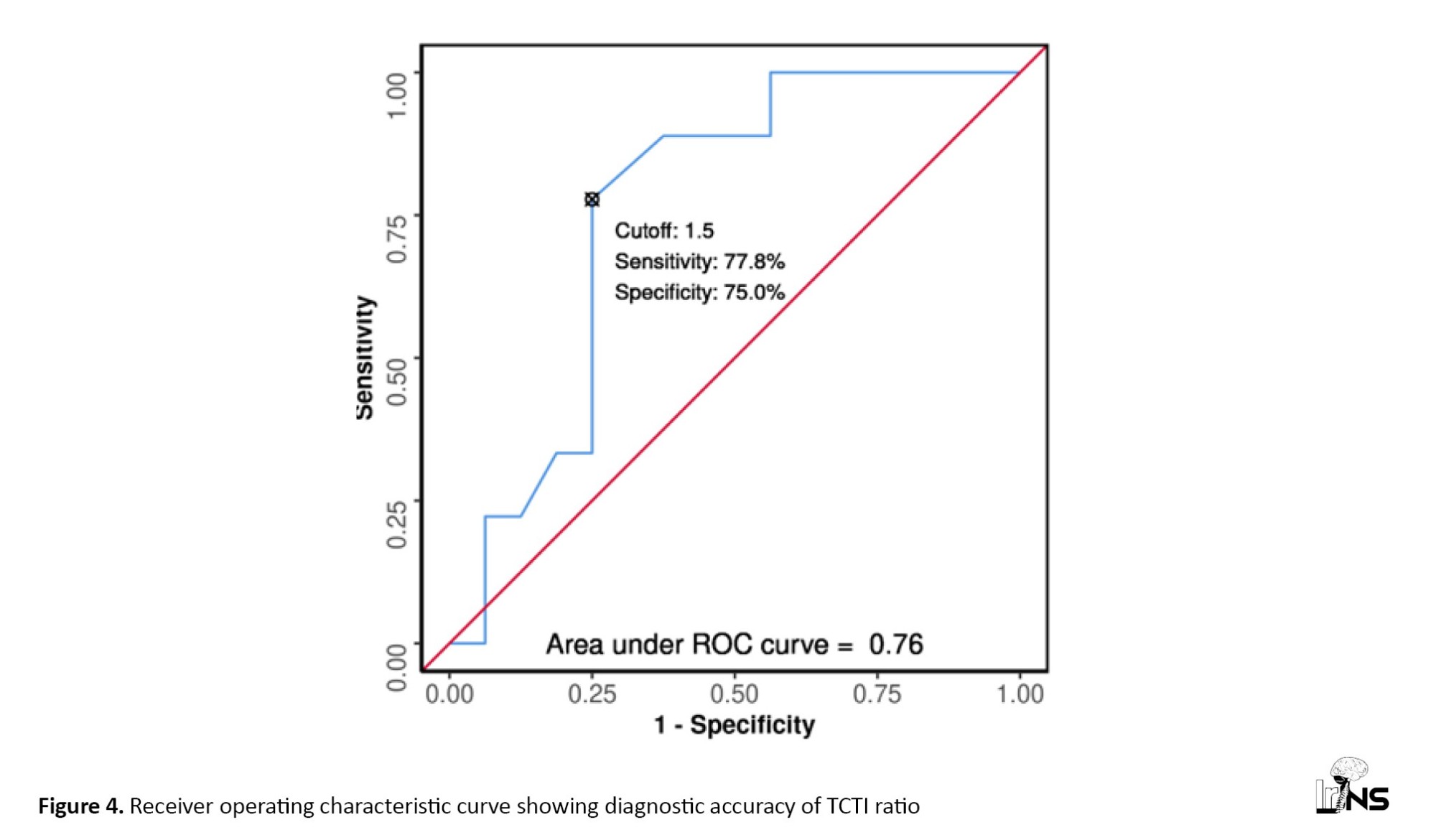
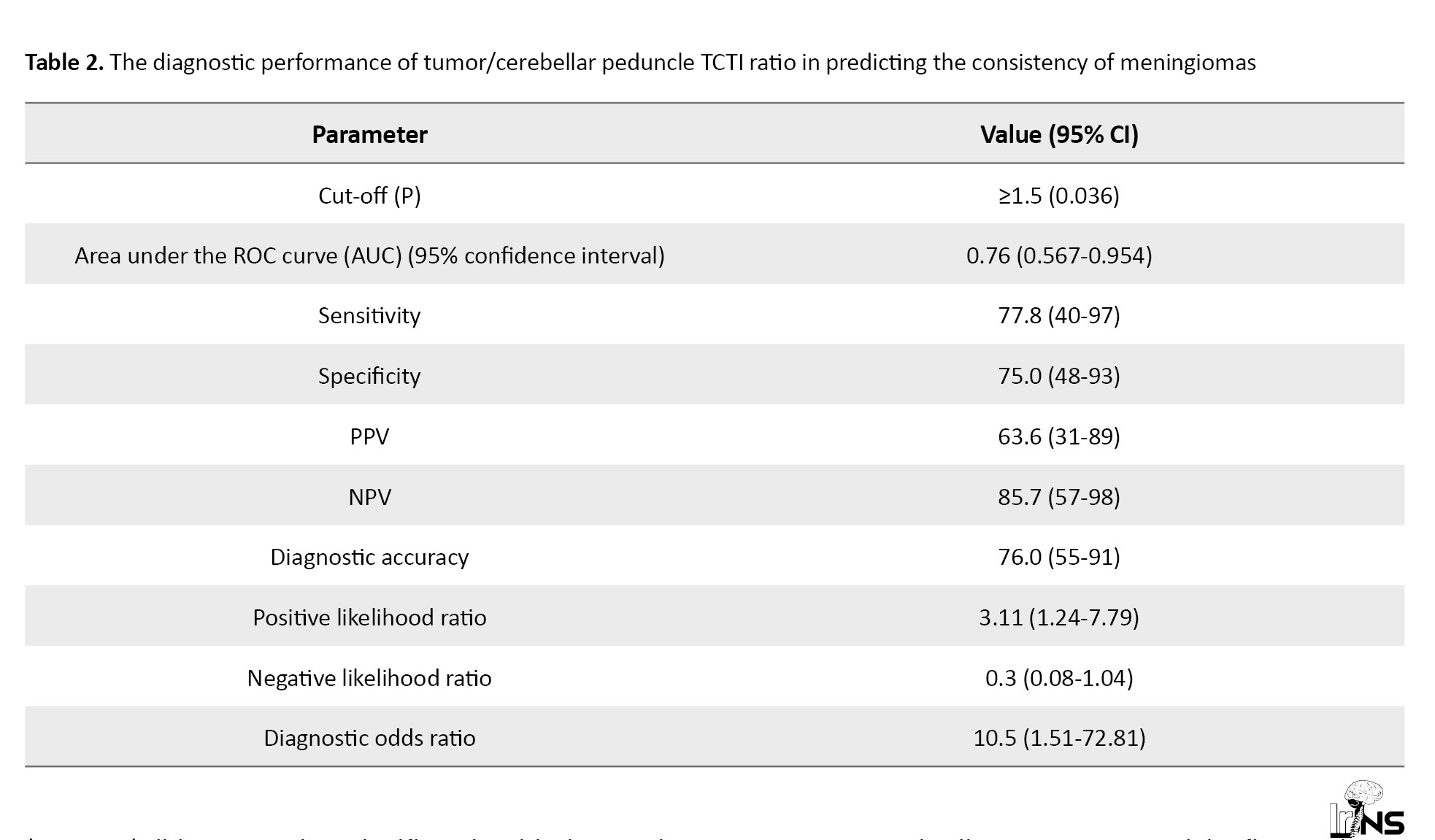
The area under the ROC curve (Figure 4) for the TCTI ratio predicting soft versus firm consistency was 0.76 (95% CI, 0.567%, 0.954%), demonstrating fair diagnostic performance. At a cut-off of TCTI ratio ≥1.5, soft consistency of meningiomas was predicted with a sensitivity of 78%, and a specificity of 75%. Table 2 shows the diagnostic performance of the TCTI in predicting the consistency of meningiomas.
There was no significant difference between the MT ratio of soft and firm meningiomas (P=0.375). Likewise, FA (P=0.169), MD (P=0.978), AD (P=0.590), and RD (P=0.522) did not correlate significantly with the consistency of meningiomas.
3.1 Correlation of histopathological types with the consistency of meningiomas
There were 11 meningothelial, five transitional, six fibroblastic, and three angiomatous meningiomas. There was a significant association between consistency and histopathological type (P=0.03) (Table 3).
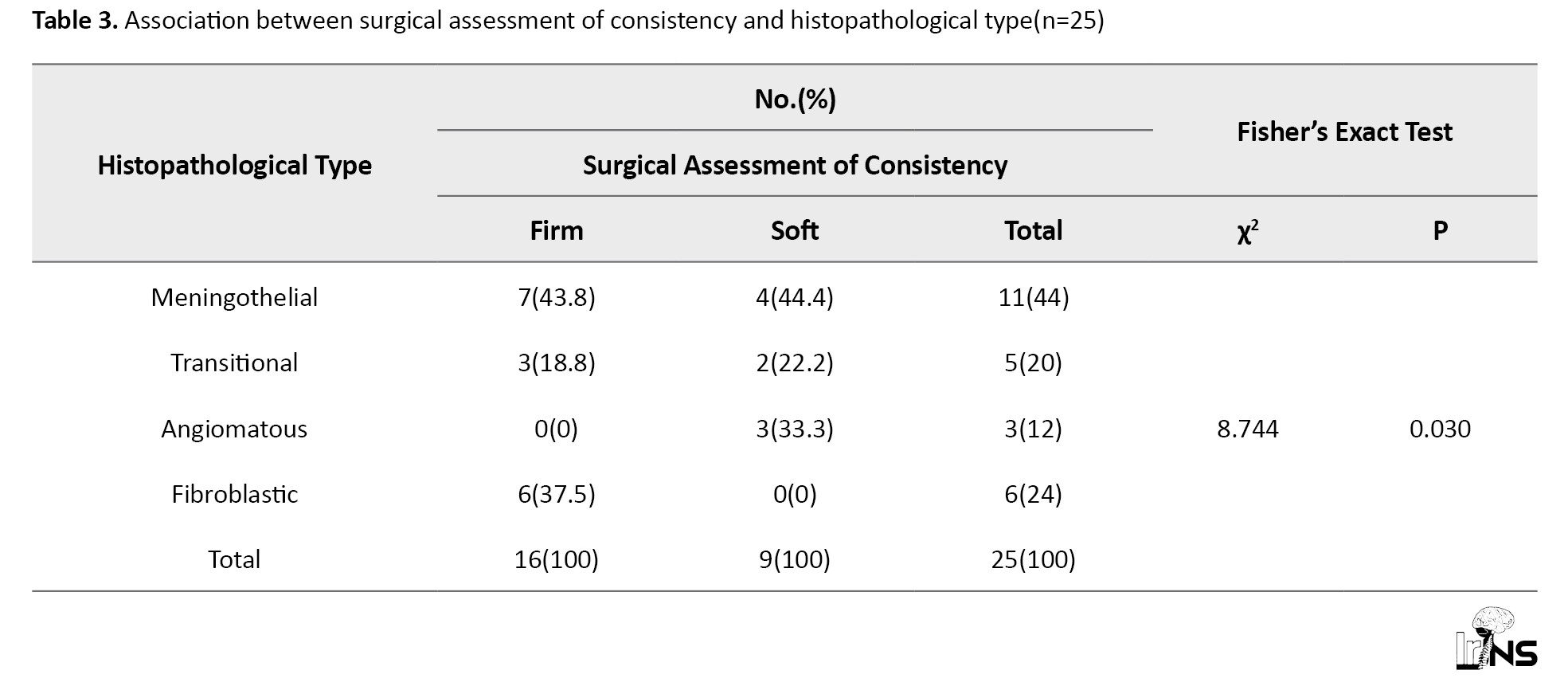
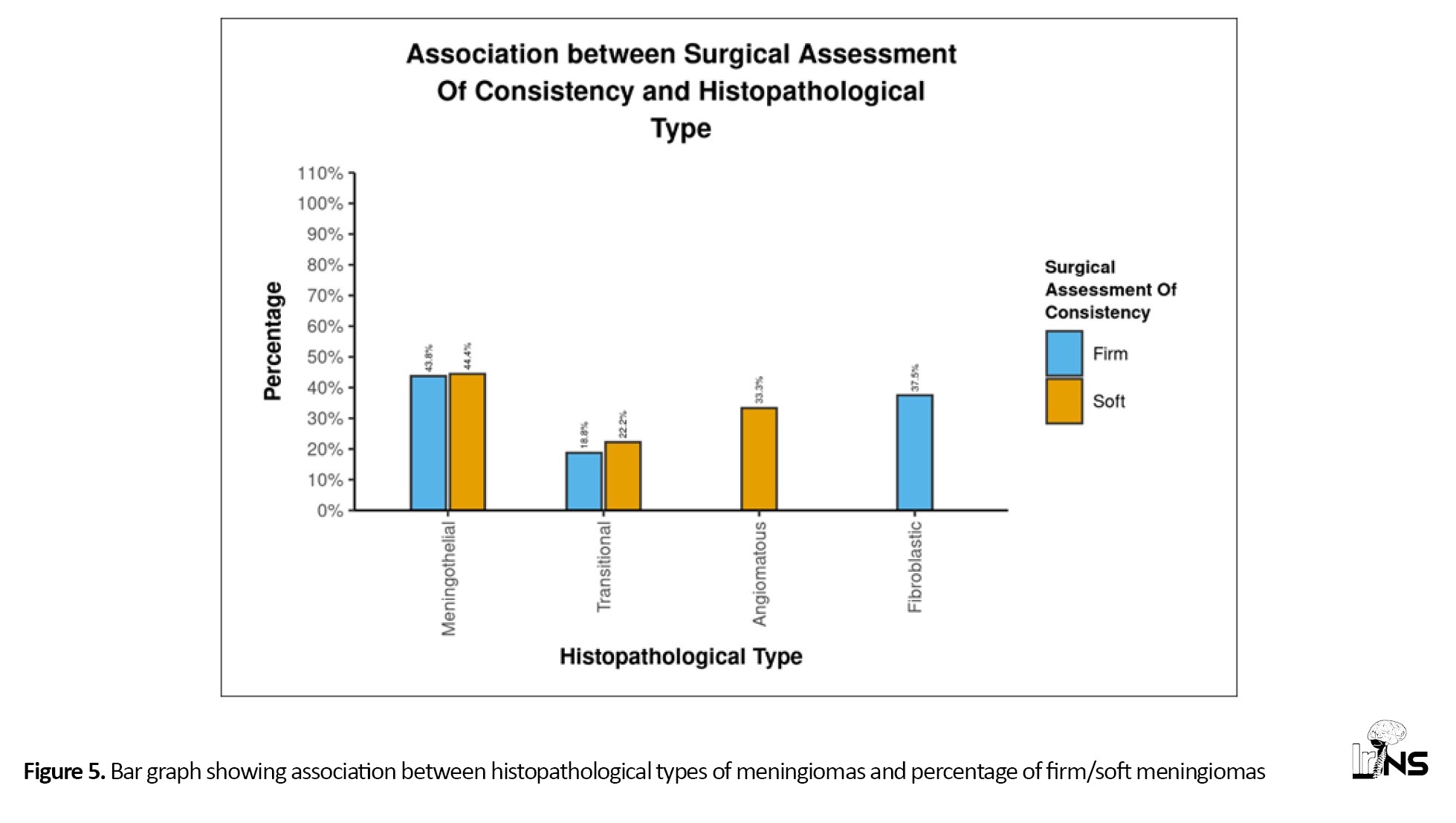
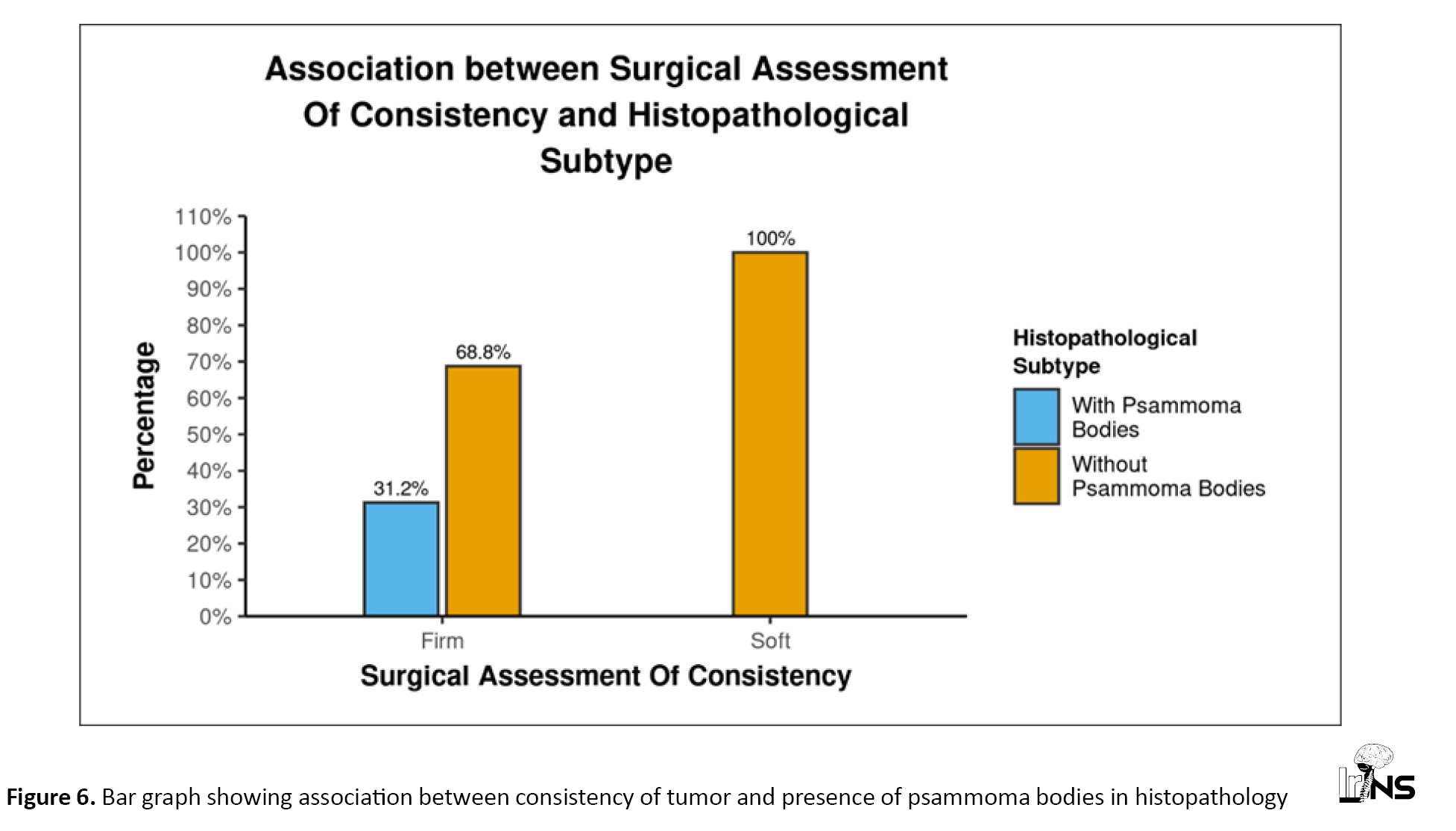
All three angiomatous meningiomas were soft, while all six fibroblastic meningiomas were firm. However, meningothelial and transitional meningiomas did not show this strong correlation (Figure 5). Psammoma bodies were reported in five patients. Although there was no significant correlation between the presence of psammoma bodies and the consistency of meningiomas (P=0.123), none of the meningiomas with psammoma bodies had soft consistency, and none of the soft tumors had psammoma bodies. This indicates that while not all firm tumors have psammoma bodies, the presence of psammoma bodies is an indicator of firmness (Figure 6). We found a significant difference between the presence and absence of psammoma bodies in terms of RD (W=17.000, P=0.024), with the median RD being highest in the meningiomas without psammoma bodies. The median RD in the meningiomas without psammoma bodies was 0.78, while in meningiomas with psammoma bodies, it was 0.64. The median (IQR) FA in meningiomas with psammoma bodies was 0.33 (0.28-0.4), while in those without psammoma bodies, it was 0.18 (0.15-0.25) (P=0.024). This can easily be explained by the fact that psammomatous calcification obstructs free diffusion, giving directionality to diffusion and thus increasing FA while decreasing RD.
We were one of the few series to study the association between the histopathological type of meningioma and the MTR. There was a significant difference among the four histopathological types in terms of MTR (χ2=8.656, P=0.034), with the mean MTR being highest in the fibroblastic type. The mean for fibroblastic type was 0.26 (0.02), which was significantly higher than that of transitional meningiomas, with a mean of 0.19 (0.09).
Correlation of the CUSA score with the consistency of tumor
The CUSA was used in 13 patients. Ten meningiomas were suckable on CUSA with different amplitudes, while three were not suckable. The CUSA score correlated significantly (P=0.037) with the consistency of meningioma (Table 4).
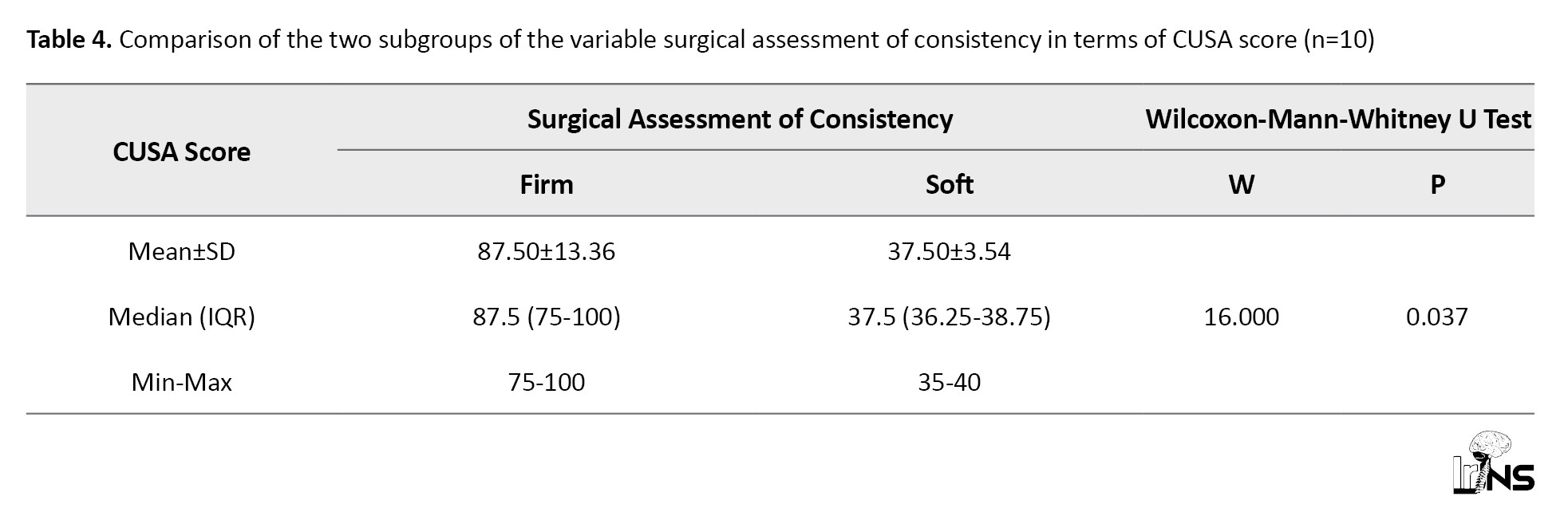
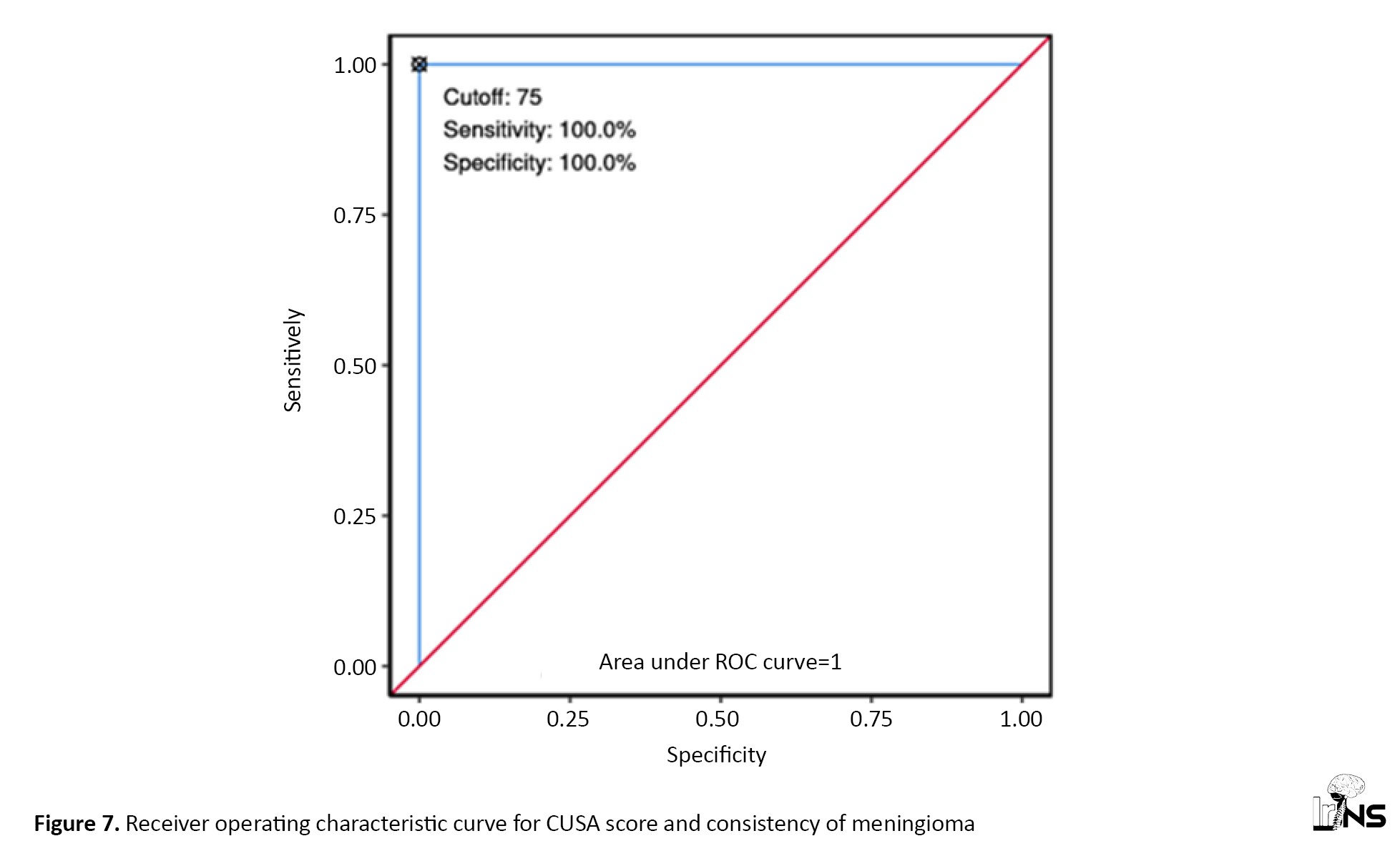
The median (IQR) of the CUSA score in the firm group was 87.50 (75-100), while in soft meningiomas it was 37.50 (36.25-38.75). The ROC curve for the CUSA score and the consistency of meningioma is shown in Figure 7. At a cutoff CUSA score of ≥75, it is possible to distinguish between firm and soft tumors with 100% sensitivity and 100% specificity.
4. Discussion
Preoperative planning is crucial for meningioma excision and is based on the position, size, and consistency of the tumor [6, 7]. Consistency is a useful predictor of resectability, surgical complexity, and the time taken for surgery. Shorter operating times can be achieved by using suction or CUSA at a low setting to remove soft tumors. Harder tumors are more challenging to remove, increasing the level of surgical complexity and lengthening the duration of the procedure. The difficulty of resection increases if tumors encase neurovascular structures. Predicting meningioma consistency before surgery would enable more effective operative planning and selection of resection techniques [7]. This is crucial for the surgical outcome of tumors located in challenging sites, like the base of the skull [6], especially when meningiomas are increasingly being resected using minimally invasive techniques and the FOV is limited.
T2-weighted imaging has emerged as the most promising modality. Suzuki et al. also found a link between T2-weighted images and meningioma consistency, with hyperintense lesions being soft and hypointense lesions being hard [8]. Hoover et al. observed that, compared to gray matter, meningiomas that were T2 hypointense were almost always firm. Meningiomas with hyperintense T2WI signals and hypointense T1WI signals were more likely to be soft, whereas meningiomas with hypointense signals on T2WI and isointense signals on T1WI were more likely to be firm [9]. However, they reported having poor sensitivity in identifying hard tumors.
Fifty-six percent of meningiomas were reported to be nearly isointense with the cortical grey matter [10]. Our study corroborated with the literature, as 48% of our tumors were isointense to the cortex. Carpeggiani et al., Kashimura et al., and Romani et al., also found no relationship between MRI signal intensity and consistency [5, 6, 11]. We also did not find any significant correlation between the consistency of meningioma with T1, T2, and FLAIR signal intensity, although the TCTI ratio was significant in this differentiation.
The intensity of reconstructed MR images is based on arbitrary units. Hence, direct comparisons between several acquisitions cannot be made. The pulse sequence or hardware design is vendor-specific. Being subjective, visual signal intensity measurement can be challenging, especially in large tumors with varied signal intensities [5, 8]. The ROI was kept as large as possible to overcome the problem of heterogeneity. The average of signal intensities in two or three ROIs was taken for tumors where heterogeneity could not be addressed by a large ROI alone. In the present study, to provide objectivity, the TCTI ratio was used. A significant correlation was observed between the TCTI ratio and tumor consistency (P=0.036). The TCTI was the most clinically significant option indicated in the present series for distinguishing between firm and soft meningiomas. This parameter also accounted for variations in T2 values in different subjects, as the own tissue acted as a reference.
The majority of studies that identified a link between consistency and conventional MRI have not provided measures of diagnostic accuracy, and do not seem to be useful in everyday clinical practice [9, 12]. In the present series, the diagnostic performance of predictors with significant correlation with the consistency of meningioma was calculated. The best predictor in terms of diagnostic accuracy has been the TCTI ratio. Our results are similar to those of Smith et al. [13], where a single cut-off TCTI value of 1.41 for soft versus firm tumors was found to be 81.9% sensitive and 84.8% specific.
Our study did not reveal any significant correlation between the consistency of meningiomas and FA, MD, AD, and RD. Kashimura et al., however, discovered that the FA values of hard tumors were substantially greater than those of soft tumors (P=0.0003) [6].
Okmura et al. found a significant difference in MTR between soft and hard tumors, although their study also included other tumors in addition to meningiomas. The MTR value for brain tumors was significantly less when compared to unaffected normal brain tissue (P<0.05). The MTR for meningioma was higher than that for other brain tumors (P<0.05). Within meningiomas, the MTR for the fibrous type was higher compared to the meningothelial type, but statistical significance could not be established [14]. However, the present study did not demonstrate any significant correlation between MTR and the consistency of meningiomas.
The key factors influencing signal intensities of the various meningioma subtypes are believed to be tumor cellularity, water content, and fibrous content [15]. The hardness of meningiomas is attributed to their high fiber content, according to several researchers, and histological appearance is one of the elements influencing tumor consistency [4, 5]. However, Yamaguchi et al. documented no significant link between histological findings and the consistency of meningiomas [10]. In the present series, the consistency of various histologic subtypes correlated significantly with consistency (P=0.03). All three angiomatous meningiomas were reported to have soft consistency, while all six fibroblastic meningiomas were noted to have firm consistency intraoperatively. The presence of psammoma bodies was correlated independently with tumor consistency. Although the correlation was not statistically significant, all 5 tumors reported to have psammoma bodies were firm, and none of the meningiomas with psammoma bodies had a soft consistency. This indicates that the relationship may indeed be significant but could not be established statistically due to insufficient power resulting from the small sample size. Over 75% of the cases studied by Elster et al. showed a good correlation between T2WI characteristics and histopathologic results [16]. Although histologic subtypes may appear differently on an MRI, Demaerel et al. suggested that the differences are insufficient to make a histologic diagnosis [17]. Our study showed a significant correlation between T2 signal intensity and histologic subtype (P=0.049). All angiomatous types had T2 isointense signal intensity, while none of the fibroblastic types exhibited hyperintense signals on T2. The mixed signal intensity, which was iso and hypointense, had the largest proportion of fibroblastic types.
Kashimura et al. found that in comparison to meningothelial meningioma, fibroblastic meningioma had considerably higher FA values (P=0.002) [6]. In the present series, the Mean±SD FA value of fibroblastic meningioma was 0.30±0.07, which was significantly higher compared to the meningothelial group, which had a value of 0.18±0.10 (P=0.05). This may be because the fibrous component in fibroblastic meningiomas offers more resistance to diffusion in the direction perpendicular to the fibrous component, thus giving directionality to diffusion. The Mean±SD FA value of transitional meningioma was 0.28±0.11, which, as expected, turned out to be intermediate, as it is a combination of both fibroblastic and meningothelial types [18].
Similar to our results, Okmura et al. also found that the MTR for fibrous-type meningiomas was higher compared to meningothelial-type meningiomas, but statistical significance could not be established for this finding [14]. This may be related to the higher collagen content in fibroblastic meningiomas.
The strength of this study is the participation of experienced neurosurgeons, radiologists, and pathologists. Objective parameters were included so that the interpretation could be reproduced. The application of the TCTI ratio improves objectivity and generalizability while reducing scanner and patient variability. This study is also the first study to consider the correlation between the consistency of meningiomas and DTI parameters other than FA, as well as to study the consistency of meningiomas in relation to their histopathologic subtypes.
The limitations of the present study include its limited sample size and interobserver bias among neurosurgeons in some cases where the CUSA score could not be assessed due to the inoperability of the CUSA for a period during the study because of equipment failure. Another limitation was that it was not a blinded study.
Conclusion
The consistency of the tumor is an important variable that affects the surgical plan and patient counseling. The neurosurgeon benefits significantly from knowing the tumor consistency prior to surgery while preparing for surgical methods. Prior knowledge of tumor consistency helps the surgeon anticipate difficulties in total tumor removal. This, in turn, influences the duration of surgery and the effectiveness of using tools, like CUSA.
Preoperative characterization of certain histopathological aspects of intracranial meningiomas may be greatly aided by MRI findings. There was a significant correlation between histopathological type and FA, MTR, and T2 signal intensity, as well as between RD and FA with the presence of psammoma bodies. Although MRI is by no means a replacement for pathological analysis, multiparametric MRI may have some predictive utility in terms of the histology of meningiomas.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the institutional review board and Institute Ethics Committee of Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, India (Code: BREC/Th/20/Radiodiag/09; dated 01.04.2021) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Written informed consent was obtained from the patients prior to the examination.
Funding
The article was extracted from the PhD dissertation of Shweta Yadav, approved by the Biomedical Research Ethics Committee of Pandit Bhagwat Dayal Sharma University, Rohtak, India.
Authors' contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
References
Meningiomas account for 16 to 20% of all cerebral tumors and are the most prevalent non-glial tumors of the central nervous system (CNS) [1]. The extent of resection and the surgical method can be affected by tumor vascularization, consistency, and surgical plane. In meningiomas of the skull base and those near neurovascular systems, these variables are more important. Hard tumors that are adherent to neighboring vital structures, such as the internal carotid artery, optic chiasm, and cranial nerves, carry a higher risk of surgical morbidity and necessitate meticulous dissection procedures for removal [2]. Surgical resection is accomplished by first devascularizing the tumor from the surrounding area of the capsule and then debulking it from the center [3]. One of the key factors in removing the entire tumor while avoiding neurological deficits is the consistency of meningiomas [4]. The surgical approach chosen may be partially determined by the ability to accurately predict the consistency of a meningioma preoperatively based on neuroimaging studies. This ability may also provide additional information about the need for staged resection, expected subtotal resection, or other challenges related to consistency and/or vascularity [5].
Most of the studies conducted previously indicate the role of T2-weighted imaging in predicting the firmness of meningiomas preoperatively. The purpose of our study was to demonstrate the effectiveness of tumor/cerebellar peduncle T2-weighted imaging intensity (TCTI) ratio, diffusion tensor imaging (DTI), and magnetization transfer ratio (MTR) in predicting the consistency of tumors in the North Indian population in an objective manner.
2. Methods and Materials/Patients
Subjects
Twenty-five patients with meningiomas were studied, and those with previous cranial surgery/radiotherapy, as well as cases where the surgeon did not define the consistency of the tumor, were excluded.
Imaging
All patients were subjected to magnetic resonance imaging (MRI) on a 3T MRI system (GE, Discovery 750w with GEM Suite, Milwaukee, WI, USA) using a dedicated head coil with 32 channels.
The following sequences were performed: T1W (Time to echo [TE]=24 ms, Time to repitition (TR)=2671.1 ms, field of view (FOV)=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=320, bandwidth=41.67 kHz); T2W (TE=114 ms, TR=8901 ms, FOV=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=512, bandwidth=50 kHz); T2W/FLAIR (TE=120 ms, TR=10000 ms, FOV=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=320, bandwidth=31.25 kHz); DWI (TE=83 ms, TR=261 ms, FOV=22 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=192, bandwidth=166.7 kHz, b=1000 s/mm²); Diffusion tensor imaging (DTI) (TE=78 ms, TR=6413 ms, FOV=22 cm, slice thickness=4.0 mm, slice spacing=1.0 mm, frequency=128, bandwidth=250 kHz, b=1000 s/mm², diffusion directions=30); and MT (TE=15 ms, TR=1300 ms, FOV=24 cm, slice thickness=5.0 mm, slice spacing=1.0 mm, frequency=256, bandwidth=14.71 kHz).
ROIs were placed on both the tumor and the cerebellar peduncle on T2W images, and the ratio of signal intensities was calculated (TCTI ratio) (Figure 1). The ROIs did not have any preset dimensions, as the size of meningioma was variable. In general, ROIs were made as large as possible to obtain the most representative sample of tumors on the axial sequence. In meningiomas with heterogeneous signal intensity, separate ROIs were placed within the tumor, and an average value was calculated. Likewise, ROIs were placed on the MT on and MT off images of the MT sequence, and the MT ratio was calculated (Figure 2). DTI data were transferred to ReadyView software, on AWS server version 3.2, where the placement of the ROI on the tumor allowed for the calculation of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) by averaging the two minor eigenvalues (Figure 3). Various parameters obtained were correlated with the intraoperative consistency of meningiomas.
Operative procedure
During surgery, the consistency of the tumor was assessed and classified as soft for tumors that could be completely removed easily with a suction cannula, and hard for those requiring the use of surgical scissors, a scalpel blade, or a cavitron ultrasonic surgical aspirator (CUSA) set at a high amplitude, or for tumors that could not be removed by gentle manipulation. After exposure of the tumor, specimens were taken from different areas of the tumor and sent for histopathological examination to confirm the diagnosis.
Statistical analysis:
Data were analyzed using SPSS software, version 23. Mean±SD, as well as medians and interquartile ranges (IQRs), were calculated for continuous variables, while frequencies and percentages were calculated for categorical variables. Group comparisons for continuously distributed data were made using an independent samples t-test when comparing two groups. In cases of non-normally distributed data, appropriate non-parametric tests, specifically the Wilcoxon test, were used. The chi-squared test was used for group comparisons of categorical data. Linear correlation between two continuous variables was explored using Pearson’s correlation (for normally distributed data) and Spearman’s correlation (for non-normally distributed data). Statistical significance was set at P<0.05. For MRI findings showing a significant correlation with tumor consistency, receiver operator characteristic (ROC) analysis was performed to predict an optimal cut-off for a continuous predictor of a binary outcome. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were calculated to assess the diagnostic performance of the predictors by creating a 2×2 cross-table with the outcome.
3. Results
There were 11 males and 14 females, ranging in age from 18 to 68 years. There was no correlation between consistency and the size or location of the tumor, except for skull base tumors, where the majority (6 out of 7) of tumors were firm. There was no significant difference between soft and firm meningiomas in terms of T1, T2, and fluid attenuated inversion recovery (FLAIR) signal intensity. However, all tumors that were hyperintense on T2W/FLAIR images were soft, while all tumors that were hypointense on T2W/FLAIR images were firm.
There was a significant difference in the TCTI ratio (P=0.036) between soft and firm tumors. Table 1 shows the consistency of meningioma with respect to the TCTI ratio.

The area under the ROC curve (Figure 4) for the TCTI ratio predicting soft versus firm consistency was 0.76 (95% CI, 0.567%, 0.954%), demonstrating fair diagnostic performance. At a cut-off of TCTI ratio ≥1.5, soft consistency of meningiomas was predicted with a sensitivity of 78%, and a specificity of 75%. Table 2 shows the diagnostic performance of the TCTI in predicting the consistency of meningiomas.

There was no significant difference between the MT ratio of soft and firm meningiomas (P=0.375). Likewise, FA (P=0.169), MD (P=0.978), AD (P=0.590), and RD (P=0.522) did not correlate significantly with the consistency of meningiomas.
3.1 Correlation of histopathological types with the consistency of meningiomas
There were 11 meningothelial, five transitional, six fibroblastic, and three angiomatous meningiomas. There was a significant association between consistency and histopathological type (P=0.03) (Table 3).

All three angiomatous meningiomas were soft, while all six fibroblastic meningiomas were firm. However, meningothelial and transitional meningiomas did not show this strong correlation (Figure 5). Psammoma bodies were reported in five patients. Although there was no significant correlation between the presence of psammoma bodies and the consistency of meningiomas (P=0.123), none of the meningiomas with psammoma bodies had soft consistency, and none of the soft tumors had psammoma bodies. This indicates that while not all firm tumors have psammoma bodies, the presence of psammoma bodies is an indicator of firmness (Figure 6). We found a significant difference between the presence and absence of psammoma bodies in terms of RD (W=17.000, P=0.024), with the median RD being highest in the meningiomas without psammoma bodies. The median RD in the meningiomas without psammoma bodies was 0.78, while in meningiomas with psammoma bodies, it was 0.64. The median (IQR) FA in meningiomas with psammoma bodies was 0.33 (0.28-0.4), while in those without psammoma bodies, it was 0.18 (0.15-0.25) (P=0.024). This can easily be explained by the fact that psammomatous calcification obstructs free diffusion, giving directionality to diffusion and thus increasing FA while decreasing RD.
We were one of the few series to study the association between the histopathological type of meningioma and the MTR. There was a significant difference among the four histopathological types in terms of MTR (χ2=8.656, P=0.034), with the mean MTR being highest in the fibroblastic type. The mean for fibroblastic type was 0.26 (0.02), which was significantly higher than that of transitional meningiomas, with a mean of 0.19 (0.09).
Correlation of the CUSA score with the consistency of tumor
The CUSA was used in 13 patients. Ten meningiomas were suckable on CUSA with different amplitudes, while three were not suckable. The CUSA score correlated significantly (P=0.037) with the consistency of meningioma (Table 4).

The median (IQR) of the CUSA score in the firm group was 87.50 (75-100), while in soft meningiomas it was 37.50 (36.25-38.75). The ROC curve for the CUSA score and the consistency of meningioma is shown in Figure 7. At a cutoff CUSA score of ≥75, it is possible to distinguish between firm and soft tumors with 100% sensitivity and 100% specificity.
4. Discussion
Preoperative planning is crucial for meningioma excision and is based on the position, size, and consistency of the tumor [6, 7]. Consistency is a useful predictor of resectability, surgical complexity, and the time taken for surgery. Shorter operating times can be achieved by using suction or CUSA at a low setting to remove soft tumors. Harder tumors are more challenging to remove, increasing the level of surgical complexity and lengthening the duration of the procedure. The difficulty of resection increases if tumors encase neurovascular structures. Predicting meningioma consistency before surgery would enable more effective operative planning and selection of resection techniques [7]. This is crucial for the surgical outcome of tumors located in challenging sites, like the base of the skull [6], especially when meningiomas are increasingly being resected using minimally invasive techniques and the FOV is limited.
T2-weighted imaging has emerged as the most promising modality. Suzuki et al. also found a link between T2-weighted images and meningioma consistency, with hyperintense lesions being soft and hypointense lesions being hard [8]. Hoover et al. observed that, compared to gray matter, meningiomas that were T2 hypointense were almost always firm. Meningiomas with hyperintense T2WI signals and hypointense T1WI signals were more likely to be soft, whereas meningiomas with hypointense signals on T2WI and isointense signals on T1WI were more likely to be firm [9]. However, they reported having poor sensitivity in identifying hard tumors.
Fifty-six percent of meningiomas were reported to be nearly isointense with the cortical grey matter [10]. Our study corroborated with the literature, as 48% of our tumors were isointense to the cortex. Carpeggiani et al., Kashimura et al., and Romani et al., also found no relationship between MRI signal intensity and consistency [5, 6, 11]. We also did not find any significant correlation between the consistency of meningioma with T1, T2, and FLAIR signal intensity, although the TCTI ratio was significant in this differentiation.
The intensity of reconstructed MR images is based on arbitrary units. Hence, direct comparisons between several acquisitions cannot be made. The pulse sequence or hardware design is vendor-specific. Being subjective, visual signal intensity measurement can be challenging, especially in large tumors with varied signal intensities [5, 8]. The ROI was kept as large as possible to overcome the problem of heterogeneity. The average of signal intensities in two or three ROIs was taken for tumors where heterogeneity could not be addressed by a large ROI alone. In the present study, to provide objectivity, the TCTI ratio was used. A significant correlation was observed between the TCTI ratio and tumor consistency (P=0.036). The TCTI was the most clinically significant option indicated in the present series for distinguishing between firm and soft meningiomas. This parameter also accounted for variations in T2 values in different subjects, as the own tissue acted as a reference.
The majority of studies that identified a link between consistency and conventional MRI have not provided measures of diagnostic accuracy, and do not seem to be useful in everyday clinical practice [9, 12]. In the present series, the diagnostic performance of predictors with significant correlation with the consistency of meningioma was calculated. The best predictor in terms of diagnostic accuracy has been the TCTI ratio. Our results are similar to those of Smith et al. [13], where a single cut-off TCTI value of 1.41 for soft versus firm tumors was found to be 81.9% sensitive and 84.8% specific.
Our study did not reveal any significant correlation between the consistency of meningiomas and FA, MD, AD, and RD. Kashimura et al., however, discovered that the FA values of hard tumors were substantially greater than those of soft tumors (P=0.0003) [6].
Okmura et al. found a significant difference in MTR between soft and hard tumors, although their study also included other tumors in addition to meningiomas. The MTR value for brain tumors was significantly less when compared to unaffected normal brain tissue (P<0.05). The MTR for meningioma was higher than that for other brain tumors (P<0.05). Within meningiomas, the MTR for the fibrous type was higher compared to the meningothelial type, but statistical significance could not be established [14]. However, the present study did not demonstrate any significant correlation between MTR and the consistency of meningiomas.
The key factors influencing signal intensities of the various meningioma subtypes are believed to be tumor cellularity, water content, and fibrous content [15]. The hardness of meningiomas is attributed to their high fiber content, according to several researchers, and histological appearance is one of the elements influencing tumor consistency [4, 5]. However, Yamaguchi et al. documented no significant link between histological findings and the consistency of meningiomas [10]. In the present series, the consistency of various histologic subtypes correlated significantly with consistency (P=0.03). All three angiomatous meningiomas were reported to have soft consistency, while all six fibroblastic meningiomas were noted to have firm consistency intraoperatively. The presence of psammoma bodies was correlated independently with tumor consistency. Although the correlation was not statistically significant, all 5 tumors reported to have psammoma bodies were firm, and none of the meningiomas with psammoma bodies had a soft consistency. This indicates that the relationship may indeed be significant but could not be established statistically due to insufficient power resulting from the small sample size. Over 75% of the cases studied by Elster et al. showed a good correlation between T2WI characteristics and histopathologic results [16]. Although histologic subtypes may appear differently on an MRI, Demaerel et al. suggested that the differences are insufficient to make a histologic diagnosis [17]. Our study showed a significant correlation between T2 signal intensity and histologic subtype (P=0.049). All angiomatous types had T2 isointense signal intensity, while none of the fibroblastic types exhibited hyperintense signals on T2. The mixed signal intensity, which was iso and hypointense, had the largest proportion of fibroblastic types.
Kashimura et al. found that in comparison to meningothelial meningioma, fibroblastic meningioma had considerably higher FA values (P=0.002) [6]. In the present series, the Mean±SD FA value of fibroblastic meningioma was 0.30±0.07, which was significantly higher compared to the meningothelial group, which had a value of 0.18±0.10 (P=0.05). This may be because the fibrous component in fibroblastic meningiomas offers more resistance to diffusion in the direction perpendicular to the fibrous component, thus giving directionality to diffusion. The Mean±SD FA value of transitional meningioma was 0.28±0.11, which, as expected, turned out to be intermediate, as it is a combination of both fibroblastic and meningothelial types [18].
Similar to our results, Okmura et al. also found that the MTR for fibrous-type meningiomas was higher compared to meningothelial-type meningiomas, but statistical significance could not be established for this finding [14]. This may be related to the higher collagen content in fibroblastic meningiomas.
The strength of this study is the participation of experienced neurosurgeons, radiologists, and pathologists. Objective parameters were included so that the interpretation could be reproduced. The application of the TCTI ratio improves objectivity and generalizability while reducing scanner and patient variability. This study is also the first study to consider the correlation between the consistency of meningiomas and DTI parameters other than FA, as well as to study the consistency of meningiomas in relation to their histopathologic subtypes.
The limitations of the present study include its limited sample size and interobserver bias among neurosurgeons in some cases where the CUSA score could not be assessed due to the inoperability of the CUSA for a period during the study because of equipment failure. Another limitation was that it was not a blinded study.
Conclusion
The consistency of the tumor is an important variable that affects the surgical plan and patient counseling. The neurosurgeon benefits significantly from knowing the tumor consistency prior to surgery while preparing for surgical methods. Prior knowledge of tumor consistency helps the surgeon anticipate difficulties in total tumor removal. This, in turn, influences the duration of surgery and the effectiveness of using tools, like CUSA.
Preoperative characterization of certain histopathological aspects of intracranial meningiomas may be greatly aided by MRI findings. There was a significant correlation between histopathological type and FA, MTR, and T2 signal intensity, as well as between RD and FA with the presence of psammoma bodies. Although MRI is by no means a replacement for pathological analysis, multiparametric MRI may have some predictive utility in terms of the histology of meningiomas.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the institutional review board and Institute Ethics Committee of Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, India (Code: BREC/Th/20/Radiodiag/09; dated 01.04.2021) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Written informed consent was obtained from the patients prior to the examination.
Funding
The article was extracted from the PhD dissertation of Shweta Yadav, approved by the Biomedical Research Ethics Committee of Pandit Bhagwat Dayal Sharma University, Rohtak, India.
Authors' contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
References
- Watts J, Box G, Galvin A, Brotchie P, Trost N, Sutherland T. Magnetic resonance imaging of meningiomas: A pictorial review. Insights into Imaging. 2014; 5:113-22. [DOI:10.1007/s13244-013-0302-4]
- Karthigeyan M, Dhandapani S, Salunke P, Singh P, Radotra BD, Gupta SK. The predictive value of conventional magnetic resonance imaging sequences on operative findings and histopathology of intracranial meningiomas: A prospective study. Neurology India. 2019; 67(6):1439-45. [DOI:10.4103/0028-3886.273632]
- Alyamany M, Alshardan MM, Jamea AA, ElBakry N, Soualmi L, Orz Y. Meningioma consistency: Correlation between magnetic resonance imaging characteristics, operative findings and histopathological features. Asian Journal of Neurosurgery. 2018; 13(02):324-8. [DOI:10.4103/1793-5482.228515]
- Chen TC, Zee CS, Miller CA, Weiss MH, Tang G, Chin L, et al. Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery. 1992; 31(6):1015-22. [DOI:10.1227/00006123-199212000-00005]
- Carpeggiani P, Crisi G, Trevisan C. MRI of intracranial meningiomas: correlation with histology and physical consistency. Neuroradiology. 1993; 35(7):532-6. [DOI:10.1007/BF00588715]
- Kashimura H, Inoue T, Ogasawara K, Arai H, Otawara Y, Kanbara Y, et al. Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. Journal of Neurosurgery. 2007; 107(4):784-7. [DOI:10.3171/JNS-07/10/0784]
- Sitthinamsuwan B, Khampalikit I, Nunta-aree S, Srirabheebhat P, Witthiwej T, Nitising A. Predictors of meningioma consistency: A study in 243 consecutive cases. Acta Neurochirurgica. 2012; 154:1383-9. [DOI:10.1007/s00701-012-1427-9]
- Suzuki Y, Sugimoto T, Shibuya M, Sugita K, Patel SJ. Meningiomas: Correlation between MRI characteristics and operative findings including consistency. Acta Neurochirurgica. 1994; 129:39-46. [DOI:10.1007/BF01400871]
- Hoover JM, Morris JM, Meyer FB. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surgical Neurology International. 2011; 2:142. [DOI:10.4103/2152-7806.85983] [PMID]
- Yamaguchi N, Kawase T, Sagoh M, Ohira T, Shiga H, Toya S. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surgical Neurology. 1997; 48(6):579-83. [DOI:10.1016/S0090-3019(96)00439-9]
- Romani R, Tang WJ, Mao Y, Wang DJ, Tang HL, Zhu FP, et al. Diffusion tensor magnetic resonance imaging for predicting the consistency of intracranial meningiomas. Acta Neurochirurgica. 2014; 156:1837-45. [DOI:10.1007/s00701-014-2149-y]
- Watanabe K, Kakeda S, Yamamoto J, Ide S, Ohnari N, Nishizawa S, et al. Prediction of hard meningiomas: Quantitative evaluation based on the magnetic resonance signal intensity. Acta Radiologica. 2016; 57(3):333-40. [DOI:10.1177/0284185115578323]
- Smith KA, Leever JD, Hylton PD, Camarata PJ, Chamoun RB. Meningioma consistency prediction utilizing tumor to cerebellar peduncle intensity on T2-weighted magnetic resonance imaging sequences: TCTI ratio. Journal of Neurosurgery. 2017; 126(1):242–8. [DOI:10.3171/2016.1.JNS152329] [PMID]
- Okumura A, Takenaka K, Nishimura Y, Asano Y, Sakai N, Kuwata K, et al. The characterization of human brain tumor using magnetization transfer technique in magnetic resonance imaging. Neurological Research. 1999; 21(3):250-4. [DOI:10.1080/01616412.1999.11740927]
- Maiuri F, Iaconetta G, de Divitiis O, Cirillo S, Di Salle F, De Caro ML. Intracranial meningiomas: Correlations between MR imaging and histology. European Journal of Radiology. 1999; 31(1):69-75. [DOI:10.1016/S0720-048X(98)00083-7] [PMID]
- Elster AD, Challa VR, Gilbert TH, Richardson DN, Contento JC. Meningiomas: MR and histopathologic features. Radiology. 1989; 170(3 Pt 1):857-62. [DOI:10.1148/radiology.170.3.2916043] [PMID]
- Demaerel P, Wilms G, Lammens M, Marchal G, Plets C, Goffin J, et al. Intracranial meningiomas: Correlation between MR imaging and histology in fifty patients. Journal of Computer Assisted Tomography. 1991; 15(1):45-51. [DOI:10.1097/00004728-199101000-00005] [PMID]
- Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. The Lancet Neurology. 2006; 15(2):1045-54. [DOI:10.1016/S1474-4422(06)70625-1] [PMID]
Type of Study: Research |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |