Thu, Nov 6, 2025
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 196-209 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Murotov T M. Effect of Hyperosmolar Combined Solution of Mannitol 15% Plus 3.5% NaCl Solution on Cerebral Edema in Patients With Traumatic Brain Injury. Iran J Neurosurg 2024; 10 : 23
URL: http://irjns.org/article-1-412-en.html
URL: http://irjns.org/article-1-412-en.html
Department of Anesthesiology and Resuscitation, Tashkent Medical Academy, Tashkent, Uzbekistan. , temurmalik_murotov@mail.ru
Keywords: Mannitol, Hypertonic
sodium chloride (NaCl), Intracranial pressure (ICP), Cerebral perfusion pressure, Hyperosmolar therapy, Craniocerebral trauma
Full Text [PDF 2304 kb]
(1622 Downloads)
| Abstract (HTML) (2998 Views)
Full Text: (636 Views)
1. Introduction
Hyperosmolar agents, such as mannitol and hypertonic saline solution (HSS) are pharmacologic options to reduce intracranial pressure (ICP), especially in patients with high-severity craniocerebral trauma [1-3]. Hypertonic sodium chloride (NaCl) solution [4] and mannitol are used for emergency treatment of acute cerebral edema and other neurologic conditions. High-quality randomized controlled trials comparing these agents are limited. Emerging evidence suggests that HSR may have a stronger effect on ICP and cerebral perfusion pressure (CPP) [5, 6, 7, 8].
In this work, we used both hyperosmolar solution colloid-mannitol 15% and crystalloid-NaCl 3.5% at the same time. Both have the same mechanism of action creating an osmotic gradient between the blood-brain barrier and brain tissue.
The present study aims to investigate the efficacy, and safety of the combined use of mannitol 15% + NaCl 3.5% in treating intracranial hypertension (ICH) in patients with isolated head injury aged ≥18 years. All patients in this group had severe isolated craniocerebral trauma caused mainly by motor vehicle accidents, falls, and sports injuries.
2. Methods and Materials/Patients
Patients were hospitalized in the immediate post-injury period and had some degree of impaired consciousness. The mean time of admission after injury was 37±8 minutes. Only patients with ICH (ICP>20 mm Hg) were included in the study. After assessment of the level of consciousness, all patients underwent head computed tomography (CT) to exclude the need for emergency neurosurgical intervention.
Study design
In this single-center, randomized clinical study, 35 patients (Retrospectively 15 patients and prospectively, 20 patients) were treated in the intensive care unit (ICU) with isolated traumatic brain injury. Their age range was from 18 to 65 years old with depression of consciousness (4-12 points on the Glasgow coma scale [GCS]), and abnormal CT brain findings of on admission.
The inclusion criteria included age >18 years, isolated traumatic brain injury, level of consciousness according to the GCS ≤12, and sustained elevation of ICP>20 mm Hg.
The exclusion criteria included:
- Need for urgent cranial or extracranial surgery
- Previous decompressive craniectomy
- Polytrauma
- Oliguric, renal failure
- Hemoglobin (Hb) level <80 g/L
- Serum osmolarity >320 mosmol/L
- Use of mannitol or HSS in the previous 6 hours
- Pregnancy
- Patients who died within 72 hours of admission to ICU
- Coagulopathies
Baseline data on admission included age, sex, weight, brain CT scan not necessary to calculate acute physiology and chronic health evaluation ii (APACHE II) severity score, mechanism of injury, pupil reactivity (reactive/non-reactive).
A set of variables was collected for each patient, including in addition to the demographic characteristics described, GCS and Glasgow outcome scale, treatment time, blood pressure (BP) monitoring, mean arterial pressure (MAP), heart rate (HR), ICP, CPP, serum osmolarity, sodium level, blood glucose, urine volume, central hemodynamic parameters (shock index (ShI), cardiac index, total peripheral resistance (TPR), hemostasis (prothrombin time, activated partial thromboplastin time (APTT), fibrinogen).
When ICP exceeded 20 mm Hg and ICH lasted more than 5 minutes, one of the mentioned hyperosmolar solutions was bolus infused through the central venous line at a rate of 5-7 mL/min (120 drops/minute), i.e. within the range of 1000-1100 mOsm/L. Solution infusion was stopped when the ICP decreased ≤20 mm Hg, which was the goal of our therapy.
ICP was invasively measured by lumbar puncture at the level of L3-L4 and noninvasively. Of 35 patients with isolated traumatic brain injury in the absence of CT signs of dislocation syndrome, lumbar puncture with manometry and obligatory filling with auto blood was performed in 8 patients. In the absolute majority and repeatedly, the ICP was non-invasively measured.
These parameters were analyzed at the following stages of the study:
- Before the infusion was started.
- After discontinuation of infusion (achieved ICP<20 mm Hg).
- 30, 60, and 120 minutes after discontinuation of infusion (after ICP<20 mm Hg).
Serum sodium level, serum osmolarity, blood glucose, blood glucose, hematocrit, and diuresis were assessed before and after therapy.
Ultrasound diagnostics
We used it for the determination of M-echopulsation in the third ventricle of the brain diagnostic complex of “Complexmed 1.2”, which allows echoencephaloscopy, extra, and transcranial dopplerography (Figure 1).
Hyperosmolar agents, such as mannitol and hypertonic saline solution (HSS) are pharmacologic options to reduce intracranial pressure (ICP), especially in patients with high-severity craniocerebral trauma [1-3]. Hypertonic sodium chloride (NaCl) solution [4] and mannitol are used for emergency treatment of acute cerebral edema and other neurologic conditions. High-quality randomized controlled trials comparing these agents are limited. Emerging evidence suggests that HSR may have a stronger effect on ICP and cerebral perfusion pressure (CPP) [5, 6, 7, 8].
In this work, we used both hyperosmolar solution colloid-mannitol 15% and crystalloid-NaCl 3.5% at the same time. Both have the same mechanism of action creating an osmotic gradient between the blood-brain barrier and brain tissue.
The present study aims to investigate the efficacy, and safety of the combined use of mannitol 15% + NaCl 3.5% in treating intracranial hypertension (ICH) in patients with isolated head injury aged ≥18 years. All patients in this group had severe isolated craniocerebral trauma caused mainly by motor vehicle accidents, falls, and sports injuries.
2. Methods and Materials/Patients
Patients were hospitalized in the immediate post-injury period and had some degree of impaired consciousness. The mean time of admission after injury was 37±8 minutes. Only patients with ICH (ICP>20 mm Hg) were included in the study. After assessment of the level of consciousness, all patients underwent head computed tomography (CT) to exclude the need for emergency neurosurgical intervention.
Study design
In this single-center, randomized clinical study, 35 patients (Retrospectively 15 patients and prospectively, 20 patients) were treated in the intensive care unit (ICU) with isolated traumatic brain injury. Their age range was from 18 to 65 years old with depression of consciousness (4-12 points on the Glasgow coma scale [GCS]), and abnormal CT brain findings of on admission.
The inclusion criteria included age >18 years, isolated traumatic brain injury, level of consciousness according to the GCS ≤12, and sustained elevation of ICP>20 mm Hg.
The exclusion criteria included:
- Need for urgent cranial or extracranial surgery
- Previous decompressive craniectomy
- Polytrauma
- Oliguric, renal failure
- Hemoglobin (Hb) level <80 g/L
- Serum osmolarity >320 mosmol/L
- Use of mannitol or HSS in the previous 6 hours
- Pregnancy
- Patients who died within 72 hours of admission to ICU
- Coagulopathies
Baseline data on admission included age, sex, weight, brain CT scan not necessary to calculate acute physiology and chronic health evaluation ii (APACHE II) severity score, mechanism of injury, pupil reactivity (reactive/non-reactive).
A set of variables was collected for each patient, including in addition to the demographic characteristics described, GCS and Glasgow outcome scale, treatment time, blood pressure (BP) monitoring, mean arterial pressure (MAP), heart rate (HR), ICP, CPP, serum osmolarity, sodium level, blood glucose, urine volume, central hemodynamic parameters (shock index (ShI), cardiac index, total peripheral resistance (TPR), hemostasis (prothrombin time, activated partial thromboplastin time (APTT), fibrinogen).
When ICP exceeded 20 mm Hg and ICH lasted more than 5 minutes, one of the mentioned hyperosmolar solutions was bolus infused through the central venous line at a rate of 5-7 mL/min (120 drops/minute), i.e. within the range of 1000-1100 mOsm/L. Solution infusion was stopped when the ICP decreased ≤20 mm Hg, which was the goal of our therapy.
ICP was invasively measured by lumbar puncture at the level of L3-L4 and noninvasively. Of 35 patients with isolated traumatic brain injury in the absence of CT signs of dislocation syndrome, lumbar puncture with manometry and obligatory filling with auto blood was performed in 8 patients. In the absolute majority and repeatedly, the ICP was non-invasively measured.
These parameters were analyzed at the following stages of the study:
- Before the infusion was started.
- After discontinuation of infusion (achieved ICP<20 mm Hg).
- 30, 60, and 120 minutes after discontinuation of infusion (after ICP<20 mm Hg).
Serum sodium level, serum osmolarity, blood glucose, blood glucose, hematocrit, and diuresis were assessed before and after therapy.
Ultrasound diagnostics
We used it for the determination of M-echopulsation in the third ventricle of the brain diagnostic complex of “Complexmed 1.2”, which allows echoencephaloscopy, extra, and transcranial dopplerography (Figure 1).
Echoencephaloscopy
The essence of the method
The principle of this diagnostic method, also called the M-method, is based on the echolocation of the so-called sagittal structures of the brain, which normally occupy a median position about the temporal bones of the skull. From the ultrasound transducer in pulse mode, the echo signal through the bone penetrates the cranial cavity, reaching the contralateral bone plate and reflecting on its way from the interfaces of media with different echo densities (tissue-liquid or tissue-bone). The three most typical and repetitive signals are registered. The first signal is the reflection from the bone plate of the skull, where the ultrasound sensor is installed, the so-called initial complex. The second signal is formed due to the reflection of the ultrasound beam from the midbrain structures. These include the interhemispheric gap, the transparent septum, the III ventricle, and the epiphysis. The third signal recorded along the path of the ultrasound beam is represented by the inner surface of the temporal bone, opposite to the location of the transmitter. A unique feature of EchoEC is the possibility of registering echopulsation of the ventricular system of the brain. When ICP increases, not only the power of reflected signals changes but also the amplitude of their pulsation.
Procedure
The patient lies on the back, without a pillow, with the right hand freely and at the same time with some support on the patient’s parieto-parietal region, turning the patient’s head to the left or right, we perform echolocation and measurement of echo-distances, while with the free left hand, we carry out the necessary movements of the echo-distance meter. To determine Ps - M-echopulsation amplitude in percent after lubrication of the frontal and temporal parts of the head with contact gel, a standard transducer was placed 3-4 cm upward from the external auditory canal and 1-2 cm anterior to it, due to a particularly powerful, constant and easily detectable M-echo complex at this location (Table 1).
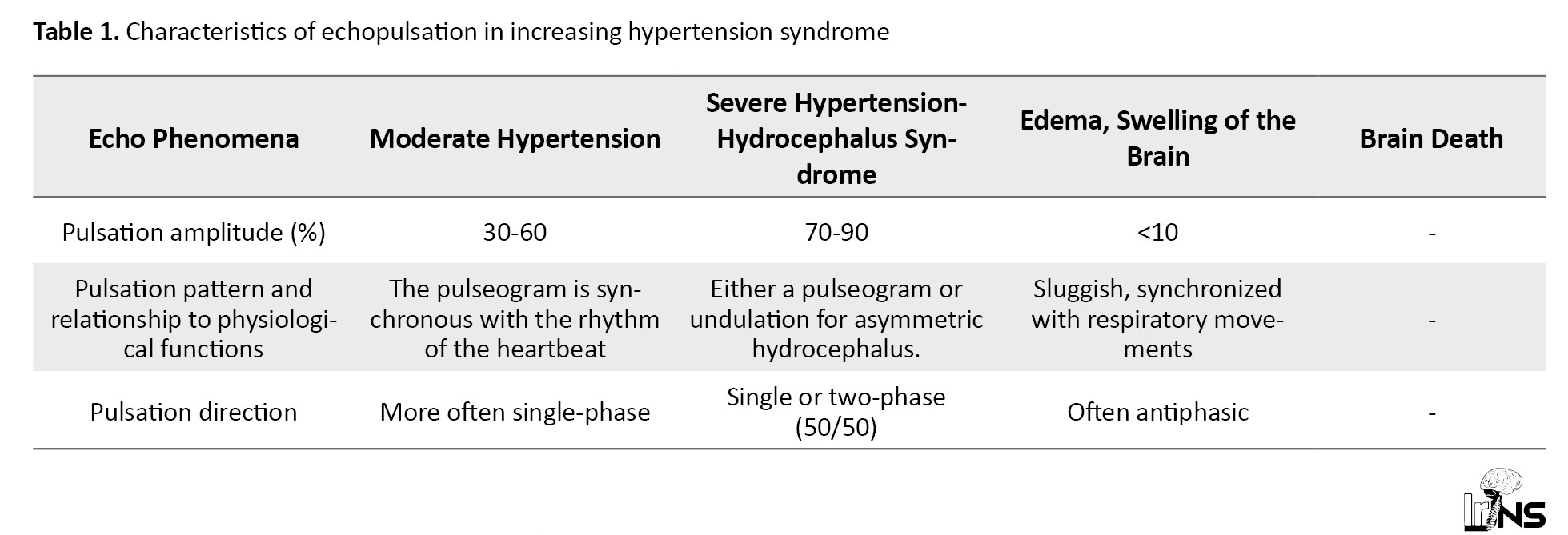
Pulsation amplitude of about 15%-25% is considered normal for the M-echo complex. As a rule, it is considered that echopulsation in 25%-50% corresponds to moderate, and above 60%-70%, pronounced ICH. We present the characteristics of echopulsation in increasing hypertension syndrome according to the “manual” for the operation of “Complexmed”.
The above data indicate that M-echopulsation of the III ventricle of the brain, obtained by using the device “Complexmed 1.2”, gives the possibility of non-invasive qualitative determination of ICP and ICH indicators (moderate, pronounced hypertension).
In our studies, we attempted to translate the resulting qualitative expressions of M-echopulsation into numerical values.
To assess the effectiveness of hyperosmolar solutions and mannitol in the treatment of traumatic brain injury, careful monitoring of ICP is necessary.
Invasive methods of its monitoring (drainage of brain ventricles) using sensors in the epi, or subdural space are accurate but are the prerogative of large neurosurgical centers. A simpler invasive method of determining ICP, intrathecal lumbar puncture with monomanometry of liquor pressure, is not always feasible in severe traumatic brain injury (wedging syndrome) and cannot serve as a method of monitoring ICP and ICH. Accepted methods for non-invasive determination of ICP do not currently exist or are in clinical trials.
The essence of our study was as follows. A total of 113 patients operated under spinal anesthesia with traumatic and degenerative changes in hip and knee joints (n=41), traumatic brain injury (brain contusion without displacement of brain structures (n=28), suspected meningitis (n=17), coma (n=27) underwent lumbar puncture with monomanometry of liquor tensions for therapeutic and diagnostic purposes. All these patients were not included in this study. In parallel, these same patients underwent qualitative noninvasive determination of IСP using M-echopulsation of the III ventricle of the brain, comparing them to the data of lumbar puncture (Figure 2).
The essence of the method
The principle of this diagnostic method, also called the M-method, is based on the echolocation of the so-called sagittal structures of the brain, which normally occupy a median position about the temporal bones of the skull. From the ultrasound transducer in pulse mode, the echo signal through the bone penetrates the cranial cavity, reaching the contralateral bone plate and reflecting on its way from the interfaces of media with different echo densities (tissue-liquid or tissue-bone). The three most typical and repetitive signals are registered. The first signal is the reflection from the bone plate of the skull, where the ultrasound sensor is installed, the so-called initial complex. The second signal is formed due to the reflection of the ultrasound beam from the midbrain structures. These include the interhemispheric gap, the transparent septum, the III ventricle, and the epiphysis. The third signal recorded along the path of the ultrasound beam is represented by the inner surface of the temporal bone, opposite to the location of the transmitter. A unique feature of EchoEC is the possibility of registering echopulsation of the ventricular system of the brain. When ICP increases, not only the power of reflected signals changes but also the amplitude of their pulsation.
Procedure
The patient lies on the back, without a pillow, with the right hand freely and at the same time with some support on the patient’s parieto-parietal region, turning the patient’s head to the left or right, we perform echolocation and measurement of echo-distances, while with the free left hand, we carry out the necessary movements of the echo-distance meter. To determine Ps - M-echopulsation amplitude in percent after lubrication of the frontal and temporal parts of the head with contact gel, a standard transducer was placed 3-4 cm upward from the external auditory canal and 1-2 cm anterior to it, due to a particularly powerful, constant and easily detectable M-echo complex at this location (Table 1).

Pulsation amplitude of about 15%-25% is considered normal for the M-echo complex. As a rule, it is considered that echopulsation in 25%-50% corresponds to moderate, and above 60%-70%, pronounced ICH. We present the characteristics of echopulsation in increasing hypertension syndrome according to the “manual” for the operation of “Complexmed”.
The above data indicate that M-echopulsation of the III ventricle of the brain, obtained by using the device “Complexmed 1.2”, gives the possibility of non-invasive qualitative determination of ICP and ICH indicators (moderate, pronounced hypertension).
In our studies, we attempted to translate the resulting qualitative expressions of M-echopulsation into numerical values.
To assess the effectiveness of hyperosmolar solutions and mannitol in the treatment of traumatic brain injury, careful monitoring of ICP is necessary.
Invasive methods of its monitoring (drainage of brain ventricles) using sensors in the epi, or subdural space are accurate but are the prerogative of large neurosurgical centers. A simpler invasive method of determining ICP, intrathecal lumbar puncture with monomanometry of liquor pressure, is not always feasible in severe traumatic brain injury (wedging syndrome) and cannot serve as a method of monitoring ICP and ICH. Accepted methods for non-invasive determination of ICP do not currently exist or are in clinical trials.
The essence of our study was as follows. A total of 113 patients operated under spinal anesthesia with traumatic and degenerative changes in hip and knee joints (n=41), traumatic brain injury (brain contusion without displacement of brain structures (n=28), suspected meningitis (n=17), coma (n=27) underwent lumbar puncture with monomanometry of liquor tensions for therapeutic and diagnostic purposes. All these patients were not included in this study. In parallel, these same patients underwent qualitative noninvasive determination of IСP using M-echopulsation of the III ventricle of the brain, comparing them to the data of lumbar puncture (Figure 2).
The studies conducted with a high degree of representativeness showed that echopulsation between 25%-50% “moderate ICH” corresponds to invasively obtained data between 20-24 mm Hg, and ICP ≥25 mm Hg is in the range of “severe ICH”-60%-80%.
Echopulsation in the range of 15%-25% corresponded to the data of lumbar puncture with manometry in wide ranges from 4 to 19 mm Hg. The obtained comparative data allowed us to use the method of echopulsation to monitor ICH during its treatment with hyperosmolar solutions. ICP values exceeding 29 mm Hg correspond to >70% with asynchronous echopulsation of undulatory type.
In the studied patients with normal values of IAP, we determined the correlation coefficient of IAP, data obtained by noninvasive and invasive method (lumbar puncture) according to our proposed formula (Equation 1).
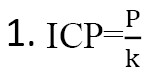
where, P-pulsogram in %, k=1.388 correction coefficient at normal pulsogram (percentage data of M-echo).
Thus, with the M-echopulsagram equal to 16.2% on average, we divide this value by the coefficient 1.388 and obtain the values of 11.7 mm Hg obtained by lumbar puncture.
Next, we determined the correction index for moderate ICH using the same formula and obtained a correction coefficient equal to k=1.677 for moderate ICH.
Thus, with M-echopulsagram equal to 37.2% on average, we divide this value by a coefficient of 1.677 and obtain values of 22.2 mm Hg obtained by lumbar puncture.
In the same way, we calculated the correction coefficient for pronounced ICH, and it amounted to k=2.339.
Thus, with M-echopulseagram equal to 64.3 on average, we divide this value by the coefficient of 2.339 and get the value of 27.5 mm Hg obtained by lumbar puncture.
Summarizing the above, we concluded that M-echopulsagram of a portable ultrasound device (Complesmed 1.2) can be used for non-invasive monitoring of ICH in trauma victims with traumatic brain injury in the process of complex therapy and to evaluate the effectiveness of the latter.
The correction indices calculated by us at normal values of IСH (k=1,388), moderate IСH (k=1,677), and pronounced IСP (k=2,339) obtained using Complexmed 1.2 when comparing them with the data of lumbar puncture correlate with Pearson correlation coefficients.
A patent for the invention “method for non-invasive assessment of ICP in patients with brain injury” (No. IAP 06573) was obtained. This method was developed to effectively treat diseases by preventing brain swelling through non-invasive assessment of ICP in patients with brain injury.
General care of patients in ICU (stage I of treatment)
We followed the recommendations of the Fond Brian Foundation (2016). All patients were sedated by continuous IV administration of propofol and opiates and underwent artificial ventilation. Patients were placed in a semi-reclined position (with the head end of the bed elevated 30-40 °C), in the absence of contraindications to this. Secondary brain damage was prevented by therapeutic craniocerebral hypothermia, covering the head and carotid vessels with cold elements with subsequent measurement of t0 in the external auditory canal, maintaining body temperature between 36-37 °C, ensuring normoglycemia, avoiding hypoxemia.
According to the protocol adopted in our clinic, basic therapy included infusion and transfusion therapy, lidocaine to close Na+ channels, nimotop (nimodipine) to block Ca2+ channels (the N-methyl-D-aspartate [NMDA] receptors), mild therapeutic craniocerebral hypothermia (4-5 °C cooling of brain structures), antioxidant therapy (α-lipoic acid, ascorbic acid, vit. E) to block reactive oxygen species, propofol, barbiturates to sedate and block transaminase activity, prophylaxis of infection, thrombotic complications and ulcerative complications and ulcerative formations in the gastrointestinal tract, early tube feeding of patients.
ICH was evaluated as susceptible (i.e. responsive to therapy, including osmotherapy, with ICP returning to <20 mm Hg) or refractory (stable ICP>20-25 mm Hg), requiring surgical decompression.
Neurologic status was assessed clinically using the Glasgow scale, and the severity of the patient’s condition and outcome were assessed using APACHE II.
A total of 35 patients were included in this study conducted over 5 years (2018-2022) in the Departments of Neurosurgery and Anesthesiology and Critical Care Medicine of Tashkent Medical Academy. All patients in this group received a mannitol infusion of 15% mannitol + NaCl 3.5% to reduce ICH. In this study, we investigated the effect of a combined solution of mannitol 15% + NaCl 3.5% on individual episodes of ICH, as well as on the time and duration of reduction of ICH peaks, the dose of these solutions reduces ICH <20 mm Hg.
Pre-hospital physiologic parameters were recorded, including post-resuscitation GCS, pupillary response to light, hemoglobin (Hb), hematocrit (Ht), and blood glucose levels. Daily data during 7 days after admission to the ICU included hourly measurements of ICP recorded noninvasively using Complexmed 1.2, and if possible by lumbar intrathecal puncture with manometry in 8 patients, serum sodium studies, pulse oximetry and measurement of daily diuresis, and plasma osmolarity. Crucial aspects of the care of trauma patients included neurosurgical operations performed when necessary (clot evacuation, decompression craniotomy), mannitol 15% + NaCl 3.5% osmotherapy, and ventilatory support. Outcome data included ICU and in-hospital mortality, length of stay in ICU, dose of combined mannitol 15% + NaCl 3.5% solution, and time required to reduce ICP<20 mm Hg.
We used descriptive statistics to examine the frequency and percentage of variables, such as gender, pupillary responses, CT findings, mechanism of injury, and level of consciousness in the patient groups, we studied as an indicator of the representativeness of these groups.
Methods of intensive therapy
All patients received standard complex intensive therapy according to international recommendations for the treatment of traumatic brain injury. The head end of the bed was raised by 30-40 °C. Ventilation by Wella and Drager apparatus with the respiratory volume of 8-10 mL/kg of ideal body weight in synchronized intermittent mandatory ventilation mode and positive end-expiratory pressure +2-10 cm of the water column. Infusion therapy was performed, combining colloid and crystalloid solutions. We tried to maintain normovolemia (central venous pressure [CVP] 8-12 cm of water column). Enteral tube feeding was started from the first day of the patient’s stay in the ICU at the rate of 20-25 kcal per kg of body weight per day after stabilization of vital parameters of the organism. Daily protein requirement was estimated according to the nitrogen balance calculation. If necessary, parenteral nutrition was added. To prevent infectious complications, all patients were treated with monotherapy with cephalosporins (ceftriaxone 2-4 g/day) or fluoroquinolones (ciprofloxacin 0.2-0.4 g/day) from the first day after surgery or in the presence of respiratory support. To prevent thrombosis of deep veins of the lower extremities (in the absence of signs of external and internal bleeding, low-molecular-weight heparin, clexane 0.4 thousand units per day subcutaneously was administered from 2-3 days). In patients who underwent ICP measurement, in case of clinical signs of dislocation syndrome (anisocoria, upward gaze paresis, Gerdwig-Majandi syndrome combined with bradycardia, arterial hypertension), CT of the brain was performed and the question of surgical intervention was decided. Blood plasma osmolarity was monitored. To control psychomotor agitation, we used medication sedation with a combination of narcotic analgesics and benzodiazepines. Hyperthermia was not allowed by us. At t>37.5 °C, antipyretics were administered and physical methods of cooling were used. In case of progressive worsening of the level of consciousness, despite conservative therapy, a CT scan of the brain was performed.
ICP was sought to be maintained within 15-20 mm Hg or less. Analgesia and sedatives were used during invasive procedures (tracheostomy, vascular catheterization) and when it was necessary to control the psychomotor agitation of the patient. Hyperosmolar solutions under the control of blood plasma osmolality were used to reduce elevated ICP. If blood plasma osmolality increased more than 320 mOsm/L, administration of hyperosmolar preparations was stopped. In the presence of persistent ICH difficult to correct by conservative methods of therapy (ICP more than 20 mm Hg for 6-12 hours), decompressive cranial trepanation was performed.
Methods of statistical analysis
Statistical processing of the obtained data was performed on a personal computer using the JASP program package. Statistical processing of the material provided for obtaining combination tables, graphs, and analytical indicators included structure, mean values and their standard errors, and student’s criterion with calculation of the probability of error. Differences in mean values were considered reliable at a significance level of P<0.05.
ICP and M-echopulsagrams were checked with Pearson’s correlation coefficient, and their reliability was checked with the student’s t-test.
3. Results
When the ICP exceeded 20 mm Hg for more than 5 minutes (two to three times measured by ultrasound (US) M-echopulsation of the cerebral III ventricle), bolus mannitol 15% + NaCl 3.5% was administered via the central vein at a rate of 6-8 mL/min (120-130 cap/min). The infusion was stopped when the ICP dropped below 20 mm Hg. We recorded the values of ICP, and CPP before and after infusion of mannitol 15% + NaCl 3.5%. We recorded ICP and CPP 15, 30, 60, and 120 minutes after drip infusion of combined mannitol 15% + NaCl 3.5% solution. The episodes of ICH requiring administration of hyperosmolar solutions per patient, the number of ICH episodes per day, and the dose of each infusion of mannitol 15% + NaCl 3.5% were recorded by us.
The age of patients in this group ranged from 20-82 years old (Mean±SD 41.5±1.7 years). The trauma was associated with road traffic accidents (n=19; 54.3%), falls (n=12; 34.3%) compression (n=2; 5.7%), and 2 patients (5.7%) had sports trauma with a bleeding wound in the frontal-temporal part of the head without damage to the skull bones. Vomiting of gastric contents occurred in 16 patients, three of whom were diagnosed with aspiration syndrome, for which sanitation bronchoscopy with lavage of airways was performed (Table 2).
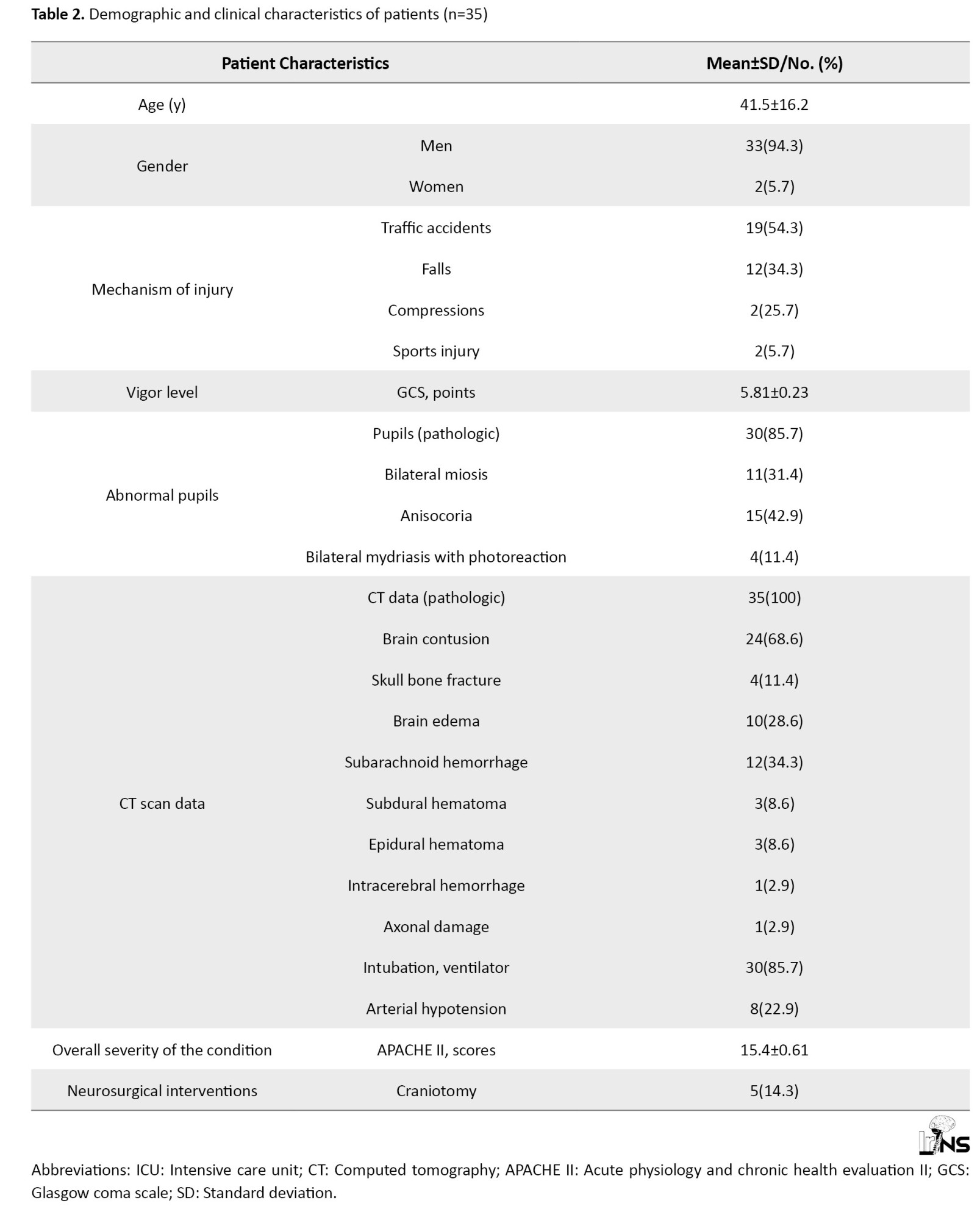
Consciousness disorder according to GCS averaged 5.81±0.23 (rang: 4-11). The severity of the condition according to APACHE II averaged 15.4±0.61 points.
Equal-sized pupils of medium size with good photoreaction were registered in 5 patients of this group, bilateral miosis, in 11 patients, anisocoria, in 15 patients, and 4 patients had bilateral mydriasis with photoreaction.
The results of the CT scan diagnosed were subarachnoid hemorrhage (n=12; 34.3%), cerebral edema (n=10; 28.6%), epidural hemorrhage (n=3; 8.6%), linear skull fracture in the occipital-parietal region without fragment separation (n=4; 11.4%), subdural hematoma (n=3; 8.6%), epidural hematoma (n=3; 8.6%) (Table 2).
Table 3 presents the clinical examination data in the patients of this group on admission to the clinic.
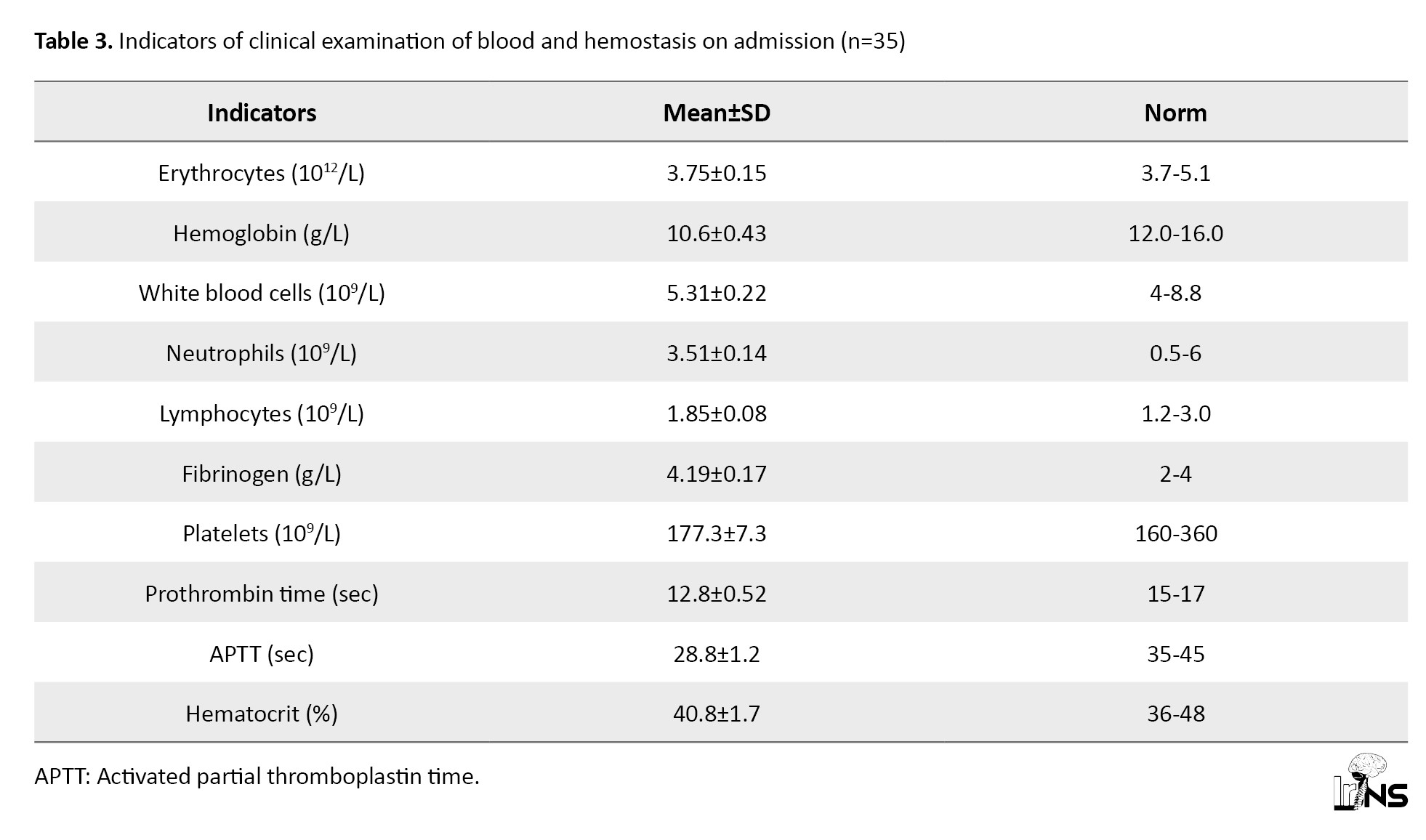
The presented data testify to moderate anemia of traumatic genesis and activity of the blood coagulation system, as evidenced by shortened prothrombin time by 8.6%, increased values of fibrinogen and decreased kephalin-kaolin time, APTT by 7.1% from the lower limit of physiological values of this index (Table 3).
The average values of the studied parameters indicated that in patients of this group at admission, moderate arterial hypotension was observed with a decrease in systolic and diastolic pressure, which affected decreased MAP. All this indicated a decrease in the tone of resistive vessels. However, TPR values were also lower than physiologic values, indicating a decrease in tone in the low-pressure system (capillaries, venules). The decrease in TPR was 9.2% of the proper values of TPR during this period (1511.1 dyne×s×cm-5) (Table 4).
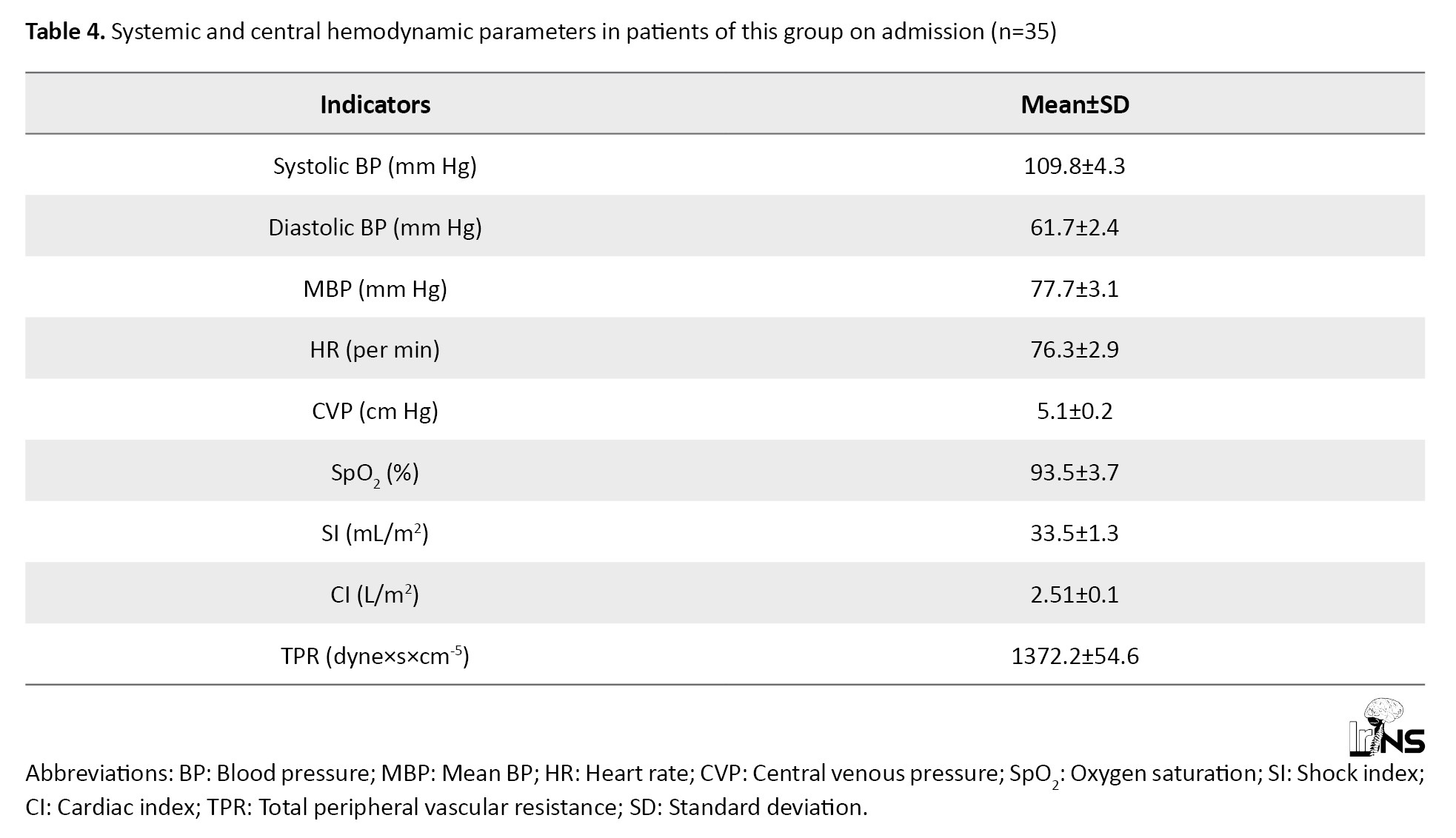
The proper values of MAP in this age group of the studied patients were 85 mm Hg. CVP was 36.25% lower than physiologic values. All these factors contributed to the decrease of single and minute cardiac output, which were at the borderline values of normo, and hypodynamic mode of blood circulation and indicated deterioration of cerebral circulation (Table 4).
Table 5 presents the initial values of blood electrolytes and plasma osmolarity.
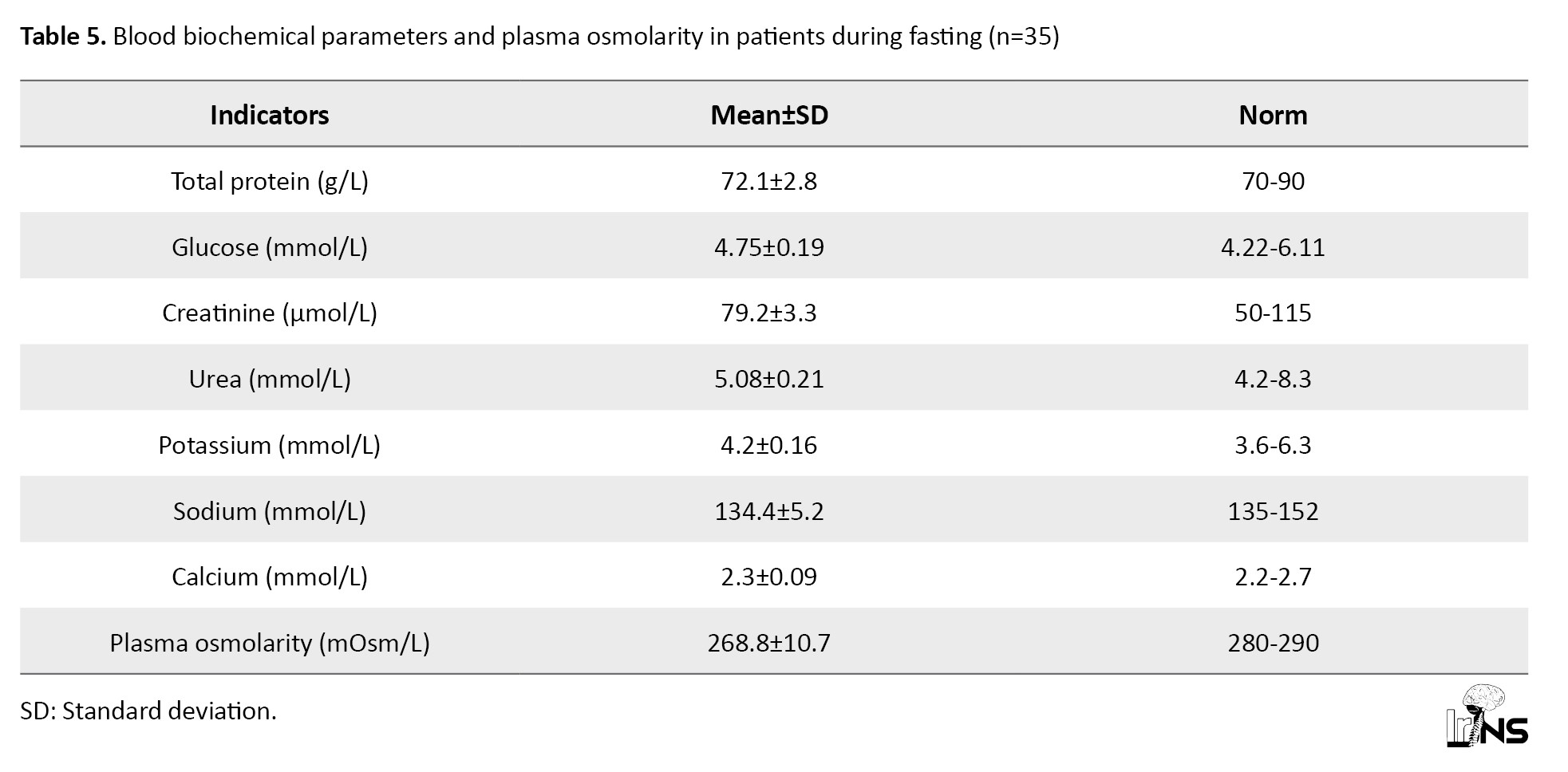
Analyzing the presented data, it can be noted that all the studied parameters practically did not exceed the physiological norm for adults. However, in this group of patients with insignificant hyponatremia (according to the mean values), a large difference is observed in the mean square deviation in the variation series, which made us study Na+ concentration in patients in more detail. Thus, in 7 patients from this group, the plasma sodium concentration exceeded 145 mmol and averaged 149.3±4.5 mmol/L, while in 28 patients, the Na+ level in plasma was below 135 mmol/L, averaging 129.5±7.2 mmol/L. Relative hyponatremia with normal blood glucose and urea values resulted in a 4.0% decrease in plasma osmolarity compared to the physiologic norm (Table 5).
The effect of combined solution of mannitol 15% + NaCl 3.5% on systemic and central Hemodynamics
The mean baseline HR, 76.3±2.9 per minute (range: 60-88 per minute), practically did not undergo clinically significant changes during the study stages. A tendency existed for HR increase (Table 6).
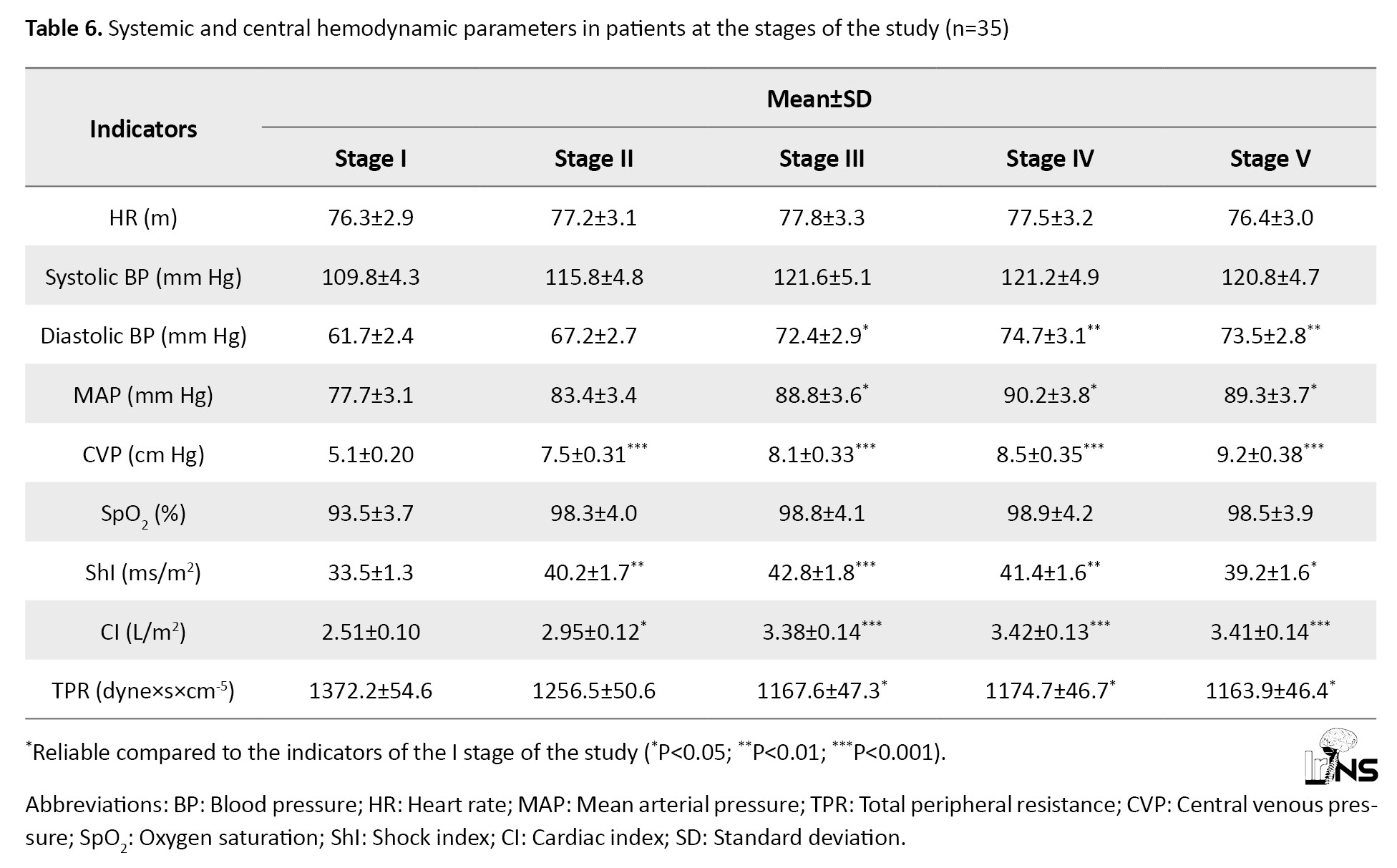
Its maximum values were observed in the third stage (15-30 minutes after administration of a bolus of mannitol 15% + NaCl 3.5%). BP increased almost due to both systolic (10.7%) and a more pronounced increase in diastolic (21.0%) components (Table 6).
Initial values of MAP, 77.7±3.1 mm Hg (range: 65-88 mm Hg), also showed a tendency to increase. It reached its maximum in the fourth stage of the study (60 minutes after administration of mannitol 15% + NaCl 3.5% bolus) and exceeded the initial data by 16.1% (P<0.05).
In 8 patients of this group (22.8%) on admission, the severity of arterial hypotension required inotropic support (pressors, hormones) for correction. The indices of pulse oximetry and CVP significantly improved, indicating improved blood gas composition (its oxygenation) and increased venous blood return to the heart, leading to increased cardiac output, both due to single heart output (by 5.1% in the second stage) and increased HR (by 1.2%). The maximum values of ShI and cardiac index (CI) were registered by us already in the third and fourth stages of the study, respectively, when they exceeded the initial values by 27.7% and 36.2%, respectively (Table 6).
The increase in cardiac performance was promoted by the decrease in TPR, which was traced at all stages of the study bolus mannitol 15% + NaCl 3.5%, although it was not statistically significant. The maximum decrease in TPR was observed already in the second stage (after HS administration). This value was 1256.5 dyne×s×cm-5, 8.5% lower than the initial data and 3.4% lower than the proper values of TPR in this period (1300.1 dyne×s×cm-5). All of these indicated improved peripheral blood circulation (Table 6).
The effect of combined mannitol 15% + NaCl 3.5% solution on ICP and cerebral perfusion pressure
Table 7 presents the dynamics of M-echopulsation, IСP, and CPP data during the study stages.
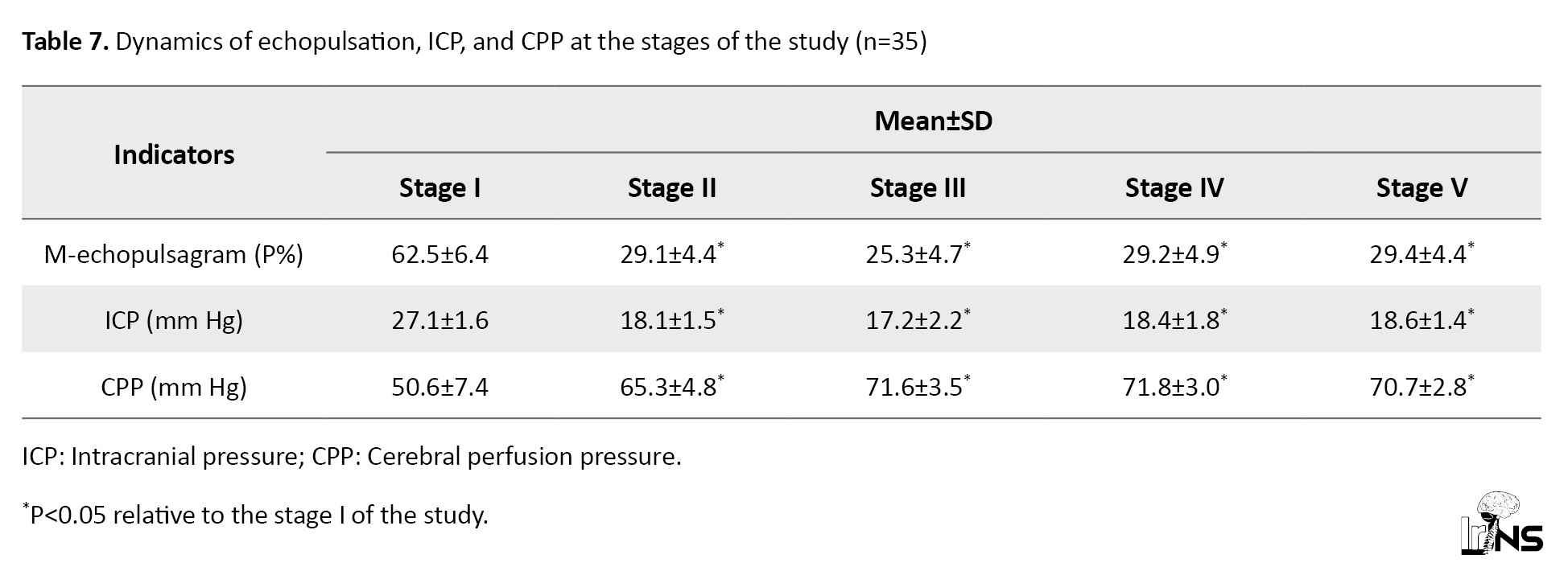
In all patients of this group on admission to the ICU, increased IСP was registered, the average value of which was 27.1±1.1 mm Hg. Average values of CPP were 50.6±1.9 mm Hg, which confirmed the deterioration of cerebral blood circulation (Table 7).
The presented data demonstrated the positive effect of mannitol 15%+NaCl 3.5% on ICP and cerebral blood flow. At 20-25 minutes after administration of a bolus of mannitol 15%+NaCl 3.5%, ICP decreased (stage 2) below 20 mm Hg, reaching a mean of 18.1±0.72 mm Hg, which was a 33.2% decrease from baseline (33.2%)(P<0.05). The maximum decrease in ICP was observed at the third stage of the study (after 30 minutes), which was 17.2±0.68 mm Hg, 36.5% (P<0.05) lower than baseline values. Currently, it showed a tendency to increase from 60 minutes after injection, and up to 120 minutes, however remaining below 20 mm Hg. Decreased ICP contributed to the increase in CPP. Therefore, in the second stage, 29.0% exceeded the initial values (P<0.05). The maximum values of CPP in this group were registered in the fourth stage (60 minutes after bolus administration), where it was 71.8 mm Hg, 41.9% higher than in the first stage of the study (Table 7).
The effect of mannitol 15% + NaCl 3.5% combined solution on blood osmolarity and hematocrit
Table 8 reflects the dynamics of electrolytes, plasma osmolarity in the stages of the study after administration of a bolus of Mannitol 15% + NaCl 3.5%.
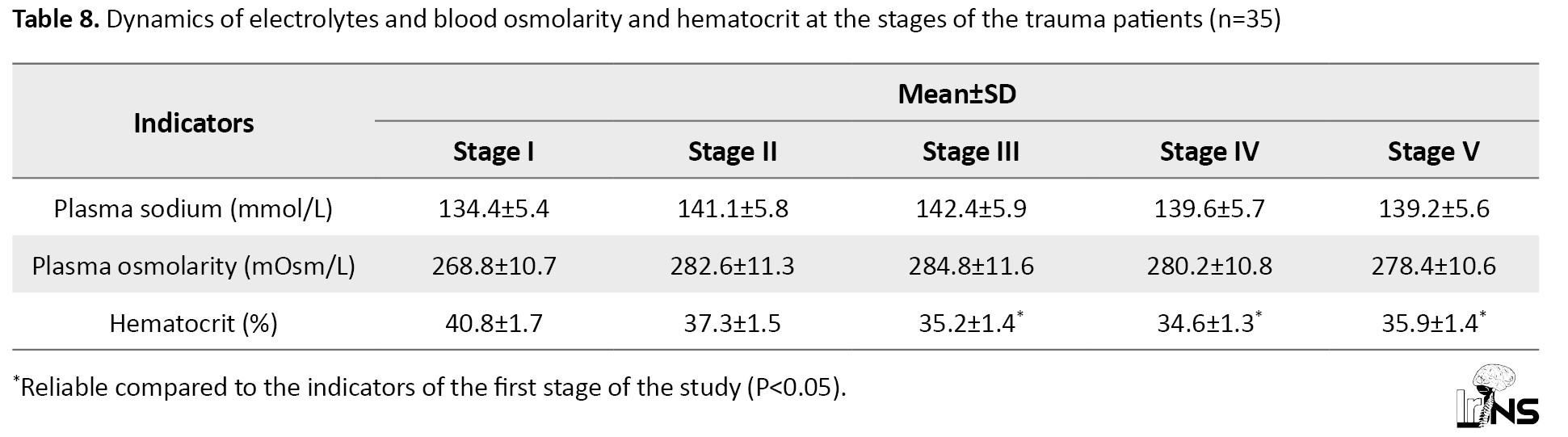
At 20-25 minutes after administration of a bolus of mannitol 15% + NaCl 3.5%, the values of sodium and plasma osmolarity (stage 2) increased by 4.9% and 5.1%, respectively (P>0.05). The maximum increase in blood sodium and osmolarity was observed at 30 minutes (stage 3), exceeding their initial values by 5.9% in both cases.
The average values of Na+ concentration and plasma osmolarity were observed. In 7 patients of this group, who had initial hypernatremia (149.3±4.5 mmol/L) after administration of bolus dose of mannitol 15% + NaCl 3.5%, increased plasma Na+ level in the second stage of the study was observed up to 157.2±2.3 mmol/L (by 5.3%) (Table 7). The osmodiuretic effect of mannitol was accompanied by an increase of hourly diuresis in them up to 110-120 mL/hour, while in the group as a whole diuresis increased up to 92-95 mL/hour. In no case in this group, we did not pay attention to the development of renal failure. On the contrary, renal compensation of hypernatremia was satisfactory. Diuresis was 100.2±10.8 mL/h.
In this group, we recorded 195 episodes of ICH, which forced the administration of another bolus of mannitol 15% + NaCl 3.5%. On average, 5 episodes (4-7) of ICH were observed per patient.
The mean interval between baseline (standard) therapy and the start of mannitol 15% + NaCl 3.5% infusion was 4.4±0.5 hours in this group. The dose of mannitol 15% + NaCl 3.5% was adjusted in each episode of ICH, starting at 5 mL/kg/hour and ending bolus infusion at ICP<20 mm Hg. This dose was 3.5±0.2 mL/kg for the whole group (Table 9).
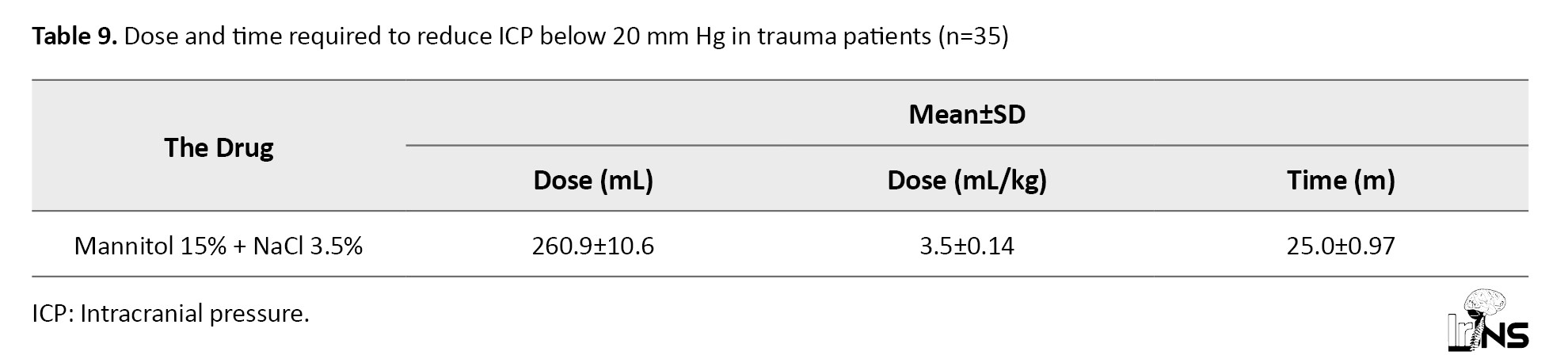
In 5 patients of this group, rather refractory ICH was observed, which was not relieved by 3.5% HSS in combination with 15% mannitol. These patients underwent neurosurgical interventions (decompression trepanation, clot evacuation).
The mean number of days of ICP measurement in this group was 5.4±0.2.
The mean number of days of patients’ stay in ICU in this group was 10.8±0.6, and in the clinic, it was 18.1±1.9 days.
The lethality in this group was 17.1%. Four deceased patients in this group had more serious primary trauma (falls from a height, blows to the head) with lower GCS scores (4-5) and higher ICP indices. In one patient in this group, the fatal outcome was associated with acute myocardial infarction. Another patient had abscessed pneumonia and sepsis.
4. Discussion
The combination of mannitol + 3.5% NaCl leads to significant and sustained improvement in systemic and cerebral blood flow. In the group, 284.8±11.6 mOsm/L with a slight decrease due to natriuresis under the effect of mannitol. However, both drugs complemented each other, and increased plasma osmolarity, leading to the movement of fluid from the cells and interstitial space into the vascular bed. The results indicated that the group of patients showed the most marked decrease in hematocrit due to hemodilution and optimal increase in CVP to maintain adequate hemodynamics.
According to Taha et al. [9], those who received combined therapy with mannitol and 3% NaCl had the lowest mortality rate, which may indicate more effective methods of treatment of increased ICP. In our study, the rate of IСP reduction in the second stage was 18.1±0.72 mm Hg. The combination of mannitol 15% + NaCl 3.5% rapidly reduced the IСP, and the duration of the decrease was maintained even by 120 minutes, by 18.6±0.76 mm Hg. The lethality in this group was 17.1%.
Narayan et al. [10] demonstrated that mannitol plus hypertonic saline did not increase the risk of renal dysfunction. In our study, we did not observe cases of ricochet syndrome and or acute renal failure in patients in the group.
Thus, the use of a combined solution of mannitol 15%+NaCl 3.5% by a more prolonged decrease in ICP revealed a persistent increase in MAP and CPP. These data showed that combined hyperosmolar solutions of mannitol 15% + NaCl 3.5% can be used in clinical practice.
5. Conclusion
Bolus infusion of a combined solution of 15% mannitol and 3.5% NaCl HSS at a rate of 3.5±0.2 mL/kg body weight leads to a rapid (20-25 minutes) and prolonged (>120 minutes) decrease in ICH (by 36.5% of baseline) and a significant (41.8%) increase in CPP.
Combined use of mannitol 15% + NaCl 3.5% can be recommended in the treatment of ICH in patients with isolated craniocerebral 3.5±0.2 mL/kg in patients with baseline hypovolemia and hyponatremia.
Ethical Considerations
Compliance with ethical guidelines
This study was carried out in accordance with the plan of research works of the Tashkent Medical Academy, Tashkent, Uzbekistan (State Registration No.: 011800230). The present study was approved by the Medical Ethics Committee of the Ministry of Health of the Republic of Uzbekistan (No. 1.12-1486). Informed consent was obtained from the relatives of all patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author express his gratitude for the support of the management of the Tashkent Medical Academy, the Department of Anesthesiology and Reanimation of the Tashkent Medical Academy, the management of the multidisciplinary clinic of the Tashkent Medical Academy, the staff of the drug supply department, the laboratory department and all the staff of the Surgical Reanimation Department N0. 1 and No. 2.
Echopulsation in the range of 15%-25% corresponded to the data of lumbar puncture with manometry in wide ranges from 4 to 19 mm Hg. The obtained comparative data allowed us to use the method of echopulsation to monitor ICH during its treatment with hyperosmolar solutions. ICP values exceeding 29 mm Hg correspond to >70% with asynchronous echopulsation of undulatory type.
In the studied patients with normal values of IAP, we determined the correlation coefficient of IAP, data obtained by noninvasive and invasive method (lumbar puncture) according to our proposed formula (Equation 1).
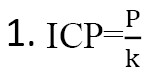
where, P-pulsogram in %, k=1.388 correction coefficient at normal pulsogram (percentage data of M-echo).
Thus, with the M-echopulsagram equal to 16.2% on average, we divide this value by the coefficient 1.388 and obtain the values of 11.7 mm Hg obtained by lumbar puncture.
Next, we determined the correction index for moderate ICH using the same formula and obtained a correction coefficient equal to k=1.677 for moderate ICH.
Thus, with M-echopulsagram equal to 37.2% on average, we divide this value by a coefficient of 1.677 and obtain values of 22.2 mm Hg obtained by lumbar puncture.
In the same way, we calculated the correction coefficient for pronounced ICH, and it amounted to k=2.339.
Thus, with M-echopulseagram equal to 64.3 on average, we divide this value by the coefficient of 2.339 and get the value of 27.5 mm Hg obtained by lumbar puncture.
Summarizing the above, we concluded that M-echopulsagram of a portable ultrasound device (Complesmed 1.2) can be used for non-invasive monitoring of ICH in trauma victims with traumatic brain injury in the process of complex therapy and to evaluate the effectiveness of the latter.
The correction indices calculated by us at normal values of IСH (k=1,388), moderate IСH (k=1,677), and pronounced IСP (k=2,339) obtained using Complexmed 1.2 when comparing them with the data of lumbar puncture correlate with Pearson correlation coefficients.
A patent for the invention “method for non-invasive assessment of ICP in patients with brain injury” (No. IAP 06573) was obtained. This method was developed to effectively treat diseases by preventing brain swelling through non-invasive assessment of ICP in patients with brain injury.
General care of patients in ICU (stage I of treatment)
We followed the recommendations of the Fond Brian Foundation (2016). All patients were sedated by continuous IV administration of propofol and opiates and underwent artificial ventilation. Patients were placed in a semi-reclined position (with the head end of the bed elevated 30-40 °C), in the absence of contraindications to this. Secondary brain damage was prevented by therapeutic craniocerebral hypothermia, covering the head and carotid vessels with cold elements with subsequent measurement of t0 in the external auditory canal, maintaining body temperature between 36-37 °C, ensuring normoglycemia, avoiding hypoxemia.
According to the protocol adopted in our clinic, basic therapy included infusion and transfusion therapy, lidocaine to close Na+ channels, nimotop (nimodipine) to block Ca2+ channels (the N-methyl-D-aspartate [NMDA] receptors), mild therapeutic craniocerebral hypothermia (4-5 °C cooling of brain structures), antioxidant therapy (α-lipoic acid, ascorbic acid, vit. E) to block reactive oxygen species, propofol, barbiturates to sedate and block transaminase activity, prophylaxis of infection, thrombotic complications and ulcerative complications and ulcerative formations in the gastrointestinal tract, early tube feeding of patients.
ICH was evaluated as susceptible (i.e. responsive to therapy, including osmotherapy, with ICP returning to <20 mm Hg) or refractory (stable ICP>20-25 mm Hg), requiring surgical decompression.
Neurologic status was assessed clinically using the Glasgow scale, and the severity of the patient’s condition and outcome were assessed using APACHE II.
A total of 35 patients were included in this study conducted over 5 years (2018-2022) in the Departments of Neurosurgery and Anesthesiology and Critical Care Medicine of Tashkent Medical Academy. All patients in this group received a mannitol infusion of 15% mannitol + NaCl 3.5% to reduce ICH. In this study, we investigated the effect of a combined solution of mannitol 15% + NaCl 3.5% on individual episodes of ICH, as well as on the time and duration of reduction of ICH peaks, the dose of these solutions reduces ICH <20 mm Hg.
Pre-hospital physiologic parameters were recorded, including post-resuscitation GCS, pupillary response to light, hemoglobin (Hb), hematocrit (Ht), and blood glucose levels. Daily data during 7 days after admission to the ICU included hourly measurements of ICP recorded noninvasively using Complexmed 1.2, and if possible by lumbar intrathecal puncture with manometry in 8 patients, serum sodium studies, pulse oximetry and measurement of daily diuresis, and plasma osmolarity. Crucial aspects of the care of trauma patients included neurosurgical operations performed when necessary (clot evacuation, decompression craniotomy), mannitol 15% + NaCl 3.5% osmotherapy, and ventilatory support. Outcome data included ICU and in-hospital mortality, length of stay in ICU, dose of combined mannitol 15% + NaCl 3.5% solution, and time required to reduce ICP<20 mm Hg.
We used descriptive statistics to examine the frequency and percentage of variables, such as gender, pupillary responses, CT findings, mechanism of injury, and level of consciousness in the patient groups, we studied as an indicator of the representativeness of these groups.
Methods of intensive therapy
All patients received standard complex intensive therapy according to international recommendations for the treatment of traumatic brain injury. The head end of the bed was raised by 30-40 °C. Ventilation by Wella and Drager apparatus with the respiratory volume of 8-10 mL/kg of ideal body weight in synchronized intermittent mandatory ventilation mode and positive end-expiratory pressure +2-10 cm of the water column. Infusion therapy was performed, combining colloid and crystalloid solutions. We tried to maintain normovolemia (central venous pressure [CVP] 8-12 cm of water column). Enteral tube feeding was started from the first day of the patient’s stay in the ICU at the rate of 20-25 kcal per kg of body weight per day after stabilization of vital parameters of the organism. Daily protein requirement was estimated according to the nitrogen balance calculation. If necessary, parenteral nutrition was added. To prevent infectious complications, all patients were treated with monotherapy with cephalosporins (ceftriaxone 2-4 g/day) or fluoroquinolones (ciprofloxacin 0.2-0.4 g/day) from the first day after surgery or in the presence of respiratory support. To prevent thrombosis of deep veins of the lower extremities (in the absence of signs of external and internal bleeding, low-molecular-weight heparin, clexane 0.4 thousand units per day subcutaneously was administered from 2-3 days). In patients who underwent ICP measurement, in case of clinical signs of dislocation syndrome (anisocoria, upward gaze paresis, Gerdwig-Majandi syndrome combined with bradycardia, arterial hypertension), CT of the brain was performed and the question of surgical intervention was decided. Blood plasma osmolarity was monitored. To control psychomotor agitation, we used medication sedation with a combination of narcotic analgesics and benzodiazepines. Hyperthermia was not allowed by us. At t>37.5 °C, antipyretics were administered and physical methods of cooling were used. In case of progressive worsening of the level of consciousness, despite conservative therapy, a CT scan of the brain was performed.
ICP was sought to be maintained within 15-20 mm Hg or less. Analgesia and sedatives were used during invasive procedures (tracheostomy, vascular catheterization) and when it was necessary to control the psychomotor agitation of the patient. Hyperosmolar solutions under the control of blood plasma osmolality were used to reduce elevated ICP. If blood plasma osmolality increased more than 320 mOsm/L, administration of hyperosmolar preparations was stopped. In the presence of persistent ICH difficult to correct by conservative methods of therapy (ICP more than 20 mm Hg for 6-12 hours), decompressive cranial trepanation was performed.
Methods of statistical analysis
Statistical processing of the obtained data was performed on a personal computer using the JASP program package. Statistical processing of the material provided for obtaining combination tables, graphs, and analytical indicators included structure, mean values and their standard errors, and student’s criterion with calculation of the probability of error. Differences in mean values were considered reliable at a significance level of P<0.05.
ICP and M-echopulsagrams were checked with Pearson’s correlation coefficient, and their reliability was checked with the student’s t-test.
3. Results
When the ICP exceeded 20 mm Hg for more than 5 minutes (two to three times measured by ultrasound (US) M-echopulsation of the cerebral III ventricle), bolus mannitol 15% + NaCl 3.5% was administered via the central vein at a rate of 6-8 mL/min (120-130 cap/min). The infusion was stopped when the ICP dropped below 20 mm Hg. We recorded the values of ICP, and CPP before and after infusion of mannitol 15% + NaCl 3.5%. We recorded ICP and CPP 15, 30, 60, and 120 minutes after drip infusion of combined mannitol 15% + NaCl 3.5% solution. The episodes of ICH requiring administration of hyperosmolar solutions per patient, the number of ICH episodes per day, and the dose of each infusion of mannitol 15% + NaCl 3.5% were recorded by us.
The age of patients in this group ranged from 20-82 years old (Mean±SD 41.5±1.7 years). The trauma was associated with road traffic accidents (n=19; 54.3%), falls (n=12; 34.3%) compression (n=2; 5.7%), and 2 patients (5.7%) had sports trauma with a bleeding wound in the frontal-temporal part of the head without damage to the skull bones. Vomiting of gastric contents occurred in 16 patients, three of whom were diagnosed with aspiration syndrome, for which sanitation bronchoscopy with lavage of airways was performed (Table 2).

Consciousness disorder according to GCS averaged 5.81±0.23 (rang: 4-11). The severity of the condition according to APACHE II averaged 15.4±0.61 points.
Equal-sized pupils of medium size with good photoreaction were registered in 5 patients of this group, bilateral miosis, in 11 patients, anisocoria, in 15 patients, and 4 patients had bilateral mydriasis with photoreaction.
The results of the CT scan diagnosed were subarachnoid hemorrhage (n=12; 34.3%), cerebral edema (n=10; 28.6%), epidural hemorrhage (n=3; 8.6%), linear skull fracture in the occipital-parietal region without fragment separation (n=4; 11.4%), subdural hematoma (n=3; 8.6%), epidural hematoma (n=3; 8.6%) (Table 2).
Table 3 presents the clinical examination data in the patients of this group on admission to the clinic.

The presented data testify to moderate anemia of traumatic genesis and activity of the blood coagulation system, as evidenced by shortened prothrombin time by 8.6%, increased values of fibrinogen and decreased kephalin-kaolin time, APTT by 7.1% from the lower limit of physiological values of this index (Table 3).
The average values of the studied parameters indicated that in patients of this group at admission, moderate arterial hypotension was observed with a decrease in systolic and diastolic pressure, which affected decreased MAP. All this indicated a decrease in the tone of resistive vessels. However, TPR values were also lower than physiologic values, indicating a decrease in tone in the low-pressure system (capillaries, venules). The decrease in TPR was 9.2% of the proper values of TPR during this period (1511.1 dyne×s×cm-5) (Table 4).

The proper values of MAP in this age group of the studied patients were 85 mm Hg. CVP was 36.25% lower than physiologic values. All these factors contributed to the decrease of single and minute cardiac output, which were at the borderline values of normo, and hypodynamic mode of blood circulation and indicated deterioration of cerebral circulation (Table 4).
Table 5 presents the initial values of blood electrolytes and plasma osmolarity.

Analyzing the presented data, it can be noted that all the studied parameters practically did not exceed the physiological norm for adults. However, in this group of patients with insignificant hyponatremia (according to the mean values), a large difference is observed in the mean square deviation in the variation series, which made us study Na+ concentration in patients in more detail. Thus, in 7 patients from this group, the plasma sodium concentration exceeded 145 mmol and averaged 149.3±4.5 mmol/L, while in 28 patients, the Na+ level in plasma was below 135 mmol/L, averaging 129.5±7.2 mmol/L. Relative hyponatremia with normal blood glucose and urea values resulted in a 4.0% decrease in plasma osmolarity compared to the physiologic norm (Table 5).
The effect of combined solution of mannitol 15% + NaCl 3.5% on systemic and central Hemodynamics
The mean baseline HR, 76.3±2.9 per minute (range: 60-88 per minute), practically did not undergo clinically significant changes during the study stages. A tendency existed for HR increase (Table 6).

Its maximum values were observed in the third stage (15-30 minutes after administration of a bolus of mannitol 15% + NaCl 3.5%). BP increased almost due to both systolic (10.7%) and a more pronounced increase in diastolic (21.0%) components (Table 6).
Initial values of MAP, 77.7±3.1 mm Hg (range: 65-88 mm Hg), also showed a tendency to increase. It reached its maximum in the fourth stage of the study (60 minutes after administration of mannitol 15% + NaCl 3.5% bolus) and exceeded the initial data by 16.1% (P<0.05).
In 8 patients of this group (22.8%) on admission, the severity of arterial hypotension required inotropic support (pressors, hormones) for correction. The indices of pulse oximetry and CVP significantly improved, indicating improved blood gas composition (its oxygenation) and increased venous blood return to the heart, leading to increased cardiac output, both due to single heart output (by 5.1% in the second stage) and increased HR (by 1.2%). The maximum values of ShI and cardiac index (CI) were registered by us already in the third and fourth stages of the study, respectively, when they exceeded the initial values by 27.7% and 36.2%, respectively (Table 6).
The increase in cardiac performance was promoted by the decrease in TPR, which was traced at all stages of the study bolus mannitol 15% + NaCl 3.5%, although it was not statistically significant. The maximum decrease in TPR was observed already in the second stage (after HS administration). This value was 1256.5 dyne×s×cm-5, 8.5% lower than the initial data and 3.4% lower than the proper values of TPR in this period (1300.1 dyne×s×cm-5). All of these indicated improved peripheral blood circulation (Table 6).
The effect of combined mannitol 15% + NaCl 3.5% solution on ICP and cerebral perfusion pressure
Table 7 presents the dynamics of M-echopulsation, IСP, and CPP data during the study stages.

In all patients of this group on admission to the ICU, increased IСP was registered, the average value of which was 27.1±1.1 mm Hg. Average values of CPP were 50.6±1.9 mm Hg, which confirmed the deterioration of cerebral blood circulation (Table 7).
The presented data demonstrated the positive effect of mannitol 15%+NaCl 3.5% on ICP and cerebral blood flow. At 20-25 minutes after administration of a bolus of mannitol 15%+NaCl 3.5%, ICP decreased (stage 2) below 20 mm Hg, reaching a mean of 18.1±0.72 mm Hg, which was a 33.2% decrease from baseline (33.2%)(P<0.05). The maximum decrease in ICP was observed at the third stage of the study (after 30 minutes), which was 17.2±0.68 mm Hg, 36.5% (P<0.05) lower than baseline values. Currently, it showed a tendency to increase from 60 minutes after injection, and up to 120 minutes, however remaining below 20 mm Hg. Decreased ICP contributed to the increase in CPP. Therefore, in the second stage, 29.0% exceeded the initial values (P<0.05). The maximum values of CPP in this group were registered in the fourth stage (60 minutes after bolus administration), where it was 71.8 mm Hg, 41.9% higher than in the first stage of the study (Table 7).
The effect of mannitol 15% + NaCl 3.5% combined solution on blood osmolarity and hematocrit
Table 8 reflects the dynamics of electrolytes, plasma osmolarity in the stages of the study after administration of a bolus of Mannitol 15% + NaCl 3.5%.

At 20-25 minutes after administration of a bolus of mannitol 15% + NaCl 3.5%, the values of sodium and plasma osmolarity (stage 2) increased by 4.9% and 5.1%, respectively (P>0.05). The maximum increase in blood sodium and osmolarity was observed at 30 minutes (stage 3), exceeding their initial values by 5.9% in both cases.
The average values of Na+ concentration and plasma osmolarity were observed. In 7 patients of this group, who had initial hypernatremia (149.3±4.5 mmol/L) after administration of bolus dose of mannitol 15% + NaCl 3.5%, increased plasma Na+ level in the second stage of the study was observed up to 157.2±2.3 mmol/L (by 5.3%) (Table 7). The osmodiuretic effect of mannitol was accompanied by an increase of hourly diuresis in them up to 110-120 mL/hour, while in the group as a whole diuresis increased up to 92-95 mL/hour. In no case in this group, we did not pay attention to the development of renal failure. On the contrary, renal compensation of hypernatremia was satisfactory. Diuresis was 100.2±10.8 mL/h.
In this group, we recorded 195 episodes of ICH, which forced the administration of another bolus of mannitol 15% + NaCl 3.5%. On average, 5 episodes (4-7) of ICH were observed per patient.
The mean interval between baseline (standard) therapy and the start of mannitol 15% + NaCl 3.5% infusion was 4.4±0.5 hours in this group. The dose of mannitol 15% + NaCl 3.5% was adjusted in each episode of ICH, starting at 5 mL/kg/hour and ending bolus infusion at ICP<20 mm Hg. This dose was 3.5±0.2 mL/kg for the whole group (Table 9).

In 5 patients of this group, rather refractory ICH was observed, which was not relieved by 3.5% HSS in combination with 15% mannitol. These patients underwent neurosurgical interventions (decompression trepanation, clot evacuation).
The mean number of days of ICP measurement in this group was 5.4±0.2.
The mean number of days of patients’ stay in ICU in this group was 10.8±0.6, and in the clinic, it was 18.1±1.9 days.
The lethality in this group was 17.1%. Four deceased patients in this group had more serious primary trauma (falls from a height, blows to the head) with lower GCS scores (4-5) and higher ICP indices. In one patient in this group, the fatal outcome was associated with acute myocardial infarction. Another patient had abscessed pneumonia and sepsis.
4. Discussion
The combination of mannitol + 3.5% NaCl leads to significant and sustained improvement in systemic and cerebral blood flow. In the group, 284.8±11.6 mOsm/L with a slight decrease due to natriuresis under the effect of mannitol. However, both drugs complemented each other, and increased plasma osmolarity, leading to the movement of fluid from the cells and interstitial space into the vascular bed. The results indicated that the group of patients showed the most marked decrease in hematocrit due to hemodilution and optimal increase in CVP to maintain adequate hemodynamics.
According to Taha et al. [9], those who received combined therapy with mannitol and 3% NaCl had the lowest mortality rate, which may indicate more effective methods of treatment of increased ICP. In our study, the rate of IСP reduction in the second stage was 18.1±0.72 mm Hg. The combination of mannitol 15% + NaCl 3.5% rapidly reduced the IСP, and the duration of the decrease was maintained even by 120 minutes, by 18.6±0.76 mm Hg. The lethality in this group was 17.1%.
Narayan et al. [10] demonstrated that mannitol plus hypertonic saline did not increase the risk of renal dysfunction. In our study, we did not observe cases of ricochet syndrome and or acute renal failure in patients in the group.
Thus, the use of a combined solution of mannitol 15%+NaCl 3.5% by a more prolonged decrease in ICP revealed a persistent increase in MAP and CPP. These data showed that combined hyperosmolar solutions of mannitol 15% + NaCl 3.5% can be used in clinical practice.
5. Conclusion
Bolus infusion of a combined solution of 15% mannitol and 3.5% NaCl HSS at a rate of 3.5±0.2 mL/kg body weight leads to a rapid (20-25 minutes) and prolonged (>120 minutes) decrease in ICH (by 36.5% of baseline) and a significant (41.8%) increase in CPP.
Combined use of mannitol 15% + NaCl 3.5% can be recommended in the treatment of ICH in patients with isolated craniocerebral 3.5±0.2 mL/kg in patients with baseline hypovolemia and hyponatremia.
Ethical Considerations
Compliance with ethical guidelines
This study was carried out in accordance with the plan of research works of the Tashkent Medical Academy, Tashkent, Uzbekistan (State Registration No.: 011800230). The present study was approved by the Medical Ethics Committee of the Ministry of Health of the Republic of Uzbekistan (No. 1.12-1486). Informed consent was obtained from the relatives of all patients.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgements
The author express his gratitude for the support of the management of the Tashkent Medical Academy, the Department of Anesthesiology and Reanimation of the Tashkent Medical Academy, the management of the multidisciplinary clinic of the Tashkent Medical Academy, the staff of the drug supply department, the laboratory department and all the staff of the Surgical Reanimation Department N0. 1 and No. 2.
References
- Alnemari AM, Krafcik BM, Mansour TR, Gaudin D. A comparison of pharmacologic therapeutic agents used for the reduction of intracranial pressure after traumatic brain injury. World Neurosurgery. 2017; 106:509-28. [DOI:10.1016/j.wneu.2017.07.009] [PMID]
- Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P, et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Medicine. 2018; 44(4):449-63. [DOI:10.1007/s00134-018-5086-z] [PMID]
- Wang H, Cao H, Zhang X, Ge L, Bie L. The effect of hypertonic saline and mannitol on coagulation in moderate traumatic brain injury patients. The American Journal of Emergency Medicine. 2017; 35(10):1404-7. [DOI:10.1016/j.ajem.2017.04.020] [PMID]
- Brown CS, Rabinstein AA, Zhao Y, Wieruszewski ED. Safety of peripheral 3% hypertonic saline bolus administration for neurologic emergency. The American Journal of Emergency Medicine. 2023; 69:83-6. [DOI:10.1016/j.ajem.2023.04.007] [PMID]
- Boone MD, Oren-Grinberg A, Robinson TM, Chen CC, Kasper EM. Mannitol or hypertonic saline in the setting of traumatic brain injury: What have we learned? Surgical Neurology International. 2015; 6:177. [DOI:10.4103/2152-7806.170248] [PMID] [PMCID]
- Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Critical Care Medicine. 2008; 36(3):795-800. [DOI:10.1097/CCM.0B013E3181643B41] [PMID]
- Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: A meta-analysis of randomized clinical trials. Critical Care Medicine. 2011; 39(3):554-9. [DOI:10.1097/CCM.0b013e318206b9be] [PMID]
- Mangat HS, Wu X, Gerber LM, Schwarz JT, Fakhar M, Murthy SB, et al. Hypertonic saline is superior to mannitol for the combined effect on intracranial pressure and cerebral perfusion pressure burdens in patients with severe traumatic brain injury. Neurosurgery. 2020; 86(2):221-30. [DOI:10.1093/neuros/nyz046] [PMID] [PMCID]
- Taha AA, Westlake C, Badr L, Mathur M. Mannitol versus 3% NaCl for management of severe pediatric traumatic brain injury. The Journal for Nurse Practitioners. 2015; 11(5):505-10. [DOI:10.1016/j.nurpra.2015.03.006]
- Narayan SW, Castelino R, Hammond N, Patanwala AE. Effect of mannitol plus hypertonic saline combination versus hypertonic saline monotherapy on acute kidney injury after traumatic brain injury. Journal of Critical Care. 2020; 57:220-4. [DOI:10.1016/j.jcrc.2020.03.006] [PMID]
Type of Study: Research |
Subject:
Neurotrauma
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |