Fri, Aug 1, 2025
Volume 11 - Continuous Publishing
Iran J Neurosurg 2025, 11 - Continuous Publishing: 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shafizad M, Ehteshami S, Sobhanian P, Hosseinzade M. Artificial Intelligence for Improved Diagnosis of Spinal
Stenosis: Implications for Neurosurgical Practice. Iran J Neurosurg 2025; 11 : 5
URL: http://irjns.org/article-1-461-en.html
URL: http://irjns.org/article-1-461-en.html
1- Department of Neurosurgery, Orthopedic Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Neurosurgery, Orthopedic Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. ,s.ehteshami@mazums.ac.ir
3- Student Research Committee, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Neurosurgery, Orthopedic Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. ,
3- Student Research Committee, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Keywords: Artificial intelligence (AI), Spinal stenosis, Magnetic
resonance imaging (MRI), Cervical spine, Lumbar
vertebrae
Full Text [PDF 1076 kb]
(624 Downloads)
| Abstract (HTML) (867 Views)
Full Text: (657 Views)
1. Introduction
Spinal stenosis is a common disease in the elderly, and its incidence has gradually increased in an aging society [1]. Lumbar and cervical canal stenoses are conditions characterized by spinal canal narrowing, leading to spinal cord and nerve root compression [1-3]. The pathophysiology of these conditions involves degenerative changes in the spine, such as disc herniation, ligamentum flavum hypertrophy, and osteophyte formation. These degenerative processes can lead to significant neurological deficits, as the narrowing of the canal restricts space for the spinal cord and nerves [4, 5]. Degenerative lumbar spinal stenosis is a progressive disease involving all spinal movement segments [6].
Nerve root compression may result from direct mechanical compression or indirectly from increased intrathecal pressure, which rises as the canal area is reduced [7]. Increased intrathecal pressure can cause venous congestion, reduced arterial blood flow, and reduced impulse conduction along nerve roots [8-10]. Evidence suggests that multilevel stenosis is necessary for this process. Spondylosis, or degenerative arthritis affecting the spine, is the most common cause of lumbar spine stenosis (LSS) and typically affects individuals over the age of 60 years [11]. Obesity and family history may also be risk factors [12, 13]. In cervical stenosis, compression can result in neck pain, radiculopathy, and myelopathy, which may manifest as weakness, numbness, or coordination difficulties [14]. Conversely, lumbar stenosis often presents with lower back pain and neurogenic claudication, wherein patients experience leg pain or weakness during prolonged standing or walking [15, 16].
Magnetic resonance imaging (MRI) is crucial in diagnosing spinal stenosis because it provides detailed images of both soft tissue structures and bone anatomy. MRI is particularly effective in visualizing the spinal cord, nerve roots, intervertebral discs, and surrounding ligaments [17, 18]. It allows clinicians to assess the degree of canal narrowing and identify potential causes of compression advanced MRI techniques, such as T2-weighted imaging and diffusion-weighted imaging, enhance the visualization of spinal structures and can help differentiate between various causes of stenosis [19-22]. These imaging methods are essential for understanding the dynamic nature of spinal canal stenosis and its impact on neurological function [23-25].
Artificial intelligence (AI) is increasingly integrated into MRI analysis to enhance diagnostic accuracy [26, 27]. AI algorithms, particularly deep-learning (DL) models, such as convolutional neural networks (CNNs), can analyze large datasets of MRI images to identify patterns associated with stenosis [28, 29]. These models can be trained on pre-labeled images to recognize features indicative of varying degrees of stenosis. Techniques, such as data augmentation, help improve model robustness by increasing the diversity of training data [30, 31]. This narrative review aims to provide a comprehensive analysis of the latest findings and published articles on the application of AI in enhancing the diagnostic accuracy of lumbar canal stenosis. It evaluates various AI technologies, particularly DL models, to assess their effectiveness in analyzing medical imaging data and discusses the clinical implications for treatment planning and patient outcomes in neurosurgery.
2. Methods and Materials/Patients
A literature search was conducted using Google Scholar, MEDLINE, and PubMed to identify original studies on the application of AI in diagnosing lumbar and cervical spinal stenosis. The search utilized keywords, such as “deep learning”, “artificial intelligence”, “machine learning”, “neural network”, “lumbar spinal stenosis”, “cervical spine”, “canal stenosis”, and “MRI”. The inclusion criteria focused on original research articles published in English that addressed AI’s role in diagnosing spinal stenosis while excluding reviews, case reports, and studies primarily focused on predicting surgical outcomes. Ultimately, 14 studies were included after thorough screening of titles, abstracts, and full texts. Data extraction was performed using a pre-designed Excel spreadsheet, with relevant information systematically recorded and validated by two neurosurgeons for accuracy. The results were analyzed through thematic analysis and categorization to identify trends in AI applications to enhance diagnostic accuracy. Emphasis was placed on including high-quality articles from reputable journals to ensure clarity in methodologies and results.
3. Results
Current diagnostic methods
Diagnosing spinal canal stenosis, particularly in the lumbar and cervical regions, begins with a thorough patient history and comprehensive physical examination [32]. Clinicians must carefully evaluate the diverse range of symptoms presented by the patient. Common presentations include pain, numbness, and weakness in the extremities, often accompanied by specific signs, such as neurogenic claudication in lumbar stenosis or myelopathy in cervical stenosis [33]. Clinical tests, including the straight leg raise and Hoffmann’s tests, can help identify nerve root involvement or spinal cord disorder [34, 35]. Following clinical evaluation, imaging studies are essential to confirm the diagnosis [36]. X-ray imaging is an essential and cost-effective initial diagnostic tool for evaluating cervical and lumbar compression [37]. It provides critical insights into spinal abnormalities, particularly in suspected stenosis cases. For instance, a cervical canal diameter of less than 13 mm on a lateral x-ray view indicates cervical stenosis [38]. Hartman et al. emphasized that X-rays and dynamic imaging techniques are crucial for assessing lumbar deformities and measuring spinal canal dimensions [39]. Studies have indicated that computed tomography (CT) is a standard diagnostic tool for cervical and lumbar canal stenosis [40, 41]. CT imaging precisely measures the sagittal and transverse dimensions of the lumbar spinal canal [42]. In a study conducted by Stafira et al., the evaluation of CT myelogram images for diagnosing cervical canal stenosis revealed that the inter-observer agreement in determining the level, degree, and cause of stenosis had κ values of 0.50, 0.26, and 0.32, respectively [43]. These results indicate significant variations in the interpretation of CT images, which may impact clinical decision-making.
MRI is the gold standard for diagnosing cervical and lumbar spinal canal stenosis due to its superior ability to visualize soft tissue structures, including the spinal cord and nerve roots [44, 45]. Various MRI techniques, such as T1-weighted imaging, allow clinicians to assess compression by comparing T1 relaxation times in compressed spinal cord regions with non-compressed areas, revealing significant differences that aid diagnosis [46]. T2-weighted imaging is crucial for highlighting edema and soft tissue involvement around the spinal canal, while short tau inversion recovery enhances the detection of edema in soft tissues [47]. Advanced techniques, such as dynamic and kinetic MRI, further improve diagnostic capabilities. Dynamic MRI captures images while the patient flexes or extends their spine, revealing how these movements may exacerbate stenosis [48]. Kinetic MRI provides real-time assessment of spinal motion, offering insights into stability or instability that may contribute to symptoms [49]. Several grading systems have been developed to evaluate the severity of stenosis based on MRI findings. For instance, Park et al. introduced a novel grading system using oblique sagittal T2-weighted sequences that classifies neural foraminal narrowing into four distinct grades, demonstrating high interobserver reliability with kappa values ranging from 0.71 to 0.99 [50]. The Schizas grading system evaluates the ratio of nerve rootlets to cerebrospinal fluid and the morphology of the dural sac in T2 axial images [51]. In contrast, the Lee grading system categorizes central canal and foraminal stenosis based on pathophysiological changes and radiological findings, facilitating communication among specialists [44]. Additionally, the Kang grading system assesses stenosis severity by measuring the percentage reduction in the diameter of the spinal canal and foraminal spaces [52]. An updated six-point grading system for lumbar foraminal stenosis based on Lee’s classification was proposed in 2021, offering a more precise description of stenosis using high-resolution MRI [53]. Despite these advancements, challenges in diagnosing spinal canal stenosis through radiology, including a lack of consensus on criteria selection, inconsistencies in their application by specialists, and poor clinical-morphological correlations that complicate interpretation and communication among professionals [54].
In summary, a comprehensive approach that combines thorough patient history, physical examination, and enhanced imaging techniques are necessary for accurately diagnosing spinal canal stenosis.
AI applications in diagnosing lumbar stenosis
AI is increasingly being recognized as a transformative tool for diagnosing LSS [55, 56]. Despite their relative accuracy, traditional imaging methods often encounter challenges, such as misinterpretation of images and reliance on the experience of physicians [57]. Over the past decade, numerous AI algorithms based on radiographs, CT, and MRI have been developed, significantly improving diagnostic accuracy and facilitating imaging processes [58]. Radiomics, an advanced approach in medical imaging, involves the extraction of quantitative features from medical images and utilizes AI to identify disease characteristics that are not visible to the naked eye, thereby providing valuable information for diagnosis and predicting treatment outcomes [59]. Recent findings have highlighted that some AI architectures achieve performance levels comparable to human experts in assessing LSS [55, 56].
AI has numerous applications, such as machine learning (ML), natural language processing (NLP), and DL [60]. ML significantly enhances the diagnosis of LSS by creating previously unavailable quantitative imaging biomarkers. A recent study in the USA has trained ML algorithms to accurately segment lumbar spinal canal areas from axial and sagittal MRI scans at each lumbar level (L1-L5). The results showed that machine-generated delineations closely matched those created by human raters, confirming the reliability of these techniques [61]. Notably, ML techniques can automate the diagnosis of LSS based on self-reported questionnaires with remarkable accuracy [62]. As a branch of AI, NLP is the automated extraction of structured data from unstructured free text data. NLP-based ML algorithms are emerging as effective tools for diagnosing spinal diseases, particularly lumbar spinal stenoses. Ren et al. demonstrated that the DL model long short-term memory (LSTM) outperformed the ensemble model XGBoost in differentiating between disc herniation and lumbar spinal stenosis using positive symptoms [63]. These results highlight the significant potential of NLP to enhance the accuracy of pre-diagnosis in spinal conditions and can serve as a foundation for future research in this area. Moreover, DL-based methods have demonstrated state-of-the-art performance in various image analysis tasks related to spinal stenosis grading [29]. A CNN is a typical model used in DL applications to extract image features. It operates as an end-to-end network model that takes input images and category labels, enabling automatic hierarchical learning of image characteristics and deeper feature extraction through increased network layers [64]. Research indicates that CNNs have been widely employed in AI approaches for diagnosing LSS, with studies utilizing various architectures, such as ResNet, RegNet, and EfficientNet-B1 (Table 1).
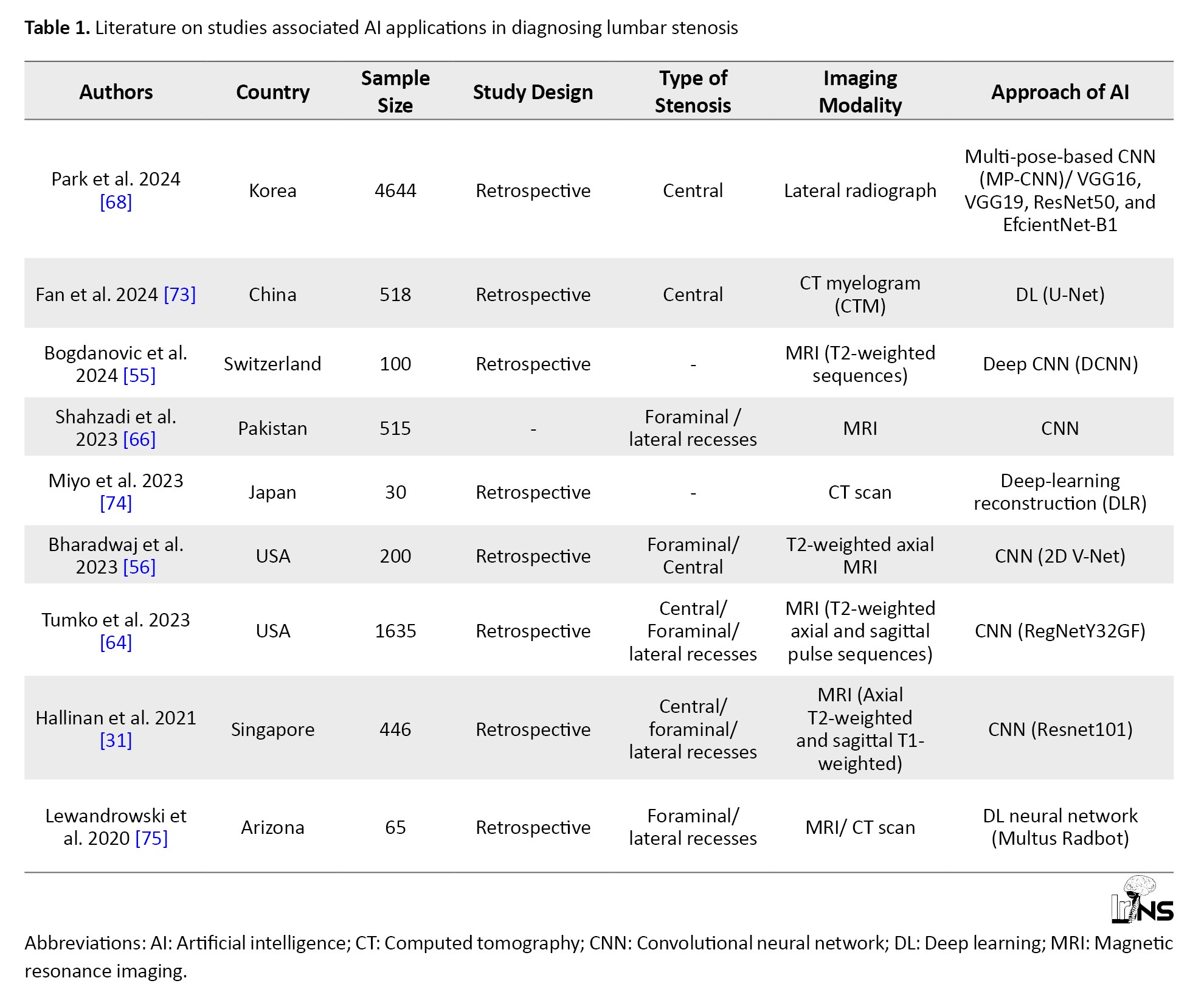
Recent advancements in CNN have evolved from one-component models for binary classification(absence/present) of LSS to more complex multi-component approaches [31]. Tumko et al. introduced a three-stage CNN that segments anatomical structures, classifies LSS presence, and assesses its severity [65]. Additionally, custom CNN models can detect and classify different types of LSS, including central canal, lateral recess, and foraminal stenoses [31, 66]. In two consecutive studies, Park et al. demonstrated that DL-based models, such as single-pose-CNN and multi-pose-CNN, showed high diagnostic accuracy when predicting LSS using lumbar radiographs [67, 68]. Although most DL studies have focused on MRI data, recent developments have also seen algorithms created for diagnosing lumbar central canal stenosis using abdominal and lumbar spine CT scans [69]. Furthermore, Suzuki et al. demonstrated that AI can automatically detect lumbar spinal canal stenosis from plain radiographs, facilitating diagnosis in medical facilities lacking MRI capabilities or specialists [70].
The potential applications of AI extend beyond diagnosis; they include automating the evaluation of surgical candidacy for LSS with performance comparable to a multidisciplinary panel of physicians [71]. Moreover, there is potential for integrating tools, such as ChatGPT, into clinical settings to support decision-making processes for LSS diagnosis and treatment [72].
AI applications in diagnosing cervical stenosis
The advent of AI has significantly reshaped the diagnostic landscape for cervical stenosis, a condition marked by the narrowing of the spinal canal that can lead to severe complications, such as spinal cord compression and neurological deficits. Traditional imaging modalities, including MRI and CT, have long been the cornerstone for diagnosing this condition. However, these methods are fraught with limitations that can hinder effective patient management [76-79]. Variability in interpretation among radiologists can lead to inconsistent diagnoses, which may result in delayed treatment and adverse outcomes [57, 80, 81]. Furthermore, the inherently time-consuming nature of these imaging techniques can prolong the diagnostic process, thereby delaying critical intervention [82]. In light of these challenges, there is a growing interest in leveraging AI technologies, particularly DL algorithms, to enhance diagnostic accuracy and efficiency in detecting cervical stenosis (Table 2).
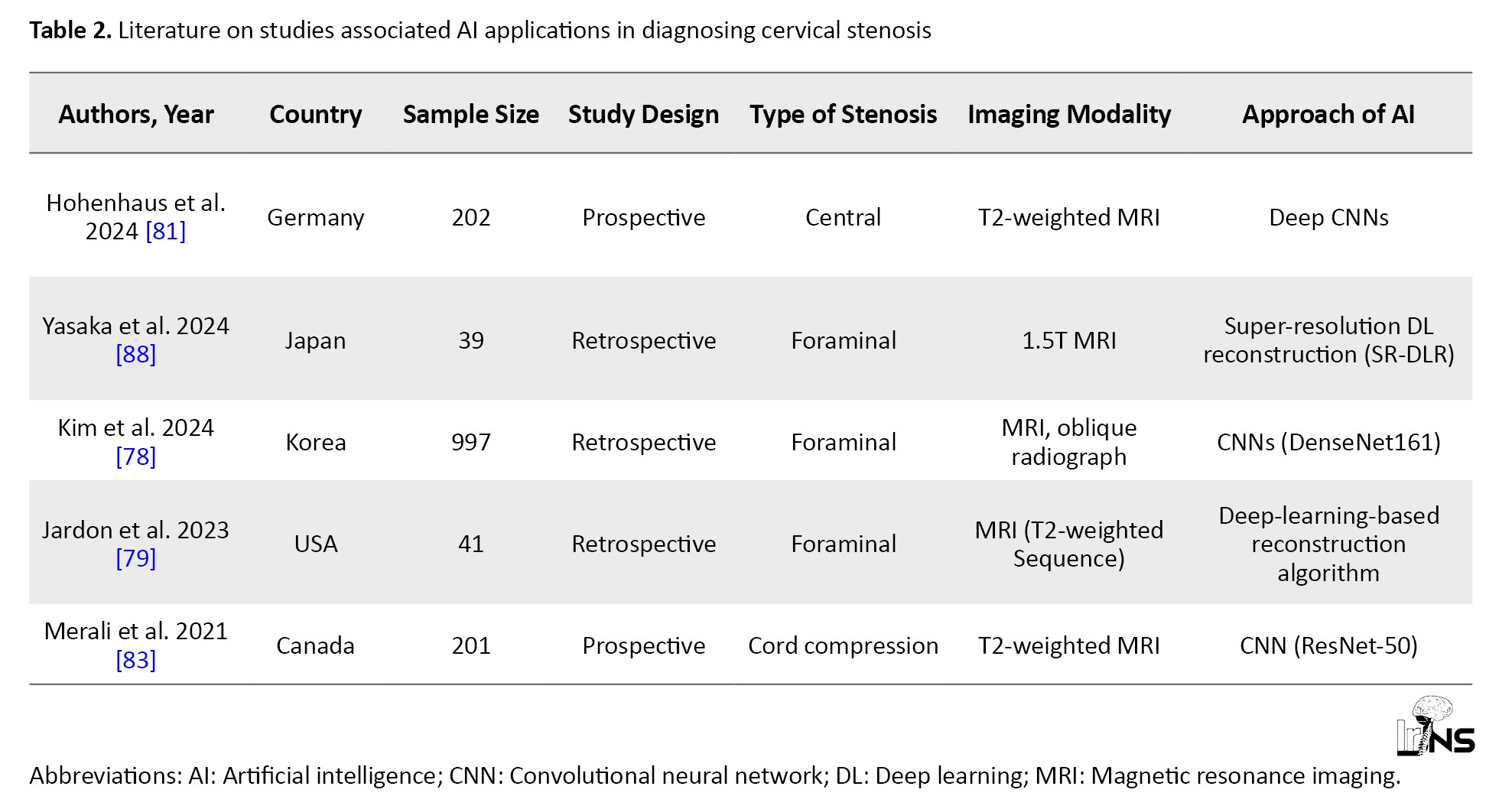
DL algorithms, especially CNN, have shown remarkable promise in analyzing imaging data for diagnosing cervical stenosis [78, 81, 83]. These models are designed to learn from extensive datasets, enabling the identification of complex patterns indicative of stenosis [64]. For instance, Kim et al. developed a CNN model that achieved an area under the curve (AUC) of 0.889 for diagnosing foraminal stenosis from oblique radiographs [78]. This performance significantly surpassed traditional evaluation methods conducted by orthopedic surgeons, highlighting AI’s potential to enhance diagnostic capabilities. Similarly, Merali et al. reported an area AUC of 0.94 for their DL model focused on detecting spinal cord compression using T2-weighted MRI scans [83]. These results underscore AI’s ability to detect subtle signs of stenosis that may elude human observers, thus facilitating early diagnosis and intervention. Moreover, Hohenhaus et al. introduced automated 3D MRI segmentation techniques that provide standardized metrics for quantifying spinal canal compromise and enhancing objectivity in clinical assessments [81]. Integrating AI into clinical practice offers numerous advantages for diagnosing cervical stenosis that extend beyond mere accuracy; it also encompasses significant improvements in workflow efficiency. AI-driven technologies can streamline diagnostic processes by substantially reducing the time required for image interpretation, allowing healthcare providers to make quicker and more informed decisions regarding patient management. For example, Jardon et al. demonstrated that deep-learning-reconstructed 3D MRI sequences yielded excellent inter-observer agreement for foraminal and central stenosis assessments, indicating that AI can provide reliable analyses across diverse patient populations [79]. This consistency is crucial for prioritizing cases based on risk stratification and ensuring timely intervention, ultimately improving patient outcome and satisfaction. Additionally, enhanced diagnostic accuracy through AI applications can lead to better treatment planning and resource allocation within healthcare systems [81, 84]. As we look toward the future, ongoing research efforts aim to refine AI algorithms to improve predictive capabilities specific to cervical stenosis. Addressing challenges, such as data bias, algorithm transparency, and ethical considerations is essential for optimizing AI integration into clinical practice [85]. For instance, while current models have shown promise in detecting cervical stenosis with high accuracy, they must be validated across diverse populations to ensure their effectiveness in real-world clinical settings [86]. Future studies should explore incorporating multimodal data, combining imaging data with clinical information, such as patient demographics and symptomatology, into AI systems to enhance predictive modeling [83, 87]. This integrative approach can lead to personalized diagnostics and treatment plans tailored to individual patient needs. Moreover, collaboration among radiologists, neurosurgeons, and technologists is critical to ensure that AI tools are effectively utilized in everyday practice [26]. The successful implementation of these technologies requires a multidisciplinary approach in which insights from various specialties converge to optimize patient care pathways. Continuous education and training of healthcare professionals on using AI tools is also essential to foster acceptance and improve outcomes. As these technologies continue to evolve, they hold significant potential to enhance diagnostic accuracy and to improve overall patient outcomes in managing cervical stenosis. In conclusion, the application of AI, particularly DL techniques, has the potential to revolutionize the diagnosis of cervical stenosis. AI can significantly impact patient care and outcomes by enhancing diagnostic accuracy and efficiency through advanced imaging analysis. As ongoing research continues to refine these technologies and address existing challenges, such as algorithm transparency and data bias, we anticipate a future in which AI is integral in effectively managing cervical stenosis while ensuring high patient safety and care standards.
Implications for neurosurgical practice
Integrating AI and DL algorithms in diagnosing and managing spinal stenosis signifies a shift in neurosurgical practice. One of the most notable advantages of these technologies is their ability to rapidly analyze extensive volumes of imaging data, enabling the identification of subtle patterns that may escape human observers [89]. Studies utilizing CNNs have shown remarkable efficacy in detecting foraminal stenosis using cervical MRI [81, 90]. These AI models can process images significantly faster than traditional methods, achieving diagnostic accuracies that frequently surpass those of seasoned clinicians. This rapid analysis enhances the precision of diagnoses and is critical in preventing complications and ensuring timely intervention, which are paramount in neurosurgical care [82]. However, it is essential to recognize that while AI can significantly aid diagnosis, it should complement rather than replace clinical expertise. Despite AI’s promising capabilities, there are inherent limitations and ethical considerations must be addressed as these technologies become more prevalent in clinical settings [85, 82]. One significant concern is the potential for bias in training data, which raises crucial questions about the fairness and equity of AI-driven diagnostics. If an AI model is predominantly trained on data from a homogenous patient population, its applicability to diverse groups may be compromised, leading to disparities in care [91]. Moreover, the interpretability of AI algorithms poses challenges; while they can provide accurate results, understanding the rationale behind their decisions remains complex. This lack of transparency can foster skepticism among healthcare providers and patients, potentially hindering the acceptance and integration of AI tools in routine practice [92, 93]. It is essential for neurosurgeons and radiologists to engage in ongoing discussions about these challenges, advocating rigorous validation studies that ensure AI tools are effective across various patient demographics while maintaining ethical standards [26, 94]. Looking ahead, the future of AI applications in diagnosing spinal stenosis and related deformities appears bright but requires careful navigation. As research continues to advance in this field, there is significant potential for AI algorithms to evolve into indispensable tools for enhancing patient care [95]. The ability to predict surgical outcomes based on comprehensive data analysis could lead to personalized treatment plans tailored to individual patient characteristics [96]. Furthermore, as larger datasets become available through electronic health records and imaging repositories, ML models will likely refine their predictive capabilities and identify previously unrecognized factors that influence surgical success. The path forward will involve harnessing these technological advancements and ensuring they are implemented responsibly within clinical practice. By addressing the limitations and ethical concerns associated with AI technology, neurosurgeons can optimize its use to improve patient outcomes while safeguarding the integrity of clinical decision-making processes.
4. Conclusion
In conclusion, integrating AI into diagnosing lumbar and cervical spinal stenoses represents a significant advancement in medical imaging and patient care. This narrative review highlights the transformative potential of AI technologies, particularly deep-learning algorithms, in enhancing diagnostic accuracy and efficiency. By analyzing large datasets of MRI and CT images, AI can identify subtle patterns indicative of stenosis that may be missed by human observers, thereby facilitating early intervention and improving patient outcome. As ongoing research continues to refine these technologies and address existing challenges, AI is poised to play a crucial role in revolutionizing the diagnostic process for spinal stenosis, ultimately leading to better treatment planning and enhanced quality of care for patients.
Ethical Considerations
Compliance with ethical guidelines
This study is a narrative review and does not require ethical considerations.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception, design, data collection, interpretation of results, and manuscript preparation. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the scientific support provided by the Department of Neurosurgery at Mazandaran University of Medical Sciences, Sari. Iran.
References
Spinal stenosis is a common disease in the elderly, and its incidence has gradually increased in an aging society [1]. Lumbar and cervical canal stenoses are conditions characterized by spinal canal narrowing, leading to spinal cord and nerve root compression [1-3]. The pathophysiology of these conditions involves degenerative changes in the spine, such as disc herniation, ligamentum flavum hypertrophy, and osteophyte formation. These degenerative processes can lead to significant neurological deficits, as the narrowing of the canal restricts space for the spinal cord and nerves [4, 5]. Degenerative lumbar spinal stenosis is a progressive disease involving all spinal movement segments [6].
Nerve root compression may result from direct mechanical compression or indirectly from increased intrathecal pressure, which rises as the canal area is reduced [7]. Increased intrathecal pressure can cause venous congestion, reduced arterial blood flow, and reduced impulse conduction along nerve roots [8-10]. Evidence suggests that multilevel stenosis is necessary for this process. Spondylosis, or degenerative arthritis affecting the spine, is the most common cause of lumbar spine stenosis (LSS) and typically affects individuals over the age of 60 years [11]. Obesity and family history may also be risk factors [12, 13]. In cervical stenosis, compression can result in neck pain, radiculopathy, and myelopathy, which may manifest as weakness, numbness, or coordination difficulties [14]. Conversely, lumbar stenosis often presents with lower back pain and neurogenic claudication, wherein patients experience leg pain or weakness during prolonged standing or walking [15, 16].
Magnetic resonance imaging (MRI) is crucial in diagnosing spinal stenosis because it provides detailed images of both soft tissue structures and bone anatomy. MRI is particularly effective in visualizing the spinal cord, nerve roots, intervertebral discs, and surrounding ligaments [17, 18]. It allows clinicians to assess the degree of canal narrowing and identify potential causes of compression advanced MRI techniques, such as T2-weighted imaging and diffusion-weighted imaging, enhance the visualization of spinal structures and can help differentiate between various causes of stenosis [19-22]. These imaging methods are essential for understanding the dynamic nature of spinal canal stenosis and its impact on neurological function [23-25].
Artificial intelligence (AI) is increasingly integrated into MRI analysis to enhance diagnostic accuracy [26, 27]. AI algorithms, particularly deep-learning (DL) models, such as convolutional neural networks (CNNs), can analyze large datasets of MRI images to identify patterns associated with stenosis [28, 29]. These models can be trained on pre-labeled images to recognize features indicative of varying degrees of stenosis. Techniques, such as data augmentation, help improve model robustness by increasing the diversity of training data [30, 31]. This narrative review aims to provide a comprehensive analysis of the latest findings and published articles on the application of AI in enhancing the diagnostic accuracy of lumbar canal stenosis. It evaluates various AI technologies, particularly DL models, to assess their effectiveness in analyzing medical imaging data and discusses the clinical implications for treatment planning and patient outcomes in neurosurgery.
2. Methods and Materials/Patients
A literature search was conducted using Google Scholar, MEDLINE, and PubMed to identify original studies on the application of AI in diagnosing lumbar and cervical spinal stenosis. The search utilized keywords, such as “deep learning”, “artificial intelligence”, “machine learning”, “neural network”, “lumbar spinal stenosis”, “cervical spine”, “canal stenosis”, and “MRI”. The inclusion criteria focused on original research articles published in English that addressed AI’s role in diagnosing spinal stenosis while excluding reviews, case reports, and studies primarily focused on predicting surgical outcomes. Ultimately, 14 studies were included after thorough screening of titles, abstracts, and full texts. Data extraction was performed using a pre-designed Excel spreadsheet, with relevant information systematically recorded and validated by two neurosurgeons for accuracy. The results were analyzed through thematic analysis and categorization to identify trends in AI applications to enhance diagnostic accuracy. Emphasis was placed on including high-quality articles from reputable journals to ensure clarity in methodologies and results.
3. Results
Current diagnostic methods
Diagnosing spinal canal stenosis, particularly in the lumbar and cervical regions, begins with a thorough patient history and comprehensive physical examination [32]. Clinicians must carefully evaluate the diverse range of symptoms presented by the patient. Common presentations include pain, numbness, and weakness in the extremities, often accompanied by specific signs, such as neurogenic claudication in lumbar stenosis or myelopathy in cervical stenosis [33]. Clinical tests, including the straight leg raise and Hoffmann’s tests, can help identify nerve root involvement or spinal cord disorder [34, 35]. Following clinical evaluation, imaging studies are essential to confirm the diagnosis [36]. X-ray imaging is an essential and cost-effective initial diagnostic tool for evaluating cervical and lumbar compression [37]. It provides critical insights into spinal abnormalities, particularly in suspected stenosis cases. For instance, a cervical canal diameter of less than 13 mm on a lateral x-ray view indicates cervical stenosis [38]. Hartman et al. emphasized that X-rays and dynamic imaging techniques are crucial for assessing lumbar deformities and measuring spinal canal dimensions [39]. Studies have indicated that computed tomography (CT) is a standard diagnostic tool for cervical and lumbar canal stenosis [40, 41]. CT imaging precisely measures the sagittal and transverse dimensions of the lumbar spinal canal [42]. In a study conducted by Stafira et al., the evaluation of CT myelogram images for diagnosing cervical canal stenosis revealed that the inter-observer agreement in determining the level, degree, and cause of stenosis had κ values of 0.50, 0.26, and 0.32, respectively [43]. These results indicate significant variations in the interpretation of CT images, which may impact clinical decision-making.
MRI is the gold standard for diagnosing cervical and lumbar spinal canal stenosis due to its superior ability to visualize soft tissue structures, including the spinal cord and nerve roots [44, 45]. Various MRI techniques, such as T1-weighted imaging, allow clinicians to assess compression by comparing T1 relaxation times in compressed spinal cord regions with non-compressed areas, revealing significant differences that aid diagnosis [46]. T2-weighted imaging is crucial for highlighting edema and soft tissue involvement around the spinal canal, while short tau inversion recovery enhances the detection of edema in soft tissues [47]. Advanced techniques, such as dynamic and kinetic MRI, further improve diagnostic capabilities. Dynamic MRI captures images while the patient flexes or extends their spine, revealing how these movements may exacerbate stenosis [48]. Kinetic MRI provides real-time assessment of spinal motion, offering insights into stability or instability that may contribute to symptoms [49]. Several grading systems have been developed to evaluate the severity of stenosis based on MRI findings. For instance, Park et al. introduced a novel grading system using oblique sagittal T2-weighted sequences that classifies neural foraminal narrowing into four distinct grades, demonstrating high interobserver reliability with kappa values ranging from 0.71 to 0.99 [50]. The Schizas grading system evaluates the ratio of nerve rootlets to cerebrospinal fluid and the morphology of the dural sac in T2 axial images [51]. In contrast, the Lee grading system categorizes central canal and foraminal stenosis based on pathophysiological changes and radiological findings, facilitating communication among specialists [44]. Additionally, the Kang grading system assesses stenosis severity by measuring the percentage reduction in the diameter of the spinal canal and foraminal spaces [52]. An updated six-point grading system for lumbar foraminal stenosis based on Lee’s classification was proposed in 2021, offering a more precise description of stenosis using high-resolution MRI [53]. Despite these advancements, challenges in diagnosing spinal canal stenosis through radiology, including a lack of consensus on criteria selection, inconsistencies in their application by specialists, and poor clinical-morphological correlations that complicate interpretation and communication among professionals [54].
In summary, a comprehensive approach that combines thorough patient history, physical examination, and enhanced imaging techniques are necessary for accurately diagnosing spinal canal stenosis.
AI applications in diagnosing lumbar stenosis
AI is increasingly being recognized as a transformative tool for diagnosing LSS [55, 56]. Despite their relative accuracy, traditional imaging methods often encounter challenges, such as misinterpretation of images and reliance on the experience of physicians [57]. Over the past decade, numerous AI algorithms based on radiographs, CT, and MRI have been developed, significantly improving diagnostic accuracy and facilitating imaging processes [58]. Radiomics, an advanced approach in medical imaging, involves the extraction of quantitative features from medical images and utilizes AI to identify disease characteristics that are not visible to the naked eye, thereby providing valuable information for diagnosis and predicting treatment outcomes [59]. Recent findings have highlighted that some AI architectures achieve performance levels comparable to human experts in assessing LSS [55, 56].
AI has numerous applications, such as machine learning (ML), natural language processing (NLP), and DL [60]. ML significantly enhances the diagnosis of LSS by creating previously unavailable quantitative imaging biomarkers. A recent study in the USA has trained ML algorithms to accurately segment lumbar spinal canal areas from axial and sagittal MRI scans at each lumbar level (L1-L5). The results showed that machine-generated delineations closely matched those created by human raters, confirming the reliability of these techniques [61]. Notably, ML techniques can automate the diagnosis of LSS based on self-reported questionnaires with remarkable accuracy [62]. As a branch of AI, NLP is the automated extraction of structured data from unstructured free text data. NLP-based ML algorithms are emerging as effective tools for diagnosing spinal diseases, particularly lumbar spinal stenoses. Ren et al. demonstrated that the DL model long short-term memory (LSTM) outperformed the ensemble model XGBoost in differentiating between disc herniation and lumbar spinal stenosis using positive symptoms [63]. These results highlight the significant potential of NLP to enhance the accuracy of pre-diagnosis in spinal conditions and can serve as a foundation for future research in this area. Moreover, DL-based methods have demonstrated state-of-the-art performance in various image analysis tasks related to spinal stenosis grading [29]. A CNN is a typical model used in DL applications to extract image features. It operates as an end-to-end network model that takes input images and category labels, enabling automatic hierarchical learning of image characteristics and deeper feature extraction through increased network layers [64]. Research indicates that CNNs have been widely employed in AI approaches for diagnosing LSS, with studies utilizing various architectures, such as ResNet, RegNet, and EfficientNet-B1 (Table 1).

Recent advancements in CNN have evolved from one-component models for binary classification(absence/present) of LSS to more complex multi-component approaches [31]. Tumko et al. introduced a three-stage CNN that segments anatomical structures, classifies LSS presence, and assesses its severity [65]. Additionally, custom CNN models can detect and classify different types of LSS, including central canal, lateral recess, and foraminal stenoses [31, 66]. In two consecutive studies, Park et al. demonstrated that DL-based models, such as single-pose-CNN and multi-pose-CNN, showed high diagnostic accuracy when predicting LSS using lumbar radiographs [67, 68]. Although most DL studies have focused on MRI data, recent developments have also seen algorithms created for diagnosing lumbar central canal stenosis using abdominal and lumbar spine CT scans [69]. Furthermore, Suzuki et al. demonstrated that AI can automatically detect lumbar spinal canal stenosis from plain radiographs, facilitating diagnosis in medical facilities lacking MRI capabilities or specialists [70].
The potential applications of AI extend beyond diagnosis; they include automating the evaluation of surgical candidacy for LSS with performance comparable to a multidisciplinary panel of physicians [71]. Moreover, there is potential for integrating tools, such as ChatGPT, into clinical settings to support decision-making processes for LSS diagnosis and treatment [72].
AI applications in diagnosing cervical stenosis
The advent of AI has significantly reshaped the diagnostic landscape for cervical stenosis, a condition marked by the narrowing of the spinal canal that can lead to severe complications, such as spinal cord compression and neurological deficits. Traditional imaging modalities, including MRI and CT, have long been the cornerstone for diagnosing this condition. However, these methods are fraught with limitations that can hinder effective patient management [76-79]. Variability in interpretation among radiologists can lead to inconsistent diagnoses, which may result in delayed treatment and adverse outcomes [57, 80, 81]. Furthermore, the inherently time-consuming nature of these imaging techniques can prolong the diagnostic process, thereby delaying critical intervention [82]. In light of these challenges, there is a growing interest in leveraging AI technologies, particularly DL algorithms, to enhance diagnostic accuracy and efficiency in detecting cervical stenosis (Table 2).

DL algorithms, especially CNN, have shown remarkable promise in analyzing imaging data for diagnosing cervical stenosis [78, 81, 83]. These models are designed to learn from extensive datasets, enabling the identification of complex patterns indicative of stenosis [64]. For instance, Kim et al. developed a CNN model that achieved an area under the curve (AUC) of 0.889 for diagnosing foraminal stenosis from oblique radiographs [78]. This performance significantly surpassed traditional evaluation methods conducted by orthopedic surgeons, highlighting AI’s potential to enhance diagnostic capabilities. Similarly, Merali et al. reported an area AUC of 0.94 for their DL model focused on detecting spinal cord compression using T2-weighted MRI scans [83]. These results underscore AI’s ability to detect subtle signs of stenosis that may elude human observers, thus facilitating early diagnosis and intervention. Moreover, Hohenhaus et al. introduced automated 3D MRI segmentation techniques that provide standardized metrics for quantifying spinal canal compromise and enhancing objectivity in clinical assessments [81]. Integrating AI into clinical practice offers numerous advantages for diagnosing cervical stenosis that extend beyond mere accuracy; it also encompasses significant improvements in workflow efficiency. AI-driven technologies can streamline diagnostic processes by substantially reducing the time required for image interpretation, allowing healthcare providers to make quicker and more informed decisions regarding patient management. For example, Jardon et al. demonstrated that deep-learning-reconstructed 3D MRI sequences yielded excellent inter-observer agreement for foraminal and central stenosis assessments, indicating that AI can provide reliable analyses across diverse patient populations [79]. This consistency is crucial for prioritizing cases based on risk stratification and ensuring timely intervention, ultimately improving patient outcome and satisfaction. Additionally, enhanced diagnostic accuracy through AI applications can lead to better treatment planning and resource allocation within healthcare systems [81, 84]. As we look toward the future, ongoing research efforts aim to refine AI algorithms to improve predictive capabilities specific to cervical stenosis. Addressing challenges, such as data bias, algorithm transparency, and ethical considerations is essential for optimizing AI integration into clinical practice [85]. For instance, while current models have shown promise in detecting cervical stenosis with high accuracy, they must be validated across diverse populations to ensure their effectiveness in real-world clinical settings [86]. Future studies should explore incorporating multimodal data, combining imaging data with clinical information, such as patient demographics and symptomatology, into AI systems to enhance predictive modeling [83, 87]. This integrative approach can lead to personalized diagnostics and treatment plans tailored to individual patient needs. Moreover, collaboration among radiologists, neurosurgeons, and technologists is critical to ensure that AI tools are effectively utilized in everyday practice [26]. The successful implementation of these technologies requires a multidisciplinary approach in which insights from various specialties converge to optimize patient care pathways. Continuous education and training of healthcare professionals on using AI tools is also essential to foster acceptance and improve outcomes. As these technologies continue to evolve, they hold significant potential to enhance diagnostic accuracy and to improve overall patient outcomes in managing cervical stenosis. In conclusion, the application of AI, particularly DL techniques, has the potential to revolutionize the diagnosis of cervical stenosis. AI can significantly impact patient care and outcomes by enhancing diagnostic accuracy and efficiency through advanced imaging analysis. As ongoing research continues to refine these technologies and address existing challenges, such as algorithm transparency and data bias, we anticipate a future in which AI is integral in effectively managing cervical stenosis while ensuring high patient safety and care standards.
Implications for neurosurgical practice
Integrating AI and DL algorithms in diagnosing and managing spinal stenosis signifies a shift in neurosurgical practice. One of the most notable advantages of these technologies is their ability to rapidly analyze extensive volumes of imaging data, enabling the identification of subtle patterns that may escape human observers [89]. Studies utilizing CNNs have shown remarkable efficacy in detecting foraminal stenosis using cervical MRI [81, 90]. These AI models can process images significantly faster than traditional methods, achieving diagnostic accuracies that frequently surpass those of seasoned clinicians. This rapid analysis enhances the precision of diagnoses and is critical in preventing complications and ensuring timely intervention, which are paramount in neurosurgical care [82]. However, it is essential to recognize that while AI can significantly aid diagnosis, it should complement rather than replace clinical expertise. Despite AI’s promising capabilities, there are inherent limitations and ethical considerations must be addressed as these technologies become more prevalent in clinical settings [85, 82]. One significant concern is the potential for bias in training data, which raises crucial questions about the fairness and equity of AI-driven diagnostics. If an AI model is predominantly trained on data from a homogenous patient population, its applicability to diverse groups may be compromised, leading to disparities in care [91]. Moreover, the interpretability of AI algorithms poses challenges; while they can provide accurate results, understanding the rationale behind their decisions remains complex. This lack of transparency can foster skepticism among healthcare providers and patients, potentially hindering the acceptance and integration of AI tools in routine practice [92, 93]. It is essential for neurosurgeons and radiologists to engage in ongoing discussions about these challenges, advocating rigorous validation studies that ensure AI tools are effective across various patient demographics while maintaining ethical standards [26, 94]. Looking ahead, the future of AI applications in diagnosing spinal stenosis and related deformities appears bright but requires careful navigation. As research continues to advance in this field, there is significant potential for AI algorithms to evolve into indispensable tools for enhancing patient care [95]. The ability to predict surgical outcomes based on comprehensive data analysis could lead to personalized treatment plans tailored to individual patient characteristics [96]. Furthermore, as larger datasets become available through electronic health records and imaging repositories, ML models will likely refine their predictive capabilities and identify previously unrecognized factors that influence surgical success. The path forward will involve harnessing these technological advancements and ensuring they are implemented responsibly within clinical practice. By addressing the limitations and ethical concerns associated with AI technology, neurosurgeons can optimize its use to improve patient outcomes while safeguarding the integrity of clinical decision-making processes.
4. Conclusion
In conclusion, integrating AI into diagnosing lumbar and cervical spinal stenoses represents a significant advancement in medical imaging and patient care. This narrative review highlights the transformative potential of AI technologies, particularly deep-learning algorithms, in enhancing diagnostic accuracy and efficiency. By analyzing large datasets of MRI and CT images, AI can identify subtle patterns indicative of stenosis that may be missed by human observers, thereby facilitating early intervention and improving patient outcome. As ongoing research continues to refine these technologies and address existing challenges, AI is poised to play a crucial role in revolutionizing the diagnostic process for spinal stenosis, ultimately leading to better treatment planning and enhanced quality of care for patients.
Ethical Considerations
Compliance with ethical guidelines
This study is a narrative review and does not require ethical considerations.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception, design, data collection, interpretation of results, and manuscript preparation. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors appreciate the scientific support provided by the Department of Neurosurgery at Mazandaran University of Medical Sciences, Sari. Iran.
References
- Jensen RK, Jensen TS, Koes B, Hartvigsen J. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. European Spine Journal. 2020; 29(9):2143-63. [DOI:10.1007/s00586-020-06339-1] [PMID]
- Katz JN, Harris MB. Lumbar spinal stenosis. The New England Journal of Medicine. 2008; 358(8):818-25. [DOI:10.1056/NEJMcp0708097] [PMID]
- Lee MJ, Cassinelli EH, Riew KD. Prevalence of cervical spine stenosis. Anatomic study in cadavers. The Journal of bone and joint Surgery. American Volume. 2007; 89(2):376-80. [DOI:10.2106/JBJS.F.00437]
- Abbas J, Peled N, Hershkovitz I, Hamoud K. Facet tropism and orientation: Risk Factors for Degenerative lumbar spinal stenosis. BioMed Research International. 2020; 2020:2453503. [DOI:10.1155/2020/2453503] [PMID]
- Lee BH, Moon SH, Suk KS, Kim HS, Yang JH, Lee HM. Lumbar spinal stenosis: pathophysiology and treatment principle: a narrative review. Asian Spine Journal. 2020; 14(5):682-93. [DOI:10.31616/asj.2020.0472] [PMID]
- Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine. 1988; 13(3):313-20. [DOI:10.1097/00007632-198803000-00015] [PMID]
- Takahashi K, Kagechika K, Takino T, Matsui T, Miyazaki T, Shima I. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine. 1995; 20(24):2746-9. [DOI:10.1097/00007632-199512150-00017] [PMID]
- Ikawa M, Atsuta Y, Tsunekawa H. Ectopic firing due to artificial venous stasis in rat lumbar spinal canal stenosis model: A possible pathogenesis of neurogenic intermittent claudication. Spine. 2005; 30(21):2393-7. [DOI:10.1097/01.brs.0000184718.56122.90] [PMID]
- Ooi Y, Mita F, Satoh Y. Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. Spine. 1990; 15(6):544-9. [DOI:10.1097/00007632-199006000-00021] [PMID]
- Rydevik B. Neurophysiology of cauda equina compression. Acta orthopaedica Scandinavica. Supplementum. 1993; 251:52-5. [DOI:10.3109/17453679309160117] [PMID]
- Atlas SJ, Delitto A. Spinal stenosis: Surgical versus nonsurgical treatment. Clinical orthopaedics and related Research. 2006; 443:198-207. [DOI:10.1097/01.blo.0000198722.70138.96] [PMID]
- Battié MC, Ortega-Alonso A, Niemelainen R, Gill K, Levalahti E, Videman T, et al. Lumbar spinal stenosis is a highly genetic condition partly mediated by disc degeneration. Arthritis & rheumatology. 2014; 66(12):3505-10. [DOI:10.1002/art.38823] [PMID]
- Venkatesan M, Uzoigwe CE, Perianayagam G, Braybrooke JR, Newey ML. Is cauda equina syndrome linked with obesity? The Journal of Bone and Joint Surgery. British Volume. 2012; 94(11):1551-6. [DOI:10.1302/0301-620X.94B11.29652] [PMID]
- Iyer S, Kim HJ. Cervical radiculopathy. Current ReViews in Musculoskeletal Medicine. 2016; 9(3):272-80. [DOI:10.1007/s12178-016-9349-4] [PMID]
- Hall S, Bartleson JD, Onofrio BM, Baker HL Jr, Okazaki H, O'Duffy JD. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Annals of Internal Medicine. 1985; 103(2):271-5. [DOI:10.7326/0003-4819-103-2-271] [PMID]
- Rauschning W. Normal and pathologic anatomy of the lumbar root canals. Spine. 1987; 12(10):1008-19. [DOI:10.1097/00007632-198712000-00012] [PMID]
- Kim GU, Chang MC, Kim TU, Lee GW. Diagnostic modality in spine disease: A review. Asian Spine Journal. 2020; 14(6):910-20. [DOI:10.31616/asj.2020.0593] [PMID]
- Pierre-Jerome C, Arslan A, Bekkelund SI. MRI of the spine and spinal cord: Imaging techniques, normal anatomy, artifacts, and pitfalls. Journal of Manipulative and Physiological Therapeutics. 2000; 23(7):470-5. [DOI:10.1067/mmt.2000.108819] [PMID]
- Aaen J, Austevoll IM, Hellum C, Storheim K, Myklebust TÅ, Banitalebi H, et al. Clinical and MRI findings in lumbar spinal stenosis: Baseline data from the NORDSTEN study. European Spine journal. 2022; 31(6):1391-8. [doi:10.1007/s00586-021-07051-4] [PMID]
- Banitalebi H, Espeland A, Anvar M, Hermansen E, Hellum C, Brox JI, et al. Reliability of preoperative MRI findings in patients with lumbar spinal stenosis. BMC Musculoskeletal Disorders. 2022; 23(1):51. [DOI:10.1186/s12891-021-04949-4] [PMID]
- Su ZH, Liu J, Yang MS, Chen ZY, You K, Shen J, et al. Automatic grading of disc herniation, central canal stenosis and nerve roots compression in lumbar magnetic resonance image diagnosis. Frontiers in Endocrinology. 2022; 13:890371. [DOI:10.3389/fendo.2022.890371] [PMID]
- Yabe Y, Hagiwara Y, Tsuchiya M, Onoda Y, Yoshida S, Onoki T, et al. Factors associated with thickening of the ligamentum flavum on magnetic resonance imaging in patients with lumbar spinal canal stenosis. Spine. 2022; 47(14):1036-41. [DOI:10.1097/BRS.0000000000004341] [PMID]
- Abdalla OY, Al-Shami H, Maghraby HM, Enayet A. The value of cervical MRI in surgical lumbar canal stenosis patients. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery. 2021; 57:10. [DOI:10.1186/s41983-020-00249-1]
- Mahdavi A, Rasti S. Dynamic Flexion-extension magnetic resonance imaging of the Cervical Spine: An evolutionary tool for diagnosis and management of Cervical Spondylotic Myelopathy. World Neurosurgery. 2024; 184:138-47. [DOI:10.1016/j.wneu.2024.01.081] [PMID]
- Zileli M, Crostelli M, Grimaldi M, Mazza O, Anania C, Fornari M, et al. Natural course and diagnosis of lumbar spinal stenosis: WFNS Spine Committee Recommendations. World neurosurgery: X. 2020; 7:100073. [DOI:10.1016/j.wnsx.2020.100073] [PMID]
- Kalani M, Anjankar A. Revolutionizing neurology: The role of artificial intelligence in advancing diagnosis and treatment. Cureus. 2024; 16(6):e61706. [DOI:10.7759/cureus.61706] [PMID]
- Rauschecker AM, Rudie JD, Xie L, Wang J, Duong MT, Botzolakis EJ, et al. Artificial intelligence system approaching neuroradiologist-level differential diagnosis accuracy at brain MRI. Radiology. 2020; 295(3):626-37. [DOI:10.1148/radiol.2020190283] [PMID]
- Baur D, Kroboth K, Heyde CE, Voelker A. Convolutional neural networks in spinal magnetic resonance imaging: a systematic review. World Neurosurgery. 2022; 166:60-70. [DOI:10.1016/j.wneu.2022.07.041] [PMID]
- Won D, Lee HJ, Lee SJ, Park SH. Spinal stenosis grading in magnetic resonance imaging using deep convolutional neural networks. Spine. 2020; 45(12):804-12. [DOI:10.1097/BRS.0000000000003377] [PMID]
- Lu JT, Pedemonte S, Bizzo B, Doyle S, Andriole KP, Michalski MH, et al. Deep spine: automated lumbar vertebral segmentation, disc-level designation, and spinal stenosis grading using deep learning. Proceedings of the 3rd Machine Learning for Healthcare Conference. 2018; 85:403-19. [Link]
- Hallinan JTPD, Zhu L, Yang K, Makmur A, Algazwi DAR, Thian YL, et al. Deep learning model for automated detection and classification of central canal, lateral recess, and neural foraminal stenosis at lumbar spine MRI. Radiology. 2021; 300(1):130-8. [DOI:10.1148/radiol.2021204289] [PMID]
- Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: A review. Jama. 2022; 327(17):1688-99. [DOI:10.1001/jama.2022.5921] [PMID]
- Maus TP. Imaging of spinal stenosis: Neurogenic intermittent claudication and cervical spondylotic myelopathy. Radiologic Clinics of North America. 2012; C50(4):651-79. [DOI:10.1016/j.rcl.2012.04.007] [PMID]
- Raja A, Hoang S, Patel P, Mesfin FB. Spinal stenosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2017. [Link]
- Fogarty A, Lenza E, Gupta G, Jarzem P, Dasgupta K, Radhakrishna M. A systematic review of the utility of the Hoffmann sign for the diagnosis of degenerative cervical myelopathy. Spine. 2018; 43(23):1664-9. [DOI:10.1097/BRS.0000000000002697] [PMID]
- Begrich D, Jäger M. [Cervical stenosis-Diagnostics and treatment of symptomatic spinal canal stenosis and neuroforaminal stenosis (German)]. Orthopadie (Heidelberg, Germany). 2024; 53(8):617-28. [DOI:10.1007/s00132-024-04526-2] [PMID]
- Green C, Butler J, Eustace S, Poynton A, O'Byrne JM. Imaging modalities for cervical Spondylotic stenosis and Myelopathy. Advances in Orthopedics. 2012; 2012:908324. [DOI:10.1155/2012/908324] [PMID]
- Edwards WC, LaRocca H. The developmental segmental sagittal diameter of the cervical spinal canal in patients with cervical spondylosis. Spine. 1983; 8(1):20-7. [DOI:10.1097/00007632-198301000-00003] [PMID]
- Hartman J, Granville M, Jacobson RE. Radiologic evaluation of lumbar spinal stenosis: The integration of Sagittal and axial views in decision making for minimally invasive surgical procedures. Cureus. 2019; 11(3):e4268. [DOI:10.7759/cureus.4268]
- Gaskill MF, Lukin R, Wiot JG. Lumbar disc disease and stenosis. Radiologic Clinics of North America. 1991; 29(4):753-64. [DOI:10.1016/S0033-8389(22)02081-4] [PMID]
- Eun SS, Lee HY, Lee SH, Kim KH, Liu WC. MRI versus CT for the diagnosis of lumbar spinal stenosis. Journal of Neuroradiology. 2012; 39(2):104-9. [DOI:10.1016/j.neurad.2011.02.008] [PMID]
- Rapała K, Chaberek S, Truszczyńska A, Lukawski S, Walczak P. Digital computed tomography affords new measurement possibilities in lumbar stenosis. Ortop Traumatol Rehabil. 2009; 11(1):13-26. [PMID]
- Stafira JS, Sonnad JR, Yuh WT, Huard DR, Acker RE, Nguyen DL, et al. Qualitative assessment of cervical spinal stenosis: Observer variability on CT and MR images. American Journal of Neuroradiology. 2003; 24(4):766-9. [PMID]
- Seo J, Lee JW. Magnetic resonance imaging grading systems for central canal and neural foraminal stenoses of the lumbar and cervical spines with a focus on the Lee grading system. Korean Journal of Radiology. 2023; 24(3):224-34. [DOI:10.3348/kjr.2022.0351] [PMID]
- Provenzale J. MR imaging of spinal trauma. Emergency Radiology. 2007; 13(6):289-97. [DOI:10.1007/s10140-006-0568-7] [PMID]
- Maier IL, Hofer S, Joseph AA, Merboldt KD, Eggert E, Behme D, et al. Quantification of spinal cord compression using T1 mapping in patients with cervical spinal canal stenosis-Preliminary experience. NeuroImage: Clinical. 2019; 21:101639. [DOI:10.1016/j.nicl.2018.101639] [PMID]
- Shah LM, Ross JS. Imaging of Spine Trauma. Neurosurgery. 2016; 79(5):626-42. [DOI:10.1227/NEU.0000000000001336] [PMID]
- Abdalhak MAM, Sakr HM, Shalaby MH, El diasty SE. Added value of dynamic MRI in assessment of cervical spondylodegenerative diseases. Egyptian Journal of Radiology and Nuclear Medicine. 2023; 54:100. [DOI:10.1186/s43055-023-01046-5]
- Jinkins JR, Dworkin JS, Damadian RV. Upright, weight-bearing, dynamic-kinetic MRI of the spine: initial results. European Radiology. 2005; 15(9):1815-25. [DOI:10.1007/s00330-005-2666-4] [PMID]
- Park HJ, Kim SS, Lee SY, Park NH, Chung EC, Rho MH, et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. The British Journal of Radiology. 2013; 86(1025):20120515. [DOI:10.1259/bjr.20120515] [PMID]
- Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine. 2010; 35(21):1919-24. [DOI:10.1097/BRS.0b013e3181d359bd] [PMID]
- Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. American Journal of Roentgenology. 2011; 197(1):W134-40. [DOI:10.2214/AJR.10.5560] [PMID]
- Sartoretti E, Wyss M, Alfieri A, Binkert CA, Erne C, Sartoretti-Schefer S, et al. Introduction and reproducibility of an updated practical grading system for lumbar foraminal stenosis based on high-resolution MR imaging. Scientific Reports. 2021; 11(1):12000. [DOI:10.1038/s41598-021-98469-9] [PMID]
- Winklhofer S, Held U, Burgstaller JM, Finkenstaedt T, Bolog N, Ulrich N, et al. Degenerative lumbar spinal canal stenosis: intra-and inter-reader agreement for magnetic resonance imaging parameters. European Spine Journal. 2017; 26(2):353-61. [DOI:10.1007/s00586-016-4667-1] [PMID]
- Bogdanovic S, Staib M, Schleiniger M, Steiner L, Schwarz L, Germann C, et al. AI-based measurement of lumbar spinal stenosis on MRI: External evaluation of a fully automated model. Investigative Radiology. 2024; 59(9):656-66. [DOI:10.1097/RLI.0000000000001070] [PMID]
- Bharadwaj UU, Christine M, Li S, Chou D, Pedoia V, Link TM, et al. Deep learning for automated, interpretable classification of lumbar spinal stenosis and facet arthropathy from axial MRI. European Radiology. 2023; 33(5):3435-43. [DOI:10.1007/s00330-023-09483-6] [PMID]
- Li J, Jiang P, An Q, Wang GG, Kong HF. Medical image identification methods: A review. Computers in Biology and Medicine. 2024; 169:107777. [DOI:10.1016/j.compbiomed.2023.107777] [PMID]
- Martín-Noguerol T, Oñate Miranda M, Amrhein TJ, Paulano-Godino F, Xiberta P, Vilanova JC, et al. The role of Artificial intelligence in the assessment of the spine and spinal cord. European Journal of Radiology. 2023; 161:110726. [DOI:10.1016/j.ejrad.2023.110726] [PMID]
- Tang X. The role of artificial intelligence in medical imaging research. BJR open. 2019; 2(1):20190031. [DOI:10.1259/bjro.20190031]
- Kapoor N, Lacson R, Khorasani R. Workflow applications of artificial intelligence in radiology and an overview of available tools. Journal of the American College of Radiology. 2020; 17(11):1363-70. [DOI:10.1016/j.jacr.2020.08.016] [PMID]
- Gaonkar B, Villaroman D, Beckett J, Ahn C, Attiah M, Babayan D, et al. Quantitative analysis of spinal canal areas in the lumbar spine: an imaging informatics and machine learning study. American Journal of Neuroradiology. 2019; 40(9):1586-91. [DOI:10.3174/ajnr.A6174] [PMID]
- Abel F, Garcia E, Andreeva V, Nikolaev NS, Kolisnyk S, Sarbaev R, et al. An artificial intelligence-based support tool for lumbar spinal stenosis diagnosis from Self-Reported History Questionnaire. World Neurosurg. 2024; 181:e953-62. [DOI:10.1016/j.wneu.2023.11.020] [PMID]
- Ren G, Yu K, Xie Z, Liu L, Wang P, Zhang W, et al. Differentiation of lumbar disc herniation and lumbar spinal stenosis using natural language processing-based machine learning based on positive symptoms. Neurosurgical Focus. 2022; 52(4):E7. [DOI:10.3171/2022.1.FOCUS21561] [PMID]
- Cui Y, Zhu J, Duan Z, Liao Z, Wang S, Liu W. Artificial intelligence in spinal imaging: Current status and future directions. International Journal of Environmental Research and Public Health. 2022; 19(18):11708. [DOI:10.3390/ijerph191811708] [PMID]
- Tumko V, Kim J, Uspenskaia N, Honig S, Abel F, Lebl DR, et al. A neural network model for detection and classification of lumbar spinal stenosis on MRI. European Spine Journal. 2024; 33(3):941-8. [DOI:10.1007/s00586-023-08089-2] [PMID]
- Shahzadi T, Ali MU, Majeed F, Sana MU, Diaz RM, Samad MA, et al. Nerve root compression analysis to find lumbar spine stenosis on MRI using CNN. Diagnostics. 2023; 13(18):2975. [DOI:10.3390/diagnostics13182975] [PMID]
- Kim T, Kim YG, Park S, Lee JK, Lee CH, Hyun SJ, et al. Diagnostic triage in patients with central lumbar spinal stenosis using a deep learning system of radiographs. Journal of Neurosurgery. Spine. 2022; 37(1):104-11. [DOI:10.3171/2021.11.SPINE211136] [PMID]
- Park S, Kim JH, Ahn Y, Lee CH, Kim YG, Yuh WT, et al. Multi-pose-based convolutional neural network model for diagnosis of patients with central lumbar spinal stenosis. Scientific Reports. 2024; 14(1):203. [DOI:10.1038/s41598-023-50885-9] [PMID]
- Jeon Y, Kim BR, Choi HI, Lee E, Kim DW, Choi B, et al. Feasibility of deep learning algorithm in diagnosing lumbar central canal stenosis using abdominal CT. Skeletal Radiology. 2024. [DOI:10.1007/s00256-024-04796-z]
- Suzuki H, Kokabu T, Yamada K, Ishikawa Y, Yabu A, Yanagihashi Y, et al. Deep learning-based detection of lumbar spinal canal stenosis using convolutional neural networks. The Spine Journal. 2024; 24(11):2086-101. [DOI:10.1016/j.spinee.2024.06.009] [PMID]
- Mourad R, Kolisnyk S, Baiun Y, Falk A, Yuriy T, Valerii F, et al. Performance of hybrid artificial intelligence in determining candidacy for lumbar stenosis surgery. European Spine Journal. 2022; 31(8):2149-55. [DOI:10.1007/s00586-022-07307-7] [PMID]
- Rajjoub R, Kurapatti M, Kim JS, Villada JSA, Mejia MR, Ahmed W, et al. P233. ChatGPT in the decision-making for the diagnosis and treatment of lumbar spinal stenosis: a comparative analysis and systematic review. The Spine Journal. 2024; 24(S 9):S179. [DOI:10.1016/j.spinee.2024.06.356]
- Fan G, Li Y, Wang D, Zhang J, Du X, Liu H, et al. Automatic segmentation of dura for quantitative analysis of lumbar stenosis: A deep learning study with 518 CT myelograms. Journal of Applied Clinical Medical Physics. 2024; 25(7):e14378. [DOI:10.1002/acm2.14378] [PMID]
- Miyo R, Yasaka K, Hamada A, Sakamoto N, Hosoi R, Mizuki M, et al. Deep-learning reconstruction for the evaluation of lumbar spinal stenosis in computed tomography. Medicine. 2023; 102(23):e33910. [DOI:10.1097/MD.0000000000033910] [PMID]
- Lewandrowski KU, Muraleedharan N, Eddy SA, Sobti V, Reece BD, Ramírez León JF, et al. Artificial intelligence comparison of the radiologist report with endoscopic predictors of successful transforaminal decompression for painful conditions of the lumber spine: Application of deep learning algorithm interpretation of routine lumbar magnetic resonance imaging scan. International Journal of Spine Surgery. 2020; 14(S 3):S75-85. [DOI:10.14444/7130] [PMID]
- Meacock J, Schramm M, Selvanathan S, Currie S, Stocken D, Jayne D, et al. Systematic review of radiological cervical foraminal grading systems. Neuroradiology. 2021; 63(3):305-16. [DOI:10.1007/s00234-020-02596-5] [PMID]
- Schell A, Rhee JM, Holbrook J, Lenehan E, Park KY. Assessing foraminal stenosis in the cervical spine: a comparison of three-dimensional computed tomographic surface reconstruction to two-dimensional modalities. Global Spine Journal. 2017; 7(3):266-71. [DOI:10.1177/2192568217699190] [PMID]
- Kim J, Yang JJ, Song J, Jo S, Kim Y, Park J, et al. Detection of cervical foraminal stenosis from oblique radiograph using Convolutional Neural Network Algorithm. Yonsei Medical Journal. 2024; 65(7):389-96. [DOI:10.3349/ymj.2023.0091] [PMID]
- Jardon M, Tan ET, Chazen JL, Sahr M, Wen Y, Schneider B, et al. Deep-learning-reconstructed high-resolution 3D cervical spine MRI for foraminal stenosis evaluation. Skeletal Radiology. 2023; 52(4):725-32. [DOI:10.1007/s00256-022-04211-5] [PMID]
- Ko S, Choi W, Chae S. Comparison of inter- and intra-observer reliability among the three classification systems for cervical spinal canal stenosis. European Spine Journal. 2017; 26(9):2290-6. [DOI:10.1007/s00586-017-5187-3] [PMID]
- Hohenhaus M, Klingler JH, Scholz C, Watzlawick R, Hubbe U, Beck J, et al. Quantification of cervical spinal stenosis by automated 3D MRI segmentation of spinal cord and cerebrospinal fluid space. Spinal Cord. 2024; 62(7):371-7. [DOI:10.1038/s41393-024-00993-8] [PMID]
- Muyskens K, Ma Y, Menikoff J, Hallinan J, Savulescu J. When can we kick (some) humans "out of the loop"? An examination of the use of AI in medical imaging for lumbar spinal stenosis. Asian Bioethics Review. 2024; 17(1):207-23.[DOI:10.1007/s41649-024-00290-9] [PMID]
- Merali Z, Wang JZ, Badhiwala JH, Witiw CD, Wilson JR, Fehlings MG. A deep learning model for detection of cervical spinal cord compression in MRI scans. Scientific Reports. 2021; 11(1):10473. [DOI:10.1038/s41598-021-89848-3] [PMID]
- Khalifa M, Albadawy M. AI in diagnostic imaging: Revolutionising accuracy and efficiency. Computer Methods and Programs in Biomedicine Update. 2024; 5:100146. [DOI:10.1016/j.cmpbup.2024.100146]
- Harishbhai Tilala M, Kumar Chenchala P, Choppadandi A, Kaur J, Naguri S, Saoji R, et al. Ethical considerations in the use of artificial intelligence and machine learning in health care: A comprehensive review. Cureus. 2024; 16(6):e62443. [DOI:10.7759/cureus.62443] [PMCID]
- Lee S, Jung JY, Mahatthanatrakul A, Kim JS. Artificial intelligence in spinal imaging and patient care: A review of recent advances. Neurospine. 2024; 21(2):474-86. [DOI:10.14245/ns.2448388.194] [PMID]
- Mohsen F, Ali H, El Hajj N, Shah Z. Artificial intelligence-based methods for fusion of electronic health records and imaging data. Scientific Reports. 2022; 12(1):17981. [DOI:10.1038/s41598-022-22514-4] [PMID]
- Yasaka K, Uehara S, Kato S, Watanabe Y, Tajima T, Akai H, et al. Super-resolution deep learning reconstruction cervical spine 1.5 T MRI: improved interobserver agreement in evaluations of neuroforaminal stenosis compared to conventional deep learning reconstruction. Journal of Imaging Informatics in Medicine. 2024; 37(5):2466-73. [DOI:10.1007/s10278-024-01112-y] [PMID]
- Chen K, Zheng L, Zhao H, Wang Z. Deep learning-based intelligent diagnosis of lumbar diseases with multi-angle view of intervertebral disc. Mathematics. 2024; 12(13):2062. [DOI:10.3390/math12132062]
- Limantara R, Kristian Y, Setiawan EI, Cahyadi D, Artha IGLNA, Deslivia MF. SpinalAI: Adeep learning approach to predict vertebrae-column level, structure, and foraminal on cervical spine axial MRI images. Paper presented: 7th International Conference on Informatics and Computational Sciences (ICICoS). 17-18 July 2024; Semarang, Indonesia. [DOI:10.1109/ICICoS62600.2024.10636887]
- Chinta SV, Wang Z, Zhang X, Viet TD, Kashif A, Smith MA, et al. Ai-driven healthcare: A survey on ensuring fairness and mitigating bias. arXiv[preprint]. 2024. [Link]
- Hurd TC, Cobb Payton F, Hood DB. Targeting machine learning and artificial intelligence algorithms in health care to reduce bias and improve population health. The Milbank Quarterly. 2024; 102(3):577-604. [DOI:10.1111/1468-0009.12712] [PMID]
- Lambert SI, Madi M, Sopka S, Lenes A, Stange H, Buszello CP, et al. An integrative review on the acceptance of artificial intelligence among healthcare professionals in hospitals. NPJ Digital Medicine. 2023; 6(1):111. [DOI:10.1038/s41746-023-00874-z] [PMID]
- Sobhanian P, Shafizad M, Karami S, Mozaffari F, Arab A, Razani G, et al. Artificial intelligence applications in clinical neurosurgery. Precision Medicine and Clinical OMICS. 2022; 2(1):e133563. [DOI:10.5812/pmco-133563]
- Yagi M, Yamanouchi K, Fujita N, Funao H, Ebata S. Revolutionizing spinal care: Current applications and future directions of artificial intelligence and machine learning. Journal of Clinical Medicine. 2023; 12(13):4188. [DOI:10.3390/jcm12134188] [PMID]
- Benítez-Andrades JA, Ordás-Reyes N, García AS, Blanco ME, Nicolás JB, Gutiérrez JV. Machine learning in predicting the success of spine surgery: A multivariable study. paper presented: 37th International Symposium on Computer-Based Medical Systems (CBMS). 26-28 June 2024; Guadalajara, Mexico. [DOI:10.1109/CBMS61543.2024.00057]
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




