Sun, Feb 22, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 188-195 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pandey S, Chakraborty S, Kumar P, Sharma N, Gupta L N, Mittal A et al . Serum Superoxide Dismutase as A Biomarker in Severe Traumatic Brain Injury: A Case-Control Study. Iran J Neurosurg 2024; 10 : 22
URL: http://irjns.org/article-1-419-en.html
URL: http://irjns.org/article-1-419-en.html
Sharad Pandey *1 
 , Sourabh Chakraborty2
, Sourabh Chakraborty2 
 , Pankaj Kumar2
, Pankaj Kumar2 
 , Neera Sharma3
, Neera Sharma3 
 , L. N. Gupta2
, L. N. Gupta2 
 , Amol Mittal2
, Amol Mittal2 
 , Achal Saxena2
, Achal Saxena2 


 , Sourabh Chakraborty2
, Sourabh Chakraborty2 
 , Pankaj Kumar2
, Pankaj Kumar2 
 , Neera Sharma3
, Neera Sharma3 
 , L. N. Gupta2
, L. N. Gupta2 
 , Amol Mittal2
, Amol Mittal2 
 , Achal Saxena2
, Achal Saxena2 

1- Department of Neurosurgery , A.B.V.I.M.S. and Dr Ram Manohar Lohia Hospital, New Delhi , drsharad23@yahoo.com
2- Department of Neurosurgery , A.B.V.I.M.S. and Dr Ram Manohar Lohia Hospital, New Delhi
3- Department of Biochemistry, A.B.V.I.M.S. and Dr Ram Manohar Lohia Hospital, New Delhi
2- Department of Neurosurgery , A.B.V.I.M.S. and Dr Ram Manohar Lohia Hospital, New Delhi
3- Department of Biochemistry, A.B.V.I.M.S. and Dr Ram Manohar Lohia Hospital, New Delhi
Full Text [PDF 1026 kb]
(581 Downloads)
| Abstract (HTML) (2567 Views)
Full Text: (213 Views)
1. Introduction
Measuring tissue or body fluid biomarkers can determine specific biological or disease states, with changes in enzyme activity, protein expression, gene expression, and metabolites playing key roles. Traumatic brain injury (TBI), often called a “hidden epidemic”, is a significant global health and socio-economic challenge [1] leading to high rates of death and disability. Quantifying TBI is complex due to varying definitions and underreporting, but Asia, particularly India [2], has observed a rise in incidence, with many cases going unreported or untreated.
Biochemical markers correlating with cerebral damage severity and prognosis are crucial in identifying high-risk patients and guiding preventive measures. Brain-specific markers measurable in serum are particularly promising for patient care, with limited studies examining cerebrospinal fluid. Recent research suggests that human superoxide dismutase (SOD) may serve as a serum marker for TBI [3], becoming inactivated post-injury, with increased microvascular superoxide radical production following TBI. This study aimed to estimate the level of serum SOD in patients with severe TBI and compare the levels of serum SOD in severe TBI cases with a control population.
2. Methods and Materials/Patients
This case-control study was conducted on patients with TBI presenting to a tertiary care Hospital in New Delhi, India from 1st December 2019 to 15th January 2021.
Inclusion criteria
The inclusion criteria included patients aged 18 years, a closed head injury presenting up to 6 hours from injury, a Glasgow coma scale (GCS) at the time of presentation less than 8, and family or next of kin available to provide written informed consent.
Exclusion criteria
The exclusion criteria included patients younger than 18 years old, severe coagulopathy like excessive bleeding, platelet count <100,000, international normalized ratio >1.4, or partial thromboplastin time >50, clinical indication for long-term anticoagulant therapy like life-threatening deep vein thrombus, pulmonary embolism, cardiac lesions, lack of informed consent, history of chronic alcohol abuse or any other substance addiction, or known psychiatric illness requiring sedatives or neuroleptics, any central nervous infection during hospitalization and pregnant women.
Sample size
The sample size was calculated using the Equation 1, reached 72 people with a prevalence of 20% and a margin of error of 10% in a minimum sample size.
(1) n = Z2 [P(1-P)]/D2
Study protocol
Patients who presented within 6 hours of injury were included in this study. The patient’s demographic information was recorded at the time of enrolment. The GCS score was calculated at the time of admission, on the third and seventh day.
The time of injury, associated injuries, and arrival of the patient at the hospital emergency and cranial computed tomography (CT) imaging results were recorded. The clinical outcome was assessed using the Glasgow outcome scale (GOS).
The mechanism of injury was categorized as a fall from height, RTA, assaults, a fall of an object on the head, or any other mode, depending on the case. The patients were called back for follow-up after six months.
Venous blood samples of enrolled patients were collected in a 10 mL serum separator tube at the time of presentation. After collection, the sample was coagulated at room temperature for 10 to 20 minutes or to sleep at 4 °C and then centrifuged at 3000 rpm for 20 minutes. If sedimentation occurs during storage, centrifugation is repeated. The serum samples were frozen and later assayed for serum SOD using the ELISA technique.
Statistical analysis
Data was entered into MS Excel and analyzed using SPSS software, version 16. Continuous parametric data was reported as Mean±SD, while continuous non-parametric data was reported as median and interquartile range. Categorical data was reported in percentages. The categorical data between the groups was compared using the chi-square test. Continuous data between two groups was compared using an independent t-test and the Friedman test between three groups.
3. Results
In this study, 500 patients were admitted to the emergency department of neurosurgery. Following the selection criteria, finally, 40 patients with severe head injuries were included in the study. The control group also included 40 patients.
A flowchart shows patients screened and included in the study group for severe TBI (Figure 1).
The most common age group was 28-37 years (33.2%), followed by 18-27 years and 38-47 years (22.5%) in each group. Out of 40 patients with severe head injuries, 28 (70%) patients were men, and 12(30%) patients were women.
The most common type of injury was road traffic accidents (RTA) (70%), followed by height falls (12.5%), physical assault (12.5 %), and sports injury (5%).
The most common CT imaging result was intracranial hematoma (55%), followed by sub-arachnoid hemorrhage (SAH) (10%), skull fracture (10%), subdural hemorrhage (SDH) (7.5%), and extradural hemorrhage (EDH) (5%).
The mean serum SOD value in the severe head injury group was 23.23 U/mL, and in the control group, it was 135.93 U/mL. The difference was statistically significant (P=0.001)
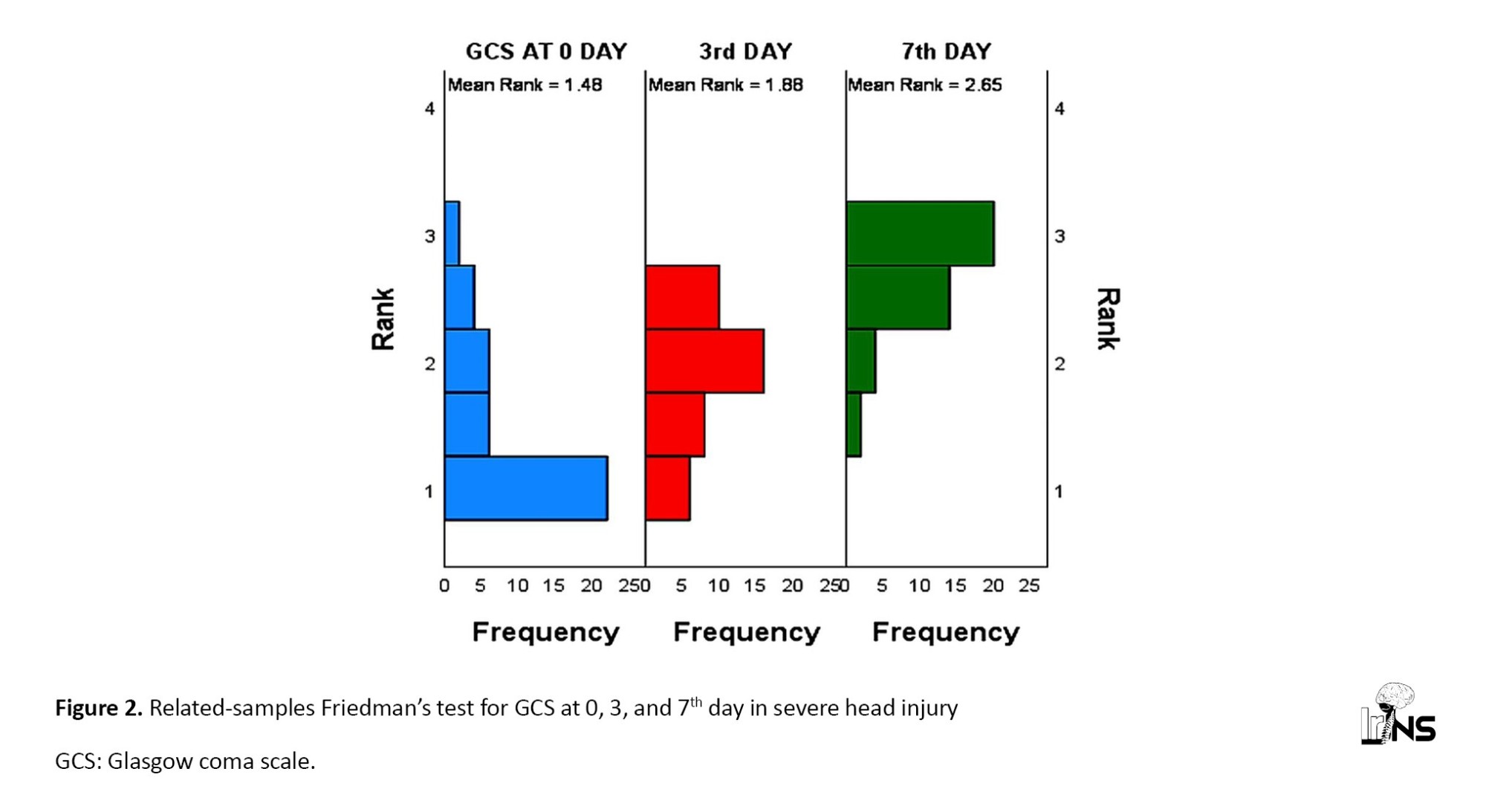
The mean GCS score on day 0 was 6; on day three, it was 9.8; and on day seven, it was 10.08.
A pairwise comparison of GCS differences on day 0, day 3, and day 7 showed significant improvements in GCS from day 0 to day 7 and from day 0 to day 3. But no significant difference in GCS was observed between day 3 and day 7 (Figure 2).
Out of 40 patients, 24(60%) had good GOS at discharge, and 16(40%) had poor outcomes during discharge. At six months of follow-up, 20 patients had good GOS, and four had poor GOS. The rest of the patients were lost in follow-up. A significant improvement was observed in outcome after six months compared to GOS at discharge.
4. Discussion
Bayir et al. [3] reported that the age range in patients with TBI was 21 to 62 years, compared to 16 to 77 years in control groups. This study showed that severe TBI predominantly affected individuals in the prime of their lives, with the most common age group being 28-37 years. This result is consistent with the previous research, highlighting that TBI disproportionately impacts younger adults who are more actively engaged in outdoor activities, resulting in significant socio-economic challenges. The male predominance in TBI cases is consistent with global data, emphasizing the need for targeted interventions to reduce risk in this demographic.
A study on mice conducted by Igarashi et al. [4] reported gender differences in the loss of cortical and subcortical neurons after TBI in animals with higher copper-zinc (CuZn) SOD. The study showed that male transgenic mice (Tg-M) significantly reduced the volume of the cortex lesion compared to male non-transgenic mice (nTg-M). However, compared to non-transgenic female mice, no similar protection was observed in female transgenic mice (Tg-F). It is hypothesized that the female ischemia brain benefits from the antioxidant properties of estrogen. A similar beneficial effect can explain the neuroprotection in traumatized female brains.
In our study, in the severe TBI group, the most common type of injury was RTA, followed by fall from height, assault, and sports injury. The mean serum SOD levels in RTA cases were 22.65±11.02 ng/mL, fall from height 24.00±15.29 ng/mL, assault 22.28±11.38 ng/mL, sports injury 31.77±9.57 ng/mL (P=0.750). In severe TBI, the serum SOD values did not show statistical significance in variation with the mode of injury.
In an epidemiological study by National Institute of Mental Health and Neurosciences (NIMHANS), the most common cause of TBI was falls (20%), assaults (10%), and falls of objects. Gururaj [2] reviewed RTA and found that in developing nations, pedestrians, motorcyclists, and bicyclists were at high risk of sustaining head injuries. This also fits well in the Indian context but not in Western countries, where motor vehicle occupants are at a greater risk than motorcyclists and bicyclists [2].
In severe TBI patients, serum SOD was (23.22±11.31 ng/mL) lower than the control group (135.93±33.27 ng/mL), which was statistically significance (P<0.001) in our study.
Cernak et al. [5], to investigate the plasma SOD activity of patients with severe TBI, reported an initial significant increase in plasma SOD activity followed by a substantial reduction in activity by the end of the posttraumatic period.
Nayak et al. [6], to evaluate and compare the oxidative changes in patients with varying severity of TBI in the early posttraumatic period using erythrocyte indicators, reported that the SOD activity is significantly increased only in severe head injury (SHI) patients and remained unchanged in moderate head injury (MHI) patients as compared to controls. They concluded that early oxidative changes may reflect the severity of neurological insult and provide an early indication of patient outcome in TBI.
Paolin et al. [7], to establish the time course of reactive oxygen species, reported that SOD activity increases significantly at 12 and 24 hrs post-trauma as compared with the time of onset of head injury. They concluded that reactive oxygen species-mediated oxidative damage can play an essential role in determining the prognosis of severe brain injury in humans.
A study conducted by Lvovskaya et al. [8] on the prognostic value of the parameters of free radical oxidation in TBI reported an increase in the level of total antioxidant activity, accompanied by the growth of glutathione peroxidases and catalase activity against the background of a decrease in SOD activity from 1 to 7-day post-trauma.
In our study, the mean GCS was 6.00 on day 0, 9.80 on day 3, and 10.08 on day 7. The distribution of serum SOD in the severe TBI group varied in all categories of GCS at zero days (P=0.721). The mean serum SOD levels in patients with GCS 3, 4, 5, 6, 7, and 8 were 16.11±5.49 ng/mL, 18.29±10.91, 22.72±14.98 ng/mL, 25.01±7.21 ng/mL, 24.15±13.88 ng/mL and 26.64±13.83 ng/mL respectively. Paired comparison of different GCS scores about serum SOD levels showed varied results.
This issue shows that with the changes in GCS, the level of serum SOD varies. In our study, patients with lower GCS had significantly lower levels of serum SOD level, which was further associated with poorer outcomes. This difference in levels may be studied in further detail with a large sample size focused on temporal serum SOD measurements along with GCS scores. Correlating the serum SOD levels and the GCS at which it is significant may help us to guide further in qualitatively analyzing the significant serum SOD levels and may shed some light on its role in projecting TBI prognosis.
Kasprzak et al. [9] showed that enhanced lipid peroxidation, as assessed by cerebrospinal fluid (CSF), in 30 patients with brain contusion, is correlated with the severity of head injury in adults with a contusion. Compared to controls, during the 10-day follow-up, patients with brain contusion had significantly increased erythrocyte SOD activity. The highest CSF SOD concentrations were observed in 5 patients who died 2, 7, or 8 days after the head injury. However, although CSF samples may more directly reflect changes in brain injury, in clinical practice, CSF sampling is not easy.
In a study conducted by Nwachuku et al. [10] on CSF biomarkers in TBI, CSF concentrations of inflammatory biomarkers had a significant relationship with the 6-month neurological outcome (P≤0.05 for each marker), with the favorable outcome group having lower concentrations of these biomarkers on average, compared to the poor neurologic outcome group over the first five days after TBI.
In the current study, serum SOD levels were correlated with the outcome at discharge, but no significant difference was observed (P=0.991)
Hamm et al. [11] reported the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated SOD (PEG-SOD) using the GOS at 3 and 6 months post-injury in 91 and 93 patients, respectively, by blinded observers not involved in the clinical management of the patients. At three months, 44% of patients in the placebo group were vegetative or died, while only 20% of patients in the group receiving 10,000 U/kg of PEG-SOD were in these outcome categories (P<0.03, multiple logistic regression test); at six months, these figures were 36% and 21%, respectively (P=0.04). Though differences in outcome between the placebo group and either of the other two groups were not statistically significant, it was concluded that PEG-SOD was well tolerated and appeared promising in improving outcomes after severe head injury.
5. Conclusion
Based on the results of this study, serum SOD levels appear to be significantly lower in patients with severe TBI compared to healthy controls. This suggests that serum SOD may serve as a valuable biomarker to assess the severity of TBI and potentially guide clinical management. Further research with larger cohorts and more comprehensive follow-up is required to fully understand the role of SOD and other biomarkers in TBI prognosis and treatment.
Limitations
The study had a small sample size, limiting the generalizability of results. It also did not include CSF measurements of SOD, which might have provided additional insights into disease pathogenesis. The study also lacked repeated measurements which could have helped us understand the dynamics of SOD levels over time and their correlation with the progression of TBI. A significant number of patients were lost to follow-up due to decreased willingness to return for a visit, especially as they started feeling better. These limitations highlight the need for further research with larger, more diverse populations and more comprehensive data collection methods to validate and expand upon the study results.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Institutional Review Board of Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. RML Hospital, New Delhi, India (Code: TP(DM/MCH) (30/2019)/IEC/ABVIMS/RMLH/863). Written informed consent was taken from all patients/participants.
Funding
The present article was Funded by the Thesis Committee of Guru Gobind Singh Indraprastha (GGSIP) University, New Delhi, India and was conducted at Dr. RML Hospital, New Delhi, India.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
References
Measuring tissue or body fluid biomarkers can determine specific biological or disease states, with changes in enzyme activity, protein expression, gene expression, and metabolites playing key roles. Traumatic brain injury (TBI), often called a “hidden epidemic”, is a significant global health and socio-economic challenge [1] leading to high rates of death and disability. Quantifying TBI is complex due to varying definitions and underreporting, but Asia, particularly India [2], has observed a rise in incidence, with many cases going unreported or untreated.
Biochemical markers correlating with cerebral damage severity and prognosis are crucial in identifying high-risk patients and guiding preventive measures. Brain-specific markers measurable in serum are particularly promising for patient care, with limited studies examining cerebrospinal fluid. Recent research suggests that human superoxide dismutase (SOD) may serve as a serum marker for TBI [3], becoming inactivated post-injury, with increased microvascular superoxide radical production following TBI. This study aimed to estimate the level of serum SOD in patients with severe TBI and compare the levels of serum SOD in severe TBI cases with a control population.
2. Methods and Materials/Patients
This case-control study was conducted on patients with TBI presenting to a tertiary care Hospital in New Delhi, India from 1st December 2019 to 15th January 2021.
Inclusion criteria
The inclusion criteria included patients aged 18 years, a closed head injury presenting up to 6 hours from injury, a Glasgow coma scale (GCS) at the time of presentation less than 8, and family or next of kin available to provide written informed consent.
Exclusion criteria
The exclusion criteria included patients younger than 18 years old, severe coagulopathy like excessive bleeding, platelet count <100,000, international normalized ratio >1.4, or partial thromboplastin time >50, clinical indication for long-term anticoagulant therapy like life-threatening deep vein thrombus, pulmonary embolism, cardiac lesions, lack of informed consent, history of chronic alcohol abuse or any other substance addiction, or known psychiatric illness requiring sedatives or neuroleptics, any central nervous infection during hospitalization and pregnant women.
Sample size
The sample size was calculated using the Equation 1, reached 72 people with a prevalence of 20% and a margin of error of 10% in a minimum sample size.
(1) n = Z2 [P(1-P)]/D2
Study protocol
Patients who presented within 6 hours of injury were included in this study. The patient’s demographic information was recorded at the time of enrolment. The GCS score was calculated at the time of admission, on the third and seventh day.
The time of injury, associated injuries, and arrival of the patient at the hospital emergency and cranial computed tomography (CT) imaging results were recorded. The clinical outcome was assessed using the Glasgow outcome scale (GOS).
The mechanism of injury was categorized as a fall from height, RTA, assaults, a fall of an object on the head, or any other mode, depending on the case. The patients were called back for follow-up after six months.
Venous blood samples of enrolled patients were collected in a 10 mL serum separator tube at the time of presentation. After collection, the sample was coagulated at room temperature for 10 to 20 minutes or to sleep at 4 °C and then centrifuged at 3000 rpm for 20 minutes. If sedimentation occurs during storage, centrifugation is repeated. The serum samples were frozen and later assayed for serum SOD using the ELISA technique.
Statistical analysis
Data was entered into MS Excel and analyzed using SPSS software, version 16. Continuous parametric data was reported as Mean±SD, while continuous non-parametric data was reported as median and interquartile range. Categorical data was reported in percentages. The categorical data between the groups was compared using the chi-square test. Continuous data between two groups was compared using an independent t-test and the Friedman test between three groups.
3. Results
In this study, 500 patients were admitted to the emergency department of neurosurgery. Following the selection criteria, finally, 40 patients with severe head injuries were included in the study. The control group also included 40 patients.
A flowchart shows patients screened and included in the study group for severe TBI (Figure 1).

The most common age group was 28-37 years (33.2%), followed by 18-27 years and 38-47 years (22.5%) in each group. Out of 40 patients with severe head injuries, 28 (70%) patients were men, and 12(30%) patients were women.
The most common type of injury was road traffic accidents (RTA) (70%), followed by height falls (12.5%), physical assault (12.5 %), and sports injury (5%).
The most common CT imaging result was intracranial hematoma (55%), followed by sub-arachnoid hemorrhage (SAH) (10%), skull fracture (10%), subdural hemorrhage (SDH) (7.5%), and extradural hemorrhage (EDH) (5%).
The mean serum SOD value in the severe head injury group was 23.23 U/mL, and in the control group, it was 135.93 U/mL. The difference was statistically significant (P=0.001)
The mean GCS score on day 0 was 6; on day three, it was 9.8; and on day seven, it was 10.08.
A pairwise comparison of GCS differences on day 0, day 3, and day 7 showed significant improvements in GCS from day 0 to day 7 and from day 0 to day 3. But no significant difference in GCS was observed between day 3 and day 7 (Figure 2).

Out of 40 patients, 24(60%) had good GOS at discharge, and 16(40%) had poor outcomes during discharge. At six months of follow-up, 20 patients had good GOS, and four had poor GOS. The rest of the patients were lost in follow-up. A significant improvement was observed in outcome after six months compared to GOS at discharge.
4. Discussion
Bayir et al. [3] reported that the age range in patients with TBI was 21 to 62 years, compared to 16 to 77 years in control groups. This study showed that severe TBI predominantly affected individuals in the prime of their lives, with the most common age group being 28-37 years. This result is consistent with the previous research, highlighting that TBI disproportionately impacts younger adults who are more actively engaged in outdoor activities, resulting in significant socio-economic challenges. The male predominance in TBI cases is consistent with global data, emphasizing the need for targeted interventions to reduce risk in this demographic.
A study on mice conducted by Igarashi et al. [4] reported gender differences in the loss of cortical and subcortical neurons after TBI in animals with higher copper-zinc (CuZn) SOD. The study showed that male transgenic mice (Tg-M) significantly reduced the volume of the cortex lesion compared to male non-transgenic mice (nTg-M). However, compared to non-transgenic female mice, no similar protection was observed in female transgenic mice (Tg-F). It is hypothesized that the female ischemia brain benefits from the antioxidant properties of estrogen. A similar beneficial effect can explain the neuroprotection in traumatized female brains.
In our study, in the severe TBI group, the most common type of injury was RTA, followed by fall from height, assault, and sports injury. The mean serum SOD levels in RTA cases were 22.65±11.02 ng/mL, fall from height 24.00±15.29 ng/mL, assault 22.28±11.38 ng/mL, sports injury 31.77±9.57 ng/mL (P=0.750). In severe TBI, the serum SOD values did not show statistical significance in variation with the mode of injury.
In an epidemiological study by National Institute of Mental Health and Neurosciences (NIMHANS), the most common cause of TBI was falls (20%), assaults (10%), and falls of objects. Gururaj [2] reviewed RTA and found that in developing nations, pedestrians, motorcyclists, and bicyclists were at high risk of sustaining head injuries. This also fits well in the Indian context but not in Western countries, where motor vehicle occupants are at a greater risk than motorcyclists and bicyclists [2].
In severe TBI patients, serum SOD was (23.22±11.31 ng/mL) lower than the control group (135.93±33.27 ng/mL), which was statistically significance (P<0.001) in our study.
Cernak et al. [5], to investigate the plasma SOD activity of patients with severe TBI, reported an initial significant increase in plasma SOD activity followed by a substantial reduction in activity by the end of the posttraumatic period.
Nayak et al. [6], to evaluate and compare the oxidative changes in patients with varying severity of TBI in the early posttraumatic period using erythrocyte indicators, reported that the SOD activity is significantly increased only in severe head injury (SHI) patients and remained unchanged in moderate head injury (MHI) patients as compared to controls. They concluded that early oxidative changes may reflect the severity of neurological insult and provide an early indication of patient outcome in TBI.
Paolin et al. [7], to establish the time course of reactive oxygen species, reported that SOD activity increases significantly at 12 and 24 hrs post-trauma as compared with the time of onset of head injury. They concluded that reactive oxygen species-mediated oxidative damage can play an essential role in determining the prognosis of severe brain injury in humans.
A study conducted by Lvovskaya et al. [8] on the prognostic value of the parameters of free radical oxidation in TBI reported an increase in the level of total antioxidant activity, accompanied by the growth of glutathione peroxidases and catalase activity against the background of a decrease in SOD activity from 1 to 7-day post-trauma.
In our study, the mean GCS was 6.00 on day 0, 9.80 on day 3, and 10.08 on day 7. The distribution of serum SOD in the severe TBI group varied in all categories of GCS at zero days (P=0.721). The mean serum SOD levels in patients with GCS 3, 4, 5, 6, 7, and 8 were 16.11±5.49 ng/mL, 18.29±10.91, 22.72±14.98 ng/mL, 25.01±7.21 ng/mL, 24.15±13.88 ng/mL and 26.64±13.83 ng/mL respectively. Paired comparison of different GCS scores about serum SOD levels showed varied results.
This issue shows that with the changes in GCS, the level of serum SOD varies. In our study, patients with lower GCS had significantly lower levels of serum SOD level, which was further associated with poorer outcomes. This difference in levels may be studied in further detail with a large sample size focused on temporal serum SOD measurements along with GCS scores. Correlating the serum SOD levels and the GCS at which it is significant may help us to guide further in qualitatively analyzing the significant serum SOD levels and may shed some light on its role in projecting TBI prognosis.
Kasprzak et al. [9] showed that enhanced lipid peroxidation, as assessed by cerebrospinal fluid (CSF), in 30 patients with brain contusion, is correlated with the severity of head injury in adults with a contusion. Compared to controls, during the 10-day follow-up, patients with brain contusion had significantly increased erythrocyte SOD activity. The highest CSF SOD concentrations were observed in 5 patients who died 2, 7, or 8 days after the head injury. However, although CSF samples may more directly reflect changes in brain injury, in clinical practice, CSF sampling is not easy.
In a study conducted by Nwachuku et al. [10] on CSF biomarkers in TBI, CSF concentrations of inflammatory biomarkers had a significant relationship with the 6-month neurological outcome (P≤0.05 for each marker), with the favorable outcome group having lower concentrations of these biomarkers on average, compared to the poor neurologic outcome group over the first five days after TBI.
In the current study, serum SOD levels were correlated with the outcome at discharge, but no significant difference was observed (P=0.991)
Hamm et al. [11] reported the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated SOD (PEG-SOD) using the GOS at 3 and 6 months post-injury in 91 and 93 patients, respectively, by blinded observers not involved in the clinical management of the patients. At three months, 44% of patients in the placebo group were vegetative or died, while only 20% of patients in the group receiving 10,000 U/kg of PEG-SOD were in these outcome categories (P<0.03, multiple logistic regression test); at six months, these figures were 36% and 21%, respectively (P=0.04). Though differences in outcome between the placebo group and either of the other two groups were not statistically significant, it was concluded that PEG-SOD was well tolerated and appeared promising in improving outcomes after severe head injury.
5. Conclusion
Based on the results of this study, serum SOD levels appear to be significantly lower in patients with severe TBI compared to healthy controls. This suggests that serum SOD may serve as a valuable biomarker to assess the severity of TBI and potentially guide clinical management. Further research with larger cohorts and more comprehensive follow-up is required to fully understand the role of SOD and other biomarkers in TBI prognosis and treatment.
Limitations
The study had a small sample size, limiting the generalizability of results. It also did not include CSF measurements of SOD, which might have provided additional insights into disease pathogenesis. The study also lacked repeated measurements which could have helped us understand the dynamics of SOD levels over time and their correlation with the progression of TBI. A significant number of patients were lost to follow-up due to decreased willingness to return for a visit, especially as they started feeling better. These limitations highlight the need for further research with larger, more diverse populations and more comprehensive data collection methods to validate and expand upon the study results.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Institutional Review Board of Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS) and Dr. RML Hospital, New Delhi, India (Code: TP(DM/MCH) (30/2019)/IEC/ABVIMS/RMLH/863). Written informed consent was taken from all patients/participants.
Funding
The present article was Funded by the Thesis Committee of Guru Gobind Singh Indraprastha (GGSIP) University, New Delhi, India and was conducted at Dr. RML Hospital, New Delhi, India.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
References
- Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003; 44(s10):2-10. [DOI:10.1046/j.1528-1157.44.s10.3.x] [PMID]
- Gururaj G. Epidemiology of traumatic brain injuries: Indian scenario. Neurological Research. 2002; 24(1):24-8. [DOI:10.1179/016164102101199503] [PMID]
- Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatric Research. 2002; 51(5):571-8. [DOI:10.1203/00006450-200205000-00005] [PMID]
- Igarashi T, Huang TT, Noble LJ. Regional vulnerability after traumatic brain injury: Gender differences in mice that overexpress human copper, zinc superoxide dismutase. Experimental Neurology. 2001; 172(2):332-41. [DOI:10.1006/exnr.2001.7820] [PMID]
- Cernak I, Savic VJ, Kotur J, Prokic V, Veljovic M, Grbovic D. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. Journal of Neurotrauma. 2000; 17(1):53-68. [DOI:10.1089/neu.2000.17.53] [PMID]
- Nayak CD, Nayak DM, Raja A, Rao A. Erythrocyte indicators of oxidative changes in patients with graded traumatic head injury. Neurology India. 2008; 56(1):31-5. [DOI:10.4103/0028-3886.39309] [PMID]
- Paolin A, Nardin L, Gaetani P, Rodriguez Y Baena R, Pansarasa O, et al. Oxidative damage after severe head injury and its relationship to neurological outcome. Neurosurgery. 2002; 51(4):949-54. [DOI:10.1227/00006123-200210000-00018] [PMID]
- Lvovskaya EI, Derginskyi NV, Sadova VA, Symnaya DB. [Prognostic value of the parameters of free radical oxidation in traumatic brain injury (Russian)]. Biomeditsinskaia Khimiia. 2016; 62(1):107-11. [DOI:10.18097/PBMC20166201107] [PMID]
- Kasprzak HA, Woźniak A, Drewa G, Woźniak B. Enhanced lipid peroxidation processes in patients after brain contusion. Journal of Neurotrauma. 2001; 18(8):793-7. [DOI:10.1089/089771501316919157] [PMID]
- Nwachuku EL, Puccio AM, Adeboye A, Chang YF, Kim J, Okonkwo DO. Time course of cerebrospinal fluid inflammatory biomarkers and relationship to 6-month neurologic outcome in adult severe traumatic brain injury. Clinical neurology and Neurosurgery. 2016; 149:1-5. [DOI:10.1016/j.clineuro.2016.06.009] [PMID]
- Hamm RJ, Temple MD, Pike BR, Ellis EF. The effect of postinjury administration of polyethylene glycol-conjugated superoxide dismutase (pegorgotein, Dismutec) or lidocaine on behavioral function following fluid-percussion brain injury in rats. Journal of Neurotrauma. 1996; 13(6):325-32. [DOI:10.1089/neu.1996.13.325] [PMID]
Type of Study: Research |
Subject:
Neurotrauma
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

