Wed, Feb 4, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 257-264 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pakseresht Mogharab M, Behzadnia H, Alijani B, Reihanian Z, Andalib S. Impact of Age on Neurosurgical Outcomes and Complications in Intracranial Meningioma. Iran J Neurosurg 2024; 10 : 30
URL: http://irjns.org/article-1-457-en.html
URL: http://irjns.org/article-1-457-en.html
Mahsa Pakseresht Mogharab1 

 , Hamid Behzadnia1
, Hamid Behzadnia1 

 , Babak Alijani1
, Babak Alijani1 

 , Zoheir Reihanian1
, Zoheir Reihanian1 

 , Sasan Andalib *2
, Sasan Andalib *2 




 , Hamid Behzadnia1
, Hamid Behzadnia1 

 , Babak Alijani1
, Babak Alijani1 

 , Zoheir Reihanian1
, Zoheir Reihanian1 

 , Sasan Andalib *2
, Sasan Andalib *2 


1- Department of Neurosurgery, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Research Unit of Neurology, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. & Department of Neurology, Odense University Hospital, Odense, Denmark. & Neuroscience Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran. & Department of Neurosurgery, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,sasan.andalib@health.sdu.dk
2- Research Unit of Neurology, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. & Department of Neurology, Odense University Hospital, Odense, Denmark. & Neuroscience Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran. & Department of Neurosurgery, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ,
Full Text [PDF 2990 kb]
(685 Downloads)
| Abstract (HTML) (2535 Views)
Full Text: (1743 Views)
1. Introduction
Meningioma arises from the arachnoid cells of the leptomeninges throughout the central nervous system (CNS) [1]. According to the latest report of the Central Brain Tumor Registry of the United States (CBTRUS) (CBTRUS: 2016-2020), meningioma, as the most commonly documented histopathology, comprised 40.8% of all of CNS tumors and 56.2% of all non-malignant CNS tumors [2]. Based on the United Nations 2024 world population prospects report, by mid-2030, the global population of 80 years and older will surpass that of infants (one year or younger) [3]. CBTRUS 2016-2020 report showed that the annual incidence rate of meningioma rose as people age. In this report, no individuals in the age group of 0-14 were diagnosed with meningioma, while the incidence rate was 20.9 in the 40+ age group [2]. The prevalence of incidental findings of meningioma also increases with age, possibly related to increased imaging indications in the elderly. Based on a 2021 meta-analysis, the pooled prevalence of incidental meningioma from 36 studies was 0.52%; however, the prevalence increased with age which showed a 3% prevalence at 90 years of age [4].
Surgery is the primary standard treatment for most of the patients with symptomatic or enlarging tumors [5]; however, it is not without complications. Meningioma surgery can lead to complications such as cerebral hemorrhage, infection, neurological deficit [6], postoperative hydrocephalus [7], peritumoral edema [8], and seizure [9]. It was shown previously that advancing age has been reported to be an independent risk factor for worse outcomes after surgical procedures [10]. Regarding meningioma surgery, the mortality and morbidity rate of meningioma in elderly patients showed considerable variation among different studies [11]. One-year and five-year mortality rates after tumor resection range from 0 to 16.7% and 7 to 27% in elderly patients, respectively [12]. Although it has been evidenced in some studies that mortality or morbidity after meningioma surgery was significantly higher in older than younger patients [13-15], it was also argued there was no significant difference between older and younger age groups regarding the rate of mortality [13, 16, 17] or complications [17] after surgery. There has been a growing body of evidence to support that age should not be considered as a sole prognostic factor before choosing a patient for surgical removal of meningioma [12, 18-21]. Instead, individualized patient selection has been proposed [22]. Therefore, identifying the relevant prognostic indicators of meningioma is crucial for developing a standard preoperative predictive score for the outcome of surgery. Previous inconsistent findings regarding the role of age in mortality and morbidity following meningioma removal necessitated more investigations.
This study aimed to investigate whether age influences the rates of complications and outcomes of meningioma surgery. The main objectives were to compare the short- and long-term complications and outcomes of surgical treatment for intracranial meningioma in two age groups.
2. Materials and Methods
Participants
In this retrospective cohort study, medical records of 62 patients with the diagnosis of meningioma who had undergone surgery at an educational hospital affiliated with the Guilan University of Medical Sciences, Rasht, Iran, involving 31 aged 18 to 65 (group 1) and 31 aged 65 or older (group 2) were selected. The inclusion criteria were patients with confirmed World Health Organization (WHO) grade I or II intracranial meningioma who had the American Society of Anesthesiologists (ASA) classification score of I or II [23]. The exclusion criteria were patients younger than 18 years of age, patients with debilitating comorbidities, such as advanced diabetes mellitus, stroke, neurofibromatosis, and other intracranial lesions, patients needing immediate radiotherapy after surgery, and patients with tumor location in the skull base (tuberculum sellae, cavernous sinus, sphenoid wing, and clinoid process) and motor cortex (precentral gyrus) on neuroimaging findings.
Baseline demographic data, complications of surgery until discharge, the discharge outcome of the patients, and long-term follow-up after six months outcome were collected via a checklist through their medical records.
Data collection
Baseline demographic and clinical data were collected from the medical records. Age at the time of surgery, gender, and tumor location (convexity, falx/tentorium, olfactory, parasagittal) were recorded for all patients. Also, information about the following complications of surgery was obtained; peritumoral edema, cerebral hemorrhage, surgical wound infection, seizure, hydrocephalus, cerebrospinal fluid (CSF) leakage, surgical wound dehiscence, deep vein thrombosis (DVT), and pressure ulcer. Peritumoral edema, cerebral hemorrhage, and hydrocephalus were evaluated using post-surgery neuroimaging. DVT was assessed using post-surgery Doppler ultrasound. The outcome of the surgery upon discharge and the long-term outcome six months after surgery was assessed using the Glasgow Outcome Scale (GOS). GOS is a five-point scale designed to evaluate disability outcomes. It comprises five descriptive categories (death, persistent vegetative state, severe disability, moderate disability, and good recovery) [24]. A favorable outcome was defined as a GOS score of 4 or 5 and an unfavorable outcome was met when a GOS score was 2 or 3 [24]. The mortality rate was calculated as the number of deaths for each group at discharge and within 6 months following surgery.
Sample size
Based on the study by Slot et al. [17] and the favorable GOS percentage of 93% in the young adults’ group and 64% in the elderly group, α=0.05, β=0.02, and group ratio (r=1), at least 31 young adults and 31 elderly participants were required for our research.
Statistical methods
Statistical analyses were performed using SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). Age, the sole continuous quantitative variable, was reported as Mean and standard deviation (Mean±SD). All other variables, being categorical, were presented as frequencies and percentages. Comparisons between study groups were conducted using the chi-square test or Fisher’s exact test, as appropriate. A significant level of 0.05 was applied for all statistical tests.
3. Results
Baseline demographics of the study groups are presented in Table 1.
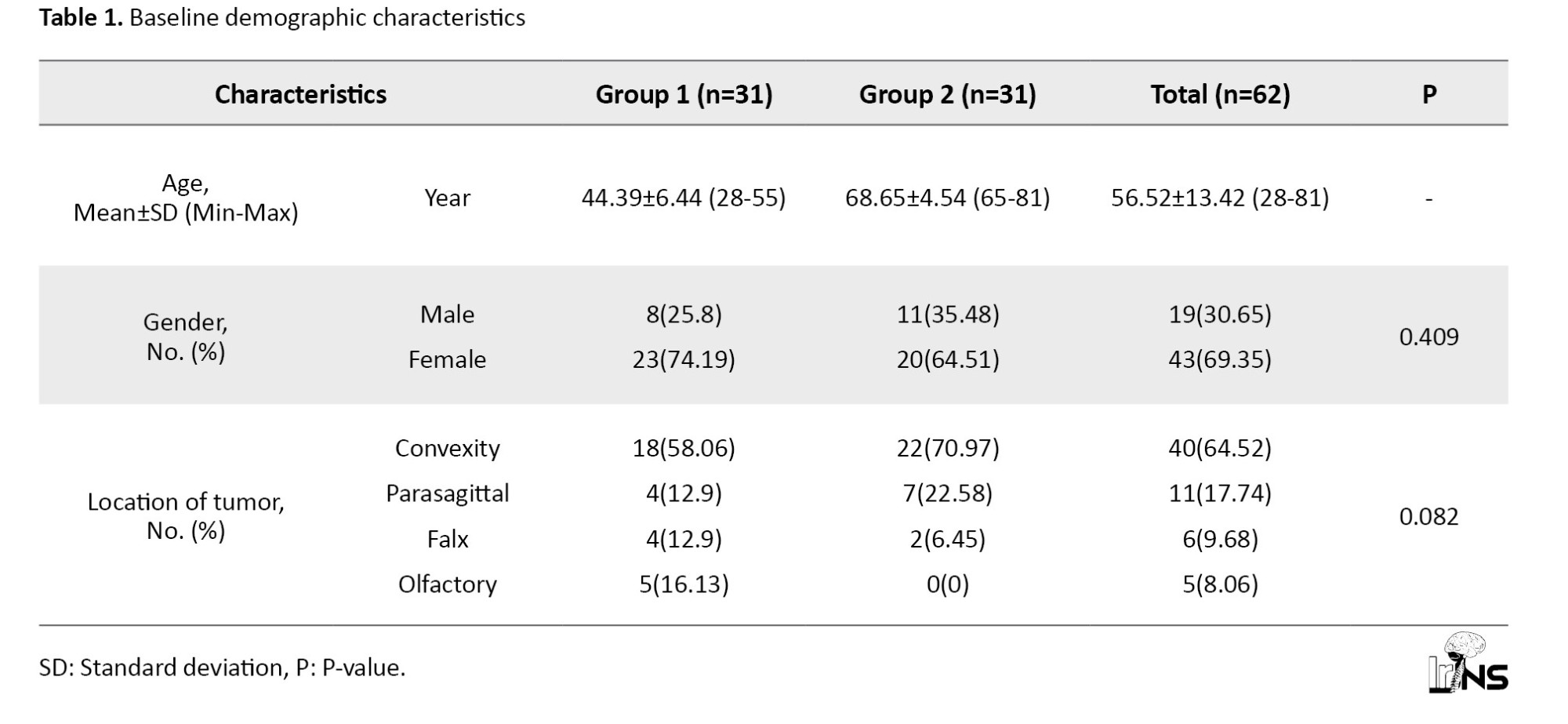
Group 1 included the younger patients with a Mean±SD of 44.39±6.44 and group 2 consisted of the older patients with a Mean±SD age of 68.65±4.54. The table shows that the frequency of females was higher than males in both groups: 23(74.19%) vs 8(25.8%) in group 1 and 20(64.51%) vs 11(35.48%) in group 2. However, the gender distribution did not show any significant difference across study groups (P=0.409). The most frequent location of the tumor was convexity among all participants (64.52%) and both groups, but the olfactory tumor was only present in group 1. The tumor location was not statistically different between groups (P=0.082).
The outcomes and complications of study participants are represented in Table 2.
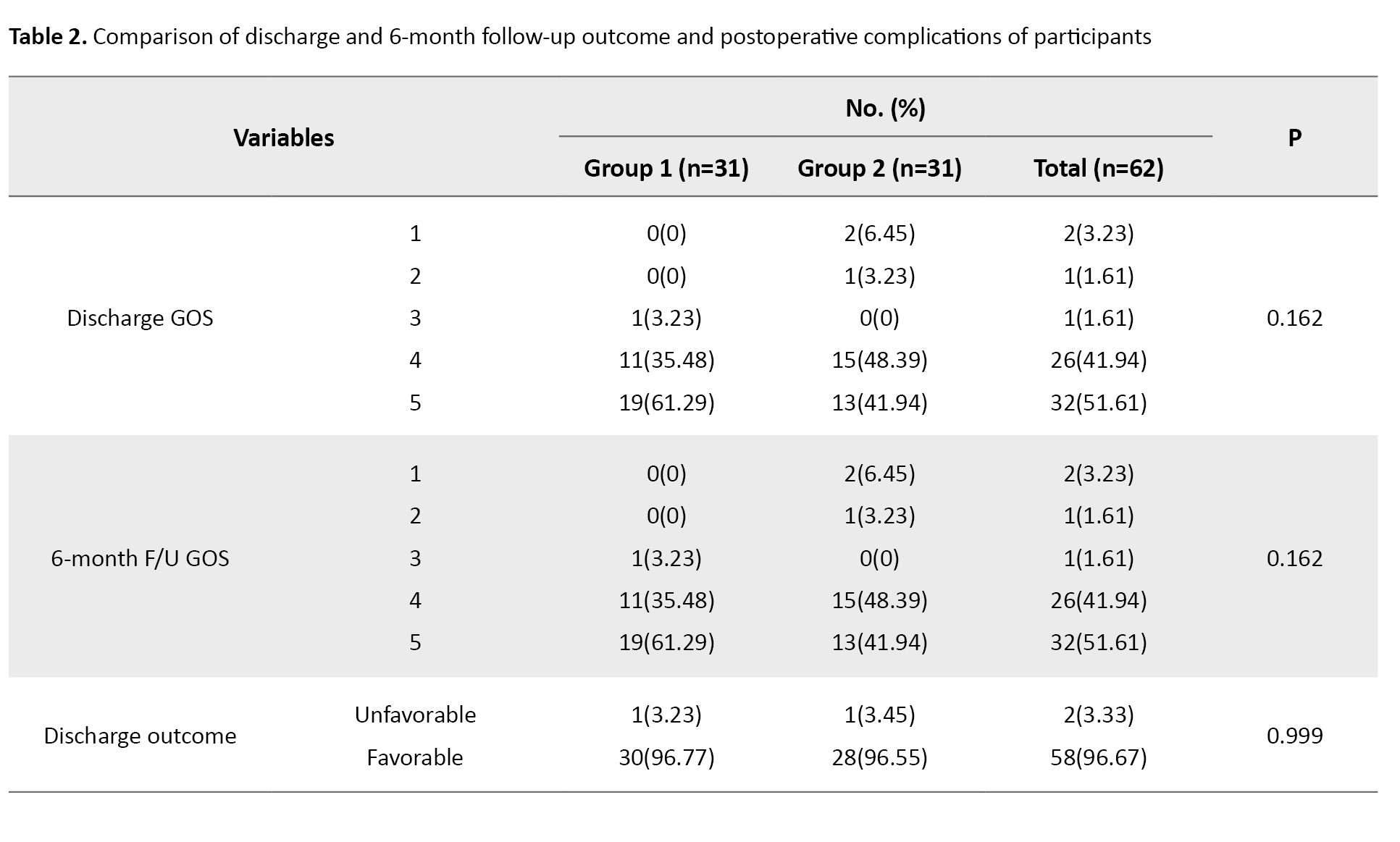
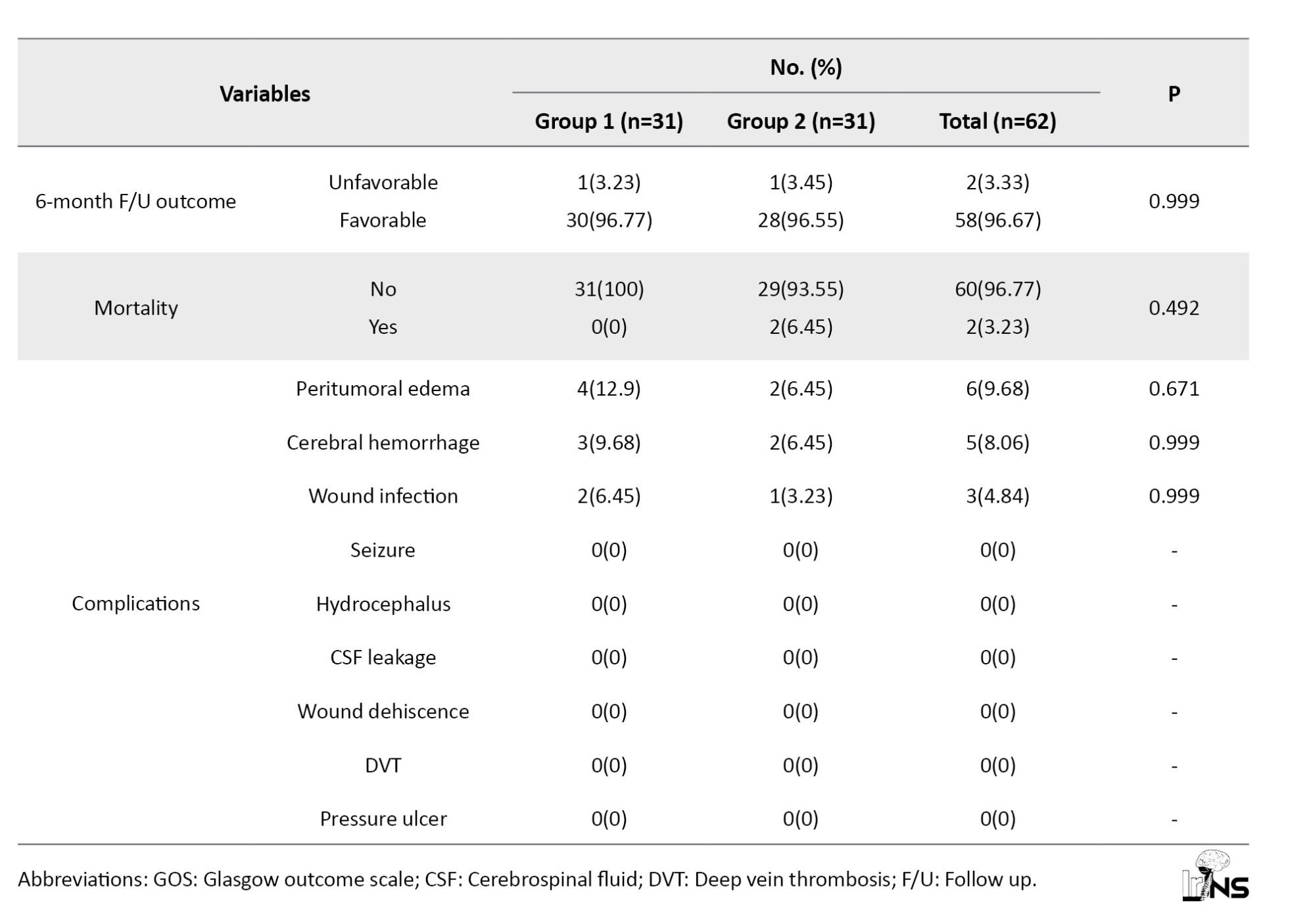
The outcome frequency of postoperative complications and discharge and 6-month follow-up outcome were the same in both groups except for mortality. Mortality occurred in two participants of group 2 at discharge while none of the patients in group 1 died; however, the mortality rate did not show any significant difference among groups (P=0.492). Most participants showed favorable GOS upon discharge and 6-month follow-up (96.67%). Of the 31 patients in group 1, 30 showed favorable outcomes, while in group 2, 28 out of 29 patients showed favorable outcomes, with only one patient having unfavorable outcomes in both groups. There was no significant difference between study groups regarding discharge and 6-month follow-up GOS and outcome. Complications, including seizures, CSF leakage, hydrocephalus, surgical wound dehiscence, DVT, and pressure ulcers were not found among participants in the study groups. Other complications, including peritumoral edema, cerebral hemorrhage, and surgical wound infection were present in both groups without any statistical difference between groups.
4. Discussion
In the present study, we found that the overall mortality rate within six months after surgical resection of meningioma was higher in older patients (6.4%) compared with the younger group (0%); however, it was not statistically significant. Also, there was no difference between the two age groups regarding unfavorable outcomes upon discharge, 6-month follow-up, and postoperative complications in this study. These findings imply that elderly patients could benefit from surgical removal of meningioma.
The literature review revealed a varied mortality rate in the elderly (defined as age ≥60-70) ranging from 0% to 27% within an interval from immediately after surgery until a 5-year follow-up [12, 16, 18, 25-27].
Consistent with our study, some of the previous studies reported that there was no significant difference between older and younger patients regarding mortality [13, 18, 28] or general surgical outcome [29, 30] and elderly patients benefit from surgery.
There are also contradictory reports showing a higher probability for older patients to die or develop worse functional outcomes when compared to youngsters [16, 20, 31]. One fact that should be considered in the interpretation of these studies is that the elderly may need more time to regain functional dependence, so longer follow-ups may be needed. It was shown that at 6-12-month follow-up, functional outcome was better in younger; however, there was no significant difference between groups among the remaining participants for 12-18-month follow-up [17]. In addition, in the study by Brokinkel et al. [2] the three-month postoperative mortality was higher in the elderly, and their overall survival was much shorter. But when compared with the age- and sex-matched general population, the overall survival did not show any difference [20] which supports the surgical treatment of elderly patients with meningioma enabling them to live at their expected age.
The same inconsistencies exist for surgical complications. Our results, showing no difference between age groups, are in agreement with the findings of the previous studies [22, 28, 29, 32, 33]; however, some studies reported a higher risk of complications in older patients [13, 16, 31, 34, 35]. Postoperative CNS complications, comprising peritumoral edema and cerebral hemorrhage were recorded for 4(12.9%) vs 2(6.45%) and 3 (9.68%) vs 2 (6.45%) in younger and older groups, respectively. In terms of general surgical complications, only surgical wound infection occurred in 2(6.45%) younger patients and 1(3.23%) in older patients. The overall prevalence of complications in this study was 17.7% CNS and 4.8% general complications. Other neurological complications were not reported in our patients. According to a review of 24 papers comprising elderly patients ≥60-80 a range of 2.7% to 49.4% was seen for CNS complications, as was 2.7% to 28.6% for the general complications [12], and based on another systematic review incorporating five studies, an overall complication rate of 2.7% to 29.8% was seen [30] and CNS complications ranged from 45.2% 15 to 100% reported by two studies [30].
Heterogenous patient populations in relatively small cohorts and the comparisons of groups that are not matched for preoperative health status and tumor characteristics might be causes of those inconsistent results. To give an example, Poon et al. [16] observed a higher perioperative complication in the elderly. Notably, those complications were minor and only observed in the subgroup of skull base tumors [16] which were excluded from our study. Therefore, their overall results are consistent with ours. Mastronardi et al. [22] conducted a study on two age groups (≥ and <70) following posterior cranial fossa meningiomas. They reported no difference regarding postoperative complications between groups. Consistent results with our study could be due to the exclusion of patients with ASA >3 [22]. Therefore, regardless of age, it seems that the preoperative health conditions of the patients are crucial in postoperative outcomes. Larger-scale studies with standardized patient selection are necessary for future studies which enable proper comparisons and recognition of relevant prognostic factors.
5. Conclusion
This study suggests that, if selected wisely, postoperative complications from intracranial meningioma removal and outcomes of elderly patients could be comparable to younger patients. The definition of age, comorbidities, preoperative health status, surgical techniques, and different follow-up intervals were possible factors influencing the inconsistent outcomes observed between the two age groups in previous studies. Future studies should include a more homogenous patient population and increase the duration of follow-ups to delineate the appropriate prognostic factors helping the patient selection for surgical removal of meningioma.
Strength and limitations
The present study conducted a comparison of homogenous groups in terms of gender, tumor location, and preoperative health status measured by ASA score including a broad range of complications. The other strength of our study is that we measured the postoperative outcome using the standard and reliable score of GOS. Using this approach, we shed light on a critical clinical challenge in meningioma surgery.
The present study also had certain limitations that should be acknowledged. First, similar to all other small cohorts, results from this study might have diminished statistical power. Second, we did not adjust the groups based on the surgical techniques or tumor volume, both of which could influence the results. Third, the retrospective data collection is subject to typical limitations such as missing or incomplete information and lack of control over data quality decreasing the accuracy of the results. Also, we did not select a specific age group over the age of 75 years, and using medical records prevented the authors from using a more detailed functional outcome scale to have a better understanding of postoperative recovery.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1400.359).
Funding
This research was financially supported by Guilan University of Medical Sciences, Rasht, Iran.
Authors' contributions
All authors contributed to the study and preparation of this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Saeideh Aghayari Sheikh Neshin who assisted in the writing and technical editing of this manuscript.
References
Meningioma arises from the arachnoid cells of the leptomeninges throughout the central nervous system (CNS) [1]. According to the latest report of the Central Brain Tumor Registry of the United States (CBTRUS) (CBTRUS: 2016-2020), meningioma, as the most commonly documented histopathology, comprised 40.8% of all of CNS tumors and 56.2% of all non-malignant CNS tumors [2]. Based on the United Nations 2024 world population prospects report, by mid-2030, the global population of 80 years and older will surpass that of infants (one year or younger) [3]. CBTRUS 2016-2020 report showed that the annual incidence rate of meningioma rose as people age. In this report, no individuals in the age group of 0-14 were diagnosed with meningioma, while the incidence rate was 20.9 in the 40+ age group [2]. The prevalence of incidental findings of meningioma also increases with age, possibly related to increased imaging indications in the elderly. Based on a 2021 meta-analysis, the pooled prevalence of incidental meningioma from 36 studies was 0.52%; however, the prevalence increased with age which showed a 3% prevalence at 90 years of age [4].
Surgery is the primary standard treatment for most of the patients with symptomatic or enlarging tumors [5]; however, it is not without complications. Meningioma surgery can lead to complications such as cerebral hemorrhage, infection, neurological deficit [6], postoperative hydrocephalus [7], peritumoral edema [8], and seizure [9]. It was shown previously that advancing age has been reported to be an independent risk factor for worse outcomes after surgical procedures [10]. Regarding meningioma surgery, the mortality and morbidity rate of meningioma in elderly patients showed considerable variation among different studies [11]. One-year and five-year mortality rates after tumor resection range from 0 to 16.7% and 7 to 27% in elderly patients, respectively [12]. Although it has been evidenced in some studies that mortality or morbidity after meningioma surgery was significantly higher in older than younger patients [13-15], it was also argued there was no significant difference between older and younger age groups regarding the rate of mortality [13, 16, 17] or complications [17] after surgery. There has been a growing body of evidence to support that age should not be considered as a sole prognostic factor before choosing a patient for surgical removal of meningioma [12, 18-21]. Instead, individualized patient selection has been proposed [22]. Therefore, identifying the relevant prognostic indicators of meningioma is crucial for developing a standard preoperative predictive score for the outcome of surgery. Previous inconsistent findings regarding the role of age in mortality and morbidity following meningioma removal necessitated more investigations.
This study aimed to investigate whether age influences the rates of complications and outcomes of meningioma surgery. The main objectives were to compare the short- and long-term complications and outcomes of surgical treatment for intracranial meningioma in two age groups.
2. Materials and Methods
Participants
In this retrospective cohort study, medical records of 62 patients with the diagnosis of meningioma who had undergone surgery at an educational hospital affiliated with the Guilan University of Medical Sciences, Rasht, Iran, involving 31 aged 18 to 65 (group 1) and 31 aged 65 or older (group 2) were selected. The inclusion criteria were patients with confirmed World Health Organization (WHO) grade I or II intracranial meningioma who had the American Society of Anesthesiologists (ASA) classification score of I or II [23]. The exclusion criteria were patients younger than 18 years of age, patients with debilitating comorbidities, such as advanced diabetes mellitus, stroke, neurofibromatosis, and other intracranial lesions, patients needing immediate radiotherapy after surgery, and patients with tumor location in the skull base (tuberculum sellae, cavernous sinus, sphenoid wing, and clinoid process) and motor cortex (precentral gyrus) on neuroimaging findings.
Baseline demographic data, complications of surgery until discharge, the discharge outcome of the patients, and long-term follow-up after six months outcome were collected via a checklist through their medical records.
Data collection
Baseline demographic and clinical data were collected from the medical records. Age at the time of surgery, gender, and tumor location (convexity, falx/tentorium, olfactory, parasagittal) were recorded for all patients. Also, information about the following complications of surgery was obtained; peritumoral edema, cerebral hemorrhage, surgical wound infection, seizure, hydrocephalus, cerebrospinal fluid (CSF) leakage, surgical wound dehiscence, deep vein thrombosis (DVT), and pressure ulcer. Peritumoral edema, cerebral hemorrhage, and hydrocephalus were evaluated using post-surgery neuroimaging. DVT was assessed using post-surgery Doppler ultrasound. The outcome of the surgery upon discharge and the long-term outcome six months after surgery was assessed using the Glasgow Outcome Scale (GOS). GOS is a five-point scale designed to evaluate disability outcomes. It comprises five descriptive categories (death, persistent vegetative state, severe disability, moderate disability, and good recovery) [24]. A favorable outcome was defined as a GOS score of 4 or 5 and an unfavorable outcome was met when a GOS score was 2 or 3 [24]. The mortality rate was calculated as the number of deaths for each group at discharge and within 6 months following surgery.
Sample size
Based on the study by Slot et al. [17] and the favorable GOS percentage of 93% in the young adults’ group and 64% in the elderly group, α=0.05, β=0.02, and group ratio (r=1), at least 31 young adults and 31 elderly participants were required for our research.
Statistical methods
Statistical analyses were performed using SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). Age, the sole continuous quantitative variable, was reported as Mean and standard deviation (Mean±SD). All other variables, being categorical, were presented as frequencies and percentages. Comparisons between study groups were conducted using the chi-square test or Fisher’s exact test, as appropriate. A significant level of 0.05 was applied for all statistical tests.
3. Results
Baseline demographics of the study groups are presented in Table 1.

Group 1 included the younger patients with a Mean±SD of 44.39±6.44 and group 2 consisted of the older patients with a Mean±SD age of 68.65±4.54. The table shows that the frequency of females was higher than males in both groups: 23(74.19%) vs 8(25.8%) in group 1 and 20(64.51%) vs 11(35.48%) in group 2. However, the gender distribution did not show any significant difference across study groups (P=0.409). The most frequent location of the tumor was convexity among all participants (64.52%) and both groups, but the olfactory tumor was only present in group 1. The tumor location was not statistically different between groups (P=0.082).
The outcomes and complications of study participants are represented in Table 2.


The outcome frequency of postoperative complications and discharge and 6-month follow-up outcome were the same in both groups except for mortality. Mortality occurred in two participants of group 2 at discharge while none of the patients in group 1 died; however, the mortality rate did not show any significant difference among groups (P=0.492). Most participants showed favorable GOS upon discharge and 6-month follow-up (96.67%). Of the 31 patients in group 1, 30 showed favorable outcomes, while in group 2, 28 out of 29 patients showed favorable outcomes, with only one patient having unfavorable outcomes in both groups. There was no significant difference between study groups regarding discharge and 6-month follow-up GOS and outcome. Complications, including seizures, CSF leakage, hydrocephalus, surgical wound dehiscence, DVT, and pressure ulcers were not found among participants in the study groups. Other complications, including peritumoral edema, cerebral hemorrhage, and surgical wound infection were present in both groups without any statistical difference between groups.
4. Discussion
In the present study, we found that the overall mortality rate within six months after surgical resection of meningioma was higher in older patients (6.4%) compared with the younger group (0%); however, it was not statistically significant. Also, there was no difference between the two age groups regarding unfavorable outcomes upon discharge, 6-month follow-up, and postoperative complications in this study. These findings imply that elderly patients could benefit from surgical removal of meningioma.
The literature review revealed a varied mortality rate in the elderly (defined as age ≥60-70) ranging from 0% to 27% within an interval from immediately after surgery until a 5-year follow-up [12, 16, 18, 25-27].
Consistent with our study, some of the previous studies reported that there was no significant difference between older and younger patients regarding mortality [13, 18, 28] or general surgical outcome [29, 30] and elderly patients benefit from surgery.
There are also contradictory reports showing a higher probability for older patients to die or develop worse functional outcomes when compared to youngsters [16, 20, 31]. One fact that should be considered in the interpretation of these studies is that the elderly may need more time to regain functional dependence, so longer follow-ups may be needed. It was shown that at 6-12-month follow-up, functional outcome was better in younger; however, there was no significant difference between groups among the remaining participants for 12-18-month follow-up [17]. In addition, in the study by Brokinkel et al. [2] the three-month postoperative mortality was higher in the elderly, and their overall survival was much shorter. But when compared with the age- and sex-matched general population, the overall survival did not show any difference [20] which supports the surgical treatment of elderly patients with meningioma enabling them to live at their expected age.
The same inconsistencies exist for surgical complications. Our results, showing no difference between age groups, are in agreement with the findings of the previous studies [22, 28, 29, 32, 33]; however, some studies reported a higher risk of complications in older patients [13, 16, 31, 34, 35]. Postoperative CNS complications, comprising peritumoral edema and cerebral hemorrhage were recorded for 4(12.9%) vs 2(6.45%) and 3 (9.68%) vs 2 (6.45%) in younger and older groups, respectively. In terms of general surgical complications, only surgical wound infection occurred in 2(6.45%) younger patients and 1(3.23%) in older patients. The overall prevalence of complications in this study was 17.7% CNS and 4.8% general complications. Other neurological complications were not reported in our patients. According to a review of 24 papers comprising elderly patients ≥60-80 a range of 2.7% to 49.4% was seen for CNS complications, as was 2.7% to 28.6% for the general complications [12], and based on another systematic review incorporating five studies, an overall complication rate of 2.7% to 29.8% was seen [30] and CNS complications ranged from 45.2% 15 to 100% reported by two studies [30].
Heterogenous patient populations in relatively small cohorts and the comparisons of groups that are not matched for preoperative health status and tumor characteristics might be causes of those inconsistent results. To give an example, Poon et al. [16] observed a higher perioperative complication in the elderly. Notably, those complications were minor and only observed in the subgroup of skull base tumors [16] which were excluded from our study. Therefore, their overall results are consistent with ours. Mastronardi et al. [22] conducted a study on two age groups (≥ and <70) following posterior cranial fossa meningiomas. They reported no difference regarding postoperative complications between groups. Consistent results with our study could be due to the exclusion of patients with ASA >3 [22]. Therefore, regardless of age, it seems that the preoperative health conditions of the patients are crucial in postoperative outcomes. Larger-scale studies with standardized patient selection are necessary for future studies which enable proper comparisons and recognition of relevant prognostic factors.
5. Conclusion
This study suggests that, if selected wisely, postoperative complications from intracranial meningioma removal and outcomes of elderly patients could be comparable to younger patients. The definition of age, comorbidities, preoperative health status, surgical techniques, and different follow-up intervals were possible factors influencing the inconsistent outcomes observed between the two age groups in previous studies. Future studies should include a more homogenous patient population and increase the duration of follow-ups to delineate the appropriate prognostic factors helping the patient selection for surgical removal of meningioma.
Strength and limitations
The present study conducted a comparison of homogenous groups in terms of gender, tumor location, and preoperative health status measured by ASA score including a broad range of complications. The other strength of our study is that we measured the postoperative outcome using the standard and reliable score of GOS. Using this approach, we shed light on a critical clinical challenge in meningioma surgery.
The present study also had certain limitations that should be acknowledged. First, similar to all other small cohorts, results from this study might have diminished statistical power. Second, we did not adjust the groups based on the surgical techniques or tumor volume, both of which could influence the results. Third, the retrospective data collection is subject to typical limitations such as missing or incomplete information and lack of control over data quality decreasing the accuracy of the results. Also, we did not select a specific age group over the age of 75 years, and using medical records prevented the authors from using a more detailed functional outcome scale to have a better understanding of postoperative recovery.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1400.359).
Funding
This research was financially supported by Guilan University of Medical Sciences, Rasht, Iran.
Authors' contributions
All authors contributed to the study and preparation of this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Saeideh Aghayari Sheikh Neshin who assisted in the writing and technical editing of this manuscript.
References
- Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. The Oncologist. 2011; 16(11):1604-13. [DOI:10.1634/theoncologist.2011-0193] [PMID]
- Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2016-2020. Neuro-Oncology. 2023; 25(12 Suppl 2):iv1-iv99. [DOI:10.1093/neuonc/noad149] [PMID]
- United Nations. World Population Prospects 2024: Ten Key Messages. New York: United Nations, Department of Economic and Social Affairs, Population Division; 2024. [Link]
- Nakasu S, Notsu A, Nakasu Y. Prevalence of incidental meningiomas and gliomas on MRI: A meta-analysis and meta-regression analysis. Acta Neurochirurgica. 2021; 163(12):3401-15. [DOI:10.1007/s00701-021-04919-8] [PMID]
- Caruso G, Ferrarotto R, Curcio A, Metro L, Pasqualetti F, Gaviani P, et al. Novel advances in treatment of meningiomas: Prognostic and therapeutic implications. Cancers (Basel). 2023; 15(18):4521. [DOI:10.3390/cancers15184521] [PMID]
- Lemée JM, Corniola MV, Da Broi M, Schaller K, Meling TR. Early postoperative complications in meningioma: Predictive factors and impact on outcome. World Neurosurgery. 2019; 128:e851–8. [DOI:10.1016/j.wneu.2019.05.010] [PMID]
- Burkhardt JK, Zinn PO, Graenicher M, Santillan A, Bozinov O, Kasper EM, et al. Predicting postoperative hydrocephalus in 227 patients with skull base meningioma. Neurosurgical Focus. 2011; 30(5):E9. [DOI:10.3171/2011.3.FOCUS117] [PMID]
- Li LM, Zheng WJ, Chen YZ, Hu ZH, Liao W, Lin QC, et al. Predictive factors of postoperative peritumoral brain edema after meningioma resection. Neurology India. 2021; 69(6):1682-7. [DOI:10.4103/0028-3886.333500] [PMID]
- Ghazou A, Yassin A, Aljabali AS, Al-Zamer YS, Alawajneh M, Al-Akhras A, et al. Predictors of early and late postoperative seizures in meningioma patients: A systematic review and meta-analysis. Neurosurgical Review. 2024; 47(1):242. [DOI:10.1007/s10143-024-02487-w] [PMID]
- Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. Journal of the American College of Surgeons. 2006; 203(6):865-77. [DOI:10.1016/j.jamcollsurg.2006.08.026] [PMID]
- Ius T, Raffa G, Aiudi D, Panciani PP, Della Pepa GM, Pessina F, et al. From data to practice: Brain meningioma treatment in elderly patients - a survey of the Italian Society of Neurosurgery (SINch®) and systematic review and meta-analysis. Neurosurgical Review. 2024; 47(1):373. [DOI:10.1007/s10143-024-02524-8] [PMID]
- Ikawa F, Kinoshita Y, Takeda M, Saito T, Yamaguchi S, Yamasaki F, et al. Review of current evidence regarding surgery in elderly patients with meningioma. Neurologia Medico-Chirurgica. 2017; 57(10):521-33. [DOI:10.2176/nmc.ra.2017-0011] [PMID]
- Boviatsis EJ, Bouras TI, Kouyialis AT, Themistocleous MS, Sakas DE. Impact of age on complications and outcome in meningioma surgery. Surgical Neurology. 2007; 68(4):407-11. [DOI:10.1016/j.surneu.2006.11.071] [PMID]
- Bartek J Jr, Sjåvik K, Förander P, Solheim O, Gulati S, Weber C, et al. Predictors of severe complications in intracranial meningioma surgery: A population-based multicenter study. World Neurosurgery. 2015; 83(5):673-8. [DOI:10.1016/j.wneu.2015.01.022] [PMID]
- Zhao X, Zhao D, Wu Y, Gao W, Cui H, Wang Y, et al. Meningioma in the elderly: Characteristics, prognostic factors, and surgical strategy. Journal of Clinical Neuroscience. 2018; 56:143-9. [DOI:10.1016/j.jocn.2018.06.011] [PMID]
- Poon MT, Fung LH, Pu JK, Leung GK. Outcome comparison between younger and older patients undergoing intracranial meningioma resections. Journal of Neuro-Oncology. 2013; 114(2):219-27. [DOI:10.1007/s11060-013-1173-8] [PMID]
- Slot KM, Peters JVM, Vandertop WP, Verbaan D, Peerdeman SM. Meningioma surgery in younger and older adults: Patient profile and surgical outcomes. European Geriatric Medicine. 2018; 9(1):95-101. [DOI:10.1007/s41999-017-0015-1] [PMID]
- Schul DB, Wolf S, Krammer MJ, Landscheidt JF, Tomasino A, Lumenta CB. Meningioma surgery in the elderly: Outcome and validation of 2 proposed grading score systems. Neurosurgery. 2012; 70(3):555-65. [DOI:10.1227/NEU.0b013e318233a99a] [PMID]
- Amano T, Nakamizo A, Michiwaki Y, Matsuo S, Fujioka Y, Nagata S. Surgical outcome in elderly patients with intracranial meningioma. Journal of Clinical Neuroscience. 2018; 56:63-6. [DOI:10.1016/j.jocn.2018.07.009] [PMID]
- Brokinkel B, Holling M, Spille DC, Heß K, Sauerland C, Bleimüller C, et al. Surgery for meningioma in the elderly and long-term survival: Comparison with an age- and sex-matched general population and with younger patients. Journal of Neurosurgery. 2017; 126(4):1201-11. [DOI:10.3171/2016.2.JNS152611] [PMID]
- Meling TR, Da Broi M, Scheie D, Helseth E. Skull base versus non-skull base meningioma surgery in the elderly. Neurosurgical Review. 2019; 42(4):961-72. [DOI:10.1007/s10143-018-1005-6] [PMID]
- Mastronardi L, Campione A, Alomari AA. Posterior cranial fossa meningiomas: Comparison of results between patients older and younger than 70 years. Brain & Spine. 2024; 4:102790. [DOI:10.1016/j.bas.2024.102790] [PMID]
- American Society of Anesthesiologists (ASAHQ). Statement on ASA Physical Status Classification System. Illinois: American Society of Anesthesiologists; 2020. [Link]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1(7905):480-4. [DOI:10.1016/S0140-6736(75)92830-5] [PMID]
- Caroli M, Locatelli M, Prada F, Beretta F, Martinelli-Boneschi F, Campanella R, et al. Surgery for intracranial meningiomas in the elderly: A clinical-radiological grading system as a predictor of outcome. Journal of Neurosurgery. 2005; 102(2):290-4. [DOI:10.3171/jns.2005.102.2.0290] [PMID]
- Chen ZY, Zheng CH, Tang Li, Su XY, Lu GH, Zhang CY, et al. Intracranial meningioma surgery in the elderly (over 65 years): Prognostic factors and outcome. Acta Neurochirurgica. 2015; 157(9):1549-57. [DOI:10.1007/s00701-015-2502-9] [PMID]
- Konglund A, Rogne SG, Lund-Johansen M, Scheie D, Helseth E, Meling TR. Outcome following surgery for intracranial meningiomas in the aging. Acta Neurologica Scandinavica. 2013; 127(3):161-9. [DOI:10.1111/j.1600-0404.2012.01692.x] [PMID]
- Armocida D, Catapano A, Palmieri M, Arcidiacono UA, Pesce A, Cofano F, et al. The surgical risk factors of giant intracranial meningiomas: A multi-centric retrospective analysis of large case serie. Brain Sciences. 2022; 12(7):817. [DOI:10.3390/brainsci12070817] [PMID]
- Engel DC, Gawellek L, Peraio S, Stanojevic M, Tatagiba M, Ebner FH. Spinal meningioma surgery in the elderly: Who can benefit from it? Journal of Neurosurgical Sciences. 2021; 65(4):408-13. [DOI:10.23736/S0390-5616.18.04582-4] [PMID]
- Poon MT, Fung LH, Pu JK, Leung GK. Outcome of elderly patients undergoing intracranial meningioma resection--a systematic review and meta-analysis. British Journal of Neurosurgery. 2014; 28(3):303-9. [DOI:10.3109/02688697.2013.841857] [PMID]
- Monden D, Raimann FJ, Neef V, Dubinski D, Gessler F, Keil F, et al. Meningioma surgery in patients ≥70 years of age: Clinical outcome and validation of the SKALE Score. Journal of Clinical Medicine. 2021; 10(9):1820. [DOI:10.3390/jcm10091820] [PMID]
- Yamamoto J, Takahashi M, Idei M, Nakano Y, Soejima Y, Akiba D, et al. Clinical features and surgical management of intracranial meningiomas in the elderly. Oncology Letters. 2017; 14(1):909-17. [DOI:10.3892/ol.2017.6174] [PMID]
- Löfgren D, Valachis A, Olivecrona M. Older meningioma patients: A retrospective population-based study of risk factors for morbidity and mortality after neurosurgery. Acta Neurochirurgica. 2022; 164(11):2987-97. [DOI:10.1007/s00701-022-05336-1] [PMID]
- Ahmeti H, Borzikowsky C, Hollander D, Röcken C, Jansen O, Synowitz M, et al. Risks and neurological benefits of meningioma surgery in elderly patients compared to young patients.Journal of Neuro-Oncology. 2021; 154(3):335-44. [DOI:10.1007/s11060-021-03832-5] [PMID]
- Ekşi MŞ, Canbolat Ç, Akbaş A, Özmen BB, Akpınar E, Usseli Mİ, et al. Elderly patients with intracranial meningioma: surgical considerations in 228 patients with a comprehensive analysis of the literature. World Neurosurgery. 2019; 132:e350-e65. [DOI:10.1016/j.wneu.2019.08.150] [PMID]
Type of Study: Research |
Subject:
Brain Tumors
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



