Sun, Oct 19, 2025
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 1-11 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haddadi K, Tahmasbi N, Alaee A, Alipour A, Ehteshami S. Comparison of Vicious Effect of Oral Pantoprazole and
Famotidine on New Bone Formation in Patients With
Lumbar Spine Fusion Surgery: A Randomized Control Trial. Iran J Neurosurg 2024; 10 : 1
URL: http://irjns.org/article-1-366-en.html
URL: http://irjns.org/article-1-366-en.html
1- Department of Neurosurgery, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Orthopedics Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
3- Orthopedics Research Center, Mazandaran University of Medical Sciences, Sari, Iran. ,paper87@yahoo.com
2- Orthopedics Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
3- Orthopedics Research Center, Mazandaran University of Medical Sciences, Sari, Iran. ,
Full Text [PDF 1234 kb]
(1519 Downloads)
| Abstract (HTML) (3680 Views)
Full Text: (1283 Views)
1. Introduction
Arthrodesis or fusion in the spine is one of the most common surgical processes used to treat several spinal diseases, such as spondylolisthesis, lumbar stenosis, and spinal deformity. An epidemiologic study in the United States revealed a growth of 2.4 times in the number of fusion processes of the spine starting from 1998 to 2008 [1]. Numerous methods, as well as several surgical procedures, different types of grafts, and instrumentation, have been used for the reconstruction of the spine. Regardless of the technique used to operate on the spine, the goal is a bone insertion (allogeneic or artificial) in the moving segment, and prompting bone restoration. Conditions, like dysentery, arthritis, and osteoarthritis of the spine can impair the process of repair and quality of life [2-6].
Medications for suppressing stomach acid production and secretion that are commonly suggested to the general public consist of proton pump inhibitors (PPIs), which constrain the gastric proton pump and prevent the secretion of acid from the gastric wall cells, and are widely used in several gastrointestinal disorders [7-9]. Other gastric acid inhibitors include histamine H2 receptor antagonists, like cimetidine and famotidine. Previous studies have shown that cimetidine improves symptoms of calcification of the shoulder tendons [10]. The prolonged use of PPIs can cause side effects, such as the inhibited reabsorption of vitamin B12 and absorption of calcium [11-13], decreased bone resorption, osteoporosis, and amplified chance of fractures [14, 15]. Pantoprazole was introduced to the pharmaceutical market in 1997, following other PPIs, like omeprazole and lansoprazole [16]. The persistent use of pantoprazole delays fracture healing by disturbing new bone formation and bone restoration as a result of decreased osteoblast activity and proliferation [17].
Due to the potentially common paths between fracture repair and lumbar fusion, it is theorized that the risk factors of delayed bone repair and non-:union: in trauma are comparable to those of the lumbar fusion process [18]. Lumbar fusion is a surgical decision preferred for patients with spondylolisthesis, severe spinal stenosis, spinal instability, or degenerative scoliosis since the lumbar vertebrae remain stable in this procedure and enhance vertebral consistency and alignment [18, 19]. Lumbar fusion was first performed in the early 1900s on the thoracolumbar spine to treat Pott’s disease caused by tuberculosis [19, 20]. Even with the developments in surgical techniques and instruments, non-:union: still occurs in 24% of patients, who end up needing reoperation [19, 20]. Various risk factors, including infections, smoking, bone metabolism disorders, and vitamin D deficiency, are involved in pseudoarthrosis [21, 22]. Pseudoarthrosis is asymptomatic in about 30% of the patients while 45% to 56% complain of refractory pain following surgery [18, 19].
Due to the increasing consumption of PPIs and their potential side effects for bone repair and the high percentage of therapy failure in lumbar fusion surgery, various studies have been done on the influence of PPIs on lumbar fusion. However, they have only assessed animal models to investigate the effect of these drugs on bone formation [18]. Since no studies have yet been led to assess the effects of PPIs on the success rate and complications after lumbar fusion surgery in human models, this study intended to determine the effect of oral pantoprazole and famotidine on new bone formation in patients undergoing surgery for spine degenerative diseases.
2. Materials and Methods
Protocol review
This study is a randomized clinical trial that involves a twelve-month evaluation of the effects of famotidine and pantoprazole on new bone marrow formation in patients undergoing lumbar fusion surgery at Imam Khomeini Hospital in Sari, Iran.
Each patient’s legally authorized representative signed an informed consent form, and the researchers also obtained consent from the patient’s primary caregivers before starting medication therapy. The patients’ information was kept confidential, and the researchers adhered to the principles outlined in the Declaration of Helsinki. Demographic information, including age, sex, weight, height, type of surgery, and primary disease diagnosis, was recorded in a dedicated questionnaire.
Subjects
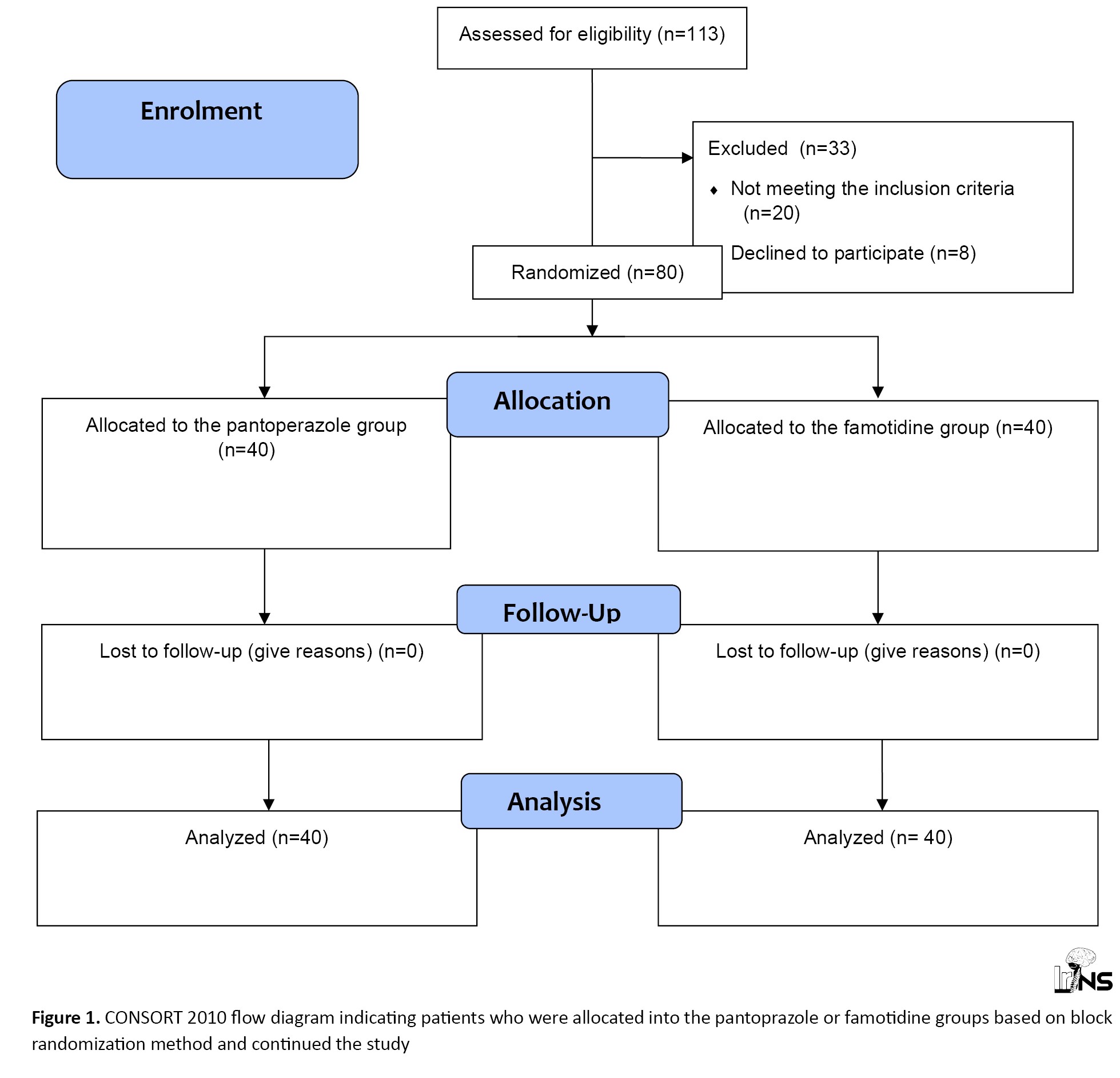
A total of 113 patients admitted to the neuro-spine department of the hospital with degenerative spinal disorder requiring spinal decompression, instrumentation, and interbody fusion were enrolled in this randomized clinical trial over a three-year period. According to our estimated sample size determined by G-power software version 3.1.9.7, out of the 80 patients who were allocated to the pantoprazole or famotidine groups based on the block randomization method, no patient missed the follow-up during the study. Overall, 80 patients completed the intervention, and their data were fully analyzed (Figure 1).
Inclusion criteria
Patients with a body mass index (BMI) of 20 to 35 and degenerative diseases requiring lumbar fusion surgery, including spondylolisthesis, lumbar spinal canal stenosis, spinal deformity, lumbar disc disease, and age between 30-65 years were included in the study.
Exclusion criteria
The exclusion criteria were as follows: 1) Spine injury due to infection, tumor, inflammatory diseases, and trauma, 2) Diabetes, 3) Heavy smokers, 4) History of severe osteoporosis, 5) Not consenting to participation in the study. Participants receiving PPIs or famotidine six months before the study.
Pharmacological agents
The patients were divided into two groups through block randomization. One group received oral pantoprazole (group P) (n=40), and the other received famotidine (group F) (n=40), with each group receiving a daily dose of 40 mg. Both groups underwent an eight-week treatment following their lumbar fusion surgery. The prescription drugs, including painkillers and antibiotics, were identical for all patients. Autografts were used during the surgery to enhance fusion in all cases.
Upon admission, the primary outcome measures included the assessment of pain using the visual analogue scale (VAS) and disability using the Oswestry disability index (ODI). A comprehensive medical history was obtained from each patient, and all patients underwent a detailed physical and neurological examination.
The primary endpoint for measuring bone-related outcomes included subsidence, loosening, and the Brantigan, Steffee, Fraser (BSF), and Lenke scores, which were determined based on spinal CT scans [22].
BSF as the classification of interbody fusion success
BSF-1: Radiographic pseudarthrosis is defined as the collapse of the construct, loss of disc height, vertebral slip, broken screws, displacement of the carbon cage, or significant resorption of the bone. Also, graft or lucency is visible around the periphery of the graft or cage.
BSF-2: Radiographical locked pseudarthrosis is defined as lucency visible in the middle of the cages with solid bone growing into the cage from each vertebral endplate.
BSF-3: Radiographic fusion is defined as bone bridges at least half of the fusion area with at least the density originally achieved at surgery. Radiographic fusion through one cage (half of the fusion area) indicates a mechanically solid fusion even if there is lucency on the opposite side.
Lenke’s classification of posterolateral fusion success
Grade A: Definitely solid with bilateral trabeculated stout fusion masses; grade B: Possibly solid with a unilateral large fusion mass and a contralateral small fusion mass; grade C: Probably not solid with a small fusion mass bilaterally; grade D: Definitely not solid with bone graft resorption or obvious pseudarthrosis
bilaterally.
Statistical analysis
The Shapiro-Wilk test was utilized to assess the normal distribution of the data. Descriptive statistics for both groups (pantoprazole and famotidine) were compared and presented as percentages, medians (interquartile range) and Mean±SD. The collected data were then subjected to a comparative analysis between the two groups, utilizing statistical tests such as the chi-square or Fisher’s exact tests for categorical data and the t-test or Mann-Whitney U-test for continuous data. Furthermore, repeated-measures regression analysis, specifically the generalized estimated equation (GEE), was employed to evaluate the trend of changes in the outcome parameters between the two treatment modalities. A significance level of P<0.05 was considered statistically significant. Statistical analyses were conducted using IBM SPSS software, version 24.
3. Results
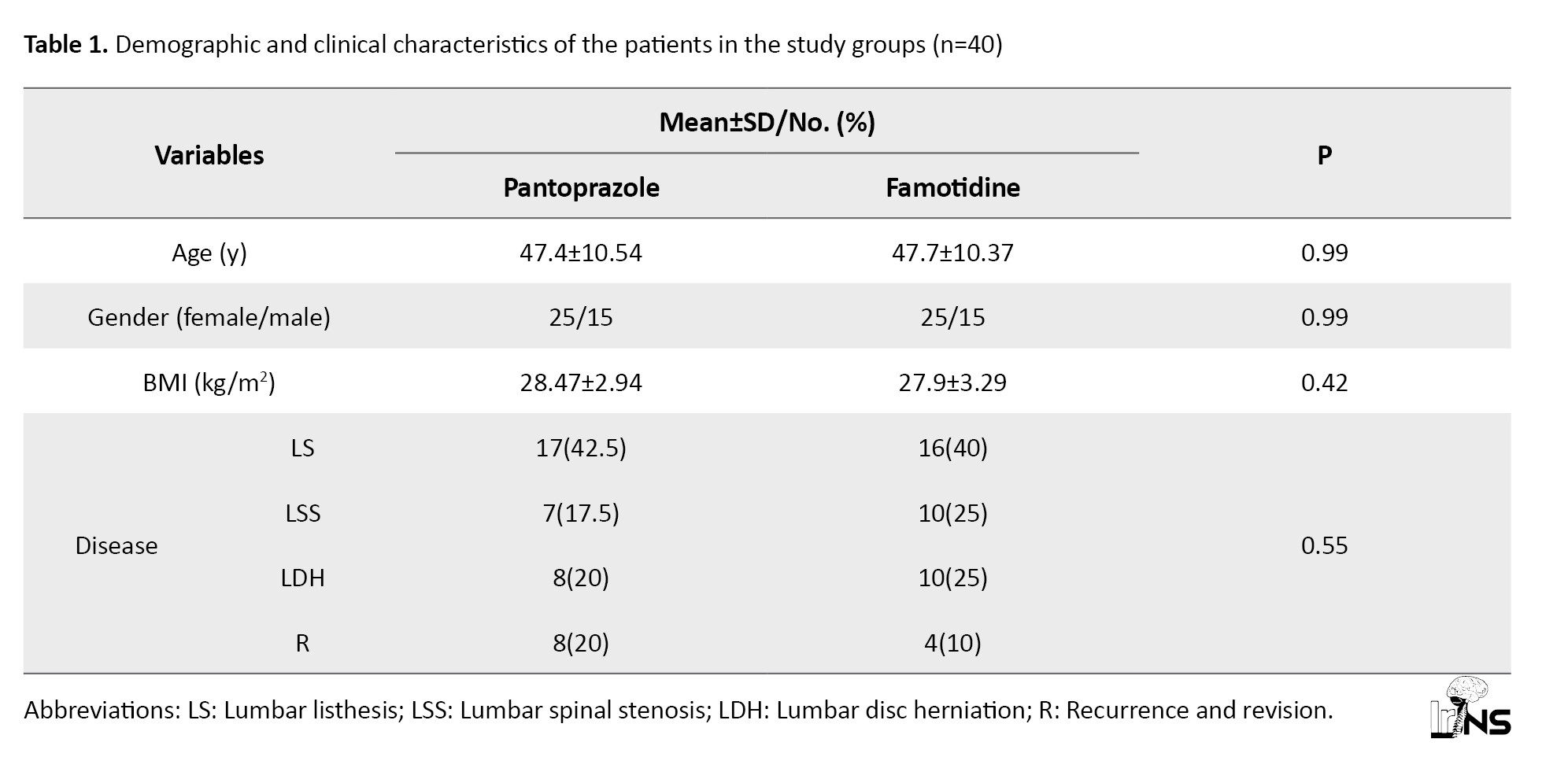
Eighty patients were included in this study based on specific inclusion and exclusion criteria. Among them, 40 received pantoprazole (group P), and 40 received famotidine (group F). The Mean±SD age was 47.4±10.54 years in group P and 47.7±10.37 years in group F, with no significant age difference between the two groups (P=0.99).
In group P, 62.5% of the participants were female, and 37.5% were male. Similarly, in group F, 62.5% were female, and 37.5% were male, with no significant gender difference between the two groups (P>0.99).
The Mean±SD BMI was 28.47±2.94 in group P and 27.9±3.29 in Group F, respectively. There was no significant difference in BMI between the two groups (P=0.42). The surgical indications, including spondylolisthesis, lumbar spinal canal stenosis, and herniated disc, were the most common in both groups, and there was no significant difference in surgical indications between the two groups (P=0.55).
Table 1 provides detailed information on the basic clinical characteristics and demographic features of the patients in both groups.
The observed differences in demographic characteristics, such as age (P=0.9), BMI (P=0.42), and the primary disease requiring surgery (P=0.55), were not statistically significant.
VAS score: Table 2 presents the mean VAS scores in the two groups.
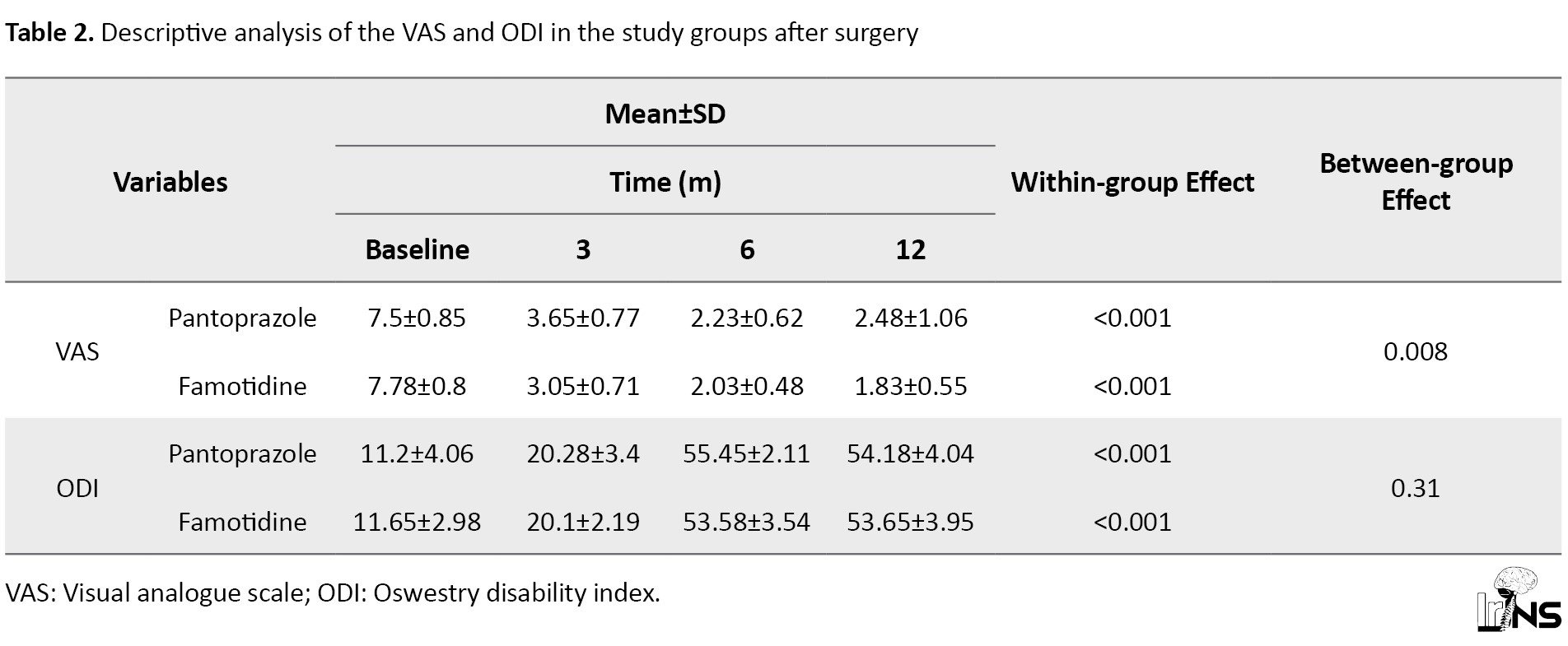
This score decreased significantly after six and twelve months with both therapies. Twelve months after the therapies, the mean percentage of reduction in VAS score from baseline was 66.93% in group P and 76.48% in group F. The GEE used for analysis of the influence of the treatments after adjusting for the VAS score at baseline and age showed a difference in the mean VAS scores between the two groups (a significant reduction was perceived in the VAS score of group F in comparison with group P; P=0.0.008).
ODI score: Table 2 presents the mean ODI scores in both groups. The mean ODI score increased significantly after six and twelve months with both therapies. Twelve months after the therapies, the mean percentage of improvement in ODI scores from baseline was 383.75% in group P and 360.52% in group F. The GEE used for testing the effect of the therapies after adjusting for the ODI score at baseline and age revealed no difference in the mean ODI scores between the two groups (no significant improvement was observed in the ODI score of group F in comparison with group P; P=0.31).
Subsidence: Table 3 presents the prevalence of positive subsidence scores in both groups.
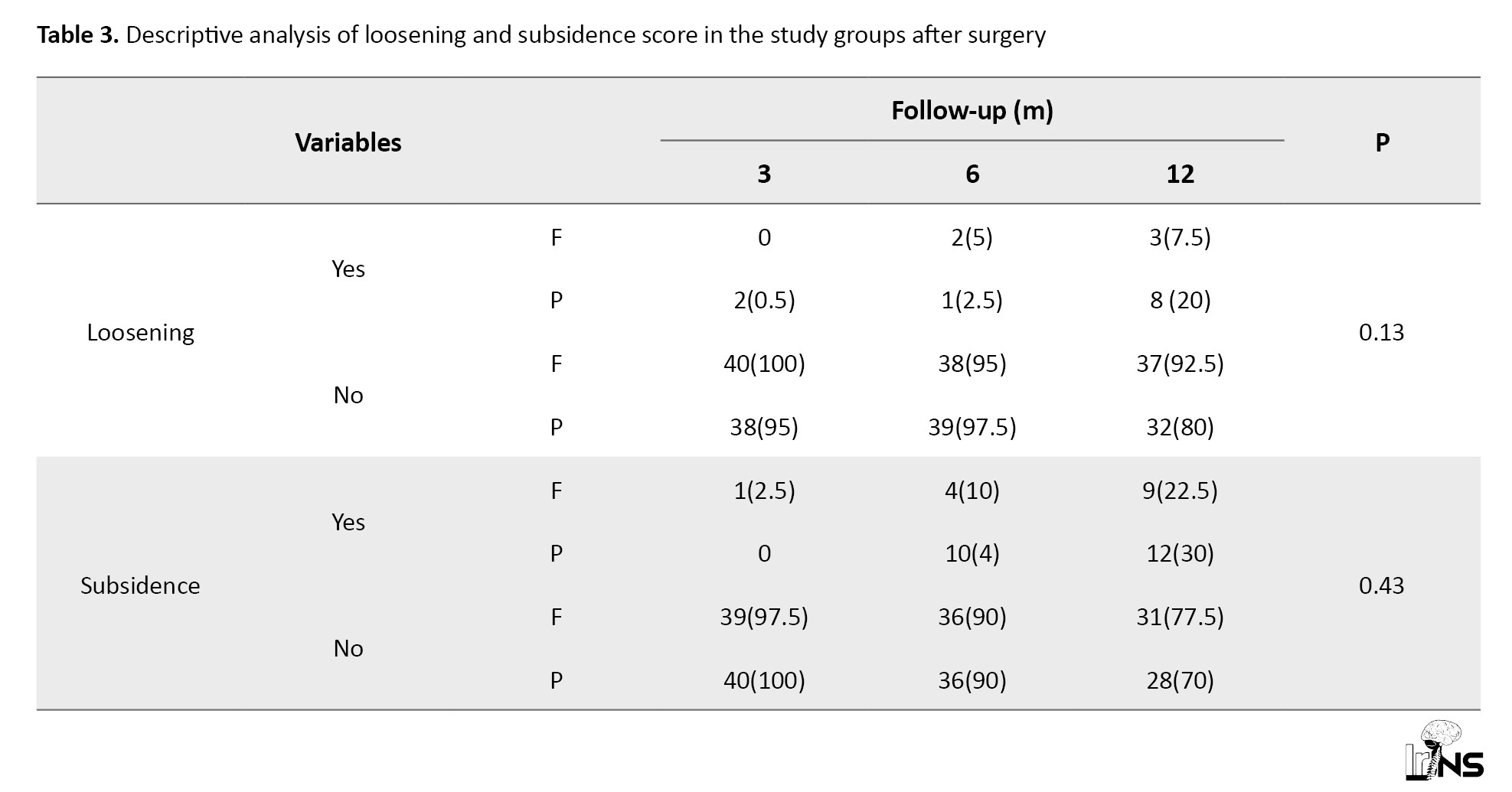
The prevalence of positive subsidence scores increased significantly after six and twelve months in both groups. After 12 months, the increase in the prevalence of positive subsidence size from baseline was 30% in group P and 20% in group F. The difference observed between the two groups was not statistically significant (P=0.43).
Loosening: The prevalence of positive loosening scores increased significantly after six and twelve months with both therapies, but this increasing trend was significant only in group P (P=0.005). After 12 months of treatment, the mean proportion of improvement in the LOOS scores from baseline was 15% in group P and 7.5% in group F. The GEE used for analysis of the effect of the therapies after adjusting for the LOOS score at baseline and age revealed no difference in the mean LOOS scores between the two groups (no significant improvement was observed in the LOOS score in group P in comparison with group F; P=0.13; Table 3).
BSF score: Table 4 presents the prevalence of the BSF grades in the two groups.
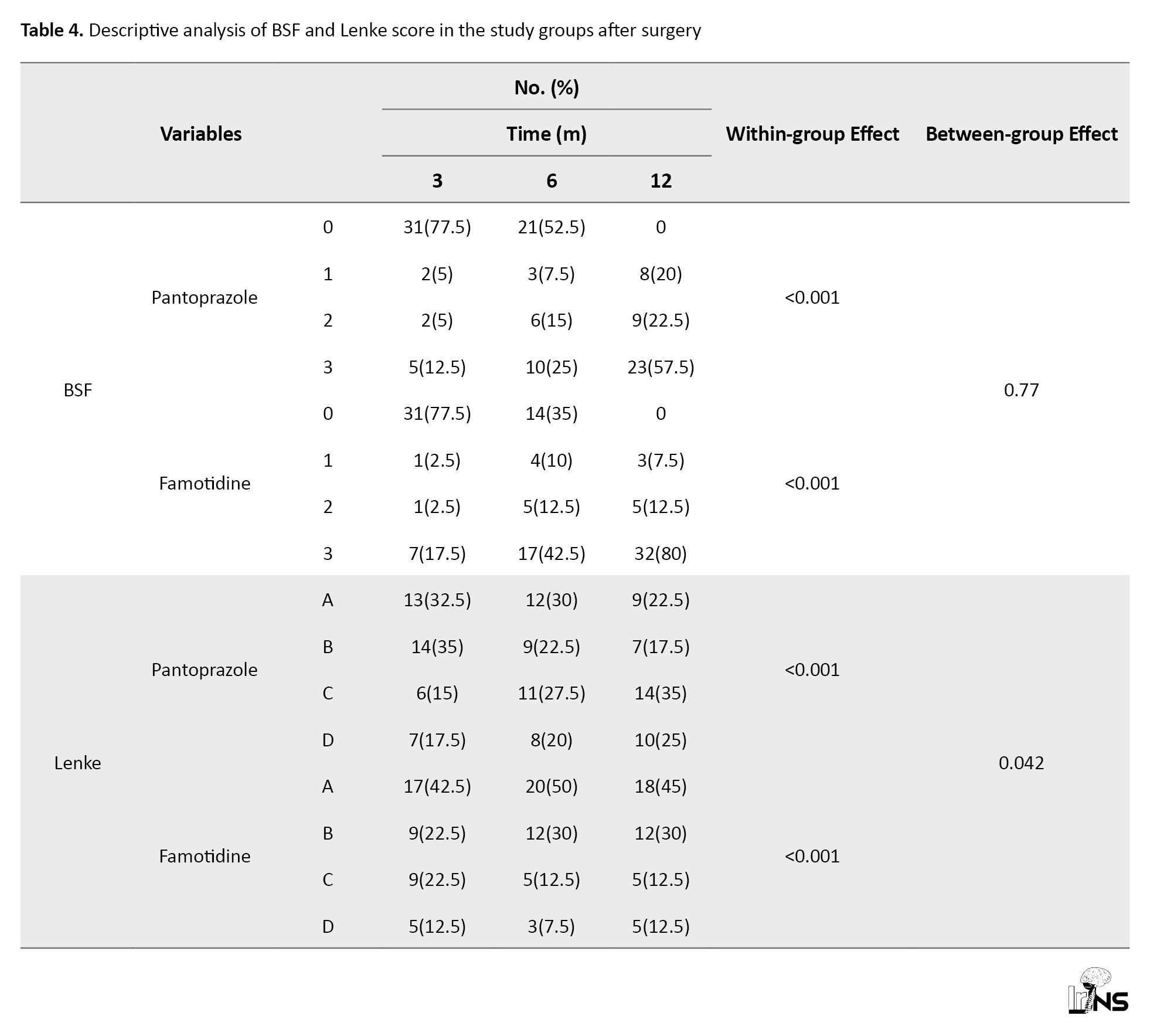
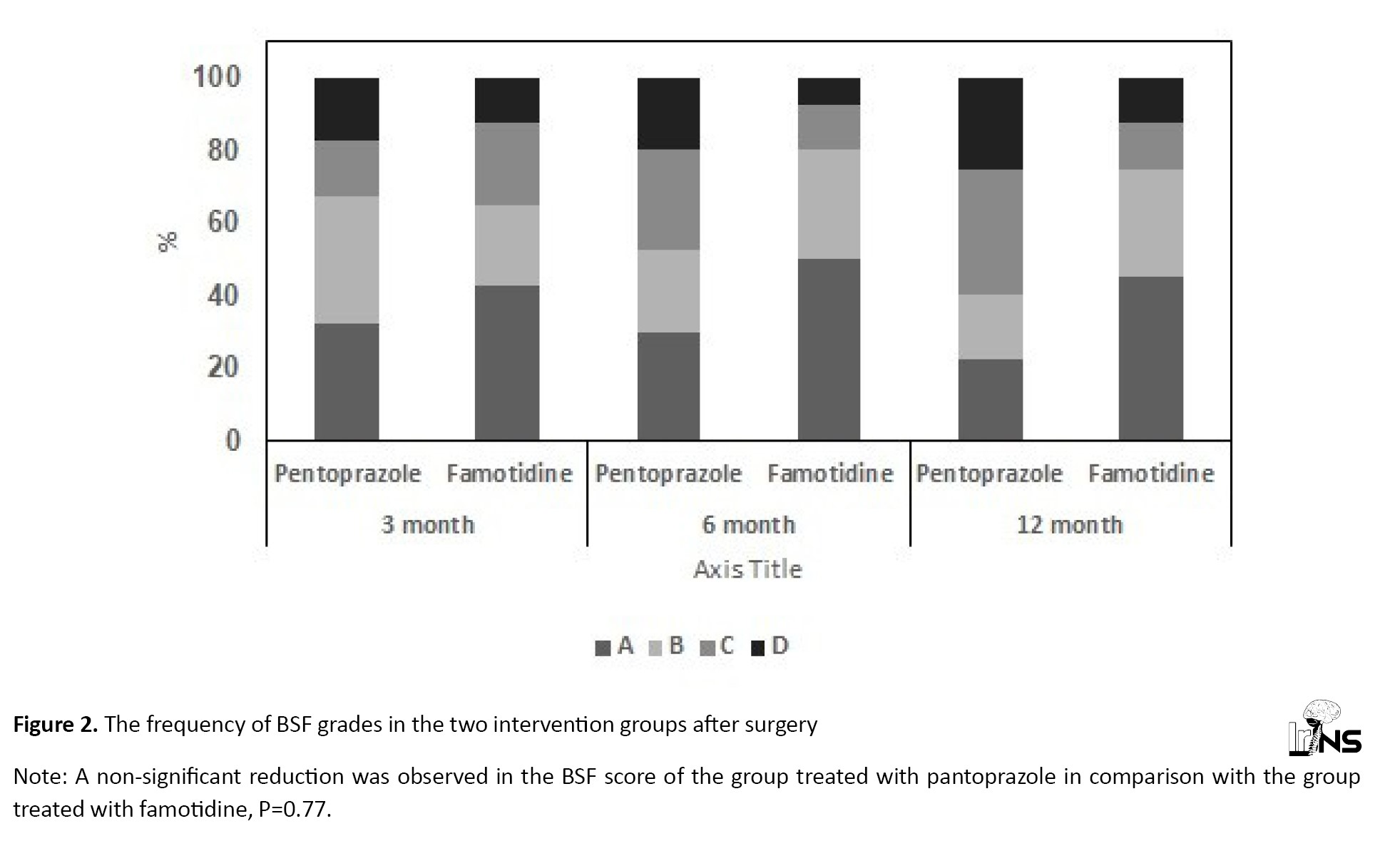
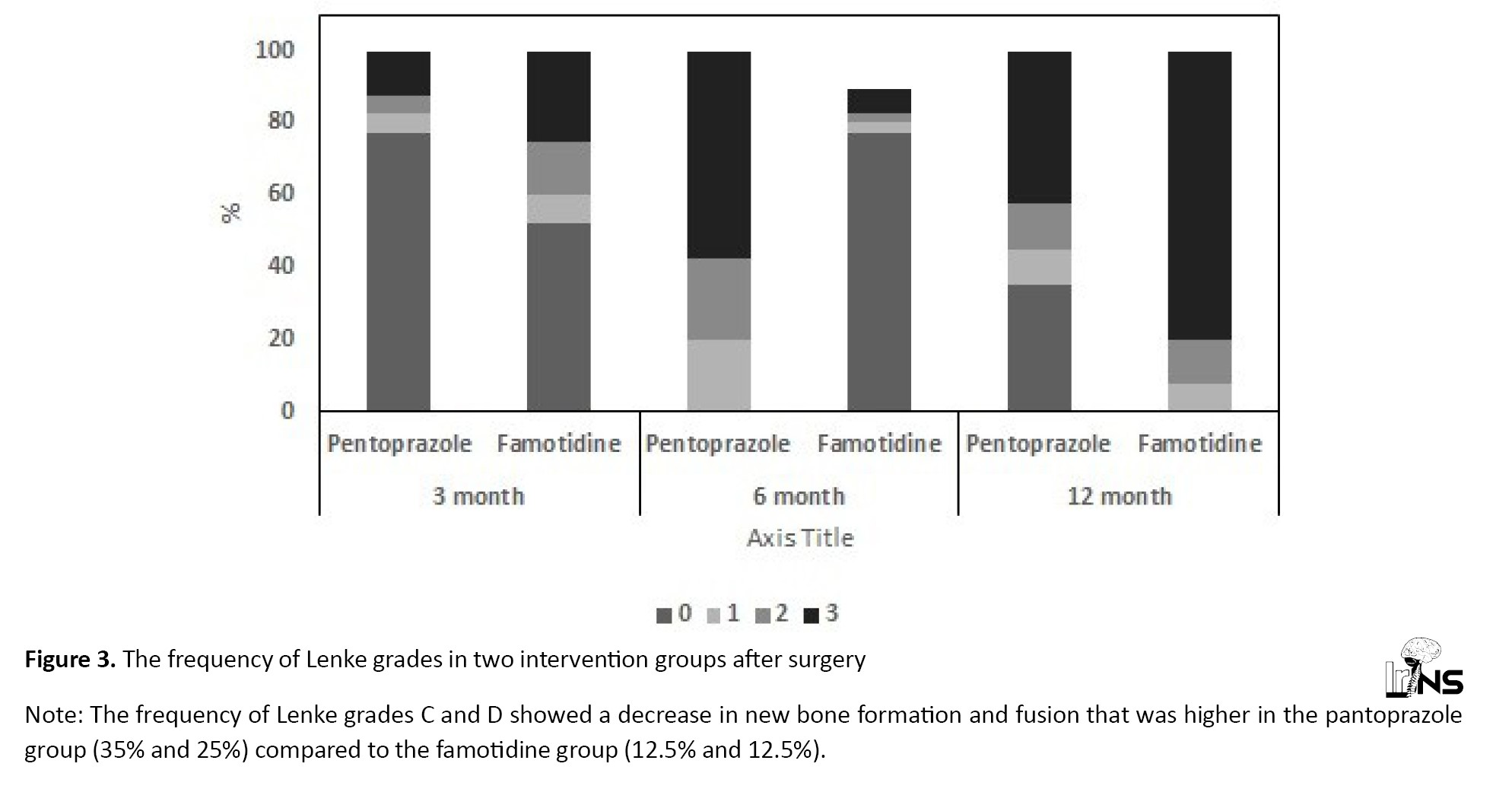
The prevalence of low-grade BSF decreased significantly after six and twelve months with both therapies. After 12 months of therapy, the mean percentage of reduction in BSF grade from baseline was 77.5% in both groups. The GEE used for testing the effect of the therapies after adjusting for the BSF score at baseline and age showed no difference in the mean BSF scores between the two groups (no significant reduction was detected in the BSF score of group P in comparison with group F; P=0.77 Figure 2). Lenke score: Table 4 presents the prevalence of the Lenke grades in both groups. The prevalence of low-grade Lenke decreased significantly after six and twelve months with both therapies. After twelve months, 52.5% of the patients in group P and 67.5% in group F were classified in grade A of the Lenke classification. The GEE used to measure the effect of the treatments after adjusting for the Lenke grade at baseline and time showed a significant difference between the two treatments. The frequency of Lenke grade C and D showed a decrease in new bone formation and fusion that was higher in group P (35% and 25%, respectively) compared to group F (12.5% and 12.5%, respectively) (P=0.042; Figure 3). Both drugs were well tolerated at the dosage used during the study. There were no thoughtful adverse effects, and both seemed quite safe. No patient mandatory changed the dosage throughout the trial for even a minor adverse effect. There were no significant changes in laboratory standards during the study or in any of the safety parameters.
4. Discussion
Prior to planning spinal fusion surgery, spinal surgeons meticulously optimize all preoperative, intraoperative, and postoperative conditions to maximize the likelihood of achieving a successful fusion rate. Significant advancements have also been made in surgical techniques, precision instruments, and biological supplements that contribute to the prospects of a successful fusion. Despite these efforts, pseudoarthrosis still occurs in 25% of patients, necessitating reoperation in some cases, with figures as high as 50% reported [23-25].
Non-:union: and pseudoarthrosis are complex complications with multifactorial origins, necessitating the identification of their risk factors. PPIs play a role in inhibiting the absorption of calcium and vitamin B12, resulting in reduced bone mineral density and an increased risk of fractures [25-27].
In a rat femur fracture model, Histing et al. [17] demonstrated bone non-:union: in the treatment of fractures. They attributed this finding to a decrease in bone regeneration, characterized by an increase in the OPG/RANKL ratio and a decrease in the expression of CYR61, PCNA, BMP-2, and BMP-4.
Numerous mechanisms are involved in the increased risk of pseudoarthrosis due to smoking, including the effects of nicotine and dioxin [28, 29] as they prevent osteoblast differentiation. Therefore, it is crucial to investigate other substances that undesirably disturb osteoblast differentiation. The belongings of dioxin are facilitated by the aryl hydrocarbon receptor (AHR) [28, 29].
PPIs are not only conjectured to adversely distress osteoblast differentiation but they are also identified as ligands activating AHR [29, 30]. This association proves the theory that PPIs and H2RA have an adverse effect on spinal fusion. To date, no previous human studies have evaluated the effect of PPI and H2RA administration on spinal fusion.
Assuming the global use of antacids and the substantial reduction in quality of life associated with fractures [31], studying the association between antacids and fractures is of great clinical importance. Although previous studies and FDA reports have shown an association between PPIs and fracture risk, this concern is still discussed [32].
Some studies have recently been issued on this subject, although with contradictory outcomes. A case-control study completed ten years and established that PPI use increases the risk of pelvic fractures, particularly in the long term and with recent usage [33]. An additional multicenter cohort study with 9423 members showed that there was a low risk of fractures in PPI customers [34]. Nonetheless, a case-control study conducted in the Mediterranean area stated no link between these variables [35].
In a study on animal models, 38 female rats experienced posterolateral lumbar spinal fusion. These animals were allocated into two groups: Pantoprazole-receiving cases and Normal saline controls managed daily via intraperitoneal injections. At eight weeks after surgery, the spines were appraised with histologic analysis, micro CT, and biomechanical testing. The study confirmed that the use of PPIs does not stop fusion rates and bone formation or disturb the biomechanical integrity of fusion; however, the lesser fusion scores in the PPI group recommend that an undesirable effect might exist [18].
The mechanism of the relationship between acid-suppressing medications and the danger of fracture is largely unknown [36]. PPIs are associated with impaired calcium absorption and loss of bone minerals, thus increasing the risk of fractures [37]. Nevertheless, studies evaluating the relationship between acid inhibition and bone mineral density have shown diverse results. Some studies have reported a slight decrease in bone density in PPI users; however, there have been no important differences in bone mineral density points between PPI consumers and controls in other studies [38].
Likewise, acid suppressants have been described to disturb bone modeling, which is involved in fracture progression [39]. Omeprazole has been stated to inhibit calcium absorption and decrease bone mineral density in rat models. Calcium malabsorption similarly increases parathyroidism, thus reducing bone mineral density [40-43]. Nonetheless, there is still no definite indication of the sustenance of these conceivable mechanisms.
Based on the findings of this study, a significant relationship was observed only between drug administration and VAS at 3, 6 and 12 months after fusion surgery (P<0.05) and the patients receiving famotidine had a lower VAS score compared to the group receiving pantoprazole. Taking pantoprazole could have a negative effect on the severity of postoperative pain. The results also showed the interaction of time and medication and the interaction of age and baseline VAS on patients’ VAS scores. Thus, by controlling the effect of time and baseline VAS, this effect may not be solely attributable to the drug.
There was no significant difference between the two groups in new ossification and fusion quality measures; however, according to the results, there was a significant difference in changes in Lenke grade 3, 6 and 12 months after surgery. New bone formation in the pantoprazole-receiving group was impaired significantly, although this issue did not cause many clinical referrals due to the severity of complications after surgery. Moreover, BSF grading is specific for interbody fusion and cannot be generalized to patients undergoing posterolateral fusion surgery alone.
The administration of a standard dose of intervention drugs was one of the limitations of this study. Due to the limited human studies on this subject and similar studies with animal models, the dose received in patients may be lower than the standard dose, which is probable to have a destructive consequence on fusion and new ossification. In addition, we recommend that a control group be added to future studies to design a detailed study without further debate.
5. Conclusion
In agreement with the initial findings of the study, it appears that in candidates for spinal fusion procedures who require chronic use of drugs to control their gastric acid secretion, the use of PPIs, such as pantoprazole has more detrimental effects on the bone formation process, and H2-blocking drugs, such as famotidine are preferable in these instances after further investigations. The researchers recommend conducting a study with a larger sample size and an extended follow-up.
Ethical Considerations
Compliance with ethical guidelines
This study was approved and registered by the Research Ethics Committee of Imam Khomeini Hospital (Code: IR.MAZUMS.IMAMHOSPITAL.REC.1399.039). The project was registered as a clinical trial with the Iranian Registry of Clinical Trials (IRCT) (CODE: IRCT20140915019185N4), and the study design was based on the CONSORT 2010 checklist items. Each patient’s legally authorized representative signed an informed consent form, and the researchers also obtained consent from the patient’s primary caregivers before starting medication therapy.
Funding
This study was extracted from medical dissertation of Navid Tahmasebi, approved by Orthopedic Research Center of Imam Khomeini Hospital, Mazandaran University of Medical Sciences and was financially supported by Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization and study design: Kaveh Haddadi, Abdolrasool Alaee and Abbas Alipour; Data collection and writhing the original draft: Kaveh Haddadi, Saeed Ehteshami and Navid Tahmasbi; Statistical analysis: Abbas Alipour; Final approval: Kaveh Haddadi and Abbas Alipour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express their gratitude to all the patients who participated in the project.
References
Arthrodesis or fusion in the spine is one of the most common surgical processes used to treat several spinal diseases, such as spondylolisthesis, lumbar stenosis, and spinal deformity. An epidemiologic study in the United States revealed a growth of 2.4 times in the number of fusion processes of the spine starting from 1998 to 2008 [1]. Numerous methods, as well as several surgical procedures, different types of grafts, and instrumentation, have been used for the reconstruction of the spine. Regardless of the technique used to operate on the spine, the goal is a bone insertion (allogeneic or artificial) in the moving segment, and prompting bone restoration. Conditions, like dysentery, arthritis, and osteoarthritis of the spine can impair the process of repair and quality of life [2-6].
Medications for suppressing stomach acid production and secretion that are commonly suggested to the general public consist of proton pump inhibitors (PPIs), which constrain the gastric proton pump and prevent the secretion of acid from the gastric wall cells, and are widely used in several gastrointestinal disorders [7-9]. Other gastric acid inhibitors include histamine H2 receptor antagonists, like cimetidine and famotidine. Previous studies have shown that cimetidine improves symptoms of calcification of the shoulder tendons [10]. The prolonged use of PPIs can cause side effects, such as the inhibited reabsorption of vitamin B12 and absorption of calcium [11-13], decreased bone resorption, osteoporosis, and amplified chance of fractures [14, 15]. Pantoprazole was introduced to the pharmaceutical market in 1997, following other PPIs, like omeprazole and lansoprazole [16]. The persistent use of pantoprazole delays fracture healing by disturbing new bone formation and bone restoration as a result of decreased osteoblast activity and proliferation [17].
Due to the potentially common paths between fracture repair and lumbar fusion, it is theorized that the risk factors of delayed bone repair and non-:union: in trauma are comparable to those of the lumbar fusion process [18]. Lumbar fusion is a surgical decision preferred for patients with spondylolisthesis, severe spinal stenosis, spinal instability, or degenerative scoliosis since the lumbar vertebrae remain stable in this procedure and enhance vertebral consistency and alignment [18, 19]. Lumbar fusion was first performed in the early 1900s on the thoracolumbar spine to treat Pott’s disease caused by tuberculosis [19, 20]. Even with the developments in surgical techniques and instruments, non-:union: still occurs in 24% of patients, who end up needing reoperation [19, 20]. Various risk factors, including infections, smoking, bone metabolism disorders, and vitamin D deficiency, are involved in pseudoarthrosis [21, 22]. Pseudoarthrosis is asymptomatic in about 30% of the patients while 45% to 56% complain of refractory pain following surgery [18, 19].
Due to the increasing consumption of PPIs and their potential side effects for bone repair and the high percentage of therapy failure in lumbar fusion surgery, various studies have been done on the influence of PPIs on lumbar fusion. However, they have only assessed animal models to investigate the effect of these drugs on bone formation [18]. Since no studies have yet been led to assess the effects of PPIs on the success rate and complications after lumbar fusion surgery in human models, this study intended to determine the effect of oral pantoprazole and famotidine on new bone formation in patients undergoing surgery for spine degenerative diseases.
2. Materials and Methods
Protocol review
This study is a randomized clinical trial that involves a twelve-month evaluation of the effects of famotidine and pantoprazole on new bone marrow formation in patients undergoing lumbar fusion surgery at Imam Khomeini Hospital in Sari, Iran.
Each patient’s legally authorized representative signed an informed consent form, and the researchers also obtained consent from the patient’s primary caregivers before starting medication therapy. The patients’ information was kept confidential, and the researchers adhered to the principles outlined in the Declaration of Helsinki. Demographic information, including age, sex, weight, height, type of surgery, and primary disease diagnosis, was recorded in a dedicated questionnaire.
Subjects
A total of 113 patients admitted to the neuro-spine department of the hospital with degenerative spinal disorder requiring spinal decompression, instrumentation, and interbody fusion were enrolled in this randomized clinical trial over a three-year period. According to our estimated sample size determined by G-power software version 3.1.9.7, out of the 80 patients who were allocated to the pantoprazole or famotidine groups based on the block randomization method, no patient missed the follow-up during the study. Overall, 80 patients completed the intervention, and their data were fully analyzed (Figure 1).
Inclusion criteria
Patients with a body mass index (BMI) of 20 to 35 and degenerative diseases requiring lumbar fusion surgery, including spondylolisthesis, lumbar spinal canal stenosis, spinal deformity, lumbar disc disease, and age between 30-65 years were included in the study.
Exclusion criteria
The exclusion criteria were as follows: 1) Spine injury due to infection, tumor, inflammatory diseases, and trauma, 2) Diabetes, 3) Heavy smokers, 4) History of severe osteoporosis, 5) Not consenting to participation in the study. Participants receiving PPIs or famotidine six months before the study.
Pharmacological agents
The patients were divided into two groups through block randomization. One group received oral pantoprazole (group P) (n=40), and the other received famotidine (group F) (n=40), with each group receiving a daily dose of 40 mg. Both groups underwent an eight-week treatment following their lumbar fusion surgery. The prescription drugs, including painkillers and antibiotics, were identical for all patients. Autografts were used during the surgery to enhance fusion in all cases.
Upon admission, the primary outcome measures included the assessment of pain using the visual analogue scale (VAS) and disability using the Oswestry disability index (ODI). A comprehensive medical history was obtained from each patient, and all patients underwent a detailed physical and neurological examination.
The primary endpoint for measuring bone-related outcomes included subsidence, loosening, and the Brantigan, Steffee, Fraser (BSF), and Lenke scores, which were determined based on spinal CT scans [22].
BSF as the classification of interbody fusion success
BSF-1: Radiographic pseudarthrosis is defined as the collapse of the construct, loss of disc height, vertebral slip, broken screws, displacement of the carbon cage, or significant resorption of the bone. Also, graft or lucency is visible around the periphery of the graft or cage.
BSF-2: Radiographical locked pseudarthrosis is defined as lucency visible in the middle of the cages with solid bone growing into the cage from each vertebral endplate.
BSF-3: Radiographic fusion is defined as bone bridges at least half of the fusion area with at least the density originally achieved at surgery. Radiographic fusion through one cage (half of the fusion area) indicates a mechanically solid fusion even if there is lucency on the opposite side.
Lenke’s classification of posterolateral fusion success
Grade A: Definitely solid with bilateral trabeculated stout fusion masses; grade B: Possibly solid with a unilateral large fusion mass and a contralateral small fusion mass; grade C: Probably not solid with a small fusion mass bilaterally; grade D: Definitely not solid with bone graft resorption or obvious pseudarthrosis
bilaterally.
Statistical analysis
The Shapiro-Wilk test was utilized to assess the normal distribution of the data. Descriptive statistics for both groups (pantoprazole and famotidine) were compared and presented as percentages, medians (interquartile range) and Mean±SD. The collected data were then subjected to a comparative analysis between the two groups, utilizing statistical tests such as the chi-square or Fisher’s exact tests for categorical data and the t-test or Mann-Whitney U-test for continuous data. Furthermore, repeated-measures regression analysis, specifically the generalized estimated equation (GEE), was employed to evaluate the trend of changes in the outcome parameters between the two treatment modalities. A significance level of P<0.05 was considered statistically significant. Statistical analyses were conducted using IBM SPSS software, version 24.
3. Results
Eighty patients were included in this study based on specific inclusion and exclusion criteria. Among them, 40 received pantoprazole (group P), and 40 received famotidine (group F). The Mean±SD age was 47.4±10.54 years in group P and 47.7±10.37 years in group F, with no significant age difference between the two groups (P=0.99).
In group P, 62.5% of the participants were female, and 37.5% were male. Similarly, in group F, 62.5% were female, and 37.5% were male, with no significant gender difference between the two groups (P>0.99).
The Mean±SD BMI was 28.47±2.94 in group P and 27.9±3.29 in Group F, respectively. There was no significant difference in BMI between the two groups (P=0.42). The surgical indications, including spondylolisthesis, lumbar spinal canal stenosis, and herniated disc, were the most common in both groups, and there was no significant difference in surgical indications between the two groups (P=0.55).
Table 1 provides detailed information on the basic clinical characteristics and demographic features of the patients in both groups.

The observed differences in demographic characteristics, such as age (P=0.9), BMI (P=0.42), and the primary disease requiring surgery (P=0.55), were not statistically significant.
VAS score: Table 2 presents the mean VAS scores in the two groups.

This score decreased significantly after six and twelve months with both therapies. Twelve months after the therapies, the mean percentage of reduction in VAS score from baseline was 66.93% in group P and 76.48% in group F. The GEE used for analysis of the influence of the treatments after adjusting for the VAS score at baseline and age showed a difference in the mean VAS scores between the two groups (a significant reduction was perceived in the VAS score of group F in comparison with group P; P=0.0.008).
ODI score: Table 2 presents the mean ODI scores in both groups. The mean ODI score increased significantly after six and twelve months with both therapies. Twelve months after the therapies, the mean percentage of improvement in ODI scores from baseline was 383.75% in group P and 360.52% in group F. The GEE used for testing the effect of the therapies after adjusting for the ODI score at baseline and age revealed no difference in the mean ODI scores between the two groups (no significant improvement was observed in the ODI score of group F in comparison with group P; P=0.31).
Subsidence: Table 3 presents the prevalence of positive subsidence scores in both groups.

The prevalence of positive subsidence scores increased significantly after six and twelve months in both groups. After 12 months, the increase in the prevalence of positive subsidence size from baseline was 30% in group P and 20% in group F. The difference observed between the two groups was not statistically significant (P=0.43).
Loosening: The prevalence of positive loosening scores increased significantly after six and twelve months with both therapies, but this increasing trend was significant only in group P (P=0.005). After 12 months of treatment, the mean proportion of improvement in the LOOS scores from baseline was 15% in group P and 7.5% in group F. The GEE used for analysis of the effect of the therapies after adjusting for the LOOS score at baseline and age revealed no difference in the mean LOOS scores between the two groups (no significant improvement was observed in the LOOS score in group P in comparison with group F; P=0.13; Table 3).
BSF score: Table 4 presents the prevalence of the BSF grades in the two groups.

The prevalence of low-grade BSF decreased significantly after six and twelve months with both therapies. After 12 months of therapy, the mean percentage of reduction in BSF grade from baseline was 77.5% in both groups. The GEE used for testing the effect of the therapies after adjusting for the BSF score at baseline and age showed no difference in the mean BSF scores between the two groups (no significant reduction was detected in the BSF score of group P in comparison with group F; P=0.77 Figure 2). Lenke score: Table 4 presents the prevalence of the Lenke grades in both groups. The prevalence of low-grade Lenke decreased significantly after six and twelve months with both therapies. After twelve months, 52.5% of the patients in group P and 67.5% in group F were classified in grade A of the Lenke classification. The GEE used to measure the effect of the treatments after adjusting for the Lenke grade at baseline and time showed a significant difference between the two treatments. The frequency of Lenke grade C and D showed a decrease in new bone formation and fusion that was higher in group P (35% and 25%, respectively) compared to group F (12.5% and 12.5%, respectively) (P=0.042; Figure 3). Both drugs were well tolerated at the dosage used during the study. There were no thoughtful adverse effects, and both seemed quite safe. No patient mandatory changed the dosage throughout the trial for even a minor adverse effect. There were no significant changes in laboratory standards during the study or in any of the safety parameters.
4. Discussion
Prior to planning spinal fusion surgery, spinal surgeons meticulously optimize all preoperative, intraoperative, and postoperative conditions to maximize the likelihood of achieving a successful fusion rate. Significant advancements have also been made in surgical techniques, precision instruments, and biological supplements that contribute to the prospects of a successful fusion. Despite these efforts, pseudoarthrosis still occurs in 25% of patients, necessitating reoperation in some cases, with figures as high as 50% reported [23-25].
Non-:union: and pseudoarthrosis are complex complications with multifactorial origins, necessitating the identification of their risk factors. PPIs play a role in inhibiting the absorption of calcium and vitamin B12, resulting in reduced bone mineral density and an increased risk of fractures [25-27].
In a rat femur fracture model, Histing et al. [17] demonstrated bone non-:union: in the treatment of fractures. They attributed this finding to a decrease in bone regeneration, characterized by an increase in the OPG/RANKL ratio and a decrease in the expression of CYR61, PCNA, BMP-2, and BMP-4.
Numerous mechanisms are involved in the increased risk of pseudoarthrosis due to smoking, including the effects of nicotine and dioxin [28, 29] as they prevent osteoblast differentiation. Therefore, it is crucial to investigate other substances that undesirably disturb osteoblast differentiation. The belongings of dioxin are facilitated by the aryl hydrocarbon receptor (AHR) [28, 29].
PPIs are not only conjectured to adversely distress osteoblast differentiation but they are also identified as ligands activating AHR [29, 30]. This association proves the theory that PPIs and H2RA have an adverse effect on spinal fusion. To date, no previous human studies have evaluated the effect of PPI and H2RA administration on spinal fusion.
Assuming the global use of antacids and the substantial reduction in quality of life associated with fractures [31], studying the association between antacids and fractures is of great clinical importance. Although previous studies and FDA reports have shown an association between PPIs and fracture risk, this concern is still discussed [32].
Some studies have recently been issued on this subject, although with contradictory outcomes. A case-control study completed ten years and established that PPI use increases the risk of pelvic fractures, particularly in the long term and with recent usage [33]. An additional multicenter cohort study with 9423 members showed that there was a low risk of fractures in PPI customers [34]. Nonetheless, a case-control study conducted in the Mediterranean area stated no link between these variables [35].
In a study on animal models, 38 female rats experienced posterolateral lumbar spinal fusion. These animals were allocated into two groups: Pantoprazole-receiving cases and Normal saline controls managed daily via intraperitoneal injections. At eight weeks after surgery, the spines were appraised with histologic analysis, micro CT, and biomechanical testing. The study confirmed that the use of PPIs does not stop fusion rates and bone formation or disturb the biomechanical integrity of fusion; however, the lesser fusion scores in the PPI group recommend that an undesirable effect might exist [18].
The mechanism of the relationship between acid-suppressing medications and the danger of fracture is largely unknown [36]. PPIs are associated with impaired calcium absorption and loss of bone minerals, thus increasing the risk of fractures [37]. Nevertheless, studies evaluating the relationship between acid inhibition and bone mineral density have shown diverse results. Some studies have reported a slight decrease in bone density in PPI users; however, there have been no important differences in bone mineral density points between PPI consumers and controls in other studies [38].
Likewise, acid suppressants have been described to disturb bone modeling, which is involved in fracture progression [39]. Omeprazole has been stated to inhibit calcium absorption and decrease bone mineral density in rat models. Calcium malabsorption similarly increases parathyroidism, thus reducing bone mineral density [40-43]. Nonetheless, there is still no definite indication of the sustenance of these conceivable mechanisms.
Based on the findings of this study, a significant relationship was observed only between drug administration and VAS at 3, 6 and 12 months after fusion surgery (P<0.05) and the patients receiving famotidine had a lower VAS score compared to the group receiving pantoprazole. Taking pantoprazole could have a negative effect on the severity of postoperative pain. The results also showed the interaction of time and medication and the interaction of age and baseline VAS on patients’ VAS scores. Thus, by controlling the effect of time and baseline VAS, this effect may not be solely attributable to the drug.
There was no significant difference between the two groups in new ossification and fusion quality measures; however, according to the results, there was a significant difference in changes in Lenke grade 3, 6 and 12 months after surgery. New bone formation in the pantoprazole-receiving group was impaired significantly, although this issue did not cause many clinical referrals due to the severity of complications after surgery. Moreover, BSF grading is specific for interbody fusion and cannot be generalized to patients undergoing posterolateral fusion surgery alone.
The administration of a standard dose of intervention drugs was one of the limitations of this study. Due to the limited human studies on this subject and similar studies with animal models, the dose received in patients may be lower than the standard dose, which is probable to have a destructive consequence on fusion and new ossification. In addition, we recommend that a control group be added to future studies to design a detailed study without further debate.
5. Conclusion
In agreement with the initial findings of the study, it appears that in candidates for spinal fusion procedures who require chronic use of drugs to control their gastric acid secretion, the use of PPIs, such as pantoprazole has more detrimental effects on the bone formation process, and H2-blocking drugs, such as famotidine are preferable in these instances after further investigations. The researchers recommend conducting a study with a larger sample size and an extended follow-up.
Ethical Considerations
Compliance with ethical guidelines
This study was approved and registered by the Research Ethics Committee of Imam Khomeini Hospital (Code: IR.MAZUMS.IMAMHOSPITAL.REC.1399.039). The project was registered as a clinical trial with the Iranian Registry of Clinical Trials (IRCT) (CODE: IRCT20140915019185N4), and the study design was based on the CONSORT 2010 checklist items. Each patient’s legally authorized representative signed an informed consent form, and the researchers also obtained consent from the patient’s primary caregivers before starting medication therapy.
Funding
This study was extracted from medical dissertation of Navid Tahmasebi, approved by Orthopedic Research Center of Imam Khomeini Hospital, Mazandaran University of Medical Sciences and was financially supported by Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization and study design: Kaveh Haddadi, Abdolrasool Alaee and Abbas Alipour; Data collection and writhing the original draft: Kaveh Haddadi, Saeed Ehteshami and Navid Tahmasbi; Statistical analysis: Abbas Alipour; Final approval: Kaveh Haddadi and Abbas Alipour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express their gratitude to all the patients who participated in the project.
References
- Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine. 2012; 37(1):67-76. [DOI:10.1097/BRS.0b013e31820cccfb] [PMID]
- Cannada LK, Scherping SC, Yoo JU, Jones PK, Emery SE. Pseudoarthrosis of the cervical spine: A comparison of radiographic diagnostic measures. Spine. 2003; 28(1):46-51. [DOI:10.1097/00007632-200301010-00012] [PMID]
- Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004; 29(7):726-33. [DOI:10.1097/01.BRS.0000119398.22620.92] [PMID]
- Makino T, Kaito T, Fujiwara H, Honda H, Sakai Y, Takenaka S, et al. Risk factors for poor patient-reported quality of life outcomes after posterior lumbar interbody fusion: An analysis of 2-year follow-up. Spine. 2017; 42(19):1502-10. [DOI:10.1097/BRS.0000000000002137] [PMID]
- Makino T, Kaito T, Fujiwara H, Ishii T, Iwasaki M, Yoshikawa H, et al. Does fusion status after posterior lumbar interbody fusion affect patient-based QOL outcomes? An evaluation performed using a patient-based outcome measure. Journal of Orthopaedic Science 2014; 19(5):707-12. [DOI:10.1007/s00776-014-0591-6] [PMID]
- Phillips FM, Carlson G, Emery SE, Bohlman HH. Anterior cervical pseudarthrosis. Natural history and treatment. Spine. 1997; 22(14):1585-9. [DOI:10.1097/00007632-199707150-00012] [PMID]
- Ferron GM, McKeand W, Mayer PR. Pharmacodynamic modeling of pantoprazole's irreversible effect on gastric acid secretion in humans and rats. Journal of Clinical Pharmacology. 2001; 41(2):149-56. [DOI:10.1177/00912700122009953] [PMID]
- Molloy D, Molloy A, O'Loughlin C, Falconer M, Hennessy M. Inappropriate use of proton pump inhibitors. Irish Journal of Medical Science. 2010; 179(1):73-5. [DOI:10.1007/s11845-009-0426-1] [PMID]
- Lindberg P, Nordberg P, Alminger T, Brändström A, Wallmark B. The mechanism of action of the gastric acid secretion inhibitor omeprazole. Journal of Medicinal Chemistry. 1986; 29(8):1327-9. [DOI:10.1021/jm00158a001] [PMID]
- Yamamoto K, Hojo H, Koshima I, Chung UI, Ohba S. Famotidine suppresses osteogenic differentiation of tendon cells in vitro and pathological calcification of tendon in vivo. Journal of Orthopaedic Research. 2012; 30(12):1958-62. [DOI:10.1002/jor.22146] [PMID]
- Hálfdánarson ÓÖ, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E, et al. Proton-pump inhibitors among adults: A nationwide drug-utilization study. Therapeutic Advances in Gastroenterology. 2018; 11:1756284818777943. [DOI:10.1177/1756284818777943] [PMID] [PMCID]
- O'Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. The American Journal of Medicine. 2005; 118(7):778-81. [DOI:10.1016/j.amjmed.2005.02.007] [PMID]
- Wilhelm SM, Kale-Pradhan PB. Effects of proton pump inhibitors on vitamin B12. Maturitas. 2014; 79(1):1-2. [DOI:10.1016/j.maturitas.2014.06.005] [PMID]
- Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcified Tissue International. 1993; 53(1):21-5. [DOI:10.1007/BF01352010] [PMID]
- Visentin L, Dodds RA, Valente M, Misiano P, Bradbeer JN, Oneta S, et al. A selective inhibitor of the osteoclastic V-H(+)-ATPase prevents bone loss in both thyroparathyroidectomized and ovariectomized rats. The Journal of Clinical Investigation. 2000; 106(2):309-18. [DOI:10.1172/JCI6145] [PMID] [PMCID]
- Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: Hypomagnesaemia induced by proton pump inhibition. Alimentary Pharmacology & Therapeutics. 2012; 36(5):405-13. [DOI:10.1111/j.1365-2036.2012.05201.x] [PMID]
- Histing T, Stenger D, Scheuer C, Metzger W, Garcia P, Holstein JH, et al. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcified Tissue International. 2012; 90(6):507-14. [DOI:10.1007/s00223-012-9601-x] [PMID]
- Sonn KA, Wallace SJ, Yuan FNF, Schneider AD, Hsu EL, Havey RM, et al. The effect of proton pump inhibitors on bone formation in a rat spinal arthrodesis model. Spine. 2019; 44(14):E815-22. [DOI:10.1097/BRS.0000000000002987] [PMID]
- Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007; 32(3):382-7. [DOI:10.1097/01.brs.0000254104.55716.46] [PMID]
- Brown CW, Orme TJ, Richardson HD. The rate of pseudarthrosis (surgical non:union:) in patients who are smokers and patients who are nonsmokers: A comparison study. Spine. 1986; 11(9):942-3. [DOI:10.1097/00007632-198611000-00015] [PMID]
- Bydon M, De la Garza-Ramos R, Abt NB, Gokaslan ZL, Wolinsky JP, Sciubba DM, et al. Impact of smoking on complication and pseudarthrosis rates after single- and 2-level posterolateral fusion of the lumbar spine. Spine. 2014; 39(21):1765-70. [DOI:10.1097/BRS.0000000000000527] [PMID]
- Hilibrand AS, Fye MA, Emery SE, Palumbo MA, Bohlman HH. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. The Journal of Bone and Joint Surgery. American Volume. 2001; 83(5):668-73. [DOI:10.2106/00004623-200105000-00004] [PMID]
- Lee KB, Taghavi CE, Hsu MS, Song KJ, Yoo JH, Keorochana G, et al. The efficacy of rhBMP-2 versus autograft for posterolateral lumbar spine fusion in elderly patients. European Spine Journal. 2010; 19(6):924-30. [DOI:10.1007/s00586-009-1248-6] [PMID] [PMCID]
- Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. The Spine Journal. 2008; 8(4):570-7. [DOI:10.1016/j.spinee.2007.03.013] [PMID]
- Bagheri SR, Alimohammadi E, Zamani Froushani A, Abdi A. Adjacent segment disease after posterior lumbar instrumentation surgery for degenerative disease: Incidence and risk factors. Journal of Orthopaedic Surgery. 2019; 27(2):2309499019842378. [DOI:10.1177/2309499019842378] [PMID]
- Shen HX, Buchowski JM, Yeom JS, Liu G, Lin N, Riew KD. Pseudarthrosis in multilevel anterior cervical fusion with rhBMP-2 and allograft: Analysis of one hundred twenty-seven cases with minimum two-year follow-up. Spine. 2010; 35(7):747-53. [DOI:10.1097/BRS.0b013e3181bc3420] [PMID]
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-53. [DOI:10.1001/jama.296.24.2947] [PMID]
- Yu EW, Blackwell T, Ensrud KE, Hillier TA, Lane NE, Orwoll E, Bauer DC. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcified Tissue International. 2008; 83(4):251-9. [DOI:10.1007/s00223-008-9170-1] [PMID] [PMCID]
- Wang H, Ma L, Yang D, Wang T, Yang S, Wang Y, et al. Incidence and risk factors for the progression of proximal junctional kyphosis in degenerative lumbar scoliosis following long instrumented posterior spinal fusion. Medicine. 2016; 95(32). [DOI:10.1097/MD.0000000000004443] [PMID]
- Hsu EL, Sonn K, Kannan A, Bellary S, Yun C, Hashmi S, et al. Dioxin exposure impairs BMP-2-mediated spinal fusion in a rat arthrodesis model. The Journal of Bone and Joint Surgery. American Volume. 2015; 97(12):1003-10. [DOI:10.2106/JBJS.N.01311] [PMID]
- Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. Journal of Bone and Mineral Metabolism. 2009; 27(5):555-61. [DOI:10.1007/s00774-009-0075-5] [PMID]
- Pombo M, Lamé MW, Walker NJ, Huynh DH, Tablin F. TCDD and omeprazole prime platelets through the aryl hydrocarbon receptor (AhR) non-genomic pathway. Toxicology Letters. 2015; 235(1):28-36. [DOI:10.1016/j.toxlet.2015.03.005] [PMID]
- Shen C, Chen F, Zhang Y, Guo Y, Ding M. Association between use of antiepileptic drugs and fracture risk: A systematic review and meta-analysis. Bone. 2014; 64:246-53. [DOI:10.1016/j.bone.2014.04.018] [PMID]
- FDA drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. [Internet]. 2011 [Updated 2017 March 8]. Available from: [Link]
- Adams AL, Black MH, Zhang JL, Shi JM, Jacobsen SJ. Proton-pump inhibitor use and hip fractures in men: A population-based case-control study. Annals of Epidemiology. 2014; 24(4):286-90. [DOI:10.1016/j.annepidem.2014.01.004] [PMID]
- Fraser LA, Leslie WD, Targownik LE, Papaioannou A, Adachi JD; CaMos Research Group. The effect of proton pump inhibitors on fracture risk: Report from the Canadian multicenter osteoporosis study. Osteoporosis International. 2013; 24(4):1161-8. [DOI:10.1007/s00198-012-2112-9] [PMID] [PMCID]
- Reyes C, Formiga F, Coderch M, Hoyo J, Ferriz G, Casanovas J, et al. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone. 2013; 52(2):557-61. [DOI:10.1016/j.bone.2012.09.028] [PMID]
- Cea-Soriano L, Johansson S, García Rodríguez LA. Risk factors for falls with use of acid-suppressive drugs. Epidemiology. 2013; 24(4):600-7. [DOI:10.1097/EDE.0b013e318294bec6] [PMID]
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010; 139(1):93-101. [DOI:10.1053/j.gastro.2010.03.055] [PMID] [PMCID]
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010; 138(3):896-904. [DOI:10.1053/j.gastro.2009.11.014] [PMID]
- Novotna A, Srovnalova A, Svecarova M, et al. In: Makishima M, editor. Differential effects of omeprazole and lansoprazole enantiomers on aryl hydrocarbon receptor in human hepatocytes and cell lines. PLoS ONE. 2014;9:e98711. [DOI: 10.1097/MD.0000000000004443] [PMID]
- Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. The American Journal of Gastroenterology. 2009; 104(Suppl 2):S2-4. [DOI:10.1038/ajg.2009.44] [PMID]
- Yang YX. Proton pump inhibitor therapy and osteoporosis. Current Drug Safety. 2008; 3(3):204-9. [DOI:10.2174/157488608785699414] [PMID]
Type of Study: Clinical Trial |
Subject:
Spine
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |